Abstract
Objective:
Selective degeneration of dopaminergic neurons is the pathological hallmark of Parkinson disease (PD). Enhanced oxidative stress, lipid peroxidation and susceptibility of dopaminergic neurons to apoptotic cellular death are the leading pathogenetic mechanisms. Chrysin is an active flavonoid. Its neuroprotective effects have been reported. This study examined the neuroprotective effects of chrysin in ameliorating the dopaminergic neuronal degeneration and motor behavioral changes in rotenone model of PD.
Methods:
Thirty Sprague-Dawley rats were assigned into three groups: Control, rotenone-treated, and rotenone+chrysin treated groups. Rotenone was given at a dose of 3 mg/kg daily intraperitoneally, and chrysin was given at a dose of 50 mg/kg daily intraperitoneally for 4 weeks. Using five neurobehavioral assessment tests, evaluation was done weekly to record the motor behavioral changes. After 4 weeks, animals were sacrificed, brains were removed, and section from striatum and substantia nigra were stained using hematoxylin and eosin and cresyl violet stains. Immunohistochemical sections were also prepared using anti-tyrosine hydroxylase (TH) antibody.
Results:
Rotenone-induced Parkinson like changes were evident from deteriorating motor behavior. These animals showed extensive loss of dopaminergic neurons, decreased immunoreactivity against anti-TH antibodies and number of TH positive dopaminergic neurons in the nigrostriatal region. Chrysin treated animals showed a significant reduction in motor behavioral changes, degeneration and loss of nigrostriatal dopaminergic neurons and increased immunoreactivity to anti-TH antibody.
Conclusion:
This study concludes that chrysin confers neuroprotection in rat model of PD. It attenuates the degeneration of the nigrostriatal dopaminergic neurons and motor behavioral abnormalities.
Keywords: Chrysin, immunohistochemistry, motor behavior, Parkinson disease, rotenone
Introduction
Parkinson disease (PD) is the second most common neurodegenerative disease after Alzheimer disease. It is characterized by tremor, rigidity, akinesia, and postural instability.[1] The pathological hallmark of PD is the selective degeneration of dopaminergic neuron in nigrostriatal region. Symptoms do not appear unless 70–80% of dopaminergic neurons degenerate in nigrostriatal region that causes a challenge for its treatment.[2] Available therapies such as levodopa temporarily relieves from the symptoms but unable to prevent neurodegeneration, so new strategic approaches are under consideration to stop the degeneration of neurons.[3,4]
Bioactive compounds from medicinal plants have gained interest due to their little or no toxicity.[5] Chrysin is an active bioflavonoid that naturally occurs in fruits, vegetable, and medicinal plants.[6] It showed many biological activities such as anti-inflammatory,[7] antioxidant,[8] anti-apoptotic,[9] antiasthmatic,[10] anti-hyper-cholesterolic,[11] antiatherogenic,[12] and antidiabetic.[13] Many in vitro and in vivo studies have also reported a neuroprotective effect of chrysin.[14] It improved the age-related cognitive decline by enhancing the brain-derived neurotrophic factor (BDNF), decreasing oxidative stress, and inhibition of Na+, K+ ATPase activity in mice.[15] Chrysin showed neuroprotection against 3-nitropropionic acid-induced apoptosis of striatal neurons by enhancing the mitochondrial functions and downregulating the Bax gene.[16] Recent studies have reported promising effects of chrysin when administered with solid lipid nanoparticles, against Alzheimer[17] and against inflammatory mediators in brain of hyperammonemic rats after oral administration.[18] In an experimental spinal cord injury model of Wistar rat, chrysin improved the neuronal functions by decreasing the inflammatory factors such as tumor necrosis factor-α, interleukin-6, and interleukin-1β. It also decreased the inducible nitric oxide synthase level and apoptosis in spinal cord.[19] Treatment with chrysin for 7 days ameliorated the oxidative stress and neuroinflammation after ischemia-reperfusion injury of brain. It also increased the number of glial cells and suppressed the proinflammatory cytokines activation.[20] In an in vitro model of PD, chrysin protected the mesencephalic dopamine neurons from toxic insult of 1-methyl-4-phenylpyridinium ion which was evident by enhanced tyrosine hydroxylase (TH) immunoreactivity and less DNA fragmentation.[21]
Based on the reported neuroprotective effects of chrysin in in vitro and in vivo animal models, the present study was designed to investigate the protective effect of chrysin against neuronal degenerations in nigrostriatal region and associated impairments of motor behavior, learning and memory functions in Parkinsonian Sprague-Dawley rats.
Materials and Methods
Animals
Thirty Sprague-Dawley rats were randomly selected for the present study form animal house of Baqai Medical University, Karachi. They were divided into control, rotenone, and rotenone+chrysin groups. Control group received 1 ml of dimethyl sulfoxide (DMSO) (Merck, CAT#802912) intraperitoneally daily. 300 mg of rotenone (Sigma, CAT#R8875) was dissolved in 98 ml of miglyol 812 N (medium chain triglyceride) (Axopharma, Belgium) and 2 ml of 100% DMSO to get a final concentration of 3 mg/mL rotenone in 2% DMSO with 98% miglyol 812 N.[22] 500 mg of Chrysin (Sigma, CAT#C80105) was dissolved in 10 ml of DMSO, so that final concentration was 50 mg/ml. Rotenone was given 3 mg/kg/body weight intraperitoneally daily for 4 weeks.[23] Rotenone+chrysin group received an intraperitoneal injection of chrysin at a dose of 50 mg/kg/body weight daily[24] simultaneously with rotenone for 4 weeks. Mortality and changes in body weight were recorded weekly.
