Abstract
Objectives
To pilot-test a Phone-based Postpartum Continuing Care (PPCC) protocol developed from existing evidence-based approaches to address both postpartum smoking relapse among low-income women who quit smoking during pregnancy and postpartum smoking increase among those who had cut down.
Methods
One hundred thirty low-income pregnant women who were current or recently quit tobacco smokers were recruited at their first prenatal appointment and randomized to either a Control (standard care) or Experimental (standard care + PPCC) group. An intent-to-treat analysis was conducted on biochemically-verified data from 6 in-person interviews during pregnancy and postpartum. Feasibility with regard to recruitment, randomization, assessment, and implementation of PPCC were assessed, along with acceptability among the target population.
Results
PPCC was found to be feasible and acceptable to some participants but not all. There were no significant differences in tobacco products per day at 6 months postpartum between groups; however, effect sizes differed at 6 weeks compared with 6 months postpartum. Similarly, there were no significant differences between groups in cessation rate (24% in each group) and past 90-day tobacco use (59 days vs 55 days, for Control and Experimental groups respectively).
Conclusions
The PPCC intervention did not differentially reduce tobacco use postpartum compared with a controlled comparison group, though it was found to be acceptable among a subpopulation of low-income pregnant women and feasible with regard to recruitment, randomization, assessment procedures, and implementation. Further research is needed to identify an intervention that significantly improves smoking relapse rates postpartum.
Keywords: pregnant, tobacco, smoking, postpartum, relapse, low-income
INTRODUCTION
Tobacco smoking among pregnant women is the leading preventable cause of poor pregnancy outcomes and a major public health issue. In spite of the well-established negative health implications of smoking during pregnancy on mother and neonate, 15% of pregnant women ages 12-44 report cigarette smoking in the past month in the United States (U.S. Department of Health and Human Services [USDHHS], 2014). Rates of past-month cigarette use typically decline from the first trimester to the second trimester, suggesting the occurrence of spontaneous quitting, but remain stable through the third trimester (e.g., Curtin & Matthews, 2016; SAMHSA, 2009). Evidence suggests relapse rates as high as 80% within the first six months postpartum (Colman & Joyce, 2003; Fang et al., 2004; Roske et al., 2006; SAMHSA, 2009). Several risk factors are associated with continued smoking through pregnancy and relapse postpartum, such as low education level, low income, African-American race, multiparity, unmarried, high stress, and having a partner who smokes (Coleman-Cowger et al., 2016; Piper et al., 2010; Prady et al., 2012; Rockhill et al., 2016). Several of these factors are non-modifiable so it is important to tailor an intervention to high-risk populations while also addressing the modifiable factors of partner smoking and stress. Given the difficulty some pregnant women face in quitting smoking completely, it is important to examine harm reduction through reduction in the number of cigarettes smoked per day among continuing smokers (Hughes, 2000; Nichter et al., 2008).
The United States Preventive Services Task Force (USPSTF) and the American Congress of Obstetricians and Gynecologists (ACOG) currently recommend asking all pregnant women about tobacco use and providing them with assistance to quit by utilizing the 5 A’s standardized behavioral intervention (ACOG, 2010; USPSTF, 2015). The 5 A’s includes the following steps: Ask about tobacco use, Advise to quit, Assess willingness to make a quit attempt, Assist in quit attempt, and Arrange follow-up. This low-intensity behavioral intervention achieves a modest but clinically significant effect on cessation rates, with an average risk ratio of 1.7 (Melvin, 2000). There is currently no formal recommendation for smoking cessation and relapse prevention postpartum. Prior research suggests that the 5A’s may not be effective for postpartum smoking cessation and abstinence (Chapin & Root, 2004).
Several studies have attempted to reduce postpartum relapse by teaching “relapse prevention skills” during pregnancy. With few exceptions, relapse prevention strategies are typically taught in the prenatal period or the first few weeks postpartum. Unfortunately, a recent meta-analysis found that the provision of relapse prevention training during pregnancy did not reduce actual relapse postpartum (Hajek et al., 2009). Hajek and colleagues identified additional problems with extant literature, such as: not using experimental designs best suited to the task, having limited power to detect expected small differences between interventions, low intensity of the intervention provided (i.e., too few sessions over too short of a time), short length of time into postpartum that intervention is offered, difficulty with recruitment for relapse prevention-only interventions, lack of performance monitoring, exclusion of pre-pregnancy quitters, and not addressing factors known to be associated with increased risk of relapse (e.g., partner’s smoking habits, mental health, stress associated with a new baby). To be effective, any intervention needs to address these limitations and provide on-going support for at least the first six months postpartum. Phone-based Postpartum Continuing Care (PPCC) was developed to address these limitations.
