Abstract
Previous studies have argued that enhanced activity of the epidermal growth factor receptor (EGFR) and the mitogen-activated protein kinase (MAPK) pathway can promote tumor cell survival in response to cytotoxic insults. In this study, we examined the impact of MAPK signaling on the survival of primary hepatocytes exposed to low concentrations of deoxycholic acid (DCA, 50 μM). Treatment of hepatocytes with DCA caused MAPK activation, which was dependent upon ligand independent activation of EGFR, and downstream signaling through Ras and PI3 kinase. Neither inhibition of MAPK signaling alone by MEK1/2 inhibitors, nor exposure to DCA alone, enhanced basal hepatocyte apoptosis, whereas inhibition of DCA-induced MAPK activation caused ∼25% apoptosis within 6 h. Similar data were also obtained when either dominant negative EGFR-CD533 or dominant negative Ras N17 were used to block MAPK activation. DCA-induced apoptosis correlated with sequential cleavage of procaspase 8, BID, procaspase 9, and procaspase 3. Inhibition of MAPK potentiated bile acid-induced apoptosis in hepatocytes with mutant FAS-ligand, but did not enhance in hepatocytes that were null for FAS receptor expression. These data argues that DCA is causing ligand independent activation of the FAS receptor to stimulate an apoptotic response, which is counteracted by enhanced ligand-independent EGFR/MAPK signaling. In agreement with FAS-mediated cell killing, inhibition of caspase function with the use of dominant negative Fas-associated protein with death domain, a caspase 8 inhibitor (Ile-Glu-Thr-Asp-p-nitroanilide [IETD]) or dominant negative procaspase 8 blocked the potentiation of bile acid-induced apoptosis. Inhibition of bile acid-induced MAPK signaling enhanced the cleavage of BID and release of cytochrome c from mitochondria, which were all blocked by IETD. Despite activation of caspase 8, expression of dominant negative procaspase 9 blocked procaspase 3 cleavage and the potentiation of DCA-induced apoptosis. Treatment of hepatocytes with DCA transiently increased expression of the caspase 8 inhibitor proteins c-FLIP-S and c-FLIP-L that were reduced by inhibition of MAPK or PI3 kinase. Constitutive overexpression of c-FLIP-s abolished the potentiation of bile acid-induced apoptosis. Collectively, our data argue that loss of DCA-induced EGFR/Ras/MAPK pathway function potentiates DCA-stimulated FAS-induced hepatocyte cell death via a reduction in the expression of c-FLIP isoforms.
INTRODUCTION
Bile acids are steroid molecules synthesized by the liver and are essential for the digestion and uptake of certain nutrients (Benage and O'Connor, 1990). Hydrophobic bile acids are known to have hepatocellular toxicity both in vivo and in vitro (Schmucker et al., 1990; Noto et al., 1998; Faubion et al., 1999; Miyoshi et al., 1999). Conjugation of bile acids to glycine and taurine is one mechanism by which an organism can decrease the hydrophobicity of a bile acid (Rust et al., 2000; Martinez-Diez et al., 2000). This can result in bile acid-conjugate molecules that are less cytotoxic at physiological concentrations (Patel et al., 1994). Toxic bile acids, when retained within the liver because of impaired secretion into the bile canaliculi, are believed to contribute to liver injury during cholestasis, leading to the development of primary biliary cirrhosis of the liver, cholangiocarcinoma, and liver failure (Bloomer et al., 1976; Koeppel et al., 1997; Neuberger, 1997, Celli and Que, 1998; Heathcote, 1999; Trauner et al., 1999; Poupon et al., 2000). Apart from their toxicity to the liver, bile acids have also been shown to be involved in the pathogenesis of other gastrointestinal malignancies, such as colorectal cancer (Schlottman et al., 2000). Therefore, the balance between the effects of toxic and nontoxic bile acids is one determinant for liver injury. However, the mechanisms of bile acid-induced liver injury are still not fully understood.
In cholestatic liver diseases, although bile ducts receive the initial insult from toxic bile salts, the progression of the liver disease is principally the result of hepatic parenchymal cell (hepatocyte) damage caused by toxic hydrophobic bile salts (Gores et al., 1998). Several mechanisms have been proposed to be responsible for the bile acid-induced liver injury. Previous studies have argued that mitochondria-derived free radicals may be one early event in hydrophobic bile acid-induced hepatocyte toxicity (Sokol et al., 1995, 1998). To further support this concept, antioxidants can abrogate bile acid-induced hepatocellular injury (Yerushalmi et al., 2001).
Numerous studies, from in vitro as well in vivo experiments, have shown that when hepatocytes are exposed to bile acids, two types of cellular injury can occur. Higher concentrations of bile acid induce necrosis (Spivey et al., 1993; Krahenbuhl et al., 1994; Botla et al., 1995), whereas lower concentrations lead to apoptosis (Patel et al., 1994; Kwo et al., 1995). Indeed, apoptosis is a common mode of cell death that occurs in many tissues in response to a large variety of physiological and pathological stimuli (Thompson, 1995). In the liver, apoptosis has been implicated as an important form of cell death in various liver diseases, including viral hepatitis, alchoholic liver diseases, cholestasis, toxin-induced liver diseases, allograft rejection reaction after liver transplantation, and hepatocellular carcinoma (Benedetti and Marucci, 1999; Kaplowitz, 2000).
Despite intensive investigation, the molecular events by which bile acids control the functions of intracellular signal transduction pathways remain poorly described. In addition, the mechanisms by which signaling pathways control bile acid-induced hepatocyte apoptosis have not yet been fully elucidated. Recent studies have suggested that either the death receptor FAS or alterations in mitochondrial function can be involved in bile acid-induced hepatocyte injury (Faubion et al., 1999; Miyoshi et al., 1999; Sodeman et al., 2000). Other studies have suggested that the therapeutic effect of tauroursodeoxycholate may be mediated by activation of the mitogen-activated protein kinase (MAPK) pathway (Schliess et al., 1997).
Recently, bile acids have been shown to activate multiple signaling pathways within cells and can alter their survival and proliferation (Stravitz et al., 1996; Rao et al., 1997; Webster and Answer, 1998). This may be similar to other toxic stresses such as chemotherapeutic drugs and ionizing radiation (Jarvis et al., 1998; Dent et al., 1999). In response to ionizing radiation, for example, several groups have shown that the epidermal growth factor receptor (EGFR) is activated in a ligand-independent manner in response to irradiation of carcinoma cells (Reardon et al., 1999). Radiation exposure, via activation of the EGFR, can activate the MAPK pathway to a level similar to that observed by physiological EGF concentrations (Schmidt-Ullrich et al., 1997; Jarvis et al., 1998; Reardon et al., 1999). Increased signaling by the EGFR/MAPK pathway also appears to be cytoprotective versus ionizing radiation and various cytotoxic drugs in a diverse range cancer cell lines, although the precise mechanism(s) by which this occurred were unclear (Dent et al., 1998; Schmidt-Ullrich et al., 2000). Indeed, functional inhibition of EGFR (Harari and Huang, 2001), Ras (Cohen-Jonathan et al., 2000), Raf-1 (O'Dwyer et al., 1999), and MEK1/2 (Sebolt-Leopold et al., 1999) have all been shown to have radio- and chemosensitizing properties in vitro and in vivo. That cytotoxic stresses can activate the EGFR/Ras/MAPK signaling module also supports the concept that certain stresses may have a self-limiting effect upon their toxicity due to activation of the MAPK pathway.
The studies reported in this article were performed to determine the molecular mechanism(s) by which low concentrations of the bile acid deoxycholic acid (DCA) activate the MAPK pathway in primary hepatocytes, and whether DCA-induced MAPK signaling was cytoprotective versus bile acid-induced hepatocyte cell death. We found that DCA caused ligand-independent activation of both the EGFR and FAS receptors in primary hepatocytes. DCA-induced EGFR signaling, via the MAPK pathway, counteracted DCA-induced death signaling from the FAS receptor.
MATERIALS AND METHODS
Materials
Deoxycholic acid, dimethyl sulfoxide (DMSO), bromophenol blue, Triazma base, EDTA, Triton X-100, leupeptin, pepstatin, aprotinin, phenylmethylsulfonyl fluoride, mercaptoethanol, collagenase type IV, and poly-l-lysine hydrobromide were all obtained from Sigma (St. Louis, MO). Anti-caspase 3, anti-caspase 8, anti-caspase 9, phospho-ERK (P-ERK), anti-poly-(ADP-ribose) polymerase, anti-BID, anti-Bcl-2, anti-Bcl-XL, and all the secondary antibodies (anti-rabbit-horseradish peroxidase [HRP], anti-mouse-HRP, and anti-goat-HRP) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies versus c-IAP-1 and c-FLIP isoforms were as in Stoka et al. (2001). Anti-cytochrome c antibody was from PharMingen (San Diego, CA). Enhanced chemiluminescence (ECL) kit was purchased from PerkinElmer Life Science Products (Boston, MA). Caspase inhibitor (Z-VAD-FMK), caspase 9 inhibitor (Z-LEHD-FMK), and caspase 8 inhibitor (Z-IETD-FMK) were purchased from Enzyme System Products (Livermore, CA), dissolved in DMSO, and stored at 4°C. The pan-inhibitor of PI3 kinases (LY294002) was from Calbiochem (San Diego, CA). The specific inhibitors for MEK1/2 PD98059 and PD184352, and U0126 were gifts from Parke-Davis (Ann Arbor, MI) and DuPont Pharmaceuticals (Wilmington, DE), respectively. Trypsin-EDTA, Williams medium E, and penicillin-streptomycin were purchased from Invitrogen (Carlsbad, CA). Hoechst 33342 and DiOC6 were purchased from Molecular Probes (Eugene, OR). FluroGard Antifade was purchased from Bio-Rad (Bio-Rad, Hercules, CA) (Wang et al., 1998a; Bajt et al., 2000; Park et al., 2000a,b).
Methods
Primary Culture of Rodent Hepatocytes.
Hepatocytes were isolated from adult male Sprague-Dawley rats and adult male mice C57/BL6 wild type; C57/BL6-lpr (FAS receptor null); C57/BL6-gld (FAS ligand mutant); by the two-step collagenase perfusion technique (Kamath et al., 1999; Park et al., 2000a,b). The freshly isolated cells were plated on rat-tail collagen (Vitrogen)-coated 12-well plastic dishes at a density of 2 × 105 cells/well, and cultured in Williams E medium supplemented with 250 nM insulin, 0.1 nM dexamethasone, 1 nM thyroxine, and 100 μg/ml penicillin/streptomycin, at 37°C in a humidified atmosphere containing 5% CO2. The initial medium change was performed 3–4 h after cell seeding to minimize the contamination of dead or mechanically damaged cells. The cells were further incubated in the above-mentioned condition overnight and then treated with bile acids as described below.
Human Hepatocyte Culture and Isolation.
Human hepatocytes were isolated and transported from the University of Pittsburgh to Virginia Commonwealth University on ice. Cells were warmed to 37°C and cultured in Williams E medium supplemented with 250 nM insulin, 0.1 nM dexamethasone, 1 nM thyroxine, and 100 μg/ml penicillin/streptomycin, at 37°C in a humidified atmosphere containing 5% CO2, 12 h before bile acid treatment.
Recombinant Adenoviral Vectors; Generation and Infection In Vitro.
Two adenoviral technologies were used. Replication defective adenovirus is conjugated to poly-l-lysine as described in (Auer et al., 1998). The DNA conjugated virus was added to hepatocytes at a multiplicity of infection (MOI) of 250 and the cells incubated for 4 h at 37°C. The cells were washed with media to remove virus. Cells express transduced gene products 10–24h after infection. With the use of a plasmid to express β-galactosidase under control of the CMV-promoter, we determined that 1 μg of plasmid conjugated to virus particles and infected into mouse hepatocytes before plating at an MOI of 250 gave 100% infection. Second, we generated recombinant adenoviruses with the use of recombination in bacteria. Hepatocytes were infected with recombinant adenoviruses at an approximate MOI of 30.
Infection of Primary Hepatocytes by Adenoviral Poly-l-lysine–conjugated Plasmid Vectors (Dominant Negative Procaspase 8, Dominant Negative Procaspase 9, Dominant Negative Fas-associated Protein with Death Domain [FADD], Dominant Negative MEK1, c-FLIP-s, Cytomegalovirus [CMV]; Balachandran et al., 2000; Ozoren et al., 2000; Perkins et al., 1998, 2000).