Behavioral tests
All the animals were evaluated for the behavioral changes every week in the same context and conditions between 9:00 am and 3:00 pm. Every animal was exposed and trained for 2–3 days before experiment to avoid anxiety and fear.[25] After each trial, test apparatus used were cleaned with 70% alcohol to remove any smell and residue.[26]
Motor test
Tremors
Tremors were observed on 0–5 scale as described earlier.[27] Briefly, they were ranked as zero score for no tremor, 1 for irregular twitching of muscle or slight tremor that were hardly appreciated, 2 for moderate or intermittent tremors seen at head region, 3 for visible tremors that were periodic in nature and seen at anterior region of body, 4 for continuous tremors that were seen at head and extremities, and 5 for continuous whole body tremors.
Akinesia
Akinesia was observed by noting the latency of movement of all four limbs by animals. Each animal was placed in an elevated wooden box and the time is taken to move all four limbs was observed. The cutoff time was 180 s.[27]
Catalepsy bar test
Catalepsy bar test was performed to observe the rotenone-induced rigidity as described earlier.[28] Briefly, a steel bar was placed on both forepaws of animal, and they were half rear 9 cm above the floor and stopwatch was started, and it was stopped when animal removed one of his paws from the bar [Figure 1]. The maximum latency time set was 180 s.
Figure 1.
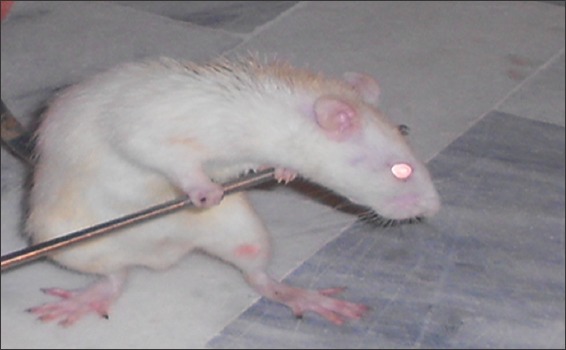
Rat with bars on forepaws during catalepsy bar test
Rearing behavior test
Animals were placed in a clear glass cylinder (40 cm high and 20 cm diameter) and number of rears in 5 min was observed.[23,27] Rear was considered as animals raise their hands above the shoulder and make contact with the wall of cylinder with their forelimb [Figure 2].
Figure 2.

Rat being assessed for rearing behavior
Learning and memory test
Morris water maze was used to assess the learning and memory impairment. The maze was a circular pool of 50 cm tall and 150 cm diameter and filled with water. By two imaginary lines, water pool was divided into four quadrants. A platform was placed 2 cm below the water level in one of the quadrants and skim milk was added into water to hide the platform. The animals memorized the platform through environmental cues. During the training session, animals were placed in one of the quadrants to find and climb on to the hidden platform within 2 min, and if they failed to find out within 2 min, they were guided toward the platform. During test session, time to reach and climb on the hidden platform was noted as escape latency time [Figure 3].[29]
Figure 3.

Morris water apparatus
Sacrifice of animals
After 4 weeks of experiment, each animal was deeply anesthetized with thiopental (50 mg/kg) intraperitoneally.[30] After exposing the chest cavity, animals were perfused through heart with 100 ml of normal saline, and re-perfused with 200 ml of neutral buffered formalin to facilitate infiltration of formalin at cellular level in living condition, after perfusion; brain was removed and fixed in 10% neutral buffered formalin.[31]
Sectioning and staining for light microscopy
After 3 days, brain was removed from normal buffered formalin, and coronal sections were made with the help of brain slicer (Zivic instrument, USA). For substantia nigra, sections were made from −2.3 to 6.04 mm from bregma, and for striatum, sections were made from 0.70 to 1.70 mm from bregma according to George Paxinos brain atlas.[32] These sections were then dehydrated in alcohol, cleared in xylene and embedded in paraffin. Five micron thick sections of striatum and substantia nigra were prepared and stained with hemotoxylin and eosin (H and E) and cresyl violet.[33]
Immunohistochemistry (IHC) for anti-TH antibody
IHC was done as described previously.[22] Sections were deparaffinized by two washes in xylene, each for 15 min, rehydrated in descending grade of alcohol followed by washing with 1× phosphate buffered saline (PBS) for 15 min. For antigen retrieval, slides were immersed in ethylenediaminetetraacetic acid solution at 90°C for 20 min. Sections were then permeabilized with 0.5% Triton X-100 for 10 min and were blocked in 5% bovine serum albumin for 2 h. Sections were then incubated in mouse monoclonal anti-TH antibody (1:25, Millipore # MAB318) for overnight at 4°C plus 2 hour at room temperature to obtain optimal antibody penetration. For detection of antigen signal, sections were washed thrice with PBS and incubated in avidin-biotin complex (Millipore) solution. After washing in PBS, the sections were developed using diaminobenzidine (DAB) (Millipore, #DAB500) to visualize the brown color precipitate at the antigen site.
Image analysis
H and E, cresyl violet, and IHC stained sections were studied under Olympus microscope equipped with DP72 camera. Images were captured at various magnifications and analyzed by Image J Software (NIH). Counting of H and E, cresyl violet, and TH positive dopaminergic neurons was done at ×40 magnified images.
Observations and Results
Mortality rate
There was no mortality in the control group while in rotenone-treated group three rats died (one in 3rd and two in 4th week) therefore mortality rate in this group was 30%. In rotenone+chrysin treated group one rat died in 4th week, therefore, the mortality rate was 10%.
Changes in body weight
Control and chrysin+rotenone treated animals showed progressive rise while rotenone-treated animals showed a progressive loss of body weight over the period [Figure 1]. There was no significant difference (P > 0.05) in the means of the body weights between the groups in 0 and 1st weeks. The mean body weights of chrysin+rotenone treated animals were significantly higher in second (P < 0.05), 3rd and 4th week (P < 0.001) as compare to rotenone-treated animals and insignificantly (P > 0.05) lower in 2nd, 3rd, and 4th week when compared with control animals [Figure 4].
