Abstract
Sweetened oral medications are widely used for children to facilitate compliance. A variety of natural and artificial sweeteners are used in these drug formulations to augment the sweetness and thereby palatability of the product. There is growing concern among dentists about the increased consumption of sugars in these medications by children, especially those who are chronically ill as it may contribute to diabetes mellitus, dental erosion, and dental caries. This literature review provides information about the sweetener content and cariogenic potential of commonly prescribed pediatric oral medications that are used for managing acute and chronic conditions in children and measures for oral health prevention.
Keywords: Dental caries, oral health, pediatric oral medications, sweetened medications
Introduction
Dental caries is caused by cariogenic bacteria that are found in dental plaque biofilm adhering to the tooth surface.[1] High-carbohydrate diet, along with a high frequency and prolonged duration of intake of sugary foods and drinks, provides the optimal conditions for the initiation of the caries process. Harmful effects occur when sucrose is metabolized by oral bacteria, particularly Streptococcus mutans, into weak organic acids.[2] These acids cause pH to fall below a critical value (5.5) resulting in demineralization of enamel and disease progression to dental caries.[2,3] During the past decades, the literature has documented a relationship between sugar-containing medications and dental caries. This relationship has been established particularly in chronically ill children who need medications for long periods of time.[4,5]
The aim of this review is to summarize the data related to sweetener content in pediatric oral medications, to identify drugs with cariogenic potential, as well as to identify common sweeteners used in liquid, chewable, and oral disintegrating medications. This review provides information intended to increase the awareness among health-care professionals and parents about the sweetener content in medications so that informed choices and personalized caries preventive protocols can be formulated for children taking long-term sweetened medications.
Material and Methods
A literature review was performed after conducting an electronic search through Medline (from 1966 to January 2017) and the Cochrane Library to identify studies relevant to the sweetener content and cariogenic potential of pediatric oral medications. The following key words were used “pediatric oral medications,” “sweetened medications,” and “dental caries”. Out of the total 2453 articles retrieved 30 articles were found to be studying the potential cariogenicity of pediatric oral medications. The search result was further narrowed down to include reviews, in vitro studies, randomized clinical trials, case–control, and cohort studies resulting in 11 studies relevant to the research question.
Pediatric medications
Pediatric patients present challenges in terms of medication compliance and therapeutic efficacy.[6] In children, drug delivery can be difficult, and the adequate method of administration is usually age-dependent. In neonates, suppositories are preferred while liquid formulations such as syrups are preferred for infants. In the 2–5 years age group oral solutions, syrups, suspensions, or effervescent dosage forms are preferred, while disintegrating tablets, chewable tablets, or thin strips are used in the ages 6–11 years. Adolescents are prescribed tablets, capsules, powders, oral disintegrating tablets (ODTs), chewable tablet, or thin strips.[4] Although most children over 6 years of age can tolerate solid forms of medication, many remain uncomfortable with this delivery system until adolescence. Among the different dosage forms, liquid preparations are popular, easily accepted by both parents and children,[5] and preferred for oral administration in infants, and children under 5 years of age.[3,6]
Solutions are preparations in which the drug substance is completely dissolved predominantly in an aqueous vehicle.[7] Solutions are of many types, simple formulations based on the constituting solvent and buffer, flavor, or preservative, and complex formulations comprising multiple solvents, solubilizing excipients, buffers, sweeteners, flavor, preservatives, and dyes.[4] Syrup is a solution that uses sucrose solution as a vehicle, resulting in a viscous preparation. Most syrups contain 60–80% sucrose, and little or no alcohol. However, other agents may substitute sucrose-based syrup in whole or in part. Solution of polyols, such as sorbitol, or a mixture of polyols, such as sorbitol and glycerin, are commonly used.[7] An elixir is a clear, sweetened, hydroalcoholic solution and because it contains a lower proportion of sugar compared to syrups, it is less viscous and less effective in masking the taste of medicinal substances. Although many elixirs are sweetened with sucrose, some use sorbitol, glycerin, and/or artificial sweeteners. Artificial sweeteners, such as saccharin, are usually incorporated in elixirs having a high alcoholic content, while sucrose is only slightly soluble in alcohol and requires greater quantities for equivalent sweetness. A disadvantage of elixirs, especially in children, is their alcohol content.[7] An oral suspension is a preparation containing finely divided, undissolved drug particles. Some suspensions are available in ready-to-use forms, while others are available as dry powders intended for suspension in liquid vehicles.[7] The solid added to a suspension is manufactured and packaged for long-term storage and transportation.[4] A chewable tablet is a smooth, rapidly dissolving when chewed or allowed to melt in the mouth. It has a creamy base,[7] usually sweetened with mannitol due to its sweetness, and soothing feel.[4] Chewable tablets are especially useful for administration in large size for children and adults who find it difficult to swallow the solid dosage forms.[6,7] An ODT is designed for patients having difficulties with swallowing whole tablets as the ODT dissolves in the presence of saliva within 1 min,[4,8] without the need for water.[4,9] This feature makes ODTs ideal for patients with dysphagia or children too young to swallow tablets or capsules.[7]
Sweeteners in pediatric medications
The role of sweeteners in pediatric medications is generally related to compliance.[6] Medication compliance in pediatric patients ranges from 11% to 93%[10] and oral medications with poor palatability may lead to non-compliance which may have a direct influence on the success and efficacy of the treatment. To overcome the palatability issues, drug manufacturers add sweeteners and flavoring agents to mask the original taste and smell of their formulations.[4,11]
Sweeteners can divided into two major groups: Natural sweeteners and artificial sweeteners as listed in Table 1. Natural sweeteners contain carbohydrate and provide energy.[12] Natural sweeteners include monosaccharide carbohydrates (glucose and fructose), disaccharide carbohydrates (sucrose and lactose), and polyols carbohydrates also known as sugar alcohols (sorbitol, xylitol, mannitol, lactitol, and maltitol).[13]
Table 1.