The infection of hepatocytes by replication-defective adenovirus was essentially performed as described previously (Auer et al., 1998). Briefly, viral vectors and plasmid DNA are conjugated to poly-l-lysine. Three to 4 h after cell seeding, the DNA-conjugated virus was added to hepatocytes at a multiplicity of infection (MOI) of 250, and the cells were incubated for 4 h at 37°C on a rocker to ensure homogenous contact of virus particles with the cells. The cells were then washed with fresh media to remove virus that are not taken-up by cells. Cells were further incubated for 24 h to ensure adequate expression of transduced gene products.
Infection of Primary Hepatocytes by Recombinant Adenoviral Vectors (Bcl-2, Bcl-XL, MEK1 EE, CMV).
The infection of hepatocytes by recombinant adenoviral vectors was also performed as described previously (Auer et al., 1998). Briefly, 3–4 h after the freshly isolated hepatocytes were incubated in the 37°C incubator, the recombinant adenoviral vectors carrying the genes of interest (Bcl-XL, Bcl-2, MEK1 EE) at the MOI of 30 were added to hepatocytes, and the cells were incubated for 4 h in a 37°C incubator on a rocker. The cells were washed with fresh media to remove virus that are not taken up by cells. Cells were incubated for 24 h before further experiments to ensure adequate expression of transduced gene products.
Hepatocyte Treatment with DCA.
DCA sodium salt was dissolved in sterile Milli-Q water at a concentration of 100 mM and stored at B201C as stock solution. Hepatocytes were treated with the indicated concentrations of DCA for the indicated times. Cells treated in the same way but without DCA were regarded as controls.
SDS-PAGE and Western Blot Analysis.
At various time points after indicated treatment, hepatocytes were lysed in whole-cell lysis buffer (0.5 M Tris-HCl, pH 6.8, 2%SDS, 10% glycerol, 1% β-mercaptoethanol, 0.02% bromophenol blue), and the samples were boiled for 30 min. The boiled samples were loaded onto 14% SDS-PAGE and electrophoresis was run overnight. Proteins were electrophoretically transferred onto 0.22-μm pure nitrocellulose (NitroBind; Osmonics, Wesborough, MA) and immunoblotted with various primary antibodies against different proteins. The membranes were washed three times, each for 10 min in Tris-buffered saline with Tween and followed by incubation with appropriate HRP-conjugated secondary antibodies. All immunoblots were visualized by ECL.
Morphological Detection of Apoptosis by H-33342 Assay.
Morphological assessment of apoptosis was performed as follows. Hepatocytes were harvested by trypsinization with Trypsin/EDTA for ∼10 min at 37°C and sedimentation at 1500 rpm for 5 min. Because some apoptotic cells detached from the culture substratum into the medium, these cells were also collected by centrifugation of the medium at 1500 rpm for 5 min. The pooled cell pellets were resuspended in phosphate-buffered saline (PBS) and a fraction of the suspension was centrifuged at 800 rpm for 10 min in a cytospinner (Cytospin 3; Shandon, Pittsburgh, PA). The slides were immediately fixed in methanol/glacial acetic acid (3:1) for 30 min at 4°C. The slides were then washed with PBS for 10 min three times. The fixed cells were stained in Hoechst 33342 (10 μg/ml) for 30 min, followed by three washes in PBS to remove excessive dye, air-dried, and mounted in FluroGard Antifade. Nuclear morphology was evaluated with the use of an Olympus fluorescent microscope at excitation and emission wavelengths of 360 and 460 nm, respectively. Apoptotic cells were identified as those whose nuclei exhibited brightly staining condensed chromatin or nuclear fragmentation or apoptotic bodies. Five hundred cells from several randomly chosen fields were counted and the number of apoptotic cells was counted and expressed as a percentage of the total number of cells counted.
Wright-Giemsa Staining.
To confirm the morphological findings by H33342 assay, we also used Wright-Giemsa staining to evaluate apoptosis. The cells were trysinized and cytospun onto the slides, as described above. The slides were fixed and stained in Diff-Quik Stain set (Dade Diagnostics, Aguada, Puerto Rico), according to the manufacturer's instruction, and viewed under light microscope. Apoptotic cells were counted and expressed as a percentage of the total number of cells counted.
Determination of Apoptosis by Terminal Deoxynucleotidyl Transferase-mediated dUTP Nick End Labeling (TUNEL).
After hepatocytes were treated with various regimes, cells were collected by trypsinization followed by cytospin onto glass slides, as described above. Cells were fixed in methanol/glacial acetic acid (3:1) for 30 min at 4°C, and TUNEL assay was performed on these cells according to the manufacturer's instructions. The slides were viewed under the fluorescence microscope and the TUNEL-positive cells were counted from five randomly selected fields, and expressed as a percentage of total cells counted.
Assessment of Mitochondrial Membrane Potential (ΔΨm).
Mitochondrial membrane potential was determined by the retention of the dye 3,3′-dihexyloxacarbocyanine (DiOC6). At the indicated intervals, cells were harvested by trypsinization and centrifugation, as described above. An aliquot of 2–4 × 105 cells were resuspended in 1 ml of the phenol red-free medium containing 1 nM DiOC6 (final concentration) and incubated for 30 min at 37°C. The level of retained DiOC6 was analyzed on a FACScan cytofluorometer with excitation and emission settings of 488 and 525 nm, respectively. The percentage of cells exhibiting low levels of DiOC6, reflecting loss of mitochondrial membrane potential, was recorded.
Cytochrome c Release
The release of cytochrome c from mitochondria was analyzed by a selective digitonin permeabilization method, as reported previously (Leist et al., 1998). Briefly, at the indicated time points, the culture medium was removed and the cells were trypsinized with the use of trypsin-EDTA. The cells were harvested by sedimentation at 2500 rpm for 5 min, washed in PBS, and counted. An aliquot of 4 × 106 cells was resuspended in 100 μl of permeabilization buffer containing 75 mM NaCl, 8 mM Na2PO4, 1 mM NaH2PO4, pH 7.4, 250 mM sucrose (added fresh before use), 1 mM EDTA, 350 μg/ml digitonin (final concentration 35 μg/4 × 106 cells). Cells were incubated in the above-described buffer for 30 s and then the permeabilization buffer was removed by centrifugation for 1 min at 13,000 × g. Protein from the supernatants of this centrifugation was mixed with equal volume of 2 × cell lysis buffer, boiled at 100°C for 15 min, and separated on a 15% SDS-PAGE. The protein was transferred to nitrocellulose membrane and probed by with the use of primary monoclonal anti-cytochrome c antibody (1:500) overnight. Cytochrome c was detected with ECL detection reagents.
Protein Tyrosine Phosphatase Assay (PTPase Activity).
Cellular PTP activity was assessed by an in vitro assay with autophosphorylated EGFR as substrate. EGFR was purified from A431 cells by affinity chromatography on lentil lectin Sepharose as previously described (Tomic et al., 1995). The affinity purified 32P-EGFR was eluted from Sepharose beads with 0.3 M mannose. Heptocytes were treated with 50 μM DCA and 5 min after treatment washed twice with ice-cold PBS, and immediately scrapped into 150 μl of degassed lysis buffer (50 mM HEPES, pH 7.4, 150 mM Na Cl, 1% Triton X-100, 1 mM EDTA, 20 mM NaF, 10% glycerol, 1 mg/ml bovine serum albumin, 1 μg/ml each aprotinin and leupeptin. Lysates were equilibrated on ice for 10 min and after a 5-min microcentrifugation, the resulting supernatants were assayed for PTPase activity. The PTP assay was initiated by adding 20 μl of EGFR substrate (∼ 4 × 104 cpm) to 20 μl lysate (∼20 μg of protein) at room temperature. After 5 and 10 min, PTP activity was terminated by the addition of ice-cold trichloroacetic acid to 10% (wt/vol) final. After microcentrifugation for 5 min, 32Pi radioactivity in the supernatants was determined by liquid scintillation spectroscopy as a measure of PTPase activity.
Assay for DNA Synthesis in Primary Hepatocytes.
For this purpose, after cells were treated with respective regimes, hepatocytes were further incubated in the presence of 4 μCi of [3H]thymidine/ml of culture media for 24 h. The cells were then lysed with 0.5 M NaOH and DNA-precipitated with 12.5% (wt/vol) trichloroacetic acid. Acid-precipitable material was recovered by high-speed centrifugation and washed three times with 5% (wt/vol) trichloroacetic acid, and [3H]thymidine incorporation into DNA was quantified by liquid scintillation spectrometry.
Protein Assay.
Protein concentration of each sample was determined by the method of Bradford (1976). Bovine serum albumin was used to generate standard curve.
Data Analysis.
Comparison of the effects of various treatments was performed with the use of one-way analysis of variance and a two-tailed t test. Differences with a p value of <0.05 were considered statistically significant. Experiments shown are the means of multiple individual points (± SEM).
RESULTS
Treatment of Primary Rat Hepatocytes with DCA Activates EGFR/Ras/MAPK Signaling Module
DCA is found in human and rodent bile ducts over a broad concentration range, from ∼10 to 100 μM (Thomas et al., 2000). Levels of DCA, however, can be much higher in the colon (Qiao et al., 2001). Treatment of primary hepatocytes with either DCA or EGF caused a rapid activation of the EGFR (Figure 1), as judged by receptor tyrosine phosphorylation. Tyrosine phosphorylation of EGFR was blocked by AG1478, a specific tyrphostin inhibitor of EGFR, and by dominant negative EGFR-CD533, as previously reported (Dent et al., 1999; Reardon et al., 1999; our unpublished results). The DCA-induced increase in EGFR tyrosine phosphorylation correlated with a reduction in total cellular protein tyrosine phosphatase activity, as measured in vitro versus purified 32P-phosphorylated-EGFR (Figure 1). Activation of the EGFR by DCA was not blocked by incubating cells with neutralizing antibodies versus autocrine ligands of EGFR, either transforming growth factor-α or EGF, arguing that DCA-induced EGFR activation is ligand-independent (our unpublished results; in agreement with Dent et al., 1999; Hagan et al., 2000).
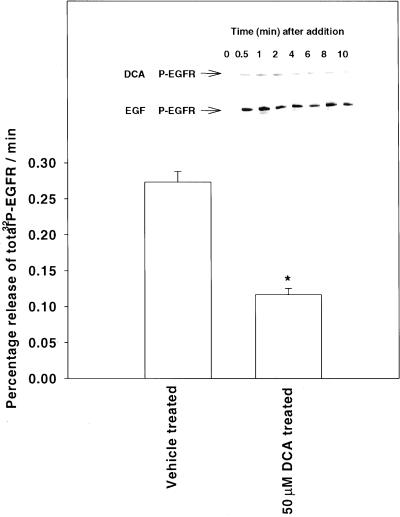
Figure 1.
Deoxycholic acid inhibits PTPase activity in hepatocytes, leading to activation of the EGFR and MAPK pathway. Cells were treated with DCA and 5 min after addition, cells were lysed and cellular PTPase activity was measured in vitro versus phospho-EGFR, as described in MATERIALS AND METHODS. Data shown represent the rate of 32P release from phospho-EGFR (per min) from the means ± SEM of four determinations. *p < 0.05 less than control. (Inset) Cells were treated with DCA and either vehicle control (VEH, DMSO), AG1478 (1 μM) or infected with a recombinant adenovirus to express dominant negative EGFR-CD533. EGFR was immunoprecipitated 0–10 min after addition. Immunoprecipitates were subjected to SDS-PAGE followed by immunoblotting versus either anti-EGFR or antiphosphotyrosine. A representative experiment is shown (n = 4).
Since exposure of hepatocytes to DCA activated the EGFR, we next investigated whether it also activated a downstream signal transduction cascade, the MAPK pathway. Growth factor signaling by the EGFR to MAPK in primary hepatocytes is mediated via the proto-oncogenes Ras and Raf-1 (Auer et al., 1998). In agreement with previous findings for growth factor stimulation and our results in Figure 1, expression of either dominant negative EGFR-CD533 or dominant negative Ras N17 blocked MAPK activation by DCA (Figure 2A). DCA caused a prolonged potent activation of MAPK for >4 h that was abolished by the free radical scavenger N-acetyl cysteine (our unpublished results). However, down-regulation of “classical” protein kinase C (PKC) isoform expression by a 24-h preincubation with bryostatin 1 did not abolish MAPK activation (Figure 2A). MAPK activation by DCA was completely blocked by multiple chemically dissimilar MEK1/2 inhibitors and by >70% for 120 min after treatment with the use of inhibitors of PI3 kinase LY294002 and wortmanin (Figure 2B, inset; our unpublished observations).
