A century ago, Walter B. Cannon, after studying battlefield casualties, concluded “… shock is a lack of homeostasis and without homeostasis the patient does not survive (1). This statement is particularly relevant to the management of trauma-induced coagulopathy (TIC)(2). Today in both military and civilian scenarios, acute hemorrhage and its sequelae are the leading cause of preventable death (3-6). TIC is the culmination of endogenous responses to hemorrhagic shock and tissue injury, and has been attributed variably to activated protein C deactivation of clotting factors V and VIII (7), auto-heparinization from glycocalyx degradation (8), fibrinogen depletion (9), platelet dysfunction (10), disseminated intravascular coagulation (11), and dysregulated fibrinolysis(12). The contribution of fibrinolysis to TIC was unappreciated until the adoption of whole blood viscoelastic assays; i.e., thrombelastography (TEG®, Haemonetics, Niles, Il) and thromboelastometry (ROTEM, TEM Systems, Munich) to guide blood component transfusion (12-15). The dominant mechanisms responsible for impaired clot formation (platelet activation, thrombin generation, fibrin cross-linking) are distinct from those regulatory clot degradation (fibrinolysis)(16, 17). Moreover, there are a multitude of additional factors implicated in impaired clot formation (Figure 1) and dysregulated clot degradation (Figure 2). Recently, we have identified nine phenotypes of post-injury fibrinolysis based on clot lysis at 30 minutes (LY30) by TEG, further stratified by tissue plasminogen activator (TPA) challenge TEG (18). Collectively, the regulatory factors and events involved in clot formation and degradation result in an array of TIC phenotypes that may be encountered in the severely injured patient (Figure 3). These phenotypes are determined by the magnitude of shock and tissue injury pattern, further modified by resuscitation and ongoing blood loss (19). The challenge is to match blood component therapy with these TIC phenotypes while achieving mechanical control of bleeding.
Figure 1. Trauma Induced Coagulopathy: Impaired Clot Formation.

Impaired clot formation is driven by both hypoxia and tissue injury. Proposed mechanisms include the activation of protein C with subsequent deactivation of factors V and VIII, and the release of heparan sulfate from the endothelial glycocalyx.
Figure 2. Trauma Induced Coagulopathy: Enhanced Clot Degradation.

Systemic hyperfibrinolysis is stimulated by hypoxia with endothial release of tPA that activates plasminogen. Inhibited fibrinolysis (shutdown), on the other hand is promoted via the byproducts of tissue injury and activation of platelets releasing antifbrinolytic agents.
Figure 3. Therapy Based on Phenotype = Personalized Medicine.

The myriad of mechanisms driving trauma induced coagulopathy result in a spectrum of postinjury coagulopathy phenotypes, emphasizing the potential benefit of a personalized medicine approach.
Goal directed management of TIC
While 10 units of red blood cells (RBC) within the first 6 hours is the best predictor of mortality due to acute blood loss(20), most deaths occur within the first 2 hours. Consequently, a number of formulas, based on early clinical measurements, have been promoted to identify the patient at risk for massive transfusions. These formulas appear reliable based on receiver operator characteristic (ROC) analyses, but the positive predictive values are typically less than 50%, due to the relatively low incidence of massive transfusion among seriously injured patients.
Irrespective of the method employed, the key question is how to manage the patient at high risk for life-threatening hemorrhage. The basic approaches are fixed ratio-based blood products or goal-directed blood components based on assessment of coagulations function (Figure 4). The current preemptive strategy is the so-called 1:1:1, representing one unit of plasma, one donor unit of platelets and one unit of red blood cells (RBC). This ratio is based on the military proposal of replacing the equivalent of whole blood lost for life threatening hemorrhage (21). While conceptually attractive, the storage, release, consumption, and necessity for this specific balance of blood components raises questions as to whether 1:1:1 is the optimal resuscitation strategy for all seriously injured patients. The only randomized prospective study to test this concept has been The Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) Trial (22). This was reported to be a comparison of 1:1:1 to 1:1:2 (plasma, platelets, RBC). In reality, however, for the first 6 units of RBC, it was 1:1:1 versus 1:0:2 as no platelets were administered until the second round of 6 units of RBC. This was due to the fact that one unit of apheresis platelets equals 6 units of single whole blood derived platelets, which could not be divided practically. Despite the lack of platelets until >6 RBC units, there was no difference in the primary endpoints of 24 hour and 30 day mortality. In a cohort study, the Ben Taub group reported improved survival following TEG-guided blood component therapy compared to 1:1:1; survival was associated with a reduction in platelet transfusion (23). A recent prospective randomized trial of platelet transfusion versus standard care after acute hemorrhagic stroke associated with antiplatelet therapy (PATCH) trial reported an adjusted odds ratio for mortality of 2.05 for patients given early empiric platelets (24). A small trial conducted in Canada comparing 1:1:1 to laboratory-guided blood component therapy showed that achieving 1:1:1 despite concerted efforts was only achieved in 57% of the patients; moreover, it resulted in increased plasma wastage (25). In sum, there is currently a lack of evidence to support empiric ratio-based blood product administration, including immediate platelet transfusion, for the seriously injured patient at risk for life-threatening hemorrhage.