List of sweetening agents with GRAS listed and/or in the FDA list of inactive ingredients for approved drug products
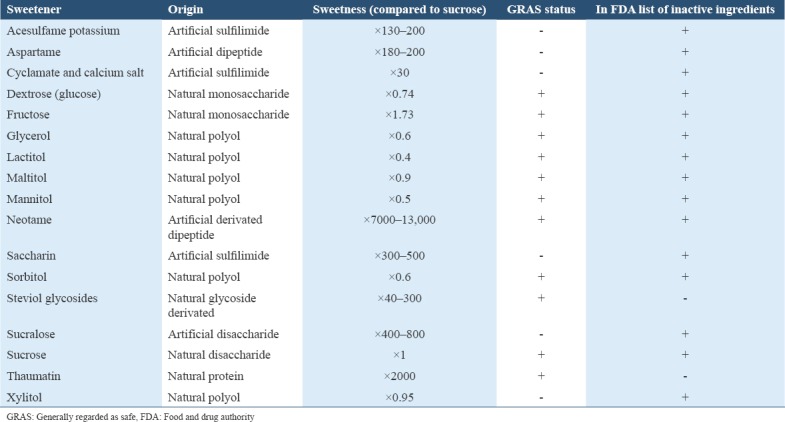
Sucrose is a disaccharide carbohydrate comprising glucose and fructose which is found naturally in fruits and vegetables.[12,13] Commercial sucrose is obtained by processing sugar cane or sugar beets.[14] Low cost and properties such as: Being a preservative, an antioxidant, a solvent, and a thickening agent,[15,16] make sucrose the most used sweetener in pediatric pharmaceutical preparations,[11] it also has been reported to be the most cariogenic sugar.[17] However, the carcinogenicity of sugars such as glucose, fructose,[18] and maltose has been reported to be very similar.[18-20] The difference in the ability of bacteria to utilize glucose, sucrose, and fructose in metabolism and consequently, produce acid is minimal.[21]
Fructose is a monosaccharide carbohydrate found naturally in fruit, honey, some vegetables, and table sugar.[12] Agave is the richest natural source of fructose, with 85% of carbohydrate as dietary fructose followed by honey with approximately 50%.[22] Limited evidence suggests that fructose may be slightly less rapidly fermented to acid than glucose. However, the difference may not be sufficient to decrease its cariogenicity and consider it as an adequate sugar alternative.[23] The sweetness response profile shows that fructose is perceived more rapidly in the mouth than sucrose and dextrose, resulting in enhancing the flavor of the syrup and fruit tablets. Hence, powdered fructose is used to coat the surface of chewable tablets, lozenges, or medical gums.[24]
Dextrose occurs in plants and is manufactured on a large scale by the acid or enzymatic hydrolysis of starch. Furthermore, dextrose is often used in solutions to adjust viscosity and as a sweetening agent.[24]
Lactose is a disaccharide formed by galactose and glucose. It is approximately 20%-40% as sweet as sucrose and is present in milk.[24,25] Lactose is used in infant formulas. It is also present in pharmaceutical preparations as filler and diluents in tablets and capsules, and in lyophilized powders, as a sweetener.[11] Since lactose elicits a smaller drop in plaque pH than glucose and fructose,[23] its cariogenicity has been reported to be less than sucrose.[18,25]
Many polyols or sugar alcohols occur naturally in plants, and they are also produced for commercial use.[14] Polyols can be divided into three types based on their chemical structure; monosaccharide-derived (e.g., sorbitol, xylitol, and erythritol); disaccharide-derived (e.g., isomalt, lactitol, and maltitol), or polysaccharide-derived mixtures (e.g., maltitol syrup). Sugar alcohols sweeten with less energy per gram (averaging 2 kcal/g), and are not fully absorbed from the gastrointestinal tract and hence less available for energy metabolism.[12,14] In general, polyols are weakly fermented by most oral bacteria,[26,27] and therefore, are considered non-cariogenic.[28] Polyols such as sorbitol and xylitol produce only a minimal plaque pH drop and are not efficient substrates for plaque bacteria, therefore, are good alternatives for sugar substitution[6,23] Polyols are metabolized by bacteria at a much slower rate than glucose or sucrose or not at all.[21] They have minimal cariogenicity compared with sugars. Park et al.[29] found that compared with sucrose or fructose, polyols resulted in significantly less acid formation in interproximal plaque than fermentable carbohydrates. Finally, when taken in excess, polyols can produce adverse effects, such as abdominal discomfort, flatulence, softened stools, and diarrhea.[14]
Glycerin (glycerol) is a clear, syrupy liquid with a sweet taste.[7] It is often used as a stabilizer and as an auxiliary solvent in conjunction with water or alcohol due to its preservative qualities.[7,30]
Maltitol is a crystalline polyhydric alcohol with 95% of the sweetness of sucrose. It is formed by the hydrolysis of starch followed by reduction of liquefied starch.[31] Its cariogenic potential is as low as that of sorbitol and mannitol.[6] Maltitol solution is used as a bulk sweetening agent, either alone or in combination with other excipients, such as sorbitol in oral formulations.[24]
Mannitol has 50–70% of the sweetness of sucrose. In some individuals, mannitol causes a laxative effect if ≥20 g of it is ingested.[12] Mannitol possesses negative heat of solution, sweetness, and mouth feel, hence it is commonly used in the production of chewable tablet formulations.[4]
Lactitol is 30–40% as sweet as sucrose.[12] It has low cariogenic potential, and is considered by bacteriological studies to be less cariogenic than mannitol and sorbitol due to lower acid and polysaccharide formation by oral microorganism.[32]
Sorbitol is the most frequently added sugar alcohol to foods, and its sweetness is about 60–70% that of sucrose.[33] Sorbitol occurs naturally in many fruits,[34] and its intake has been associated with gastrointestinal issues such as diarrhea and malabsorption.[35] Sorbitol is considered non-cariogenic in nature, although acid formation in the bacterial plaque can occur during metabolism by an oral microorganism, it is very slow.[28,34,36-38] Sorbitol is particularly useful in chewable tablets due to its pleasant sweet taste and cooling sensation. It is used as a vehicle in sugar-free formulations in liquid preparations.[24]
Xylitol is cariostatic and has anticariogenic properties which help in the prevention of dental caries.[32,36,39,40] It is sweet as sucrose and is found in fruits, such as plums and berries and in vegetables.[12] None of the predominant bacteria of dental plaque produces acid from xylitol, and its presence reduces acid production from glucose in dental plaque in vivo.[41] Xylitol is an effective flavor enhancer of tablets and syrups and has the property of masking unpleasant taste of some active ingredients in pharmaceutical formulations.[24]