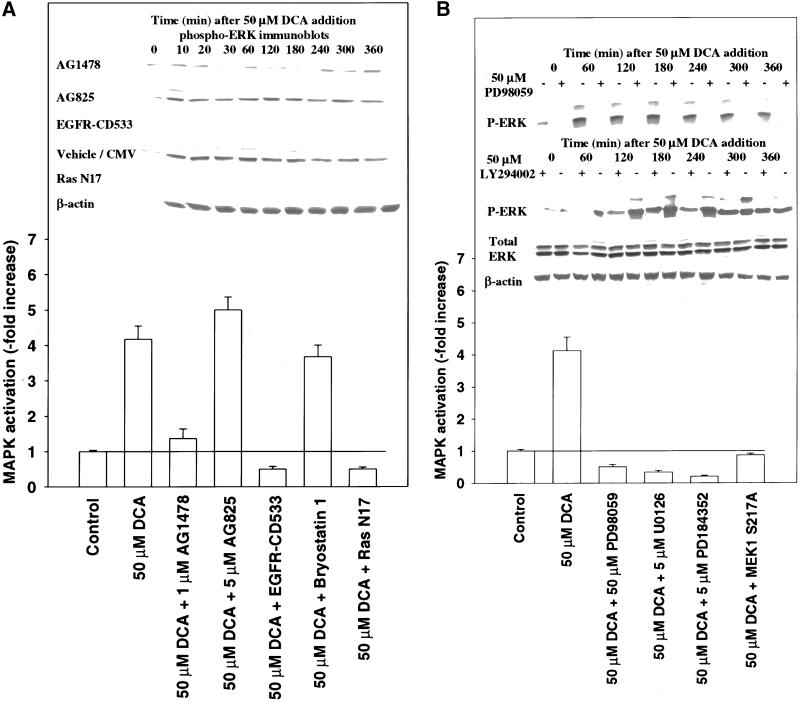
Figure 2.
DCA-induced activation of MAPK proceeds via the proto-oncogene Ras and not via classical PKC isoforms. (A) Cells were either pretreated with vehicle control (DMSO, VEH), bryostatin 1 (10 nM), or infected with either recombinant adenoviruses to express Ras N17, EGFR-CD533, or a null virus. Twenty-four hours after initial treatment/infection, cells were exposed to DCA (50 μM) and/or vehicle control (H2O, VEH), and 20 min after exposure, MAPK immunoprecipitated. Cells were lysed and portions (∼100 μg) from each plate well used to immunoprecipitate MAPK followed by immune-complex kinase assays as described in MATERIALS AND METHODS. (Inset) Cells were treated with 50 μM DCA in the presence or absence of either EGFR-CD533 or Ras N17 and MAPK activity determined >0–360 min by immunoblotting of cell lysates. Lysates were subjected to SDS-PAGE followed by immunoblotting versus antiphospho-MAPK antibody. A representative experiment is shown (n = 3). (B) DCA activates MAPK in hepatocytes, which is blocked by multiple small molecular weight chemical inhibitors of MEK1/2, dominant negative MEK1, and inhibitors of PI3 kinase. Cells were infected with either poly-l-lysine–conjugated adenoviruses to express either dominant negative MEK1 S217A or a null virus. Twenty-four hours after infection, cells were pretreated with either vehicle control (DMSO, VEH) or with PD98059 (50 μM), U0126 (5 μM), PD184352 (5 μM), wortmanin (5 μM), or LY294002 (50 μM). Cells were exposed to DCA (50 μM) and MAPK was activity determined 0–360 min after addition, via immunoblotting of cell lysates. Lysates were subjected to SDS-PAGE followed by immunoblotting versus antiphospho-MAPK antibody. A representative experiment is shown (n = 3).
MAPK Inhibition Promotes Cell Death in Primary Hepatocytes Treated with DCA
Several studies have argued that a variety of different bile acids at high concentrations (>250 μM) can cause apoptosis in primary hepatocytes (Patel et al., 1994; Miyoshi et al., 1999; Martinez-Diez et al., 2000; Rust et al., 2000). In addition, we have found that the killing of tumor cells exposed to cytotoxic stresses can frequently be enhanced by inhibition of the EGFR/Ras/MAPK pathway (Reardon et al., 1999). Because of these findings, we next examined whether inhibition of DCA-induced EGFR/Ras/MAPK signaling impacted on hepatocyte cell survival.
Exposure of primary rat hepatocytes to either 50 μM DCA or 50 μM PD98059 did not significantly increase basal apoptosis within 6 h. Combined exposure to both DCA and PD98059, however, enhanced apoptosis from ∼2 to ∼20–25% within 6 h (Figure 3A). Treatment of rat hepatocytes with higher concentrations of bile acid alone resulted in apoptosis, which was further potentiated by MAPK inhibition (Figure 3A). The apoptotic response of rat hepatocytes was also potentiated by a variety of MEK1/2 inhibitors (Figure 3B). Treatment of primary mouse and primary human hepatocytes with either 50–150 μM DCA or 50 μM PD98059 also did not significantly increase basal apoptosis within 6 h. However, combined exposure to both DCA and PD98059 in these cells enhanced apoptosis from ∼1 to ∼20% within 6 h (Figure 3C). Similar data were obtained with the use of the inhibitors of PI3 kinase, wortmanin and LY294002, in agreement with the ability of these drugs to also blunt MAPK activation (Figure 3D).
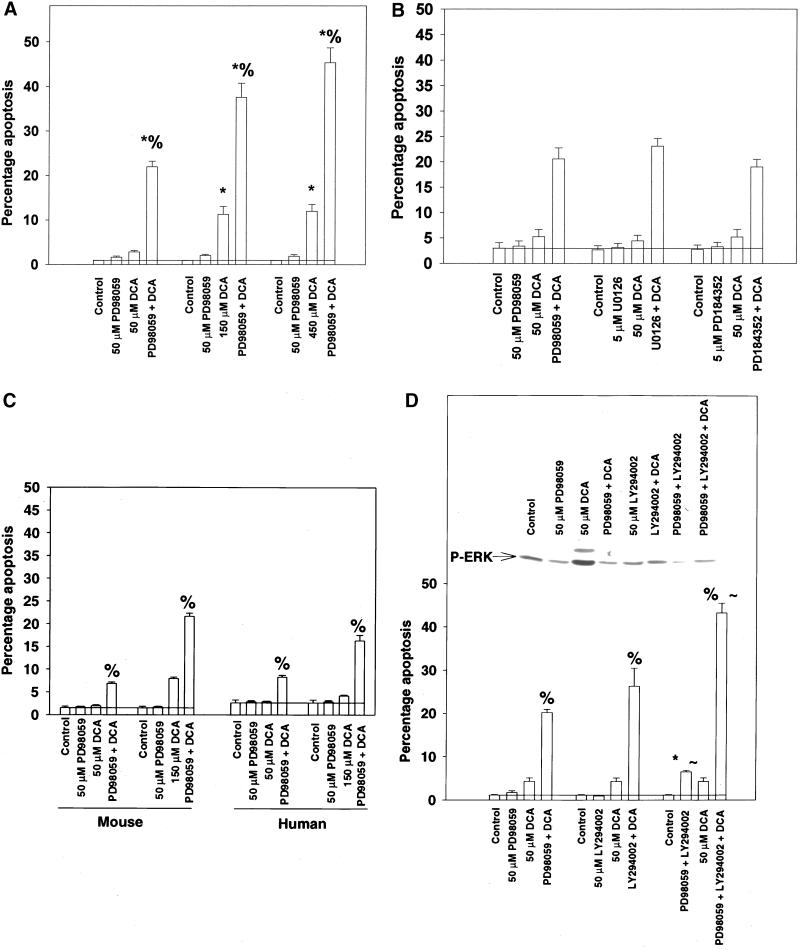
Figure 3.
Inhibition of MAPK potentiates DCA-induced apoptosis in hepatocytes within 6 h. (A) DCA increases apoptosis in hepatocytes that is potentiated by MAPK inhibition. Cells were treated with DCA (50, 150, 450 μM) and either vehicle control (DMSO, VEH) or PD98059 (50 μM) and the percentage of apoptosis determined 360 min after addition with the use of H-33342. Data are the means ± SEM of three independent experiments. *p < 0.05, greater than control value; %p < 0.05, greater than DCA alone. (B) DCA increases apoptosis in hepatocytes that is potentiated by a variety of MAPK inhibitors. Cells were pretreated with either vehicle or with PD98059 (50 μM), U0126 (5 μM), or PD184352 (5 μM). Cells were exposed to DCA (50 μM) and the percentage of apoptosis determined 360 min after addition. Data are the means ± SEM of three experiments. (C) DCA increases apoptosis in primary mouse and human hepatocytes, which is potentiated by MAPK inhibition. Cells were treated with DCA (50, 150 μM) and either vehicle control or PD98059 (50 μM) and the percentage of apoptosis determined 360 min after addition. Data are the means ± SEM of three independent experiments. (D) DCA increases apoptosis in hepatocytes, which is enhanced by inhibitors of PI3 kinase and MEK1/2. Cells were treated with DCA (50 μM) and either vehicle control (DMSO), LY294002 (50 μM), PD98059 (50 μM), or both kinase inhibitors and the percentage of apoptosis determined 360 min after addition. Data are the means ± SEM of three experiments. *p < 0.05, greater than control value; %p < 0.05, greater than DCA alone; ∼p < 0.05, greater than corresponding values in hepatocytes treated with one kinase inhibitor. (Inset) Cells were exposed to DCA and MAPK activity determined 360 min after addition via immunoblotting of cell lysates versus antiphospho-MAPK antibody.
In Figures 1 and 2, we demonstrated that inhibition of either EGFR or Ras function blocked the ability of DCA to activate MAPK. We thus examined whether EGFR-CD533 or Ras N17 could also potentiate DCA-induced apoptosis (Figure 4). Inhibition of EGFR function by EGFR-CD533 or inhibition of Ras function by Ras N17 potentiated DCA-induced apoptosis to within ∼75–100% of the value observed for direct inhibition of the MAPK pathway (Figure 4; cf. Figure 3A). Similar data were obtained in primary mouse and primary human hepatocytes (our unpublished results). Collectively, the data in Figures 3 and 4 demonstrate that DCA-induced EGFR/Ras/MAPK activity is a cytoprotective response of primary rodent and human hepatocytes to DCA exposure.
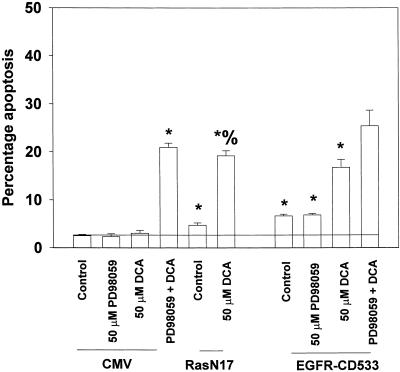
Figure 4.
Inhibition of either EGFR or Ras function, which both block MAPK activation, also potentiates DCA-induced apoptosis in hepatocytes. Cells were infected with recombinant adenoviruses to express either a null virus, a recombinant virus Ras N17, or a recombinant virus EGFR-CD533. Twenty-four hours after infection, cells were treated with either vehicle control (DMSO, VEH) or PD98059 (50 μM), as indicated, and then exposed to DCA (50 μM) and/or vehicle control, followed by determination of apoptosis 360 min afterH-33342. Data shown are the means of three independent experiments ± SEM. *p < 0.05, greater than corresponding control value; %p < 0.05, greater than DCA alone.