Phone-based continuing care has been shown to help with addiction treatment in general (McKay, 2009) and specifically with maintaining tobacco abstinence (Stead, Perera, & Lancaster, 2006). Most smoking cessation hotlines provide 24/7 services using some variation of the 5 A’s components but require the smoker to make the initial call; however, proactive staff-initiated telephone counseling is significantly more effective at increasing quit rates than passive referrals to phone based counseling (Stead, Perera, & Lancaster, 2006; Brandon et al., 2000; Lichtenstein et al., 1996; Solomon & Flynn, 2005). Studies have demonstrated the feasibility and promise of proactive phone-based counseling delivered during the postpartum period (Parker et al., 2007; Reitzel et al., 2010), though results related to the efficacy of this approach have been mixed. Testing postpartum continuing care models is of great public health importance (Coleman-Cowger, 2012), given that an effective intervention that continues to promote cessation and prevents postpartum relapse can fundamentally change the trajectory of health outcomes for mothers and their children. Smoking cessation during pregnancy improves the health of both mother and baby and is considered a priority area for research both nationally and internationally. The continuation of smoking cessation care postpartum decreases the risk of long-term health consequences of second hand smoke exposure, including asthma, SIDS, respiratory infections, behavioral problems, and an increased likelihood that the child will smoke in the future (Prady et al., 2012; Rockhill et al., 2016; Steldinger, Luck, & Nau, 1988).
A primary aim of this randomized controlled pilot trial was to test a PPCC protocol developed from existing evidence-based approaches to address high rates of smoking relapse and return to previous levels of use in the postpartum period among low-income women who quit or cut down on smoking during pregnancy. In addition, a secondary aim was to demonstrate the feasibility of recruitment, randomization, assessment, and implementation of the PPCC intervention. This study is one of the first to focus specifically on postpartum continuing care for smoking cessation and relapse prevention among low-income pregnant women.
METHODS
Participants
The sample for this pilot randomized controlled trial (RCT) was drawn from a population of low-income pregnant women attending their first prenatal visit at a single academic obstetrics clinic. Eligibility criteria included: (a) first or second trimester of pregnancy, (b) age 18 or older, and (c) self-reported tobacco use in the past 90 days. Women were initially screened by clinic staff and referred to research staff if eligible and with expressed interest in learning more about the study. All participants completed an in-person informed consent process with research staff within one week of their first prenatal visit. Data was collected at 6 time points: intake, 3 months post-intake, 6 months post-intake, 6 weeks postpartum, 3 months postpartum, and 6 months postpartum. Incentives in the form of gift cards were provided for each 20-30 minute visit completed, with increasing incentives at each visit.
Sample randomization
Randomization took place after viability had been reached (week 26). Computer-based urn randomization was utilized to assign participants to either the Control n=64 (referral to a 24/7 state quit line postpartum — the current standard of care) or the Experimental n=64 (standard of care + PPCC) group. Urn randomization conditions the probability assignment based on the cumulative assignments to date in each of the strata into which they fall. As a result, the selection process is systematically biased toward maintaining balance while continuing to retain randomization as the primary process.
The Project Coordinator informed Chestnut Global Partners (CGP) staff (via email) and participants (via mailed letter with an enclosed “Healthy Mom, Healthy Baby” booklet and pedometer) within one week of assignment to the Experimental group. CGP, located in Bloomington, Illinois, was responsible for intervention delivery. Clinic staff, located in Baltimore, Maryland, were responsible for data collection, and were blinded to condition assignment since the Experimental group received PPCC off-site. CGP staff only interacted with women in the Experimental condition group. The study was approved by the Institutional Review Boards of the recruitment site (University of Maryland Baltimore), intervention delivery site (Chestnut Global Partners), and research institution (Battelle Memorial Institute).
PPCC intervention
The study investigator developed the initial PPCC protocol, and three separate versions of the protocol were evaluated by the target population (focus groups), two expert consultants with experience in phone-based relapse prevention with pregnant and postpartum women (Brandon et al., 2012; Lopez et al., 2008; Severson et al., 1995), and an obstetrician-gynecologist boarded in addiction medicine. The final PPCC protocol went through three rounds of revisions pre-pilot and one round of revisions post-pilot. PPCC combines two evidence-based approaches [Recovery Management Checkups (RMC) and 5 A’s] to address factors associated with relapse and includes a manual for intervention delivery, patient booklet, and documentation booklet to be completed by those administering the intervention (see Supplemental Materials).
PPCC offers an individualized approach grounded in motivational interviewing (MI) that encourages abstinence foremost, but also stresses the importance of harm reduction for women who are in the contemplation stage of change and find it more difficult to quit smoking. PPCC was designed to offer ten proactive calls with the option of calling in to a 24/7 toll-free number in the event of craving, lapse, or relapse. Ten themes were offered as discussion points (Introduction to Healthy Mom, Healthy Baby; Baby’s Birth; Understanding the Process of Behavior Change; Managing Day-to-Day Stress; Partner Support; Breaking Down Common Barriers; Quit Plan; Relapse Prevention; Eating Well and Staying Healthy; Maintaining a Tobacco-Free Lifestyle) and participants choose what they wanted to focus on in every session. Further details related to the development of the PPCC protocol are reported elsewhere.