Artificial sweeteners include saccharin, cyclamate, aspartame,[42] and sucralose.[30] They are referred to as high-intensity sweeteners because they sweeten with little volume however these artificial or nonnutritive sweeteners offer no energy (or insignificant energy in the case of aspartame).[14] The intense sweeteners are considered to be non-cariogenic because, unlike fermentable carbohydrates, they cannot act as an energy source for dental plaque microorganism, and microbial acid and polysaccharides cannot be derived from them.[43]
Aspartame is a dipeptide of aspartic acid and a methyl ester of phenylalanine.[36] It is 160-220 times sweeter than sucrose, and produces a limited glycemic response.[14] Due to the fact that aspartame is a source of phenylalanine,[44] the major consideration in the use of aspartame is in those children with autosomal recessive phenylketonuria.[45] Aspartame has been reported not to have cariogenic potential,[12,43,46,47] and is abundantly present as a sweetener in chewable tablets and liquid formulations.[11]
Although it has very different characteristics in comparison to non-nutritive sweeteners, high-fructose corn syrup (HFCS) is also considered to be an artificial sweetener.[48] HFCS is similar in sweetness to sucrose.[22] Partial enzymatic isomerization of glucose to fructose results in HFCS-42, containing 42% fructose, 53% glucose, and 5% higher saccharides; HFCS-55, which contains 55% fructose and 42% glucose; and HFCS-90, which contains 90% fructose and 10% glucose.[48] Both HFCS-42 and HFCS-55 have the same monosaccharide content similar to that of sucrose. However, the monosaccharides, fructose and glucose, exist free in solution in HFCS in contrast to sucrose.[49] There is limited literature on the cariogenicity of HFCS. Ma et al.[1] compared the acidogenicity, adherence, and biofilm properties of S. mutans in the presence of HFCS to those in the presence of sucrose. They concluded that the adherence ability of S. mutans in vitro in media containing HFCS was diminished compared to the biofilms formed in the presence of sucrose. However, S. mutans exhibited stronger acidogenicity grown in the presence of HFCS relative to that grown in sucrose.[1]
Saccharin is largely used in hypocaloric food products due to its sweetening power.[11] It is 200–500 times sweeter than sucrose and non-cariogenic and noncaloric.[14,30,43] Although the carcinogenic potential of saccharin has been reported in animal studies, its carcinogenic potential is unclear for humans.[14,43,50,51] Of importance, cross-sensitivity reactions in children with sulfonamide allergy have been reported with saccharin.[30,52]
Sucralose is chemically synthesized by the reaction of sucrose with thionyl chloride.[24] Sucralose is 600 times sweeter than sucrose, noncaloric; has no nutritional value,[36] and is non-cariogenic.[30]
Cariogenic potential of pediatric medications
Sometimes, children may be required to take medications on a daily basis and several times during the day for long periods of time. Such chronic conditions include asthma, epilepsy, immune deficiencies, attention deficit hyperactivity disorder (ADHD), cystic fibrosis, chronic renal failure, leukemia, cardiac conditions, and also, children with recurrent otitis media or upper respiratory tract infection.[53-55] Therefore, chronically ill children are exposed to a greater sugar load from oral medications than healthy children[56] leading to a greater risk of developing caries as a side effect of the treatment for their medical condition.[57,58]
The association between sugar-based syrups and dental caries has been reported, especially if medications are administered at bedtime where studies report a reduced protective buffering and cleansing effects of saliva due to a fall in the salivary flow rate.[59] According to Durward and Thou.[55] several factors such as high frequency consumption, bedtime consumption, low pH, dry mouth, and high viscosity make sugar-containing medicines, potentially harmful for children’s teeth. Moreover, bedtime consumption along with diminution in the production of saliva and lack of mastication movements increase the cariogenic potential of medicines.[60] In addition, prolonged oral clearance, determined by the high viscosity of some liquid medications and poor muscular control, can put children with neurological conditions, such as cerebral palsy, at risk for dental caries.[58] Gabre et al.[61] found a higher initial saliva glucose concentration and a longer clearance time in persons with oral motor dysfunctions compared to the controls, which will result in a more pronounced pH decrease. Furthermore, young children in comparison to older children and adults have slower salivary sugar clearance and larger variation in clearance among various foodstuffs.[62]
Few studies have reported a relationship between medications and the development of dental caries [Table 2].[56,57,63-70] Feigal et al.[64] compared the changes in pH produced after a 60-second rinse with seven different liquid medications commonly prescribed for long-term treatment to those pH changes produced by a rinse with a 10% sucrose solution. Sucrose content ranged from 0 to 70 g/10 ml for the seven medications. It was concluded that dental plaque pH was significantly lowered by all medications that contained high sucrose concentrations. The pH changes approached or surpassed those caused by sucrose. Sugars added to medicines can be fermented by oral bacteria leading to acid formation and a drop in intraoral pH.[5]
Table 2.