Inhibition of MAPK Signaling Enhances Bile Acid-Induced Cleavage of Procaspases and BID, which Correlates with Loss of Mitochondrial Membrane Permeability Transition and Release of Cytochrome c
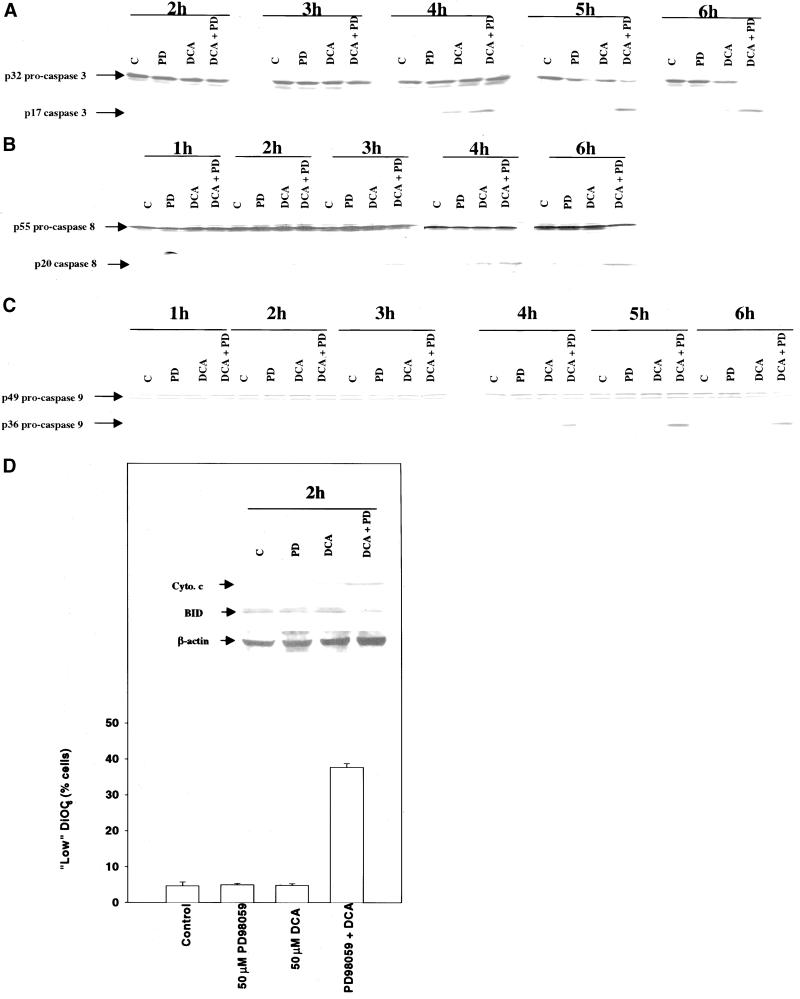
Because we had observed apoptosis in Figures 3 and 4, we next examined the activation of caspases in primary hepatocytes. Exposure of rat hepatocytes to either DCA or MEK1/2 inhibitor alone caused little alteration in the protein levels of the executioner caspase procaspase 3 over 6 h (Figure 5A). However, combined exposure to DCA and MEK1/2 inhibitor resulted in a time-dependent reduction in the protein levels of p32 procaspase 3 and an increase in the cleaved active p17 form of the molecule. The appearance of the cleaved active form of the caspase 3 molecule was readily detectable only ∼4–6h after treatment. Because of these findings, we determined the integrity of other caspase enzymes, 0–6 h after treatment, which may be potentially upstream of procaspase 3: procaspase 8 (Figure 5B) and procaspase 9 (Figure 5C). Appearance of the p20 caspase 8 cleavage product was observed as early as ∼1–2 h after exposure without an apparent large alteration in p55 procaspase 8 levels, whereas cleavage of procaspase 9 occurred later, within a similar time frame to that observed for procaspase 3 (∼3–6 h). Activated caspase 8 has been proposed to promote procaspase 9 activation via cleavage of BID, resulting in BID translocation to the mitochondria, leading to release of cytochrome c and activation of procaspase 9. Combined exposure to DCA and MEK1/2 inhibitor promoted loss of the mitochondrial membrane permeability potential 2 h after exposure (Figure 5D), which correlated with cleavage of BID (Figure 5D, inset) and release of cytochrome c into the cytosol (Figure 5D, inset).
Figure 5.
Potentiation of bile acid-induced apoptosis by MAPK inhibition correlates with cleavage of procaspase 8, BID, procaspase 9, and procaspase 3. (A) DCA causes cleavage of procaspase 3, which is potentiated by MAPK inhibition. Cells were treated with DCA (50 μM) and either vehicle control (DMSO, VEH) or PD98059 (50 μM) and the protein levels of procaspase 3 determined 0–360 min after addition. Data are from a representative experiment (n = 3). (B) DCA causes cleavage of procaspase 8, which is potentiated by MAPK inhibition. Cells were treated with DCA (50 μM) and either vehicle control (DMSO, VEH) or PD98059 (50 μM) and the protein levels of procaspase 8 determined 0–360 min after addition. Data are from a representative experiment (n = 3). (C) DCA causes cleavage of procaspase 9, which is potentiated by MAPK inhibition. Cells were treated with DCA (50 μM) and either vehicle control or PD98059 (50 μM) and the protein levels of procaspase 9 determined 0–360 min after addition. Data are from a representative experiment (n = 3). (D) DCA causes little reduction in the mitochondrial membrane potential 2 h after exposure, which is dramatically potentiated by MAPK inhibition. Cells were treated with DCA (50 μM) and PD98059 (50 μM), and the mitochondrial membrane potential was determined 120 min after addition. Data are the means of three experiments ± SEM. (Inset) DCA causes cleavage of BID and release of cytochrome c into the cytosol 2 h after exposure, which is potentiated by MAPK inhibition. Cells were treated with DCA (50 μM) and either vehicle control or PD98059 (50 μM), and total protein levels of BID and cytosolic levels of cytochrome c were determined 120 min after addition. Data are from a representative experiment (n = 3).
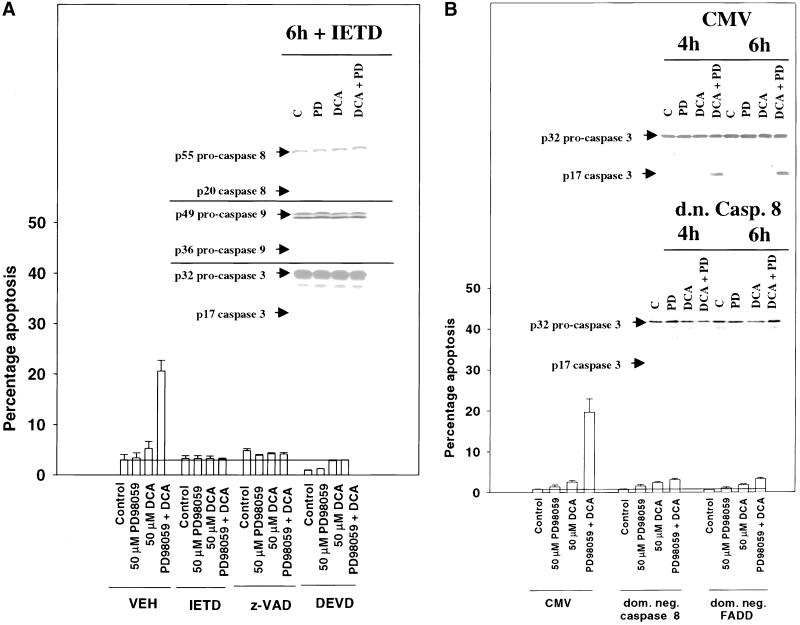
Incubation of hepatocytes with either the pan-caspase inhibitor z-VAD-fmk, the caspase 8-specific inhibitor IETD-fmk, or the caspase 3-specific inhibitor DEVD-fmk blocked the potentiation of bile acid-induced apoptosis by MAPK inhibition (Figure 6A). Furthermore, cleavage of all procaspases was blocked by incubation of cells with IETD-fmk (Figure 6A, inset; cf. Figure 5, A–C). Because peptide inhibitors of caspases may have overlapping specificities with other cytotoxic proteases, e.g., cathespins (Guicciardi et al., 2000), we also made use of dominant negative caspase molecules. Expression of either dominant negative procaspase 8 or dominant negative FADD blocked the potentiation of bile acid-induced apoptosis by MAPK inhibition (Figure 6B). Dominant negative procaspase 8 blocked cleavage of procaspase 3 (Figure 6B, inset). Collectively, these findings suggest that low concentrations of DCA rapidly activate a “death receptor”/FADD/caspase 8 pathway, whose proapoptotic function is inhibited by DCA-induced MAPK signaling.
Figure 6.
Inhibition of a death receptor/procaspase 8 pathway blocks the potentiation of bile acid-induced apoptosis and procaspase cleavage by MAPK inhibition. (A) DCA increases apoptosis in rat hepatocytes, which is potentiated by MAPK inhibition and blocked by peptide inhibitors of caspases. Cells were pretreated with either vehicle, z-VAD-fmk (20 μM), IETD-fmk (20 μM), or DEVD-fmk (20 μM) followed 30 min afterward by DCA (50 μM) and either vehicle control or PD98059 (50 μM), and the percentage of apoptosis was determined 360 min after addition H-33342. Data the means ± SEM of three independent experiments. (Inset) DCA causes cleavage of procaspase 3, procaspase 8, and procaspase 9, which is potentiated by MAPK inhibition and blocked by peptide inhibitors of caspases. Cells were pretreated with either vehicle or IETD-fmk (20 μM) followed 30 min afterward by DCA (50 μM) and either vehicle control or PD98059 (50 μM), and the protein expression levels of each procaspase were determined 360 min after addition. Data are from a representative experiment (n = 3). (B) DCA increases apoptosis in hepatocytes, which is potentiated by MAPK inhibition and blocked by expression of either dominant negative FADD or dominant negative procaspase 8. Cells were infected with poly-l-lysine–conjugated adenoviruses to express either dominant negative procaspase 8, dominant negative FADD, or a null virus. Twenty-four hours after infection, cells were treated with DCA (50 μM) and either vehicle control or PD98059 (50 μM) and the percentage of apoptosis was determined 360 min after addition. Data are the means of three experiments ± SEM. (Inset) Dominant negative procaspase 8 blocks cleavage of procaspase 3. Cells were infected with poly-l-lysine–conjugated adenoviruses to express either control plasmid or dominant negative procaspase 8. Twenty-four hours after infection, cells were treated with DCA (50 μM) and either vehicle control or PD98059 (50 μM), and the protein levels of procaspase 3 were determined 360 min after addition.
Potentiation of Bile Acid-induced Apoptosis in Primary Mouse Hepatocytes Is Mediated by Ligand-independent and Ligand-dependent Activation of FAS Receptor, which Is Related to Bile Acid Concentration
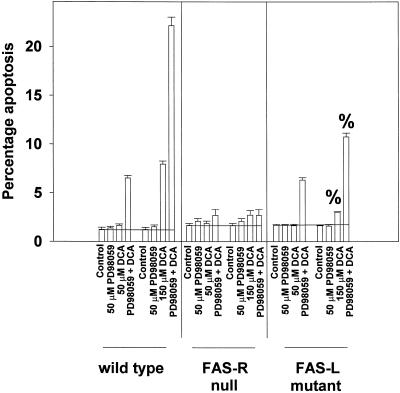
Because of our data arguing that dominant negative FADD blocked the potentiation of apoptosis, we made use of primary mouse hepatocytes expressing either a nonfunctional mutant FAS ligand or were embryonically deleted for expression of the FAS receptor. Initial studies demonstrated that mouse hepatocytes displayed a similar potentiation of DCA-induced apoptosis to rat hepatocytes when MAPK signaling was inhibited (Figure 7). Loss of FAS receptor expression abolished DCA alone and DCA plus MEK1/2 inhibitor-induced apoptosis (Figure 7). Loss of FAS ligand function did not, however, alter the ability of MAPK inhibition to potentiate apoptosis in response to treatment of cells with 50 μM DCA (Figure 7). These data argue that the potentiation of DCA-induced apoptosis by MAPK inhibition, in response to low concentrations of bile acid, is dependent upon ligand-independent activation of the FAS receptor. When hepatocytes from FAS ligand mutant mice were exposed to a higher concentration of DCA (150 μM), a significant reduction in both DCA alone and DCA plus MEK1/2 inhibitor-induced apoptosis was observed, compared with wild-type cells expressing FAS ligand. This finding argues that high concentrations of DCA use ligand-dependent and ligand-independent mechanisms to induce hepatocyte apoptosis via the FAS receptor.
Figure 7.