Figure 4.
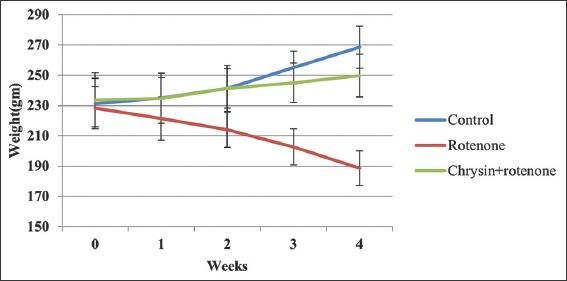
Changes in body weight
Tremors
No tremor was noted in control animals throughout the experiment and in any other groups during 0 and 1st week. In 2nd, 3rd, and 4th weeks, mean tremor scores of rotenone-treated animals were significantly higher (P < 0.001) than control animals. Mean tremor scores of chrysin+rotenone treated animals were significantly (P < 0.001) lower than rotenone-treated animals and insignificantly (P > 0.05) higher in 2nd and 3rd week but significantly (P < 0.05) higher in the 4th week as compared to control animals [Table 1].
Table 1.
Week wise comparison of behavior tests
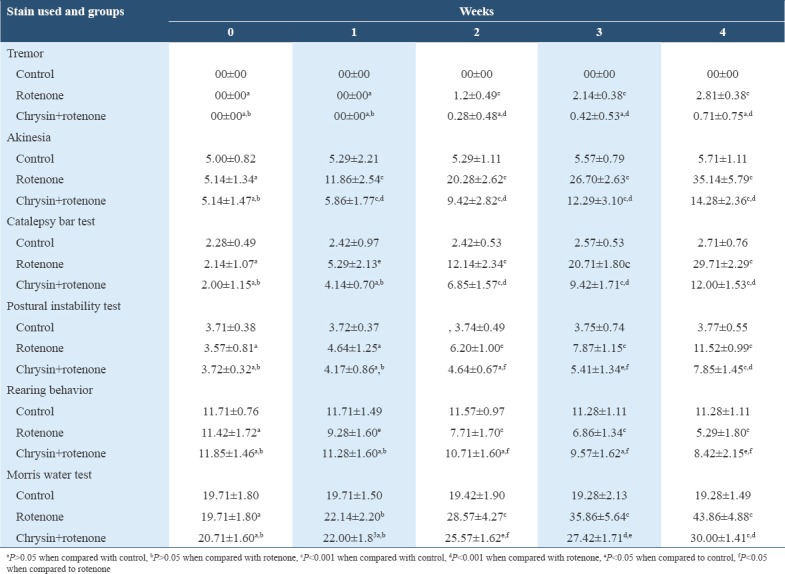
Akinesia
Statistical analysis did not show any significant difference (P > 0.05) in the akinesia scores between groups in week 0. In 1st, 2nd, 3rd, and 4th weeks, mean akinesia scores of rotenone-treated animals were significantly higher (P < 0.001) than control animals, while the mean akinesia scores of rotenone+chrysin treated animals were significantly higher (P < 0.001) than control animals and significantly (P < 0.001) lower as compared to rotenone-treated animals [Table 1].
Catalepsy bar test
Rigidity was measured by catalepsy bar test in animals. No significant difference in the descent latency time was noted between the groups in week 0. The mean descent latency time of rotenone-treated animals was significantly higher in 1st (P < 0.05), 2nd, 3rd, and 4th weeks (P < 0.001) than control animals. Mean descent latency time of chrysin+rotenone treated animals was non-significant in 1st week as compared to control and rotenone-treated animals, while in 2nd, 3rd, and 4th week were significantly (P < 0.001) lower than rotenone-treated animals and significantly (P < 0.001) higher than control animals [Table 1].
Postural instability test
Mean postural instability scores were statistically insignificant (P > 0.05) between the groups in week 0 and week 1. While in 2nd, 3rd, and 4th week, these scores of rotenone-treated group were significantly higher (P < 0.001) than control group. Mean scores of chrysin+rotenone treated animals were significantly lower in 2nd and 3rd week (P < 0.05) and 4th week (P < 0.001) than rotenone-treated animals. When compare with control it was insignificant in 2nd week (P > 0.05), significantly higher in 3rd week (P < 0.05) and 4th week (P < 0.001) [Table 1].
Rearing behavior
There was no significant difference in mean rearing scores between the groups in week 0. The mean rearing score of rotenone-treated animals was significantly lower in 1st week (P < 0.05), 2nd, 3rd, and 4th weeks (P < 0.001) than the control animals. The mean rearing score of chrysin+rotenone treated animals was insignificant in 1st week (P > 0.05) as compare to control and rotenone-treated animals. It was significantly higher in 2nd, 3rd, and 4th weeks (P < 0.05) as compare to rotenone-treated animals and insignificantly lower in 2nd and 3rd week (P > 0.05) and significantly lower in 4th week as compare to control animals [Table 1].
Morris water test
Statistically, there was no significant difference (P > 0.05) in the means of escape latency time between the groups in week 0. The means escape latency time of rotenone group was non-significantly higher in 1st week (P > 0.05) but significantly higher in 2nd, 3rd, and 4th weeks (P < 0.001) than control animals. Mean escape latency time of chrysin+rotenone animals was statistically insignificant in 1st week (P > 0.05) as compare to control and rotenone-treated animals. It was significantly lower in 2nd (P < 0.05), 3rd and 4th weeks (P < 0.001) than rotenone-treated animals and significantly higher in 2nd and 3rd week (P < 0.05) and 4th week (P > 0.001) as compare to control animals [Table 1].