Overview of studies related to sweetener content and cariogenic potential of pediatric oral medications

Roberts and Roberts.[57] compared the indices for decayed (d), extracted (e), and filled (f) surfaces in 44 children aged 9 months to 6 years, who had chronic medical disorders, had been taking sucrose-containing syrup medicines regularly for at least 6 months, and had been attending hospital outpatient clinic for 6 months to a control group of 47 children of similar ages, who either received no medication or took medication in tablet form. The results showed an increased incidence of dental caries and gingivitis with chronic administration of liquid medicines sweetened with sucrose.[57]
Kenny and Somaya.[56] determined the history of oral liquid medication use and incidence of dental caries in a group of 20 chronically ill children from birth until approximately 36 months of age. Interviews with the parents revealed daily doses of syrup medications and elixirs 3–4 times a day and at least two of these doses were administered just before or during a designated nap or bedtime. It was concluded that frequent ingestion of sugar-containing medications contribute to the caries found in these children.[56]
Hill et al.[66] gathered data on the sweetener content of most commonly prescribed pediatric medications. Medications included antibiotics, antifungal agents, bronchodilators, antihistamines, antifungal agents, anticonvulsants, and other agents. The sweetener content of 150 liquid preparations was obtained. The results revealed that antibiotics contained a wide range of sucrose content (18–80%). Only four antibiotics preparations were sucrose-free. The anticonvulsant preparations, phenytoin and phenobarbital, had low concentrations of sucrose (20% and 13%, respectively). Cimetidine was sucrose-free but contained large amounts of sorbitol. The bronchodilators and antihistamines contained sucrose, sorbitol, and saccharin. The chewable tablet preparations contained sucrose, mannitol, or saccharin. Only Augmentin® tablets were sucrose-free. It was concluded that the sweetener content of common pediatric liquid and chewable medication varies widely.[66]
Nik-Hussein et al.[67] determined the sugar content of some commonly prescribed liquid medicines in infants and young children at the pediatric clinics and the University Hospital in Kuala, Lumpur. The sugar content of the 24 liquid medicines was determined using a sugar refractometer. The authors found that all of the liquid medicines tested contained sugar. The sugar content ranged from 29.4% to 61.2%.[67] Sucrose was most commonly used; while fructose and glucose were less used.
Kumar et al.[68] studied 91 antimicrobial preparations to identify the amount of sweeteners, flavorings, and dyes used in these preparations. 87 preparations contained one or more of the following sweeteners: Mannitol, lactose, saccharin, sorbitol, and sucrose. Sucrose was found to be present in 74 (85%) of the 87 preparations, followed by saccharin in 30 (34%) preparations. Mannitol, lactose, and sorbitol were each present in 7 preparations.[68]
Passos et al.[69] determined the pH and sucrose concentrations of 71 pediatric medications of long-term use in Brazil. The highest sucrose value concentrations were found in respiratory and antibiotic preparations. The sucrose concentration values were higher in syrups than any other formulations. Glucose was found in 16 medicines and sucrose in 58 medications, and the average pH value was 5.89±2.02.69. It was concluded that the high sucrose concentration and low pH in the pediatric medicines studied varied according to therapeutic class, daily dose, and brand.[69]
Pomarico et al.[65] evaluated the pH, presence and concentration of sucrose, glucose, lactose, and fructose in 10 medications (7 antiretroviral and 3 antibacterial agents) that are commonly used for the management of HIV infection in children. Antibacterials displayed the highest sucrose concentration, ranging from 40% to 54%.[65] Glucose was detected in one of the medications, sucrose was found in seven, and none contained lactose. Fructose was not found in these medications.