MAPK potentiates apoptosis after treatment with low concentrations of DCA by a ligand-independent activation of the FAS receptor: higher concentrations cause apoptosis via both ligand-dependent and ligand-independent FAS receptor activation. Primary mouse hepatocytes from wild-type, FAS receptor null and FAS ligand mutant mice were isolated as described in MATERIALS AND METHODS. Mouse hepatocytes were treated with DCA (50, 150 μM) and either vehicle control or PD98059 (50 μM), and the percentage of apoptosis was determined 360 min after addition of H-33342. Data are the means ± SEM of three independent experiments. %p < 0.05, less than corresponding value obtained from wild-type hepatocytes.
Caspase 9 Function Is Required to Permit Potentiation of DCA-induced Apoptosis by MAPK Inhibition
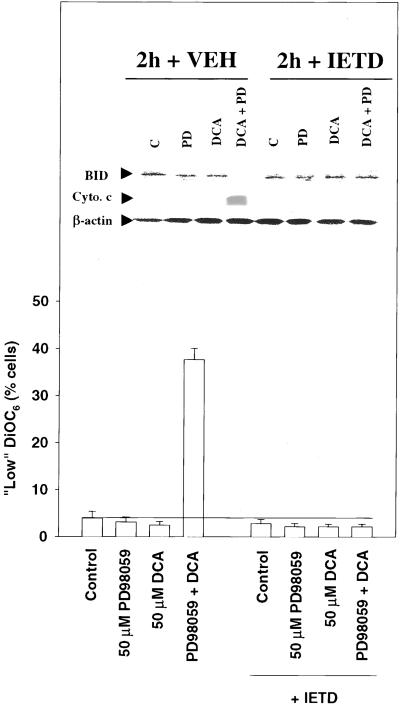
Recent studies with the use of TRAIL, a death receptor ligand, have argued that death receptor signaling toward apoptosis in hepatocytes requires caspase 8-stimulated release of cytochrome c from the mitochondrion, leading to activation of procaspase 9 (Jo et al., 2000; Ozoren et al., 2000). Furthermore, mice null for expression of BID are reported to be resistant to FAS-induced hepatocellular apoptosis, which is independent of Bax function (Yin et al., 1999; Kim et al., 2000). Inhibition of caspase 8 function maintained the mitochondrial membrane potential 2 h after exposure to DCA and MEK1/2 inhibitor (Figure 8), blocked cleavage of BID (Figure 8, inset), and abolished the release of cytochrome c into the cytosol (Figure 8, inset). These findings further suggest that the initial primary mechanism by which DCA-induced mitochondrial dysfunction occurs is via a death receptor/caspase 8 pathway.
Figure 8.
Bile acid-induced cleavage of BID, loss of mitochondrial membrane potential, and release of cytochrome c is blocked by inhibition of procaspase 8, 2 h after exposure. Rat hepatocytes were pretreated with either vehicle or IETD-fmk (20 μM) followed 30 min afterward by DCA (50 μM) and either vehicle control or PD98059 (50 μM) and the mitochondrial membrane potential determined 120 min after addition. Data are the means of three experiments ± SEM. (Inset) DCA increases BID cleavage and increases release of cytochrome c into the cytosol of hepatocytes, which is potentiated by MAPK inhibition, and blocked by a peptide inhibitor of caspase 8. Cells were pretreated with either vehicle or IETD-fmk (20 μM) followed 30 min afterward by DCA (50 μM) and either vehicle control or PD98059 (50 μM), and the expression of total BID and cytosolic cytochrome c was determined 120 min after addition. Data are representative of three experiments.
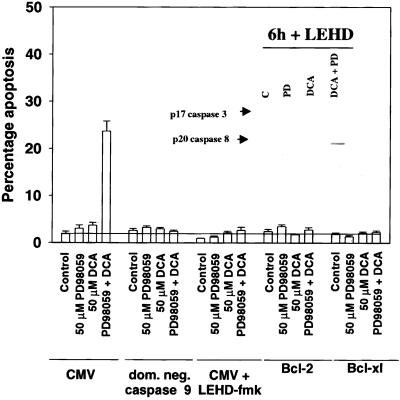
It has been proposed that active caspase 8 may mediate activation of the downstream executioner procaspase 3 via two overlapping mechanisms. Caspase 8 either directly induces procaspase 3 cleavage or induces cleavage indirectly by a mitochondrial amplification loop requiring BID cleavage, cytochrome c release, and activation of procaspase 9 (Kurosawa et al., 1997; Yin et al., 1999; Chang and Xu, 2000; Ozoren et al., 2000). Expression of dominant negative procaspase 9 or treatment of cells with a peptide inhibitor of caspase 9, LEHD-fmk, blocked the potentiation of DCA-induced apoptosis by MAPK inhibition (Figure 9) and also abolished cleavage of procaspase 3 (Figure 9, inset). This blockade occurred even though cleavage of procaspase 8 and BID were observed (Figure 9, inset; our unpublished observations). Furthermore, overexpression of either Bcl-2 or Bcl-XL also inhibited the potentiation of apoptosis (Figure 9). Overexpression of either Bcl-2 or Bcl-XL blocked the release of cytochrome c into the cytosol but did not block appearance of the p20 cleavage product of caspase 8, 2 h after exposure (our unpublished results). Collectively, these data suggest that caspase 8 requires an amplification of its activity, via the mitochondrion and procaspase 9, to achieve activation of procaspase 3 and the apoptotic execution of primary hepatocytes.
Figure 9.
Inhibition of procaspase 9 function also blocks the potentiation of bile acid-induced apoptosis by MAPK inhibition. DCA increases apoptosis in hepatocytes, which is potentiated by MAPK inhibition and blocked by expression of either Bcl-2, Bcl-XL, dominant negative procaspase 9 or the peptide inhibitor of caspase 9, LEHD-fmk. Cells were infected with poly-l-lysine–conjugated adenoviruses to express either dominant negative procaspase 9 or a null virus. Cells were infected with recombinant adenoviruses to express either Bcl-2, Bcl-XL or a null virus. Twenty-four hours after infection, cells were pretreated with either vehicle control or LEHD-fmk (40 μM) and cells were subsequently treated with DCA (50 μM) and either vehicle control or PD98059 (50 μM), and the percentage of apoptosis was determined 360 min after addition of H-33342. Data are the means of three experiments ± SEM. No difference in the apoptotic profile was observed comparing CMV poly-l-lysine virus infected to CMV recombinant virus-infected cells. (Inset) LEHD prevents cleavage of procaspase 3 but not procaspase 8 2 h after exposure. Cells were pretreated with either vehicle or LEHD-fmk (20 μM) followed 30 min afterward by DCA (50 μM) and either vehicle control or PD98059 (50 μM), and the expression of p20-cleaved caspase 8 and p17-cleaved caspase 3 determined 120 min after addition. Data are representative of two experiments.
Modulation of Caspase Inhibitor Protein Expression Levels in Rat Hepatocytes Correlates with Potentiation of DCA-induced Apoptosis by MAPK Inhibition
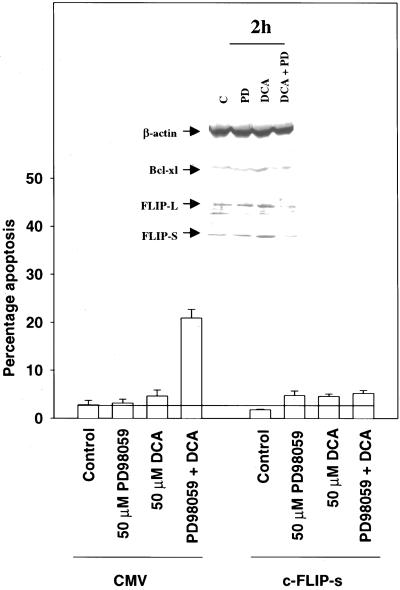
Recent studies in transformed cell types have argued that one mechanism by which MAPK signaling can blunt FAS-induced apoptosis is via modulating the expression of caspase inhibitor proteins, e.g., c-FLIP, and mitochondrial-associated antiapoptotic proteins, e.g., Mcl-1 and Bcl-XL (Yeh et al., 1998; Leu et al., 2000; Jost et al., 2001). We discovered that treatment of rat hepatocytes with DCA increased expression of c-FLIP-S and c-FLIP-L, whose expression was almost abolished in cells treated with MEK1/2 inhibitors (Figure 10, inset). In contrast, a PI3 kinase inhibitor LY294002 completely abolished expression of c-FLIP-S and c-FLIP-L, regardless of DCA exposure (our unpublished results). DCA did not alter the low total protein levels of Bcl-2, Bax, or BAD, but did, however, enhance expression of Bcl-XL that was blocked by inhibition of MAPK signaling (Figure 10; our unpublished results). This was not observed in mouse hepatocytes (our unpublished observations).
Figure 10.
Potentiation of DCA-induced apoptosis by MAPK inhibition correlates with reduced protein expression of c-FLIP-L, c-FLIP-S and Bcl-XL and is blocked by overexpression of c-FLIP-s. Primary rat hepatocytes were infected with poly-l-lysine–conjugated adenoviruses to express either c-FLIP-S or a CMV null virus. Twenty-four hours after infection, cells were treated with DCA (50 μM) and either vehicle control or PD98059 (50 μM), and the percentage of apoptosis determined 360 min after addition of H-33342. Data are the means of three experiments ± SEM. (Inset) DCA increases protein expression of Bcl-XL, c-FLIP-S, and c-FLIP-L in primary hepatocytes, which is reduced by MAPK inhibition. Cells were treated with DCA (50 μM) and either vehicle control or PD98059 (50 μM), and the protein levels Bcl-XL and c-FLIP isoforms were determined 120 min after addition. Data are a representative experiment (n = 3).
The apoptosis inhibitor c-FLIP is proposed to modulate death receptor-stimulated apoptosis via inhibition of cytosolic procaspase 8 self-processing in the DISC complex (Irmler et al., 1997; Srinivasula et al., 1997; Yeh et al., 1998). Because the potentiation of apoptosis in hepatocytes was dependent upon signaling from the FAS receptor, and c-FLIP-s expression was virtually abolished after combined DCA and MEK1/2 inhibitor exposure, we investigated whether enforced expression of c-FLIP-s could blunt the apoptotic response in hepatocytes. Constitutive overexpression of c-FLIP-s abolished the potentiation of apoptosis after combined exposure to DCA and PD98059 (Figure 10). Similar data were obtained when an inhibitor of PI3 kinase was used (our unpublished results). Collectively, our data argue that DCA induces ligand-independent activation of the FAS receptor in hepatocytes that can lead to cell death, but which is blunted by the EGFR/MAPK-dependent enhancement in the expression of c-FLIP-s.
DISCUSSION
Inhibition of the EGFR/Ras/MAPK pathway has been shown to enhance the toxicity of a variety of cellular stresses, and molecules that inhibit EGFR/Ras/MAPK pathway function are currently entering clinical trials to treat cancer. Exposure of either hepatocytes or colonic epithelial cells to bile acids is also known to cause a variety of cellular stresses, including DNA damage and cell death (Jones et al., 1997). Furthermore, previous studies have argued that conjugated bile acids can cause apoptosis in hepatocytes, and that the mechanism(s) by which this occurs may be dependent upon ligand-independent signaling from the death receptor FAS/APO-1/CD95. In contrast, other data have argued that DCA causes apoptosis in hepatocytes by impacting directly upon mitochondrial function, leading to BAX-dependent cytochrome c release into the cytosol (Rodrigues et al., 1998). The studies in this article were designed to determine the impact of unconjugated deoxycholic acid on MAPK activity and the proliferation and survival of primary rodent and human hepatocytes.
Treatment of hepatocytes with DCA caused a prolonged activation of the both the EGFR and the MAPK pathway. Activation of the EGFR was ligand-independent and increased tyrosine phosphorylation of EGFR correlated with reduced anti-EGFR protein tyrosine phosphatase activity in cells. Activation of the MAPK pathway was dependent upon EGFR signaling as judged by molecular inhibitors of EGFR function blocking the MAPK response. EGFR and MAPK activation were also blocked by pretreatment of cells with N-acetyl cysteine, which may act to protect protein tyrosine phosphatase activity in cells. Since protein phosphatases tend to have 1–2 orders of magnitude greater catalytic activity than protein kinases (Tonks, 1996), it is probable that DCA-mediated inhibition of phosphatase activity accounts for the activation of the EGFR. The mechanisms by which DCA may reduce tyrosine phosphatase activity in cells, such as the generation of reactive oxygen and nitrogen species, remain to be determined.
