ABSTRACT
The FcαR receptor (CD89) binds to the constant region of Immunoglobulin (Ig) A to mediate mucosal immunity [1–2]. FcαR consist of five exons: two that code for the signal peptide regions S1 & S2, two for the extracellular regions EC1 and EC2, and the final exon for the transmembrane/cytoplasmic tail region [3]. Previously, we reported that the EC1 region plays an essential role for extracellular membrane localization of the receptor [4], where the absence of EC1 would prevent the variants from localizing to the cell surface, even with a full signal peptide. In the case of FcαR Variant 4 (lacking the S2 region only), there was some “leakiness” to membrane surface localization.
KEYWORDS: FCAR, EC2, signal peptide
Carrying on our investigation after our previous publication [4], we investigated the role of EC2 in the extracellular membrane localization by studying two additional FcαR variants that lacked EC2: FcαR variants 3 (lacking only EC2 [3], Accession No; NM_133271.3) and 5 (lacking both S2 and EC2, Accession No; NM_133273.3). Following the same methodology [4], we transiently transfected variants 3 and 5, followed by confocal microscopy and fluorescent activated cell sorting (FACS) analysis. For confocal microscopy, HeLa cells (chosen for their non-overlapping monolayer characteristics) were seeded at 1 × 104 cell/well on glass coverslips. Cells were washed with cold PBS, fixed with 2% formaldehyde and mounted onto glass slides before staining with DAPI (Cat no: H-1500, Vector Laboratories). Similarly, FACS analyses were also performed using PE anti-FcαR/CD89 antibodies (Cat no: LS-C307768, Life Span Biosciences) on HEK293 EXPI cells (chosen for their high transfection rates). Our results showed that these FcαR splice variants (without EC2 region) were not able to be detected extracellularly (by FACS) even if they contained EC1 with or without a complete S1 + S2 signal peptide (Figure 1) when compared to the control variant 1. Thus, it is clear from the confocal microscopy and FACS analyses that both EC1 and EC2 are crucial for correct FcαR surface membrane localization. Like the EC1 region, EC2 also plays a significant role in extracellular membrane localization for FcαR. A deletion of EC2 in variants 3 and 5 gave similar findings to variants that were lacking EC1 in our previous study [4]. It is indeed surprising to find a role in membrane localization for both EC1 and EC2 domains, especially given the more distal location of EC2 from the signal peptide. Nonetheless, a recent study suggesting that the immunoglobulin (Ig) superfamily members closely resemble the CH1 domain of antibodies [5] may help explain our observations. Given that antibody CH1 domain is known to bind BiP and released only upon the light chain binding, it is possible that the Ig-like domains of EC1 and EC2 are both required for interactions in order to be released from the ER. We also observed both variants (3 and 5) to have less GFP+ve cells than those transfected with variant 1. While there are many factors for this from the nature of the transient tranfection efficiency of the plasmid to the required complete domains of FcαR for effective expression and secretion, there is the possibility of partial folding or lower protein stability. In conclusion, FcαR is indeed an enigmatic protein that does not fall within the preconceived notion of the role of signal peptide motif at the N-terminus [6] in conventional protein secretory pathways. Beyond challenging the textbook knowledge of signal peptides, our FcαR findings suggest that proteins may need to be considered as a whole rather than merely a sum of regions/domains. While certain protein regions may have the dominant responsibility for specific functions, FcαR clearly showed inter-region effects, where protein localization can go beyond single domains.
Figure 1.
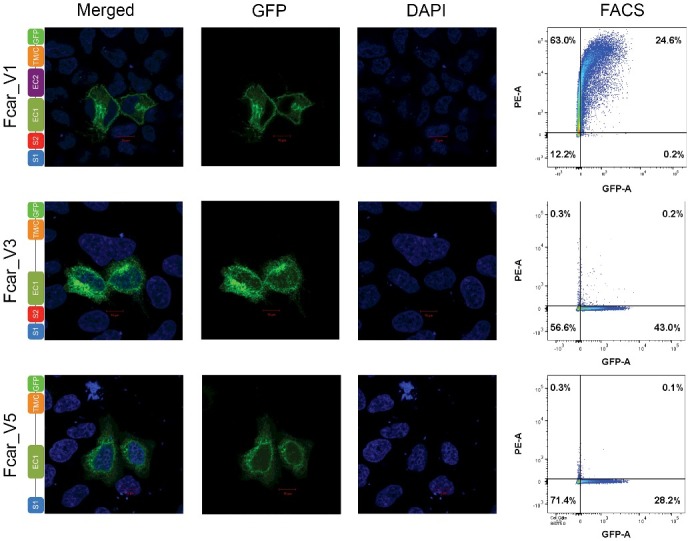
Confocal microscopy (first three columns) and FACS analysis (fourth column) of FcαR variants 1, 3 and 5. HeLa cells were transfected with FcαR variants with C-terminal GFP tags. Variant 1 showed clear fusion protein localization on the membrane whereas variants 3 and 5 showed predominant intracellular localizations. FACS plots of PE-signal on the Y-axis and GFP-signal on the X-axis of HEK293 EXPI cells transfected with GFP fused FcαR variants showed detection of FcαR on extracellular membrane by anti-FcαR-PE antibody only for variant 1.
Funding Statement
This work was supported by the JCO1334i00050 grant from Joint Council Office, Agency for Science, Technology, and Research (A*STAR) in Singapore.
Disclosure of potential conflict of interest
No potential conflict of interest were disclosed.
References
- [1].Maliszewski CR, VandenBos T, Shen L, et al. . Recombinant soluble IgA Fc receptor: generation, biochemical characterization, and functional analysis of the recombinant protein. J Leukoc Biol. 1993;53:223–232. doi: 10.1002/jlb.53.3.223. PMID:8454945 [DOI] [PubMed] [Google Scholar]
- [2].Herr AB, Maliszewski CR, White CL, et al. . Bivalent binding of IgA1 to FcalphaRI suggests a mechanism for cytokine activation of IgA phagocytosis. J Mol Biol. 2003;327:645–657. doi: 10.1016/S0022-2836(03)00149-9. PMID:12634059 [DOI] [PubMed] [Google Scholar]
- [3].Reterink TJF, Verweij CL, van Es LA, et al. . Alternative splicing of IgA Fc receptor (CD89) transcripts. Gene. 1996;175:279–280. doi: 10.1016/0378-1119(96)00152-7. PMID:8917112 [DOI] [PubMed] [Google Scholar]
- [4].Lua WH, Ling WL, Su CT, et al. . Discovery of a novel splice variant of Fcar (CD89) unravels sequence segments necessary for efficient secretion: a story of bad signal peptides and good ones that nevertheless do not make it. Cell Cycle. 2017;16:457–467. doi: 10.1080/15384101.2017.1281480. PMID:28103138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hebditch M, Curtis R, Warwicker J. Sequence composition predicts immunoglobulin superfamily members that could share the intrinsically disordered properties of antibody CH1 domains. Sci Rep. 2017;7:12404. doi: 10.1038/s41598-017-12616-9. PMID:28963509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Blobel G, Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975;67:835–851. doi: 10.1083/jcb.67.3.835. PMID:811671 [DOI] [PMC free article] [PubMed] [Google Scholar]