Subramanian et al.[5] assessed the type and sugar concentration of 10 commonly prescribed liquid medications in India. 50% of the preparations contained sucrose, glucose, and sorbitol with sucrose observed in 9 and glucose in 7 of the medications.[5]
Valinoti et al.[70] assessed the sweetener content, cariogenic and erosive potentials of 29 pediatric antibiotics and concluded that many antibiotics presented high concentration of sugars (n = 24), high titratable acidity (n = 27), pH below the critical value (n = 15) and high viscosity which can be considered risk factors for dental caries and erosion when consumed frequently. These results corroborate with the results of a recent study by Gupta and Panda,[71] where authors concluded that all the pediatric medications contain sucrose and an acidic pH. The authors further concluded that the parents and dentists are unaware of the hidden sugars and cariogenicity of these medications as reported in previous studies.[72,73]
Oral health preventive measures
It is expected that concerned physicians and the other primary caregivers be aware of the risk of oral health discrepancy due to the incessant usage of pediatric medicines. There also need to be a focus on prevention of dental caries during pediatric wellchild visits by education and motivating parents, about the importance of oral hygiene practices and awareness on sugar-containing medicines and suggest alternate options available.[55,57,58,64,72]
The oral hygiene practices should include regular brushing with toothpaste containing optimal fluoride for preventing dental caries. Oral hygiene must be insisted for all children taking sweetened medication after each dose of medication as a primary step for minimizing the risk of dental caries and dental erosion.[57,60,73] Patients should be encouraged to take medications at mealtimes only, and to avoid taking medicines before going to bed especially, due to decreased oral clearance at night time. The use of non-cariogenic substances in medicines or sugar-free medicines must be suggested whenever possible. Pediatric dentists must ensure that the parents seek regular preventive oral care for their children and follow the routine oral hygiene recommendations after every dose of medication.[55,72,73]
Awareness of the dangers posed by these medications should be promoted on the labels of the medications by the manufacturers and regulatory authorities, to bring about increased availability and judicious use of sugar-free liquid medications.
Conclusion
A case-based approach should be used for the choice of sweetened medications. For short-term treatment regimens, use of nutritive sweetener based formulations is more suitable as the minimal sweetener content is unlikely to cause complications, along with appropriate oral hygiene instructions. Caloric/nutritive sweeteners should be strictly avoided in diabetic children as they have a potential to raise plasma glucose level and cause dental caries especially if used for a long-term dosage. Drug manufacturers should explicitly state the purpose of a particular sweetening agent in pediatric medical preparations clearly described on labels in addition to drug interactions, adverse reactions, and contraindications. The potential laxative effect of polyols (e.g., sorbitol and mannitol) should be emphasized along with their osmotic properties and their potential effects on bioavailability.
As a drug class, analgesics and antibiotics were most likely to contain sweeteners with a high cariogenic potential. Psychotropic and respiratory preparations, on the other hand, were less likely to contain sweeteners with high cariogenic potential.
Despite the availability of multiple and novel sugar substitutes, many products in the market continue to include sweeteners with cariogenic potential. Alternative measures to improve the flavor or taste using techniques such as coating, complex formation, choice of vehicle, and adjustment of viscosity should be considered in the development of drug formulations. Drugs with a neutral taste should be formulated for patients undergoing treatment of chronic conditions. The recommended pediatric dosage forms worldwide are flexible oral solid dosage forms, such as orodispersible tablets and/or tablets used to prepare oral liquid preparations which are found to be suitable for younger children. Accurate and consistent administration of oral pediatric formulations can be achieved using modified feeding bottles and pacifiers with medicines placed in a reservoir, help improve the palatability of oral solutions using a dose-sipping technology and help increase product stability using a pulp-spoon with a single dry dose of medicine. These current modifications of pediatric dosing devices facilitate and assist in the oral delivery of liquids to small children.
The advent of safer natural sugars and the advances in the understanding of neurobiological mechanisms for taste perception, improved drug palatability and pediatric dosing, pediatric specialists and physicians should be able to provide a tailor-made medicine for each child with least adverse effects in the near future.