H and E staining
In control group, sections from substantia nigra and striatum showed dopaminergic neurons which were easily identified by their multipolar shape, dark stained pigmented cytoplasm, and visible nuclei. Sections from rotenone group showed neuronal damage with loss of multipolar shape and distorted nuclei of the dopaminergic neurons. Sections from, chrysin+rotenone treated group showed multipolar neurons with a polygonal shape having distinct nuclei both in striatum and substantia nigra [Figures 5 and 6].
Figure 5.
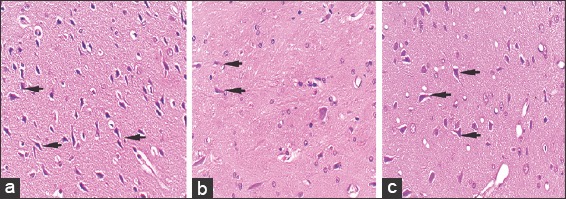
Hematoxylin and eosin stained, 5 μm thick sections of substantia nigra at ×40 showing dopaminergic neurons in control animals (a), rotenone-treated animals (b), and chrysin+rotenone treated animals (c). Section b from rotenone-treated Parkinson animals is showing few neurons of variable sizes. Section c, from animals treated with chrysin+rotenone, is showing a higher number of dopaminergic neurons comparatively, with uniform dimension
Figure 6.
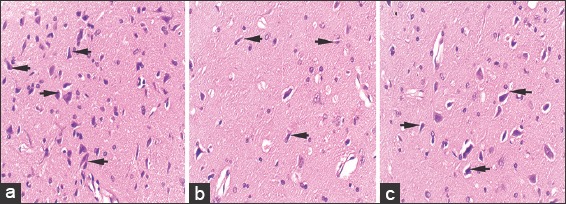
Hematoxylin and eosin stained, 5 μ thick sections of striatum at ×40 in control (a), rotenone (b), and chrysin+rotenone (c) treated animals. Sections c is showing a higher number of neurons with uniform dimensions as compared the neurons in section b from rotenone-treated animals
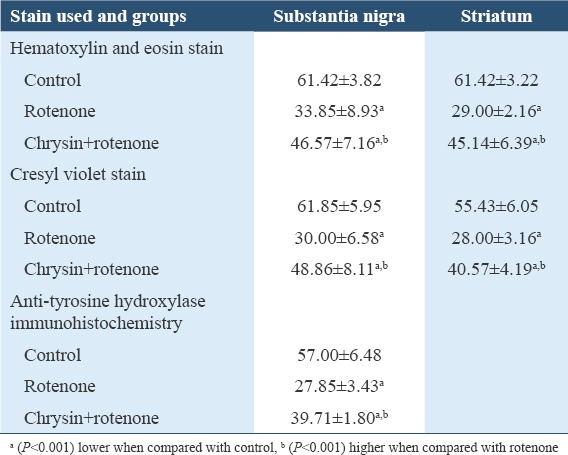
The mean number of dopaminergic neurons of substantia nigra and striatum in chrysin+rotenone was highly significantly higher (P > 0.001) than the rotenone group but significantly lower (P < 0.001) than control group [Figures 5 and 6, Table 2].
Table 2.
Number of neurons/high power field
Cresyl violet staining
Cresyl violet staining was also used to examine the morphology of dopaminergic neurons at different magnifications in SN and striatum. Normal polygonal shape of dopaminergic neurons was seen in the control group. Rotenone group showed loss of dopaminergic neurons, neurons with distorted, indistinct nuclei and many of them were contracted. Neuronal count was done at ×40 magnification. Mean number of dopaminergic neurons in SN as well as in striatum of rotenone-treated group was significantly (P < 0.001) lower as compared to control group. Chrysin+rotenone group showed significant (P > 0.001) higher count when compared with rotenone group but significantly (P > 0.001) lower than control animals [Figures 7 and 8, Table 2].
Figure 7.
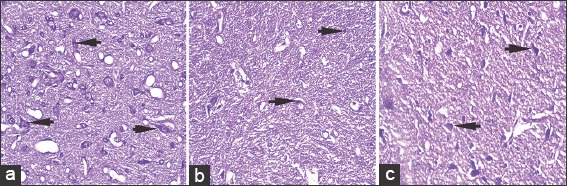
Cresyl violet stained, 5 μ thick sections of substantia nigra at ×40 in control (a), rotenone (b), and chrysin+rotenone (c) treated animals. Significantly higher numbers of cresyl violet positive dopaminergic neurons are present in section c treated with chrysin as compared to section b from rotenone only treated animals
Figure 8.
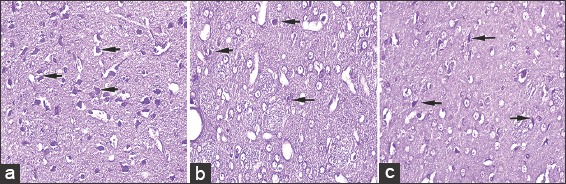
Cresyl violet stained, 5 μ thick sections of striatum at ×40 in control (a), rotenone (b), chrysin+rotenone (c) treated animals. Significantly higher numbers of cresyl violet positive dopaminergic neurons are present in section c treated with chrysin as compared to section b from rotenone alone treated animals
Anti-TH IHC
The mean neuronal count in substantia nigra of rotenone-treated animals was highly significantly (P > 0.001) lower than the control animals, while chrysin+rotenone treated were having significantly higher number of neurons as compare to rotenone alone treated animals but highly significantly (P < 0.001) lower than control animals [Figures 9 and 10, Table 2].
Figure 9.