Figure 4. Presumptive versus Goal-directed Blood Transfusions.

Transfusion of blood components for patients at risk for TIC is conceptually based on either a fixed ratio of blood component therapy approach versus transfusion directed by laboratory assessment of the patient’s coagulation status.
The alternative approach is goal-directed blood component administration based on laboratory assessments of coagulation function (Figure 4). Conventional laboratory testing consists of prothrombin time (PT) and partial thromboplastin time (PTT) with additional measures of platelet count, fibrinogen levels and D-dimers. PT and PTT are plasma-based tests that were originally designed to evaluate anticoagulant therapy and hemophilia due to isolated clotting factor deficiencies. PT assesses the extrinsic pathway, and is believed to represent the clotting activity of factor VII; whereas the PTT assesses the intrinsic pathway, reflecting the clotting activity of factors XI, IX, VIII. Both tests reflect the common pathway (factors X, V, and II). The PT, reported as the international normalized ratio (INR), has generally been used to define TIC, with thresholds values ranging from >1.2 to >1.5. Interestingly, however, serious injured patients at risk for massive transfusion present with varied profiles of isolated prolongation of the INR, isolated elevated PTT, and a combination of abnormalities. A recent report from the San Francisco General group (26) indicated prolonged INR (>1.3) in 9%, elevated PTT (> 34sec) in 43%, and combined PT and PTT abnormalities in 48 % among coagulopathic patients. In our recent analysis of patients requiring a massive transfusion (>10 RBC/first 6 hours), 2% had a prolonged INR (>1.3), 13% had an elevated PTT (>30 sec), and 72% had combined abnormalities of INR and PTT. Of note, 13% had normal INR and PTT (manuscript in preparation). Perhaps more concerning, in a preliminary study of 30 seriously injured patient in whom we measured clotting factors activity, greater than 40% of the variation in INR and PTT could not be explained by clotting factor deficiency. In sum, although modest prolongation in the INR or PTT are relatively sensitive in identifying the seriously injured patients at risk for TIC, they do not clearly indicate its cause(s), and consequently are weak guides for therapy. Moreover, platelet count is very insensitive for identifying the need for platelet transfusion. The vast majority of patients at risk for a massive transfusion present with what is currently considered a normal platelet count, i.e., greater than 150,000/mcL (22). Similarly, most patients with TIC appear to have adequate fibrinogen (>150mg/dL) levels (22). Thus, platelet counts and fibrinogen levels add little to the initial management of most patients at risk for life-threatening hemorrhage.
The alternative to conventional laboratory assessment of clotting functions is whole blood viscoelastic hemostatic assays (VHA). The currently US FDA approved methods are TEG and ROTEM (27, 28). Unlike PT and PTT, which only measure the plasma dependent enzymatic component of clotting, VHA’s reflect thrombin generation, platelet activity and fibrinogen cross-linking, providing a measurement of maximum clot strength, and subsequent clot dissolution. Recognizing these advantages, coupled with the availability of improved equipment, has resulted in progressive adoption of VHAs for management of TIC, but there are few comparative studies between VHAs and conventional coagulation tests (CCTs). The Memorial Herman group reported superiority of TEG compared PT and PTT in a large animal model (29) and the Ben Taub group suggested advantages of TEG compared to 1:1:1 in a retrospective cohort study(23).