References
- 1.Ma R, Sun M, Wang S, Kang Q, Huang L, Li T, et al. Effect of high-fructose corn syrup on the acidogenicity, adherence and biofilm formation of Streptococcus mutans. Aust Dent J. 2013;58:213–8. doi: 10.1111/adj.12074. [DOI] [PubMed] [Google Scholar]
- 2.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369:51–9. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 3.Kutsch VK, Young DA. New directions in the etiology of dental caries disease. J Calif Dent Assoc. 2011;39:716–21. [PubMed] [Google Scholar]
- 4.Strickley RG, Iwata Q, Wu S, Dahl TC. Pediatric drugs –A review of commercially available oral formulations. J Pharm Sci. 2008;97:1731–74. doi: 10.1002/jps.21101. [DOI] [PubMed] [Google Scholar]
- 5.Subramaniam P, Nandan N. Cariogenic potential of pediatric liquid medicaments –An in vitro study. J Clin Pediatr Dent. 2012;36:357–62. doi: 10.17796/jcpd.36.4.nt11584612462t84. [DOI] [PubMed] [Google Scholar]
- 6.Allen LV., Jr Dosage form design and development. Clin Ther. 2008;30:2102–11. doi: 10.1016/j.clinthera.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Allen SM, Popovich NG, Ansel HC. Ansel's Pharmaceutical Dosage Forms and Drug Delivery Systems. 9th ed. Philadelphia, PA: Walters Kluwer Health/Lippincott Williams & Wilkins; 2011. pp. 331–429. [Google Scholar]
- 8.Scadding G. Pediatric allergy medications: Review of currently available formulations. Curr Med Res Opin. 2009;25:2069–79. doi: 10.1185/03007990903116875. [DOI] [PubMed] [Google Scholar]
- 9.Badgujar BP, Mundada AS. The technologies used for developing orally disintegrating tablets: A review. Acta Pharm. 2011;61:117–39. doi: 10.2478/v10007-011-0020-8. [DOI] [PubMed] [Google Scholar]
- 10.Winnick S, Lucas DO, Hartman AL, Toll D. How do you improve compliance? Pediatrics. 2005;115:e718–24. doi: 10.1542/peds.2004-1133. [DOI] [PubMed] [Google Scholar]
- 11.Fabiano V, Mameli C, Zuccotti GV. Paediatric pharmacology: Remember the excipients. Pharmacol Res. 2011;63:362–5. doi: 10.1016/j.phrs.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Fitch C, Keim KS, Academy of Nutrition and Dietetics Position of the academy of nutrition and dietetics: Use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739–58. doi: 10.1016/j.jand.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Cummings JH, Stephen AM. Carbohydrate terminology and classification. Eur J Clin Nutr. 2007;61(Suppl 1):S5–18. doi: 10.1038/sj.ejcn.1602936. [DOI] [PubMed] [Google Scholar]
- 14.American Dietetic Association. Position of the American dietetic association: Use of nutritive and nonnutritive sweeteners. J Am Diet Assoc. 2004;104:255–75. doi: 10.1016/j.jada.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Subramaniam P, Kumar K. Cariogenic potential of medications used in treatment of children with HIV infection. Spec Care Dentist. 2014;34:127–30. doi: 10.1111/scd.12041. [DOI] [PubMed] [Google Scholar]
- 16.Xavier AF, Moura EF, Azevedo WF, Vieira FF, Abreu MH, Cavalcanti AL, et al. Erosive and carcinogenicity potential of pediatric drugs: Study of physicochemical parameters. BMC Oral Health. 2013;13:71. doi: 10.1186/1472-6831-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheinin A, Mäkinen KK, Ylitalo K. Turku sugar studies V. Final report on the effect of sucrose, fructose and xylitol diets on the caries incidence in man. Acta Odontol Scand. 1976;34:179–216. doi: 10.3109/00016357608997711. [DOI] [PubMed] [Google Scholar]
- 18.Koulourides T, Bodden R, Keller S, Manson-Hing L, Lastra J, Housch T, et al. Cariogenicity of nine sugars tested with an intraoral device in man. Caries Res. 1976;10:427–41. doi: 10.1159/000260235. [DOI] [PubMed] [Google Scholar]
- 19.Scheinin A, Makinen KK, Tammisalo E, Rekola M. Turku sugar studies I-XI. Acta Odontol Scand. 1975;33:269–78. doi: 10.3109/00016357509004632. [DOI] [PubMed] [Google Scholar]
- 20.Moynihan PJ. Update on the nomenclature of carbohydrates and their dental effects. J Dent. 1998;26:209–18. doi: 10.1016/s0300-5712(97)00010-9. [DOI] [PubMed] [Google Scholar]
- 21.Tinanoff N, Palmer CA. Dietary determinants of dental caries and dietary recommendations for preschool children. J Public Health Dent. 2000;60:197–206. doi: 10.1111/j.1752-7325.2000.tb03328.x. [DOI] [PubMed] [Google Scholar]
- 22.Bloomgarden ZT. Nonnutritive sweeteners, fructose, and other aspects of diet. Diabetes Care. 2011;34:e46–51. doi: 10.2337/dc11-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edgar WM, Dodds MW. The effect of sweeteners on acid production in plaque. Int Dent J. 1985;35:18–22. [PubMed] [Google Scholar]
- 24.Rowe RC, Sheskey PJ, Quinn ME. Handbook of Pharmaceutical Excipients. 6th ed. London: APhA/Pharmaceutical Press; 2009. 48,273,283,357,364,414,424,605. [Google Scholar]