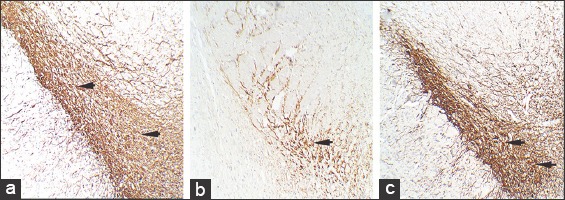
Sections showing anti-tyrosine hydroxylase (TH) immunohistochemistry of substantia nigra at ×10 magnification in control (a), rotenone (b), and chrysin+rotenone (c) treated animals. Brownish stained structures represent the immuno-reactive dopaminergic neurons. Section b from a rotenone treated Parkinsonian animal shows decreased reactivity/depleted number while sections c from a Chrysin treated animal shows enhanced reactivity/ higher number of TH positive dopaminergic neurons. Sections c from chrysin treated animals is showing enhanced immunoreactivity and a higher number of TH positive neurons as compare to rotenone-treated Parkinson animals
Figure 10.
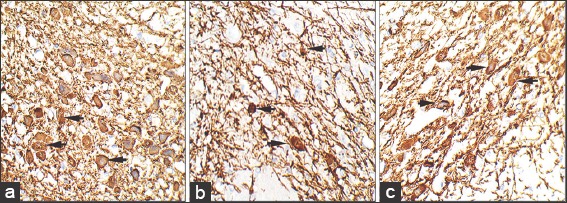
Sections are showing anti-tyrosine hydroxylase (TH) immunohistochemistry of substantia nigra at ×40 magnification in control (a), rotenone (b) and chrysin+rotenone (c), and treated animals. Sections c from chrysin treated animals are showing enhanced immunoreactivity, and a higher number of TH positive neurons as compare to rotenone only treated animals
Discussion
Many in vitro and in vivo studies have shown the neuroprotective behavior of chrysin against different disease models.[20,21,34] The present study showed a neuroprotective role of chrysin against dopaminergic neuronal degeneration and impairment in motor behavior, learning and memory impairment in Parkinson model of albino rats.
In our study, rotenone-treated Parkinson animals showed degeneration and distortion of dopaminergic neurons with a decrease in their number in the nigrostriatal region. This result is in complete agreement with previous studies done using animal models, where rotenone selectively damaged the neurons in dopamine-rich brain regions.[35-38] Loss of these neurons results in loss of TH deficiency as shown by the IHC sections stained using anti-TH antibody. Diminished immunoreactivity in this study reflects a diminished number of TH immune-positive dopaminergic neuron in substantia nigra. Similar results have also been reported by other studies.[36,39,40]
Microscopic sections from striatum and substantia nigra from the animals simultaneously treated with chrysin, in this study, have revealed attenuation in the degeneration and loss of dopaminergic neuron with a higher count of dopaminergic neurons and enhanced immunoreactivity against TH antibodies. This study is the first to report the neuroprotective efficacy of chrysin against dopaminergic degeneration and TH immunoreactivity. These neurons are considered in a state of oxidative stress and predicted to undergo autoxidation with production of reactive oxygen species that converts dopamine into reactive dopamine quinone which is highly toxic to these dopaminergic neurons.[41] Antioxidative property of chrysin appears to play a major role in the neuroprotection seen in this study.
Resting tremor, akinesia and postural instability are the cardinal sign of PD. Present study showed progressive development of these motor symptoms with passage of time in rotenone-induced Parkinson’s animals.[27,42] Postural instability also involves a disintegration of sensorimotor and proprioception functions besides motor component.[43] Basal ganglia are involved in many functions including voluntary movement of the body. Pathophysiological studies have concluded that these symptoms of Parkinson could be due the altered activity of basal ganglia, decreased dopamine levels, or dysfunction of cerebello-thalamo-cortical circuit.[44-46] The section from nigrostriatal regions in this study have also shown dopaminergic neuronal loss and decreased immunoreactivity. Simultaneous treatment with chrysin, significantly prevented these symptoms to develop. This improvement could be due to the protection of dopaminergic neurons in nigrostriatal system with improved function of basal ganglia, related circuits, and higher dopamine levels.
Rearing behavior and catalepsy tests are good indicators of general locomotors and muscular function associated with nigrostriatal neuronal loss in experimental animals.[22,27] A positive indication of the chrysin treatment on motor behavior in this study is a significant improvement in the outcome of these tests. Parkinson animals in this study developed rigidity as reflected by an increase in mean latency time to correct the externally imposed posture and which deteriorated with passage of time as shown by catalepsy bar test.[27,28,42] This test determines interference with dopaminergic transmission.[47] Rearing behavior test measures the frequency of rearing and locomotors activity in movement disorders like PD.[22] Simultaneous treatment with chrysin along with rotenone showed a reduction in rigidity and movement disability with a decrease in mean latency time to correct the imposed posture and in attempting rears in this study. This could be due to the protection of dopaminergic neurons in nigrostriatal system with improved function of basal ganglia and higher dopamine levels.
Morris Water maze was used to assess the impairment in learning and memory through visual cues in this study.[48] Parkinsonian animals showed a significant reduction in learning and memory behavior as evident by more time taken to find the hidden platform, and it deteriorated with passage of time.[49,50] A significant recovery of learning and memory was observed in chrysin+rotenone treated animals as evident by less time was taken to find out the hidden platform. This is in complete agreement with earlier studies which have reported a significant improvement in learning and memory behavior in chronically hypo-perfused[51] and diabetic rats.[52] This improvement could be due to antioxidative property of chrysin which results in attenuation of oxidative stress and improvement in BDNF levels in brain.[15]
Previous studies have reported controversial findings on the mortality rate in the rotenone-treated animals, ranging from non to 51%.[53-55] The present study finds a mortality rate of 25% among the rotenone-treated animals. This higher rate may be because systemic toxicity associated with rotenone.[56] Chrysin+rotenone treated animals showed a lower mortality as compared to rotenone-treated animals. Downregulation of mortality markers was reported by a study after lipopolysaccharide injected toxic mice treated with chrysin.[57]
Animals simultaneously treated with chrysin along with rotenone showed a reduced weight loss as compared to control and significant weight gain when compared to rotenone-treated animals. Some earlier studies have reported that chrysin prevented the body weight loss in lung cancer model of mice[58] and 2,3,7,8 tertrachlorodibenzo-p-dioxin-induced body wasting.[59]
Conclusion
In this study, chrysin has shown neuroprotection in the Parkinson model of rat. It ameliorated the degeneration of the dopaminergic neurons in nigrostriatal region and impairment of motor behavior. It also improved the learning and memory function, but still, a full recovery was not possible in this study. Further studies to investigate the role of neuroglia and apoptotic markers are urged to explore the precise protective mechanism conferred by chrysin.