DCA activated the EGFR and this signal was transduced to the MAPK pathway via Ras and the PI3 kinase pathway, as could be expected for treatment of hepatocytes with natural ligands of the EGFR. Several studies have argued that bile acids, including DCA, can activate PKC isoforms, which may play a role in MAPK activation. However, DCA-induced MAPK activation was not dependent upon “classical” PKC enzymes as judged by the inability of bryostatin 1-mediated PKC down-regulation to block MAPK activation. It is possible that other PKC isoforms, which are not down-regulated by bryostatin 1, may play a role in DCA-mediated MAPK activation (Rust et al., 2000).
Bile acids are known to cause apoptosis and as such, treatment of a hepatocyte with a bile acid can be analogized to exposure of cell to a cytotoxic stress. This is of note, because the toxicity of stresses can be amplified when EGFR/Ras/MAPK activation is reduced (Dent et al., 1999). Thus, we discovered that when DCA-induced EGFR/Ras/MAPK signaling was abolished, either by direct inhibition of the MAPK pathway, by inhibition of Ras, by inhibition of PI3 kinase, or by inhibition of the EGFR, DCA-induced apoptosis was enhanced. This effect was particularly striking at lower concentrations of DCA, which, by themselves, did not cause a significant amount of apoptosis within 6 h but in the presence of MAPK inhibition caused a >10-fold increase in morbidity above basal levels. Thus, in a similar manner to studies with the use of the drug Ara C, hydrogen peroxide, and ionizing radiation, it appears that DCA is toxic to primary hepatocytes and that DCA has a self-limiting effect on its toxicity by activating the EGFR/Ras/MAPK pathway (Wang et al., 1998a; Schmidt-Ullrich et al., 2000).
Glycine conjugates of chenodeoxycholic acid have been shown to promote apoptosis in hepatocytes and in these studies, signaling by the novel PKC isoform PKC zeta, via the PI3 kinase pathway, could play a protective role (Rust et al., 2000). We found that PI3 kinase inhibitors blunted DCA-induced MAPK activation in primary hepatocytes by ∼70% within 2 h after DCA treatment. Since it was during the first 2 h after treatment where the initial cleavage of BID and cytochrome c release occurred, it is likely that a portion of DCA-induced PI3 kinase signaling plays its cytoprotective role via the MAPK pathway.
Because of the rapid potentiation of apoptosis, we examined the impact of DCA and MAPK inhibition on the expression of procaspase molecules. DCA and MAPK inhibition promoted detectable cleavage of procaspase 8 within 2 h, and procaspase 9 and procaspase 3 within 3–6 h. Of note, however, profound cleavage of p55 procaspase 8 did not occur until 6 h after exposure, suggesting that p55 cleavage at this time is mediated by active caspase 3 rather than from its own autoprocessing in a DISC complex. Dominant negative procaspase 8 or the inhibitor of caspase 8 IETD-fmk blocked the potentiation of apoptosis, as did expression of dominant negative FADD. This finding argues that DCA is recruiting death receptor(s) upstream of procaspase 8 to initiate the apoptotic response, and that these receptors play a key role in the process by which DCA induced apoptosis. In agreement with the recruitment of death receptor signaling, hepatocytes from mice that did not express the FAS receptor, but of note that still expressed other death receptors capable of forming DISC complexes, were unable to undergo apoptosis in response to either DCA alone or DCA in combination with a MEK1/2 inhibitor. In hepatocytes that expressed a mutant FAS ligand, DCA remained competent to induce apoptosis. Thus, our data tend to favor a mechanism in which DCA caused ligand-independent activation of the FAS receptor that was a primary signaling event in the apoptotic response. How DCA enhanced FAS receptor signaling, perhaps also via the generation of reactive oxygen and nitrogen species, remains to be determined.
From these findings, it appeared that exposure to DCA increased apoptosis in hepatocytes by two overlapping mechanisms. The potentiation of DCA-induced (50 μM) apoptosis by MEK1/2 inhibitors was dependent upon a functional FAS receptor, but independent of FAS ligand expression. In contrast, although DCA-induced (150 μM) apoptosis was also dependent upon a functional FAS receptor, a reduced potentiation of the apoptotic response by MEK1/2 inhibitors was observed in cells expressing a mutant FAS ligand. This is in contrast to data with other bile acids where FAS ligand has been shown to not play any role in the apoptotic process (Faubion et al., 1999). Hepatoma cells can lose either FAS-R and/or FAS-L function during transformation. Loss of function within the FAS autocrine loop will thus enhance tumor cell survival in response to multiple toxic agents, including bile acids.
Recently, it was shown that FAS-mediated killing in primary hepatocytes required BID, arguing that active caspase 8 does not directly mediate cleavage and activation of procaspase 3 in primary hepatocytes (Yin et al., 1999), as has been proposed in other cell types (Bossy-Wetzel and Green, 1999; Engels et al., 2000, and references therein; Ozoren et al., 2000). Thus, for active caspase 8 to activate executioner procaspases, a mitochondrial amplification loop of BID, cytochrome c release and activation of procaspase 9 would be required. In agreement with this concept, BID was rapidly cleaved in response to combined exposure to DCA and MEK1/2 inhibition. BID cleavage was likely to be a causal factor in the release of cytochrome c in to the cytosol, because incubation of cells with IETD-fmk blocked BID cleavage and cytochrome c release.
Also concordant with a role for a mitochondrial amplification loop in the killing process, expression of dominant negative procaspase 9 or incubation of hepatocytes with a caspase 9 inhibitor LEHD-fmk blocked the potentiation of bile acid apoptosis, even though cleavage of procaspase 8 and BID was observed. Furthermore, treatment of cells with IETD-fmk or overexpression of either Bcl-2 or Bcl-XL also prevented both cytochrome c release and apoptosis. Collectively, these data argue that for bile acid-activated death receptors and caspase 8 to cause apoptosis in primary hepatocytes, an intact mitochondrial amplification loop is required to achieve activation of the executioner procaspase 3 (Yin, 2000).
Procaspase 8 in some cell systems, but not others, appears to be largely sequestered within the mitochondrion under unstimulated conditions (Zhivotovsky et al., 1999; Qin et al., 2001). It is possible that the profound cleavage of p55 procaspase 8 we observed 5–6 h after treatment is due to release of mitochondrial procaspase 8 into the cytosol, where it can take part in an amplification loop with active caspase 3 to enhance the apoptotic response, in agreement with the findings of Bajt et al. (2000). Proapoptotic pore formation between Bcl-2/Bcl-XL and Bax can also enhance translocation of procaspase 8 and cytochrome c into the cytosol (Yin et al., 1999; Kim et al., 2000; Qin et al., 2001). However, in other studies, Bax translocation to the mitochondria during apoptosis could be inhibited by IETD-fmk, arguing that Bax translocation is secondary to an initial activation of caspase 8 (Kim et al., 2000; Gao et al., 2001). Furthermore, FAS receptor ligation in hepatocytes can cause BID cleavage and cytochrome c release in the absence of Bax expression, although expression of Bax can synergize with BID to cause apoptosis (Kim et al., 2000; Ruffolo et al., 2000). Thus, the relative roles of proteins such as Bax in the amplification of DCA-induced FAS killing 4–6 h after exposure in our system is currently unclear and will require further study.
In agreement with a role for bile acid-induced MAPK signaling in the control of cytoprotective protein expression, DCA treatment of hepatocytes increased expression of Bcl-XL, and c-FLIP-S/L. In the instance of c-FLIP, inhibition of DCA-induced MAPK signaling almost abolished c-FLIP expression. Increased MAPK signaling has been implicated in the control of Bcl-XL expression in keratinocytes (Jost et al., 2001) and Mcl-1 expression in other cell types (Leu et al., 2000). Expression of c-FLIP isoforms has been shown to block death receptor-mediated cell killing in both T cells and E1A-transformed cells (Yeh et al., 1998). Thus, it is possible that DCA-induced PI3 kinase/MAPK signaling may inhibit parallel DCA-induced FAS death signaling by 1) blocking self-processing and activation of procaspase 8 in the DISC complex via c-FLIP-S/L (Holmstrom et al., 2000), and 2) by blocking the downstream release of cytochrome c into the cytosol via Bcl-XL (Tzung et al., 1997).
However, additional mechanisms besides those described above may also play a role in protecting hepatocytes from apoptosis. For example, it has been noted in fibroblasts that prolonged MAPK signaling can inhibit the ability of cytosolic cytochrome c to cause activation of procaspase 3 and apoptosis (Erhardt et al., 1999). In epithelial tumor cells, and in part agreement with our findings, recent studies have also argued that signaling via the PI3 kinase pathway plays a much greater role in the control of c-FLIP expression than MAPK pathway signaling (Panka et al., 2001). It is probable that both DCA-induced PI3 kinase and MAPK signaling is responsible for the increase in c-FLIP expression, as suggested by our data in Figure 3D. This effect may be due to a posttranscriptional stabilization of c-FLIP molecules, as was observed in primary hepatocytes for p21Cip-1/WAF1/Mda6 (Park et al., 2000a,b). Additional studies will be required to explore the interactions between MAPK and PI3 kinase signaling, and their relative cytoprotective roles, after DCA treatment of hepatocytes.
Note Added in Proof
Since the submission of this manuscript, Kovalovich et al. (2001) showed that cytoprotective signaling by IL6 in hepatocytes versus FAS receptor activation correlates with increased c-FLIP and Bcl-XL levels. Takikawa et al. (2001) also showed that the potentiation of bile acid-induced apoptosis by PI3 kinase inhibitors is due to a defect in cytoprotection at the level of the DISC complex, below the FAS receptor and upstream of procaspase 8, which is also suggestive of an involvement of c-FLIP expression being modulated.
ACKNOWLEDGMENTS
This work was funded by Public Health Service Grants R01-DK52825, R01-CA88906, P01-CA72955, and P01-DK38030 and a Department of Defense Career Development Award (BC980148) (to P.D.); Public Health Services Grant P01-DK38030 (to P.B.H.); and Public Health Service Grants P01-CA72955, R01-CA63753, and R01-CA77141, and a Leukemia Society of America Grant 6405-97 (to. S.G.). We thank Dr. Ross Mikkelsen for assistance with PTPase activity measurement; Dr. Craig Logsdon (University of Michigan, Ann Arbor, MI) for Ras N17 adenovirus; Drs. J.C. Reed and S. Krajewski (Burnham Institute, La Jolla, CA) for anti-FLIP, anti-XIAP, and anti-IAP antibodies; Dr. S.C. Strom (University of Pittsburgh, Pittsburgh, PA) for primary human hepatocytes; and Dr. K. Bhalla for mutant FADD and caspase proteins. K.L. is the recipient of a National Institutes of Health training grant.