We have had encouraging experience with both TEG and ROTEM in trauma management, but have done more work with TEG due to our institution’s preference. Based on an analysis of 160 healthy control patients in Denver and clinical investigations, we established transfusion thresholds for specific citrated rapid TEG measurements, i.e.: FFP plasma for an activated clotting time (ACT) >128 seconds, fibrinogen for an angle <65°, platelets for a maximum amplitude (MA) <55mm, and anti-fibrinolytic (e.g., tranexamic acid) (TXA) for a lysis at 30 minutes (LY30) >5% (30). Using this protocol, we conducted a pragmatic, randomized clinical trial (RCT) comparing TEG-guided blood component transfusion based on TEG compared to conventional laboratory coagulation assessment (INR, PTT, platelet count, fibrinogen levels, and D-dimers) (31). Over a 3-year period, patients at risk for massive transfusion were randomized to TEG versus CCT–guided resuscitation. The overall results were a 50% improvement in survival when blood components were delivered based on TEG measurements (Figure 5). These results are limited to a single institution, however, at this time, we are not aware of any additional prospective randomized trials comparing TEG (or ROTEM) guided hemostatic resuscitation to conventional laboratory testing or to a fixed ratio 1:1:1 ratio. Nevertheless, the dramatic survival benefit we observed in the trial should serve as an impetus for wider adoption of VHA-based resuscitation, enabling more definitive and generalizable studies.
Figure 5.
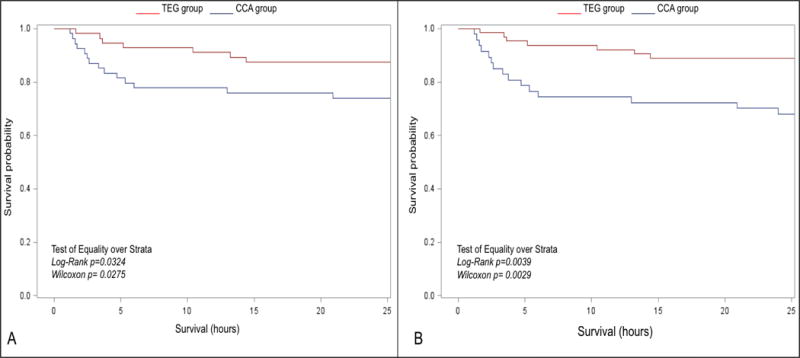
Kaplan-Meier estimates of survival by randomization group for patients analyzed as treated. Survival in the thrombelastography (TEG) guided group was significantly higher than the conventional coagulation assays (CCA) group (31). Reproduced from reference 31 with permission from Wolters Kluwer Health, Inc.
Based on our collective experience over the past decade, our current protocol for the resuscitation of the seriously injured patient is summarized in Figure 6. We believe the key to preventing life-threatening coagulopathy is rapid reversal of shock and preservation of microvascular circulation. Our first cooler of blood products consists of 4 units of RBC and 2 units of FFP. No crystalloids are administered until the patient’s systolic blood pressure (SBP) is >100 mmHg. Subsequent platelet and fibrinogen delivery is based on TEG. Platelets given during advanced shock are rendered dysfunctional due to metabolic acidosis (32) and post shock metabolites (33). White et al. (34) have reported experimental evidence indicating fibrinogen is similarly modified by the oxidative stress of shock. These facts represent the scientific underpinning of our strategy of achieving adequate resuscitation before transfusion of platelets and cryoprecipitate. An exception to the TEG-directed protocol outlined above is the patient with massive ongoing blood loss, we define as requiring 4 units of RBC/2unit of FFP within the first 30 min, thus, precluding completion of the TEG analysis. In this scenario we administer 1 apheresis unit of platelets and a 10 pack of cryoprecipitate because our data indicate >50% of these patients need both these additional products (To be presented at the Academic Surgical Congress, February 2018; manuscript in progress). Of course, we believe whole blood is the preferred approach in patients with massive ongoing blood loss, but this is currently not widely available in civilian centers.
Figure 6. Goal-directed Hemostasis.

Based on our ongoing studies, and those from others, we believe the priority in hemostatic resuscitation is minimizing the duration of shock and avoiding hemodilution with crystalloid. Goal-directed component therapy is initiated after the I first 2 U of plasma and 4 U of RBCs
The role of tranexamic acid (TXA) in a mature trauma system with rapid transport to a level 1 trauma center where there is immediate availability of blood components also appears limited (35-40). It is likely, however, that TXA is beneficial in a subpopulation of severely injured patients with a combination of poor clot strength and hyperfibrinolysis, suggested by a recent reevaluation of the MATTERS trials (41). Ongoing analysis of military experience (42) also suggests TXA may be beneficial with inherent delays in transport of combat casualties to definitive facilities, but at an increased risk of venous thromboembolism (VTE). However, the subpopulation likely to benefit has yet to be defined. The majority of severely injured patients in the civilian setting arrive at the hospital in fibrinolysis shutdown (35-40) rendering them at risk of thrombosis if TXA is given. Currently, there are large multicenter randomized civilian trials designed to determine the role of prehospital TXA in traumatic brain injury (personal communication M.A. Schreiber, October 20, 2017) and seriously injured patient requiring helicopter transport (personal communication J.L. Sperry, October 20, 2017).