Strength and Limitations
IHC staining of the SN in this study has shown substantial evidence that chrysin is an active, cheap and cost-effective natural source of neuroprotection in the rat model of Parkinsonism. TH-IHC of striatum was not performed due to a limited budget.
References
- 1.Ma MW, Wang J, Zhang Q, Wang R, Dhandapani KM, Vadlamudi RK, et al. NADPH oxidase in brain injury and neurodegenerative disorders. Mol Neurodegener. 2017;12:7. doi: 10.1186/s13024-017-0150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuter K, Kratochwil M, Berghauzen-Maciejewska K, Głowacka U, Sugawa MD, Ossowska K, et al. Adaptation within mitochondrial oxidative phosphorylation supercomplexes and membrane viscosity during degeneration of dopaminergic neurons in an animal model of early parkinson's disease. Biochim Biophys Acta. 2016;1862:741–53. doi: 10.1016/j.bbadis.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Mazo NA, Echeverria V, Cabezas R, Ávila-Rodriguez M, Aliev G, Leszek J, et al. Medicinal plants as protective strategies against parkinson's disease. Curr Pharm Des. 2017 doi: 10.2174/1381612823666170316142803. [DOI] [PubMed] [Google Scholar]
- 4.Dietrichs E, Odin P. Algorithms for the treatment of motor problems in parkinson's disease. Acta Neurol Scand. 2017;136:378–85. doi: 10.1111/ane.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yates AA, Erdman JW, Jr, Shao A, Dolan LC, Griffiths JC. Bioactive nutrients-time for tolerable upper intake levels to address safety. Regul Toxicol Pharmacol. 2017;84:94–101. doi: 10.1016/j.yrtph.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Gothai S, Ganesan P, Park SY, Fakurazi S, Choi DK, Arulselvan P, et al. Natural phyto-bioactive compounds for the treatment of Type 2 diabetes: Inflammation as a target. Nutrients. 2016:8. doi: 10.3390/nu8080461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen X, Zhao Z, Wang H, Guo Z, Hu B, Zhang G, et al. Elucidation of the anti-inflammatory mechanisms of bupleuri and scutellariae radix using system pharmacological analyses. Mediators Inflamm. 2017;2017:3709874. doi: 10.1155/2017/3709874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kandemir FM, Kucukler S, Eldutar E, Caglayan C, Gülçin İ. Chrysin protects rat kidney from paracetamol-induced oxidative stress, inflammation, apoptosis, and autophagy: A Multi-biomarker approach. Sci Pharm. 2017:85. doi: 10.3390/scipharm85010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu D, Jin J, Yu H, Zhao Z, Ma D, Zhang C, et al. Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J Exp Clin Cancer Res. 2017;36:44. doi: 10.1186/s13046-017-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao J, Jiang M, Zhang Y, Liu X, Du Q, Feng G, et al. Chrysin alleviates allergic inflammation and airway remodeling in a murine model of chronic asthma. Int Immunopharmacol. 2016;32:24–31. doi: 10.1016/j.intimp.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Ismail TA, Soliman MM, Nassan MA, Mohamed DI. Antihypercholesterolemic effects of mushroom, chrysin, curcumin and omega-3 in experimental hypercholesterolemic rats. J Food Nutr Res. 2015;3:77–87. [Google Scholar]
- 12.Basu A, Das AS, Majumder M, Mukhopadhyay R. Antiatherogenic roles of dietary flavonoids chrysin, quercetin, and luteolin. J Cardiovasc Pharmacol. 2016;68:89–96. doi: 10.1097/FJC.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 13.Lukačínová A, Mojžiš J, Beňačka R, Keller J, Maguth T, Kurila P, et al. Preventive effects of flavonoids on alloxan-induced diabetes mellitus in rats. Acta Vet Brno. 2008;77:175–82. [Google Scholar]
- 14.Nabavi SF, Braidy N, Habtemariam S, Orhan IE, Daglia M, Manayi A, et al. Neuroprotective effects of chrysin: From chemistry to medicine. Neurochem Int. 2015;90:224–31. doi: 10.1016/j.neuint.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Souza LC, Antunes MS, Filho CB, Del Fabbro L, de Gomes MG, Goes AT, et al. Flavonoid chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol Biochem Behav. 2015;134:22–30. doi: 10.1016/j.pbb.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Thangarajan S, Ramachandran S, Krishnamurthy P. Chrysin exerts neuroprotective effects against 3-nitropropionic acid induced behavioral despair-mitochondrial dysfunction and striatal apoptosis via upregulating bcl-2 gene and downregulating bax-bad genes in male wistar rats. Biomed Pharmacother. 2016;84:514–25. doi: 10.1016/j.biopha.2016.09.070. [DOI] [PubMed] [Google Scholar]