Abbreviations used:
- DCA
deoxycholic acid
- DMSO
dimethyl sulfoxide
- ECL
enhanced chemiluminescence
- DiOC6
3,3-dihexyloxacarbocyanine
- FADD
Fas-associated protein with death domain
- MAPK
mitogen-activated protein kinase
- MOI
multiplicity of infection
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
- Z-VAD
benzyloxycarbonyl-Val-Ala-Asp fluoromethyl ketone
- IETD
Ile-Glu-Thr-Asp-p-nitroanilide
REFERENCES
- Auer K, Contessa J, Brenz-Verca S, Pirola L, Rusconi S, Cooper G, Abo A, Wymann M, Davis RJ, Birrer M, Dent P. The Ras/Rac1/Cdc42/SEK/JNK/c-Jun cascade is a key pathway by which agonists stimulate DNA synthesis in primary cultures of rat hepatocytes. Mol Biol Cell. 1998;9:561–573. doi: 10.1091/mbc.9.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes, and nonparenchymal cells by a caspase-8 inhibitor in vivo. Evidence for a postmitochondrial processing of caspase-8. Toxicol Sci. 2000;58:109–117. doi: 10.1093/toxsci/58.1.109. [DOI] [PubMed] [Google Scholar]
- Balachandran S, Roberts PC, Kipperman T, Bhalla KN, Compans RW, Archer DR, Barber GN. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway. J Virol. 2000;74:1513–1523. doi: 10.1128/jvi.74.3.1513-1523.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benage D, O'Connor KW. Cholecystocolonic fistula: malabsorptive consequences of lost bile acids. J Clin Gastroenterol. 1990;12:192–194. [PubMed] [Google Scholar]
- Benedetti A, Marucci L. The significance of apoptosis in the liver. Liver. 1999;19:453–463. doi: 10.1111/j.1478-3231.1999.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Bloomer JR, Allen RM, Klatskin G. Serum bile acids in primary biliary cirrhosis. Arch Intern Med. 1976;136:57–61. [PubMed] [Google Scholar]
- Bossy-Wetzel E, Green DR. Caspases induce cytochrome c release from mitochondria by activating cytosolic factors. J Biol Chem. 1999;274:17484–17490. doi: 10.1074/jbc.274.25.17484. [DOI] [PubMed] [Google Scholar]
- Botla R, Spivey J, Aguilar H, Bronk SF, Gores G. Ursodeoxycholate (UDCA) inhibits the mitochondrial membrane permeability transition induced by glycochenodeoxycholate: a mechanism of UDCA cytoprotection. J Pharmacol Exp Ther. 1995;272:930–938. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Celli A, Que FG. Dysregulation of apoptosis in the cholangiopathies and cholangiocarcinoma. Semin Liver Dis. 1998;18:177–185. doi: 10.1055/s-2007-1007153. [DOI] [PubMed] [Google Scholar]
- Chang YC, Xu YH. Expression of Bcl-2 inhibited Fas-mediated apoptosis in human hepatocellular carcinoma BEL-7404 cells. Cell Res. 2000;10:233–242. doi: 10.1038/sj.cr.7290052. [DOI] [PubMed] [Google Scholar]
- Cohen-Jonathan E, Muschel RJ, McKenna GW, Evans SM, Cerniglia G, Mick R, Kusewitt D, Sebti SM, Hamilton AD, Oliff A, Kohl N, Gibbs JB, Bernhard EJ. Farnesyltransferase inhibitors potentiate the antitumor effect of radiation on a human tumor xenograft expressing activated HRAS. Radiat Res. 2000;154:125–132. doi: 10.1667/0033-7587(2000)154[0125:fiptae]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Dent P, Jarvis WD, Birrer MJ, Fisher PB, Schmidt-Ullrich RK, Grant S. The roles of signaling by the p42/p44 mitogen-activated protein (MAP) kinase pathway; a potential route to radio- and chemo-sensitization of tumor cells resulting in the induction of apoptosis and loss of clonogenicity. Leukemia. 1998;12:400–408. doi: 10.1038/sj.leu.2401222. [DOI] [PubMed] [Google Scholar]
- Dent P, Reardon DB, Park JS, Bowers G, Logsdon C, Valerie K, Schmidt-Ullrich RK. Radiation-induced release of transforming growth factor alpha activates the epidermal growth factor receptor and mitogen-activated protein kinase pathway in carcinoma cells, leading to increased proliferation and protection from radiation-induced cell death. Mol Biol Cell. 1999;10:2493–2506. doi: 10.1091/mbc.10.8.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels IH, Stepczynska A, Stroh C, Lauber K, Berg C, Schwenzer R, Wajant H, Janicke RU, Porter AG, Belka C, Gregor M, Schulze-Osthoff K, Wesselborg S. Caspase-8/FLICE functions as an executioner caspase in anticancer drug-induced apoptosis. Oncogene. 2000;19:4563–4573. doi: 10.1038/sj.onc.1203824. [DOI] [PubMed] [Google Scholar]
- Erhardt P, Schremser EJ, Cooper GM. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol Cell Biol. 1999;19:5308–5315. doi: 10.1128/mcb.19.8.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubion WA, Guicciardi ME, Miyoshi H, Bronk SF, Roberts PJ, Svingen PA, Kaufmann SH, Gores GJ. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest. 1999;103:137–145. doi: 10.1172/JCI4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao CF, Ren S, Zhang L, Nakajima T, Ichinose S, Hara T, Koike K, Tsuchida N. Caspase-dependent cytosolic release of cytochrome c and membrane translocation of Bax in p53-induced apoptosis. Exp Cel Res. 2001;265:145–151. doi: 10.1006/excr.2001.5171. [DOI] [PubMed] [Google Scholar]
- Gores GJ, Miyoshi H, Botla R, Aguilar HI, Bronk SF. Induction of the mitochondrial permeability transition as a mechanism of liver injury during cholestasis: a potential role for mitochondrial proteases. Biochim Biophys Acta. 1998;1366:167–175. doi: 10.1016/s0005-2728(98)00111-x. [DOI] [PubMed] [Google Scholar]
- Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, Kaufmann SH, Gores GJ. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106:1127–1137. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan M, Wang L, Hanley JR, Park JS, Dent P. Ionizing radiation-induced mitogen-activated protein (MAP) kinase activation in DU145 prostate carcinoma cells: MAP kinase inhibition enhances radiation-induced cell killing and G2/M-phase arrest. Radiat Res. 2000;153:371–383. doi: 10.1667/0033-7587(2000)153[0371:irimap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Harari PM, Huang S. Head, and neck cancer as a clinical model for molecular targeting of therapy. Combining EGFR blockade with radiation. Int J Radiat Oncol Biol Phys. 2001;49:427–433. doi: 10.1016/s0360-3016(00)01488-7. [DOI] [PubMed] [Google Scholar]
- Heathcote EJ. Evidence-based therapy of primary biliary cirrhosis. Eur J Gasteroenterol Hepatol. 1999;11:607–615. doi: 10.1097/00042737-199906000-00005. [DOI] [PubMed] [Google Scholar]
- Holmstrom TH, Schmitz I, Soderstrom TS, Poukkula M, Johnson VL, Chow SC, Krammer PH, Eriksson JE. MAPK/ERK signaling in activated T cells inhibits CD95/Fas-mediated apoptosis downstream of DISC assembly. EMBO J. 2000;19:5418–5428. doi: 10.1093/emboj/19.20.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Jarvis WD, Fornari FA, Tombes RM, Erukulla RK, Bittman R, Schwartz GK, Dent P, Grant S. Evidence for involvement of mitogen-activated protein kinase, rather than stress-activated protein kinase, in potentiation of 1-beta-D-arabinofuranosylcytosine-induced apoptosis by interruption of protein kinase C signaling. Mol Pharmacol. 1998;54:844–856. doi: 10.1124/mol.54.5.844. [DOI] [PubMed] [Google Scholar]
- Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Billiar TR, Strom SC. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000;6:564–567. doi: 10.1038/75045. [DOI] [PubMed] [Google Scholar]
- Jones BA, Rao YP, Stravitz RT, Gores GJ. Bile salt-induced apoptosis of hepatocytes involves activation of protein kinase C. Am J Physiol. 1997;272:G1109–G1115. doi: 10.1152/ajpgi.1997.272.5.G1109. [DOI] [PubMed] [Google Scholar]
- Jost M, Huggett TM, Kari C, Boise LH, Rodeck U. EGFR-dependent control of keratinocyte survival, and Bcl-xL expression through a MEK-dependent pathway. J Biol Chem. 2001;276:6320–6326. doi: 10.1074/jbc.M008210200. [DOI] [PubMed] [Google Scholar]
- Kamath AB, Camacho I, Nagarkatti PS, Nagarkatti M. Role of Fas-Fas ligand interactions in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced immunotoxicity: increased resistance of thymocytes from Fas-deficient (lpr) and Fas ligand-defective (gld) mice to TCDD-induced toxicity. Toxicol Appl Pharmacol. 1999;160:141–155. doi: 10.1006/taap.1999.8753. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N. Mechanisms of liver cell injury. J Hepatol. 2000;32(suppl 1):39–47. doi: 10.1016/s0168-8278(00)80414-6. [DOI] [PubMed] [Google Scholar]
- Kim TH, Zhao Y, Barber MJ, Kuharsky DK, Yin XM. Bid-induced cytochrome c release is mediated by a pathway independent of mitochondrial permeability transition pore, and Bax. J Biol Chem. 2000;275:39474–39481. doi: 10.1074/jbc.M003370200. [DOI] [PubMed] [Google Scholar]
- Koeppel TA, Trauner M, Baas JC, Thies JC, Schlosser SF, Post S, Gebhard MM, Herfarth C, Boyer JL, Otto G. Extrahepatic biliary obstruction impairs microvascular perfusion and increases leukocyte adhesion in rat liver. Hepatology. 1997;26:1085–1091. doi: 10.1002/hep.510260501. [DOI] [PubMed] [Google Scholar]
- Kovalovich K, Li W, Deangelis R, Greenbaum LE, Ciliberto G, Taub R. IL-6 protects against Fas-mediated death by establishing a critical level of anti-apoptotic hepatic proteins FLIP, Bcl-2, and Bcl-xL. J Biol Chem. 2001;276:26605–26613. doi: 10.1074/jbc.M100740200. [DOI] [PubMed] [Google Scholar]
- Krahenbuhl S, Talos C, Fischer S, Reichen J. Toxicity of bile acids on the electron transport chain of isolated rat liver mitochondria. Hepatology. 1994;19:471–479. doi: 10.1002/hep.1840190228. [DOI] [PubMed] [Google Scholar]
- Kurosawa H, Que FG, Roberts LR, Fesmier PJ, Gores GJ. Hepatocytes in the bile duct-ligated rat express Bcl-2. Am J Physiol. 1997;272:G1587–G1593. doi: 10.1152/ajpgi.1997.272.6.G1587. [DOI] [PubMed] [Google Scholar]
- Kwo P, Patel T, Bronk S, Gores G. Nuclear serine protease activity contributes to bile acid-induced apoptosis in hepatocytes. Am J Physiol. 1995;268:G613–G621. doi: 10.1152/ajpgi.1995.268.4.G613. [DOI] [PubMed] [Google Scholar]
- Leist M, Volbracht C, Fava E, Nicotera P. 1-Methyl-4-phenylpyridinium induces autocrine excitotoxicity, protease activation, and neuronal apoptosis. Mol Pharmacol. 1998;54:789–801. doi: 10.1124/mol.54.5.789. [DOI] [PubMed] [Google Scholar]
- Leu CM, Chang C, Hu C. Epidermal growth factor (EGF) suppresses staurosporine-induced apoptosis by inducing mcl-1 via the mitogen-activated protein kinase pathway. Oncogene. 2000;19:1665–1675. doi: 10.1038/sj.onc.1203452. [DOI] [PubMed] [Google Scholar]
- Martinez-Diez MC, Serrano MA, Monte MJ, Marin JJ. Comparison of the effects of bile acids on cell viability, and DNA synthesis by rat hepatocytes in primary culture. Biochim Biophys Acta. 2000;1500:153–160. doi: 10.1016/s0925-4439(99)00099-x. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Rust C, Roberts PJ, Burgart LJ, Gores GJ. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gasteroenterology. 1999;117:669–677. doi: 10.1016/s0016-5085(99)70461-0. [DOI] [PubMed] [Google Scholar]
- Neuberger J. Primary biliary cirrhosis. Lancet. 1997;350:875–879. doi: 10.1016/S0140-6736(97)05419-6. [DOI] [PubMed] [Google Scholar]
- Noto H, Matsushita M, Koike M, Takahashi M, Matsue H, Kimura J, Todo S. Effect of high concentrations of bile acids on cultured hepatocytes. Artif Organs. 1998;22:300–307. doi: 10.1046/j.1525-1594.1998.05071.x. [DOI] [PubMed] [Google Scholar]