- 25.Muñoz-Sandoval C, Muñoz-Cifuentes MJ, Giacaman RA, Ccahuana-Vasquez RA, Cury JA. Effect of bovine milk on Streptococcus mutans biofilm cariogenic properties and enamel and dentin demineralization. Pediatr Dent. 2012;34:e197–201. [PubMed] [Google Scholar]
- 26.Bradshaw DJ, Lynch RJ. Diet and the microbial a etiology of dental caries: New paradigms. Int Dent J. 2013;63(Suppl2):64–72. doi: 10.1111/idj.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loesche WJ. The effect of sugar alcohols on plaque and saliva level of Streptococcus mutans. Swed Dent J. 1984;8:125–35. [PubMed] [Google Scholar]
- 28.Ly KA, Milgrom P, Rothen M. Xylitol, sweeteners, and dental caries. Pediatr Dent. 2006;28:154–63. [PubMed] [Google Scholar]
- 29.Park KK, Schemehorn BR, Stookey GK, Butchko HH, Sanders PG. Acidogenicity of high-intensity sweeteners and polyols. Am J Dent. 1995;8:23–6. [PubMed] [Google Scholar]
- 30.Roberts MW, Wright JT. Food sugar substitutes: A brief review for dental clinicians. J Clin Pediatr Dent. 2002;27:1–4. doi: 10.17796/jcpd.27.1.bl98u70371655hp8. [DOI] [PubMed] [Google Scholar]
- 31.Maguire A, Rugg-Gunn AJ, Wright WG. Adaptation of dental plaque to metabolize maltitol compared with other sweeteners. J Dent. 2000;28:51–9. doi: 10.1016/s0300-5712(99)00050-0. [DOI] [PubMed] [Google Scholar]
- 32.Imfeld T. Efficacy of sweeteners and sugar substitutes in caries prevention. Caries Res. 1993;27(Suppl1):50–5. doi: 10.1159/000261603. [DOI] [PubMed] [Google Scholar]
- 33.Durso SC, Vieira LM, Cruz JN, Azevedo CS, Rodrigues PH, Simionato MR, et al. Sucrose substitutes affect the cariogenic potential of Streptococcus mutans biofilms. Caries Res. 2014;48:214–22. doi: 10.1159/000354410. [DOI] [PubMed] [Google Scholar]
- 34.Birkhed D, Edwardsson S, Kalfas S, Svensäter G. Cariogenicity of sorbitol. Swed Dent J. 1984;8:147–54. [PubMed] [Google Scholar]
- 35.Duro D, Rising R, Cedillo M, Lifshitz F. Association between infantile colic and carbohydrate malabsorption from fruit juices in infancy. Pediatrics. 2002;109:797–805. doi: 10.1542/peds.109.5.797. [DOI] [PubMed] [Google Scholar]
- 36.Hayes C. The effect of non-cariogenic sweeteners on the prevention of dental caries: A review of the evidence. J Dent Educ. 2001;65:1106–9. [PubMed] [Google Scholar]
- 37.Ritter AV, Bader JD, Leo MC, Preisser JS, Shugars DA, Vollmer WM, et al. Tooth-surface-specific effects of xylitol: Randomized trial results. J Dent Res. 2013;92:512–7. doi: 10.1177/0022034513487211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birkhed D, Bär A. Sorbitol and dental caries. World Rev Nutr Diet. 1991;65:1–37. doi: 10.1159/000419465. [DOI] [PubMed] [Google Scholar]
- 39.Matsukubo T, Takazoe I. Sucrose substitutes and their role in caries prevention. Int Dent J. 2006;56:119–30. doi: 10.1111/j.1875-595x.2006.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 40.Rugg-Gunn AJ, Edgar WM. Sweeteners and dental health. Community Dent Health. 1985;2:213–23. [PubMed] [Google Scholar]
- 41.Wåler SM, Rölla G. Effect of xylitol on dental plaque in vivo during carbohydrate challenge. Scand J Dent Res. 1983;91:256–9. doi: 10.1111/j.1600-0722.1983.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 42.Pawar S, Kumar A. Issues in the formulation of drugs for oral use in children: Role of excipients. Paediatr Drugs. 2002;4:371–9. doi: 10.2165/00128072-200204060-00004. [DOI] [PubMed] [Google Scholar]
- 43.Grenby TH. Update on low-calorie sweeteners to benefit dental health. Int Dent J. 1991;41:217–24. [PubMed] [Google Scholar]
- 44.Stegink LD, Filer LJ, Jr, Bell EF, Ziegler EE, Tephly TR, Krause WL, et al. Repeated ingestion of aspartame-sweetened beverages: Further observations in individuals heterozygous for phenylketonuria. Metabolism. 1990;39:1076–81. doi: 10.1016/0026-0495(90)90169-d. [DOI] [PubMed] [Google Scholar]
- 45.Butchko HH, Stargel WW, Comer CP, Mayhew DA, Benninger C, Blackburn GL, et al. Aspartame: Review of safety. Regul Toxicol Pharmacol. 2002;35:S1–93. doi: 10.1006/rtph.2002.1542. [DOI] [PubMed] [Google Scholar]
- 46.Lout RK, Messer LB, Soberay A, Kajander K, Rudney J. Cariogenicity of frequent aspartame and sorbitol rinsing in laboratory rats. Caries Res. 1988;22:237–41. doi: 10.1159/000261113. [DOI] [PubMed] [Google Scholar]
- 47.Das S, Das AK, Murphy RA, Worawongvasu R. Aspartame and dental caries in the rat. Pediatr Dent. 1991;13:217–20. [PubMed] [Google Scholar]
- 48.Moeller SM, Fryhofer SA, Osbahr AJ, 3rd, Robinowitz CB, Council on Science and Public Health American Medical Association The effects of high fructose syrup. J Am Coll Nutr. 2009;28:619–26. doi: 10.1080/07315724.2009.10719794. [DOI] [PubMed] [Google Scholar]