- 17.Vedagiri A, Thangarajan S. Mitigating effect of chrysin loaded solid lipid nanoparticles against amyloid β25-35 induced oxidative stress in rat hippocampal region: An efficient formulation approach for alzheimer's disease. Neuropeptides. 2016;58:111–25. doi: 10.1016/j.npep.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Mani R, Natesan V, Arumugam R. Neuroprotective effect of chrysin on hyperammonemia mediated neuroinflammatory responses and altered expression of astrocytic protein in the hippocampus. Biomed Pharmacother. 2017;88:762–9. doi: 10.1016/j.biopha.2017.01.081. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Y, Gong FL, Zhao GB, Li J. Chrysin suppressed inflammatory responses and the inducible nitric oxide synthase pathway after spinal cord injury in rats. Int J Mol Sci. 2014;15:12270–9. doi: 10.3390/ijms150712270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao Y, Chen L, Xiao J, Wang C, Jiang W, Zhang R, et al. Chrysin protects against focal cerebral ischemia/reperfusion injury in mice through attenuation of oxidative stress and inflammation. Int J Mol Sci. 2014;15:20913–26. doi: 10.3390/ijms151120913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mercer LD, Kelly BL, Horne MK, Beart PM. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: Investigations in primary rat mesencephalic cultures. Biochem Pharmacol. 2005;69:339–45. doi: 10.1016/j.bcp.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT, et al. Ahighly reproducible rotenone model of parkinson's disease. Neurobiol Dis. 2009;34:279–90. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tapias V, Cannon JR, Greenamyre JT. Pomegranate juice exacerbates oxidative stress and nigrostriatal degeneration in parkinson's disease. Neurobiol Aging. 2014;35:1162–76. doi: 10.1016/j.neurobiolaging.2013.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glory MD, Thiruvengadam D. Potential chemopreventive role of chrysin against N-nitrosodiethylamine-induced hepatocellular carcinoma in rats. Biomed Prev Nutr. 2012;2:106–12. [Google Scholar]
- 25.Xiong N, Xiong J, Khare G, Chen C, Huang J, Zhao Y, et al. Edaravone guards dopamine neurons in a rotenone model for parkinson's disease. PLoS One. 2011;6:e20677. doi: 10.1371/journal.pone.0020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Can OD, Demir Özkay U, Kıyan HT, Demirci B. Psychopharmacological profile of chamomile (Matricaria recutita L.) essential oil in mice. Phytomedicine. 2012;19:306–10. doi: 10.1016/j.phymed.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Salama M, Ellaithy A, Helmy B, El-Gamal M, Tantawy D, Mohamed M, et al. Colchicine protects dopaminergic neurons in a rat model of parkinson's disease. CNS Neurol Disord Drug Targets. 2012;11:836–43. doi: 10.2174/1871527311201070836. [DOI] [PubMed] [Google Scholar]
- 28.Sharma N, Nehru B. Beneficial effect of vitamin E in rotenone induced model of PD: Behavioural, neurochemical and biochemical study. Exp Neurobiol. 2013;22:214–23. doi: 10.5607/en.2013.22.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sriraksa N, Wattanathorn J, Muchimapura S, Tiamkao S, Brown K, Chaisiwamongkol K, et al. Cognitive-enhancing effect of quercetin in a rat model of parkinson's disease induced by 6-hydroxydopamine. Evid Based Complement Alternat Med. 2012;2012:823206. doi: 10.1155/2012/823206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soltani Hekmat A, Javanmardi K, Kouhpayeh A, Baharamali E, Farjam M. Differences in cardiovascular responses to alamandine in two-kidney, one clip hypertensive and normotensive rats. Circ J. 2017;81:405–12. doi: 10.1253/circj.CJ-16-0958. [DOI] [PubMed] [Google Scholar]
- 31.Chang YJ, Ho TY, Wu ML, Hwang SM, Chiou TW, Tsai MS, et al. Amniotic fluid stem cells with low γ-interferon response showed behavioral improvement in parkinsonism rat model. PLoS One. 2013;8:e76118. doi: 10.1371/journal.pone.0076118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates: The New Coronal Set. 5th ed. San Diego: Elsevier Academic Press; 2005. [Google Scholar]
- 33.Fathalla AM, Soliman AM, Moustafa AA. Selective A<sub>2A</sub>receptors blockade reduces degeneration of substantia nigra dopamine neurons in a rotenone-induced rat model of parkinson's disease: A histological study. Neurosci Lett. 2017;643:89–96. doi: 10.1016/j.neulet.2017.02.036. [DOI] [PubMed] [Google Scholar]
- 34.Mehri S, Karami HV, Hassani FV, Hosseinzadeh H. Chrysin reduced acrylamide-induced neurotoxicity in both in vitro and in vivo assessments. Iran Biomed J. 2014;18:101–6. doi: 10.6091/ibj.1291.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erbaş O, Oltulu F, Taşkiran D. Amelioration of rotenone-induced dopaminergic cell death in the striatum by oxytocin treatment. Peptides. 2012;38:312–7. doi: 10.1016/j.peptides.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, et al. Mechanism of toxicity in rotenone models of parkinson's disease. J Neurosci. 2003;23:10756–64. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nisticò R, Mehdawy B, Piccirilli S, Mercuri N. Paraquat- and rotenone-induced models of parkinson's disease. Int J Immunopathol Pharmacol. 2011;24:313–22. doi: 10.1177/039463201102400205. [DOI] [PubMed] [Google Scholar]
- 38.Zaitone SA, Abo-Elmatty DM, Elshazly SM. Piracetam and vinpocetine ameliorate rotenone-induced parkinsonism in rats. Indian J Pharmacol. 2012;44:774–9. doi: 10.4103/0253-7613.103300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goswami P, Gupta S, Joshi N, Sharma S, Singh S. Astrocyte activation and neurotoxicity: A study in different rat brain regions and in rat C6 astroglial cells. Environ Toxicol Pharmacol. 2015;40:122–39. doi: 10.1016/j.etap.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 40.He Y, Imam SZ, Dong Z, Jankovic J, Ali SF, Appel SH, et al. Role of nitric oxide in rotenone-induced nigro-striatal injury. J Neurochem. 2003;86:1338–45. doi: 10.1046/j.1471-4159.2003.01938.x. [DOI] [PubMed] [Google Scholar]