In sum, our collective experience, and that of a number of other investigators, indicates blood component transfusion in the seriously injured patients at risk for massive transfusions should be personalized and goal-directed, using TEG or ROTEM. The availability of new generation devices should enhance the feasibility of earlier testing and, ultimately, the proven benefit.
Acknowledgments
Supported in part by NIH grants: P50 GM 49222, T32 08315, and UM1 HL 120877
Footnotes
Presented at the 5th Annual meeting of Trauma Hemostasis and Oxygenation Research, June 25-28. 2017, Bergen, Norway
Author contributions: Concept … EEM, HBM, MPC, EG, AS; Collecting data … HBM, EG; Analyzing data … EEM, HBM, MPC, EG, and AS; First draft …EEM; Critical manuscript revision … EEM, HBM, MPC, EG, and AS
References
- 1.Cannon WB, Fraser J, Cowell EM. The preventive treatment of wound shock. JAMA. 1918;70:618–21. [Google Scholar]
- 2.Moore HB, Moore EE, Liras IN, Wade C, Huebner BR, Burlew CC, Pieracci FM, Sauaia A, Cotton BA. Targeting resuscitation to normalization of coagulating status: Hyper and hypocoagulability after severe injury are both associated with increased mortality. Am J Surg. 2017;214:1041–6. doi: 10.1016/j.amjsurg.2017.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eastridge BJ, Hardin M, Cantrell J, Oetjen-Gerdes L, Zubko T, Mallak C, Wade CE, Simmons J, Mace J, Mabry R, et al. Died of wounds on the battlefield: causation and implications for improving combat casualty care. J Trauma. 2011;71(1 Suppl):S4–S8. doi: 10.1097/TA.0b013e318221147b. [DOI] [PubMed] [Google Scholar]
- 4.Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, Friese RS. Increasing trauma deaths in the United States. Ann Surg. 2014;260:13–21. doi: 10.1097/SLA.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 5.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–93. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Tisherman SA, Schmicker RH, Brasel KJ, Bulger EM, Kerby JD, Minei JP, Powell JL, Reiff DA, Rizoli SB, Schreiber MA. Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the Resuscitation Outcomes Consortium. Ann Surg. 2015;261:586–90. doi: 10.1097/SLA.0000000000000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, Pittet JF. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64:1211–7. doi: 10.1097/TA.0b013e318169cd3c. [DOI] [PubMed] [Google Scholar]
- 8.Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg. 2012;73:60–6. doi: 10.1097/TA.0b013e31825b5c10. [DOI] [PubMed] [Google Scholar]
- 9.Schlimp CJ, Schochl H. The role of fibrinogen in trauma-induced coagulopathy. Hamostaseologie. 2014;34:29–39. doi: 10.5482/HAMO-13-07-0038. [DOI] [PubMed] [Google Scholar]
- 10.Wohlauer MV, Moore EE, Thomas S, Sauaia A, Evans E, Harr J, Silliman CC, Ploplis V, Castellino FJ, Walsh M. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg. 2012;214:739–46. doi: 10.1016/j.jamcollsurg.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gando S, Wada H, Kim HK, Kurosawa S, Nielsen JD, Thachil J, Toh CH. Comparison of disseminated intravascular coagulation in trauma with coagulopathy of trauma/acute coagulopathy of trauma-shock. Journal of thrombosis and haemostasis : J Thromb Haemost. 2012;10:2593–5. doi: 10.1111/jth.12011. [DOI] [PubMed] [Google Scholar]
- 12.Kashuk JL, Moore EE, Sawyer M, Wohlauer M, Pezold M, Barnett C, Biffl WL, Burlew CC, Johnson JL, Sauaia A. Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma. Ann Surg. 2010;252:434–42. doi: 10.1097/SLA.0b013e3181f09191. [DOI] [PubMed] [Google Scholar]
- 13.Chapman MP, Moore EE, Ramos CR, Ghasabyan A, Harr JN, Chin TL, Stringham JR, Sauaia A, Silliman CC, Banerjee A. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg. 2013;75:961–7. doi: 10.1097/TA.0b013e3182aa9c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, Schochl H, Wade CE, Holcomb JB, Matijevic N. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012;73:365–70. doi: 10.1097/TA.0b013e31825c1234. [DOI] [PubMed] [Google Scholar]