- O'Dwyer PJ, Stevenson JP, Gallagher M, Cassella A, Vasilevskaya I, Monia BP, Holmlund J, Dorr FA, Yao KS. c-raf-1 depletion and tumor responses in patients treated with the c-raf-1 antisense oligodeoxynucleotide ISIS 5132 (CGP 69846A) Clin Cancer Res. 1999;5:3977–3982. [PubMed] [Google Scholar]
- Ozoren N, Kim K, Burns TF, Dicker DT, Moscioni AD, El-Deiry WS. The caspase 9 inhibitor Z-LEHD-FMK protects human liver cells while permitting death of cancer cells exposed to tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2000;60:6259–6265. [PubMed] [Google Scholar]
- Panka DJ, Mano T, Suhara T, Walsh K, Mier JW. Phosphatidylinositol-3 kinase/Akt activity regulates c-FLIP expression in tumor cells. J Biol Chem. 2001;276:6893–6896. doi: 10.1074/jbc.C000569200. [DOI] [PubMed] [Google Scholar]
- Park JS, Boyer S, Mitchell K, Gilfor D, Birrer M, Darlington G, El Deiry W, Firestone G, Munger K, Band V, Fisher PB, Dent P. Expression of human papilloma virus E7 protein causes apoptosis, and inhibits DNA synthesis in primary hepatocytes via increased expression of p21(Cip-1/WAF1/MDA6) J Biol Chem. 2000a;274:18–28. doi: 10.1074/jbc.275.1.18. [DOI] [PubMed] [Google Scholar]
- Park JS, Qiao L, Gilfor D, Yang MY, Hylemon P, Benz C, Darlington G, Firestone G, Fisher PB, Dent P. A role for both ets and C/EBP transcription factors and mRNA stabilization in the MAPK-dependent increase in p21 (Cip-1/WAF1/mda6) protein levels in primary hepatocytes Mol. Biol Cell. 2000b;11:2915–2932. doi: 10.1091/mbc.11.9.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T, Bronk S, Gores G. Increases of intracellular magnesium promote glycodeoxycholate-induced apoptosis in rat hepatocytes. J Clin Invest. 1994;94:2183–2192. doi: 10.1172/JCI117579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins CL, Fang G, Kim CN, Bhalla KN. The role of Apaf-1, caspase-9, and bid proteins in etoposide- or paclitaxel-induced mitochondrial events during apoptosis. Cancer Res. 2000;60:1645–1653. [PubMed] [Google Scholar]
- Perkins C, Kim CN, Fang G, Bhalla KN. Overexpression of Apaf-1 promotes apoptosis of untreated and paclitaxel- or etoposide-treated HL-60 cells. Cancer Res. 1998;58:4561–4566. [PubMed] [Google Scholar]
- Poupon R, Chazouilleres O, Poupon RE. Chronic cholestatic diseases. J Hepatol. 2000;32:129–140. doi: 10.1016/s0168-8278(00)80421-3. [DOI] [PubMed] [Google Scholar]
- Qin ZH, Wang Y, Kikly KK, Sapp E, Kegel KB, Aronin N, DiFiglia M. Pro-caspase-8 is predominately localized in mitochondria and released into cytoplasm upon apoptotic stimulation. J Biol Chem. 2001;276:8079–8086. doi: 10.1074/jbc.M007028200. [DOI] [PubMed] [Google Scholar]
- Qiao D, Stratagouleas ED, Martinez JD. Activation, and role of mitogen-activated protein kinases in deoxycholic acid-induced apoptosis. Carcinogenesis. 2001;22:35–41. doi: 10.1093/carcin/22.1.35. [DOI] [PubMed] [Google Scholar]
- Rao YP, Stravitz RT, Vlahcevic ZR, Gurley EC, Sando JJ, Hylemon PB. Activation of protein kinase C alpha and delta by bile acids: correlation with bile acid structure and diacylglycerol formation. J Lipid Res. 1997;38:2446–2454. [PubMed] [Google Scholar]
- Reardon DB, Contessa JN, Mikkelsen RB, Valerie K, Dent P, Schmidt-Ullrich RK. Dominant negative EGFR-CD533 and inhibition of MAPK modify JNK1 activation and enhance radiation toxicity of human mammary carcinoma cells. Oncogene. 1999;18:4756–4766. doi: 10.1038/sj.onc.1202849. [DOI] [PubMed] [Google Scholar]
- Rodrigues CM, Fan G, Wong PY, Kren BT, Steer CJ. Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Mol Med. 1998;4:165–178. [PMC free article] [PubMed] [Google Scholar]
- Ruffolo SC, Breckenridge DG, Nguyen M, Goping IS, Gross A, Korsmeyer SJ, Li H, Yuan J, Shore GC. BID-dependent, and BID-independent pathways for BAX insertion into mitochondria. Cell Death Differ. 2000;7:1101–1108. doi: 10.1038/sj.cdd.4400739. [DOI] [PubMed] [Google Scholar]
- Rust C, Karnitz LM, Paya CV, Moscat J, Simari RD, Gores GJ. The bile acid taurochenodeoxycholate activates a phosphatidylinositol 3-kinase-dependent survival signaling cascade. J Biol Chem. 2000;275:20210–20216. doi: 10.1074/jbc.M909992199. [DOI] [PubMed] [Google Scholar]
- Schliess F, Kurz AK, von Dahl S, Haussinger D. Mitogen-activated protein kinases mediate the stimulation of bile acid secretion by tauroursodeoxycholate in rat liver. Gastroenterology. 1997;113:1306–1314. doi: 10.1053/gast.1997.v113.pm9322526. [DOI] [PubMed] [Google Scholar]
- Schlottman K, Wachs FP, Krieg RC, Kullmann F, Scholmerich J, Rogler G. Characterization of bile salt-induced apoptosis in colon cancer cell lines. Cancer Res. 2000;60:4270–4276. [PubMed] [Google Scholar]
- Schmidt-Ullrich RK, Dent P, Grant S, Mikkelsen RB, Valerie K. Signal transduction, and cellular radiation responses. Radiat Res. 2000;153:245–257. doi: 10.1667/0033-7587(2000)153[0245:stacrr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich RK, Mikkelsen RB, Dent P, Todd DG, Valerie K, Kavanagh BD, Contessa JN, Rorrer WK, Chen PB. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15:1191–1197. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- Schmucker DL, Ohta M, Kanai S, Sato Y, Kitani K. Hepatic injury induced by bile salts: correlation between biochemical and morphological events. Hepatology. 1990;12:1216–1221. doi: 10.1002/hep.1840120523. [DOI] [PubMed] [Google Scholar]
- Sebolt-Leopold JS, Dudley DT, Herrera R, Van Becelaere K, Wiland A, Gowan RC, Tecle H, Barrett SD, Bridges A, Przybranowski S, Leopold WR, Saltiel AR. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat Med. 1999;5:810–816. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- Sodeman T, Bronk SF, Roberts PJ, Miyoshi H, Gores GJ. Bile salts mediate hepatocyte apoptosis by increasing cell surface trafficking of Fas. Am J Physiol. 2000;278:G992–G999. doi: 10.1152/ajpgi.2000.278.6.G992. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, McKim JM, Jr, Goff MC, Ruyle SZ, Devereaux MW, Han D, Packer L, Everson G. Vitamin E reduces oxidant injury to mitochondria and the hepatotoxicity of taurochenodeoxycholic acid in the rat. Gastroenterology. 1998;114:164–174. doi: 10.1016/s0016-5085(98)70644-4. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Winklhofer-Roob BM, Devereaux MW, McKim JM., Jr Generation of hydroperoxides in isolated rat hepatocytes and hepatic mitochondria exposed to hydrophobic bile acids. Gastroenterology. 1995;109:1249–1256. doi: 10.1016/0016-5085(95)90585-5. [DOI] [PubMed] [Google Scholar]
- Spivey JR, Bronk S, Gores G. Glycochenodeoxycholate-induced lethal hepatocellular injury in rat hepatocytes. Role of ATP depletion and cytosolic free calcium. J Clin Invest. 1993;92:17–24. doi: 10.1172/JCI116546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasula SM, Ahmad M, Ottilie S, Bullrich F, Banks S, Wang Y, Fernandes-Alnemri T, Croce CM, Litwack G, Tomaselli KJ, Armstrong RC, Alnemri ES. F.L.A.M.E-1, a novel F.A.D.D-like anti-apoptotic molecule that regulates Fas/TNFR1-induced apoptosis. J Biol Chem. 1997;272:18542–18545. doi: 10.1074/jbc.272.30.18542. [DOI] [PubMed] [Google Scholar]
- Stoka V, Turk B, Schendel SL, Kim TH, Cirman T, Snipas SJ, Ellerby LM, Bredesen D, Freeze H, Abrahamson M, Bromme D, Krajewski S, Reed JC, Yin XM, Turk V, Salvesen GS. Lysosomal protease pathways to apoptosis. Cleavage of BID, not procaspases, is the most likely route. J Biol Chem. 2001;276:3149–3157. doi: 10.1074/jbc.M008944200. [DOI] [PubMed] [Google Scholar]
- Stravitz RT, Rao YP, Vlahcevic ZR, Gurley EC, Jarvis WD, Hylemon PB. Hepatocellular protein kinase C activation by bile acids: implications for regulation of cholesterol 7 alpha-hydroxylase. Am J Physiol. 1996;271:G293–G303. doi: 10.1152/ajpgi.1996.271.2.G293. [DOI] [PubMed] [Google Scholar]
- Takikawa Y, Miyoshi H, Rust C, Roberts P, Siegel R, Mandal PK, Millikan RE, Gores GJ. The bile acid-activated phosphatidylinositol 3-kinase pathway inhibits fas apoptosis upstream of bid in rodent hepatocytes. Gastroenterology. 2001;120:1810–1817. doi: 10.1053/gast.2001.24835. [DOI] [PubMed] [Google Scholar]
- homas LA, Veysey MJ, Bathgate T, King A, French G, Smeeton NC, Murphy GM, Dowling RH. Mechanism for the transit-induced increase in colonic deoxycholic acid formation in cholesterol cholelithiasis. Gastroenterology. 2000;119:806–815. doi: 10.1053/gast.2000.16495. [DOI] [PubMed] [Google Scholar]
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Tomic S, Greiser U, Lammers R, Kharitonenkov A, Imyanitov E, Ullrich A, Bohmer F-D. Association of SH2 domain protein tyrosine phosphatases with the epidermal growth factor receptor in human tumor cells. Phosphatidic acid activates receptor dephosphorylation by PTP1C. J Biol Chem. 1995;270:21277–21284. doi: 10.1074/jbc.270.36.21277. [DOI] [PubMed] [Google Scholar]
- Tonks NK. Protein tyrosine phosphatases and the control of cellular signaling responses. Adv Pharmacol. 1996;36:91–119. doi: 10.1016/s1054-3589(08)60578-5. [DOI] [PubMed] [Google Scholar]
- Trauner M, Meier PJ, Boyer JL. Molecular regulation of hepatocellular transport systems in cholestasis. J Hepatol. 1999;31:165–178. doi: 10.1016/s0168-8278(99)80179-2. [DOI] [PubMed] [Google Scholar]
- Tzung SP, Fausto N, Hockenbery DM. Expression of Bcl-2 family during liver regeneration and identification of Bcl-x as a delayed early response gene. Am J Pathol. 1997;50:1985–1995. [PMC free article] [PubMed] [Google Scholar]
- Wang S, Guo CY, Castillo A, Dent P, Grant S. Effect of bryostatin 1 on taxol-induced apoptosis and cytotoxicity in human leukemia cells (U937) Biochem Pharmacol. 1998a;56:635–644. doi: 10.1016/s0006-2952(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signaling pathways on cell survival. Biochem J. 1998b;333:291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster CR, Anwer MS. Cyclic adenosine monophosphate-mediated protection against bile acid-induced apoptosis in cultured rat hepatocytes. Hepatology. 1998;27:1324–1331. doi: 10.1002/hep.510270519. [DOI] [PubMed] [Google Scholar]
- Yeh JH, Hsu SC, Han SH, Lai MZ. Mitogen-activated protein kinase kinase antagonized fas-associated death domain protein-mediated apoptosis by induced FLICE-inhibitory protein expression. J Exp Med. 1998;188:1795–1802. doi: 10.1084/jem.188.10.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerushalmi B, Dahl R, Devereaux MW, Gumpricht E, Sokol RJ. Bile acid induced rat hepatocyte apoptosis is inhibited by antioxidants, and blockers of the mitochondrial permeability transition. Hepatology. 2001;33:616–626. doi: 10.1053/jhep.2001.22702. [DOI] [PubMed] [Google Scholar]
- Yin XM. Bid, a critical mediator for apoptosis induced by the activation of Fas/TNF-R1 death receptors in hepatocytes. J Mol Med. 2000;78:203–211. doi: 10.1007/s001090000099. [DOI] [PubMed] [Google Scholar]
- Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B, Samali A, Gahm A, Orrenius S. Caspases: their intracellular localization and translocation during apoptosis. Cell Death Differ. 1999;6:644–651. doi: 10.1038/sj.cdd.4400536. [DOI] [PubMed] [Google Scholar]