- 41.Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson's disease. J Parkinsons Dis. 2013;3:461–91. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salama M, Helmy B, El-Gamal M, Reda A, Ellaithy A, Tantawy D, et al. Role of L-thyroxin in counteracting rotenone induced neurotoxicity in rats. Environ Toxicol Pharmacol. 2013;35:270–7. doi: 10.1016/j.etap.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Santamato A, Ranieri M, Cinone N, Stuppiello LA, Valeno G, De Sanctis JL, et al. Postural and balance disorders in patients with parkinson's disease: A Prospective open-label feasibility study with two months of action observation treatment. Parkinsons Dis. 2015;2015:902738. doi: 10.1155/2015/902738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleming SM, Ekhator OR, Ghisays V. Assessment of sensorimotor function in mouse models of parkinson's disease. J Vis Exp. 2013;76:e50303. doi: 10.3791/50303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helmich RC, Hallett M, Deuschl G, Toni I, Bloem BR. Cerebral causes and consequences of parkinsonian resting tremor: A tale of two circuits? Brain. 2012;135:3206–26. doi: 10.1093/brain/aws023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linard-Medeiros CF, Sales VD, Ramos AC, Sereniki A, Trevisan MT, Wanderley AG, et al. Neuroprotective effect of extract of Anacardium occidentale Linn on a rotenone model of Parkinson's disease. Int J Pharm Sci Res. 2015;6:123–9. [Google Scholar]
- 47.Alvarez-Cervera FJ, Villanueva-Toledo J, Moo-Puc RE, Heredia-López FJ, Alvarez-Cervera M, Pineda JC, et al. Anovel automated rat catalepsy bar test system based on a RISC microcontroller. J Neurosci Methods. 2005;146:76–83. doi: 10.1016/j.jneumeth.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 48.D'Hooge R, De Deyn PP. Applications of the morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 49.Moreira CG, Barbiero JK, Ariza D, Dombrowski PA, Sabioni P, Bortolanza M, et al. Behavioral, neurochemical and histological alterations promoted by bilateral intranigral rotenone administration: A new approach for an old neurotoxin. Neurotox Res. 2012;21:291–301. doi: 10.1007/s12640-011-9278-3. [DOI] [PubMed] [Google Scholar]
- 50.Yy S, Jiang M, Xia Y, Chen Q-y, Wen T-q. Rotenone induces more serious learning and memory impairment than α-synuclein A30P does in Drosophila. J Shanghai Univ. 2011;15:229–34. [Google Scholar]
- 51.He XL, Wang YH, Bi MG, Du GH. Chrysin improves cognitive deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Eur J Pharmacol. 2012;680:41–8. doi: 10.1016/j.ejphar.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 52.Li R, Zang A, Zhang L, Zhang H, Zhao L, Qi Z, et al. Chrysin ameliorates diabetes-associated cognitive deficits in wistar rats. Neurol Sci. 2014;35:1527–32. doi: 10.1007/s10072-014-1784-7. [DOI] [PubMed] [Google Scholar]
- 53.Esteve-Rudd J, Fernández-Sánchez L, Lax P, De Juan E, Martín-Nieto J, Cuenca N, et al. Rotenone induces degeneration of photoreceptors and impairs the dopaminergic system in the rat retina. Neurobiol Dis. 2011;44:102–15. doi: 10.1016/j.nbd.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Binienda ZK, Sarkar S, Mohammed-Saeed L, Gough B, Beaudoin MA, Ali SF, et al. Chronic exposure to rotenone, a dopaminergic toxin, results in peripheral neuropathy associated with dopaminergic damage. Neurosci Lett. 2013;541:233–7. doi: 10.1016/j.neulet.2013.02.047. [DOI] [PubMed] [Google Scholar]
- 55.Yang Y, Liu X, Long Y, Wang F, Ding JH, Liu SY, et al. Systematic administration of iptakalim, an ATP-sensitive potassium channel opener, prevents rotenone-induced motor and neurochemical alterations in rats. J Neurosci Res. 2005;80:442–9. doi: 10.1002/jnr.20467. [DOI] [PubMed] [Google Scholar]
- 56.Johnson ME, Bobrovskaya L. An update on the rotenone models of parkinson's disease: Their ability to reproduce the features of clinical disease and model gene-environment interactions. Neurotoxicology. 2015;46:101–16. doi: 10.1016/j.neuro.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 57.Byun E-B, Jang B-S, Byun E-H, Sung N-Y. Effect of gamma irradiation on the change of solubility and anti-inflammation activity of chrysin in macrophage cells and LPS-injected endotoxemic mice. Radiat Phys Chem. 2016;127:276–85. [Google Scholar]
- 58.Kasala ER, Bodduluru LN, Barua CC, Madhana RM, Dahiya V, Budhani MK, et al. Chemopreventive effect of chrysin, a dietary flavone against benzo(a)pyrene induced lung carcinogenesis in swiss albino mice. Pharmacol Rep. 2016;68:310–8. doi: 10.1016/j.pharep.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 59.Ciftci O, Ozdemir I. Protective effects of quercetin and chrysin against 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induced oxidative stress, body wasting and altered cytokine productions in rats. Immunopharmacol Immunotoxicol. 2011;33:504–8. doi: 10.3109/08923973.2010.543686. [DOI] [PubMed] [Google Scholar]