- 15.Ives C, Inaba K, Branco BC, Okoye O, Schochl H, Talving P, Lam L, Shulman I, Nelson J, Demetriades D. Hyperfibrinolysis elicited via thromboelastography predicts mortality in trauma. J Am Coll Surg. 2012;215:496–502. doi: 10.1016/j.jamcollsurg.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Chin TL, Moore EE, Moore HB, Gonzalez E, Chapman MP, Stringham JR, Ramos CR, Banerjee A, Sauaia A. A principal component analysis of postinjury viscoelastic assays: clotting factor depletion versus fibrinolysis. Surgery. 2014;156:570–7. doi: 10.1016/j.surg.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kutcher ME, Ferguson AR, Cohen MJ. A principal component analysis of coagulation after trauma. J Trauma Acute Care Surg. 2013;74:1223–9. doi: 10.1097/TA.0b013e31828b7fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore HB, Moore EE, Huebner BR, Dzieciatkowska M, Stettler GR, Nunns GR, Lawson PJ, Ghasabyan A, Chandler J, Banerjee A, et al. Fibrinolysis Shutdown Is Associated with a Five-Fold Increase in Mortality in Trauma Patients Lacking Sensitivity to Tissue Plasminogen Activator. J Trauma Acute Care Surg. 2017;83:1014–20. doi: 10.1097/TA.0000000000001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore HB, Moore EE, Chapman MP, Gonzalez E, Slaughter AL, Morton AP, D’Alessandro A, Hansen KC, Sauaia A, Banerjee A, et al. Viscoelastic measurements of platelet function, not fibrinogen function, predicts sensitivity to tissue-type plasminogen activator in trauma patients. J Thromb Haemost. 2015;13:1878–87. doi: 10.1111/jth.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL. Post-Injury Life Threatening Coagulopathy: Is 1:1 Fresh Frozen Plasma (FFP): Packed Red Blood Cells (RBC) the Answer? J Trauma. 2008;65:261–70. doi: 10.1097/TA.0b013e31817de3e1. [DOI] [PubMed] [Google Scholar]
- 21.Armand R, Hess JR. Treating coagulopathy in trauma patients. Transfus Med Rev. 2003;17:223–31. doi: 10.1016/s0887-7963(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 22.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–82. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tapia NM, Chang A, Norman M, Welsh F, Scott B, Wall MJ, Jr, Mattox KL, Suliburk J. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013;74:378–85. doi: 10.1097/TA.0b013e31827e20e0. [DOI] [PubMed] [Google Scholar]
- 24.Baharoglu MI, Cordonnier C, Salman RA-S, de Gans K, Koopman MM, Brand A, Majoie CB, Beenen LF, Marquering HA, Vermeulen M, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet. 387:2605–13. doi: 10.1016/S0140-6736(16)30392-0. [DOI] [PubMed] [Google Scholar]
- 25.Nascimento B, Callum J, Tien H, Rubenfeld G, Pinto R, Lin Y, Rizoli S. Effect of a fixed-ratio (1:1:1) transfusion protocol versus laboratory-results-guided transfusion in patients with severe trauma: a randomized feasibility trial. CMAJ : Can Med Assoc J. 2013;185:E583–9. doi: 10.1503/cmaj.121986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christie SA, Kornblith LZ, Howard BM, Conroy AS, Kunitake RC, Nelson MF, Hendrickson CM, Calfee CS, Callcut RA, Cohen MJ. Characterization of distinct coagulopathic phenotypes in injury: Pathway-specific drivers and implications for individualized treatment. J Trauma Acute Care Surg. 2017;82:1055–62. doi: 10.1097/TA.0000000000001423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aleshnick M, Orfeo T, Brummel-Ziedins K, Gissel M, Mann K. Interchangeability of rotational elastographic instruments and reagents. J Trauma Acute Care Surg. 2014;76:107–13. doi: 10.1097/TA.0b013e3182aa80dc. [DOI] [PubMed] [Google Scholar]
- 28.Prat NJ, Meyer AD, Ingalls NK, Trichereau J, DuBose JJ, Cap AP. Rotational thromboelastometry significantly optimizes transfusion practices for damage control resuscitation in combat casualties. J Trauma Acute Care Surg. 2017;83:373–80. doi: 10.1097/TA.0000000000001568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martini WZ, Cortez DS, Dubick MA, Park MS, Holcomb JB. Thrombelastography is Better Than PT, aPTT, and Activated Clotting Time in Detecting Clinically Relevant Clotting Abnormalities After Hypothermia, Hemorrhagic Shock and Resuscitation in Pigs. J Trauma Acute Care Surg. 2008;65:535–43. doi: 10.1097/TA.0b013e31818379a6. [DOI] [PubMed] [Google Scholar]