- 49.Rippe JM, Angelopoulos TJ. Sucrose, high-fructose corn syrup, and fructose, their metabolism and potential health effects: What do we really know? Adv Nutr. 2013;4:236–45. doi: 10.3945/an.112.002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker AM, Dreyer NA, Friedlander E, Loughlin J, Rothman KJ, Kohn HI, et al. An independent analysis of the national cancer institute study on non-nutritive sweeteners and bladder cancer. Am J Public Health. 1982;72:376–81. doi: 10.2105/ajph.72.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saccharin. Review of safety issues. Council on scientific affairs. JAMA. 1985;254:2622–4. [PubMed] [Google Scholar]
- 52.Inactive Ingredients in Pharmaceutical Products: Update (Subject Review) American Academy of Pediatrics Committee on Drugs. Pediatrics. 1997;99:268–78. doi: 10.1542/peds.99.2.268. [DOI] [PubMed] [Google Scholar]
- 53.Pless IB, Douglas JW. Chronic illness in childhood. I. Epidemiological and clinical characteristics. Pediatrics. 1971;47:405–14. [PubMed] [Google Scholar]
- 54.van der Lee JH, Mokkink LB, Grootenhuis MA, Heymans HS, Offringa M. Definitions and measurement of chronic health conditions in childhood: A systematic review. JAMA. 2007;297:2741–51. doi: 10.1001/jama.297.24.2741. [DOI] [PubMed] [Google Scholar]
- 55.Durward C, Thou T. Dental caries and sugar-containing liquid medicines for children in New Zealand. N Z Dent J. 1997;93:124–9. [PubMed] [Google Scholar]
- 56.Kenny DJ, Somaya P. Sugar load of oral liquid medications on chronically ill children. J Can Dent Assoc. 1989;55:43–6. [PubMed] [Google Scholar]
- 57.Roberts IF, Roberts GJ. Relation between medicines sweetened with sucrose and dental disease. Br Med J. 1979;2:14–6. doi: 10.1136/bmj.2.6181.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaw L, Glenwright HD. The role of medications in dental caries formation: Need for sugar-free medication for children. Pediatrician. 1989;16:153–5. [PubMed] [Google Scholar]
- 59.Foster H, Fitzgerald J. Dental disease in children with chronic illness. Arch Dis Child. 2005;90:703–8. doi: 10.1136/adc.2004.058065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bigeard L. The role of medication and sugars in pediatric dental patients. Dent Clin North Am. 2000;44:443–56. [PubMed] [Google Scholar]
- 61.Gabre P, Norrman C, Birkhed D. Oral sugar clearance in individuals with oral motor dysfunctions. Caries Res. 2005;39:357–62. doi: 10.1159/000086841. [DOI] [PubMed] [Google Scholar]
- 62.Crossner CG, Hase JC, Birkhed D. Oral sugar clearance in children compared with adults. Caries Res. 1991;25:201–6. doi: 10.1159/000261368. [DOI] [PubMed] [Google Scholar]
- 63.Roberts GJ, Roberts IF. Dental disease in chronically sick children. ASDC J Dent Child. 1981;48:346–51. [PubMed] [Google Scholar]
- 64.Feigal RJ, Jensen ME, Mensing CA. Dental caries potential of liquid medications. Pediatrics. 1981;68:416–9. [PubMed] [Google Scholar]
- 65.Pomarico L, Czauski G, Portela MB, de Souza IP, Kneipp L, de Araújo Soares RM, et al. Cariogenic and erosive potential of the medication used by HIV-infected children: PH and sugar concentration. Community Dent Health. 2008;25:170–2. [PubMed] [Google Scholar]
- 66.Hill EM, Flaitz CM, Frost GR. Sweetener content of common pediatric oral liquid medications. Am J Hosp Pharm. 1988;45:135–42. [PubMed] [Google Scholar]
- 67.Nik-Hussein NN, Razak IA, Karim MN. An analysis of sugar content of commonly used pediatric liquid medicines –Its relevance to dentistry. Singapore Dent J. 1988;13:24–6. [PubMed] [Google Scholar]
- 68.Kumar A, Weatherly MR, Beaman DC. Sweeteners, flavorings, and dyes in antibiotic preparations. Pediatrics. 1991;87:352–60. [PubMed] [Google Scholar]
- 69.Passos IA, Sampaio FC, Martínez CR, Freitas CH. Sucrose concentration and pH in liquid oral pediatric medicines of long-term use for children. Rev Panam Salud Publica. 2010;27:132–7. doi: 10.1590/s1020-49892010000200007. [DOI] [PubMed] [Google Scholar]
- 70.Valinoti AC, da Costa LC, Jr, Farah A, Pereira de Sousa V, Fonseca-Gonçalves A, Maia LC, et al. Are pediatric antibiotic formulations potentials risk factors for dental caries and dental erosion? Open Dent J. 2016;10:420–30. doi: 10.2174/1874210601610010420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta M, Panda S. Cariogenic potential of the commonly prescribed pediatric liquid medicaments in kingdom of Saudi Arabia: An in vitro study. J Contemp Dent Pract. 2017;18:307–11. doi: 10.5005/jp-journals-10024-2036. [DOI] [PubMed] [Google Scholar]
- 72.Neves BG, Pierro VS, Maia LC. Pediatricians'perceptions of the use of sweetened medications related to oral health. J Clin Pediatr Dent. 2008;32:133–7. doi: 10.17796/jcpd.32.2.5773462618772x11. [DOI] [PubMed] [Google Scholar]
- 73.Nirmala SV, Popuri VD, Chilamakuri S, Nuvvula S, Veluru S, Minor Babu MS, et al. Oral health concerns with sweetened medicaments: Pediatricians'acuity. J Int Soc Prev Community Dent. 2015;5:35–9. doi: 10.4103/2231-0762.151973. [DOI] [PMC free article] [PubMed] [Google Scholar]