- 30.Einersen PM, Moore EE, Chapman MP, Moore HB, Gonzalez E, Silliman CC, Banerjee A, Sauaia A. Rapid thrombelastography thresholds for goal-directed resuscitation of patients at risk for massive transfusion. J Trauma Acute Care Surg. 2017;82:114–9. doi: 10.1097/TA.0000000000001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer M, Barnett CC, Bensard DD, Biffl WL, et al. Goal-directed Viscoelastic-Guided Hemostatic Resuscitation of Trauma Induced Coagulopathy: A Pragmatic Randomized Clinical Trial. Ann Surg. 2015;236:1051–9. doi: 10.1097/SLA.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maegele M, Spinella PC, Schochl H. The acute coagulopathy of trauma: mechanisms and tools for risk stratification. Shock. 2012;38:450–8. doi: 10.1097/SHK.0b013e31826dbd23. [DOI] [PubMed] [Google Scholar]
- 33.D’Alessandro A, Moore HB, Moore EE, Wither M, Nemkov T, Gonzalez E, Slaughter A, Fragoso M, Hansen KC, Silliman CC, et al. Early hemorrhage triggers metabolic responses that build up during prolonged shock. Am J Physiol Regul Integr Comp Physiol. 2015;308:R1034–R44. doi: 10.1152/ajpregu.00030.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White NJ, Wang Y, Fu X, Cardenas JC, Martin EJ, Brophy DF, Wade CE, Wang X, St John AE, Lim EB, et al. Post-translational oxidative modification of fibrinogen is associated with coagulopathy after traumatic injury. Free Rad Biol Med. 2016;96:181–9. doi: 10.1016/j.freeradbiomed.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leeper CM, Neal MD, McKenna C, Sperry J, Gaines BA. Abnormalities in fibrinolysis at the time of admission are associated with DVT, mortality and disability in a pediatric trauma population. J Trauma Acute Care Surg. 2017;82:27–34. doi: 10.1097/TA.0000000000001308. [DOI] [PubMed] [Google Scholar]
- 36.Meizoso JP, Karcutskie CA, Ray JJ, Namias N, Schulman CI, Proctor KG. Persistent Fibrinolysis Shutdown Associated with Increased Mortality in Severely Injured Trauma Patients. J Am Coll Surg. 2017;224:575–582. doi: 10.1016/j.jamcollsurg.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 37.Moore HB, Moore EE, Huebner BR, Stettler GR, Nunns GR, Einersen PM, Silliman CC, Sauaia A. Tranexamic acid is associated with increased mortality in patients with physiological fibrinolysis. The J Surg Res. 2017;213:166. doi: 10.1016/j.jss.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valle EJ, Allen CJ, Van Haren RM, Jouria JM, Li H, Livingstone AS, Namias N, Schulman CI, Proctor KG. Do all trauma patients benefit from tranexamic acid? J Trauma Acute Care Surg. 2014;76:1373–8. doi: 10.1097/TA.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 39.Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, Banerjee A, Sauaia A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: The spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77:811–7. doi: 10.1097/TA.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore HB, Moore EE, Liras IN, Gonzalez E, Harvin JA, Sauaia A, Holcomb JB, Cotton BA. Acute fibrinolysis shutdown following injury increases mortality: a multicenter evaluation of 2540 severely injured patients. J Am Coll Surg. 2016;222:347–55. doi: 10.1016/j.jamcollsurg.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore HB, Cotton BA, Moore EE, Sauaia A, Dorlac W, DuBose JJ, Wade C, Morrison JJ. Does Selective use of TXA MATTER-Fibrinolysis Phenotypes and Associated Outcomes. J Trauma Acute Care Surg. In Press. [Google Scholar]
- 42.Howard JT, Stockinger ZT, Cap AP, Bailey JA, Gross KR. Military use of TXA in combat trauma: Does it matter? J Trauma Acute Care Surg. 2017;83:579–588. doi: 10.1097/TA.0000000000001613. [DOI] [PubMed] [Google Scholar]