The intervention was delivered by Chestnut Global Partners (CGP) health coaches, who were trained on the PPCC protocol in a 2-day session after the protocol was finalized. Health coaches were all female, had at least a bachelor’s degree in a related field, and had previously been trained in motivational interviewing (MI) and phone-based smoking cessation counseling as part of their orientation training at CGP. CGP is one of the nation’s leading vendors of evidence-based health coaching and disease management for tobacco cessation, substance use, depression, diabetes, and a range of other issues. All PPCC sessions were digitally recorded and the Project Coordinator, a Ph.D. psychologist with previous training in MI, provided individual supervision to each health coach on a bi-weekly basis over the course of the project. Bi-weekly peer supervision provided additional opportunity for feedback and training.
PPCC was designed to offer 10 proactive calls beginning in the third trimester of pregnancy (week 36) and continuing through 6 months postpartum with the option for participants to call in to a 24/7 toll-free number, different from the standard referral state quit line, in the event of craving, lapse, or relapse. For this study, PPCC was delivered from May 2013-March 2015. Calls took place every 2 weeks for the first 3 months postpartum, then monthly for the last 3 months.
Study design and procedures
Data from in-person interviews and biochemical verification of smoking status (urine cotinine) was collected from participants at 3 time points during pregnancy and 3 time points postpartum. Attempts were made to schedule participants’ interviews on the same day as an existing clinic appointment to increase compliance. When this was not possible, participants made a research-specific appointment to complete measures. Bus tokens were offered to offset transportation costs. Measures were self-administered via iPad and conducted in a private space.
Measures
Participants were given the same measures at all 6 data collection time points: Global Appraisal of Individual Needs-Pregnant and Postpartum Women Screener (GAIN-PPWS), Tobacco Use Patterns Survey (TUPS), Edinburgh Postnatal Depression Scale (EPDS), and Perceived Stress Scale (PSS). The primary self-report measure was the GAIN-PPWS, derived from the Global Appraisal of Individual Needs, that included symptom count screeners in 5 areas (internalizing disorders, externalizing disorders, tobacco dependence, substance use disorders, psychosocial stressors), recency/frequency of tobacco use, information on pregnancy, readiness to quit (as assessed by a 0 to 100 scale), and demographics. To determine tobacco use participants were asked “When was the last time you smoked or used any kind of tobacco? Please include cigarettes, cigars, chewing tobacco and pipes.” Those with past-year smoking were then asked: “During the past 90 days, on how many days have you smoked or used any kind of tobacco?”; and “On those days, how many times per day did you usually smoke or use any kind of tobacco? (Note: A pack of cigarettes would be about 20 times.)”
Given that the focus was on harm reduction rather than solely cessation, the primary outcome was number of tobacco products smoked per day. The following dependent variables were also monitored: number of days smoked in the past 90 days, smoking status (abstinent for past 7 days vs. current smoker), times mother smoked while breast feeding, and times mother smoked in the room with the infant. Relative to the Control group, it was expected that in the 6 months following childbirth, women in the Experimental group would: a) smoke fewer cigarettes, b) smoke fewer days, c) smoke fewer times while breastfeeding, and d) smoke fewer times while in the same room as the infant.
At each measurement point, on-site urine cotinine was measured (NicCheck I Test Strips, Mossman Associates Inc.). This test has been validated within pregnancy and postpartum (Hoffman, 1997; Swamy et al., 2011). The test scale ranges from 0-14 (0: negative; 1-6: low; and 7-14: high nicotine consumer). The lowest concentrations at which a clearly discernable positive result was visible was 2.5 μg/mL for cotinine.
Intervention Delivery
All women received 5 A’s as part of standard care during their prenatal visits and were given a referral to a 24/7 state quit line (1-800-QUIT-NOW) postpartum. PPCC health coaches made initial contact with participants in the Experimental group at 36 weeks of gestation (i.e., one week prior to full-term), called again within one week after the baby’s birth, and 8 additional times over the course of the first 6 months postpartum, while utilizing a PPCC Counselor Manual and Documentation Booklet. In cases of preterm birth, the first call occurred within two weeks after the baby’s birth. Each participant was assigned an individual female health coach to be in contact with until the end of the study. All PPCC sessions were digitally recorded and logged by staff. Independent research staff re-rated a random sample of these tapes for training feedback, quality control, and fidelity evaluation.
Data Analysis
The study design involved between-group comparisons to determine differences between the Experimental and Control groups. Analysis of smoking status was performed on an intent-to-treat (ITT) basis. All participants lost to follow-up were considered to be smokers. Statistical analyses were conducted using Stata (version 13.1) and SPSS (version 24). Outcome measures were (a) binary variables (e.g., maternal primary outcome: 7-day point prevalence abstinence) assumed to follow a binomial distribution; and (b) discrete random variables (e.g., number of tobacco products used per day), assumed to follow a Poisson distribution. Time was included in the statistical model as a “repeated factor” to represent the six assessment points, thus allowing for the evaluation of differential outcome course during pregnancy and the postpartum period as a function of the “between-subjects” Experimental Condition effect. A Generalized Linear Mixed Model (GLiMM) was used to conduct all analyses of outcomes that were measured repeatedly. Significance was determined by p-value less than 0.05.
RESULTS
Participant Characteristics
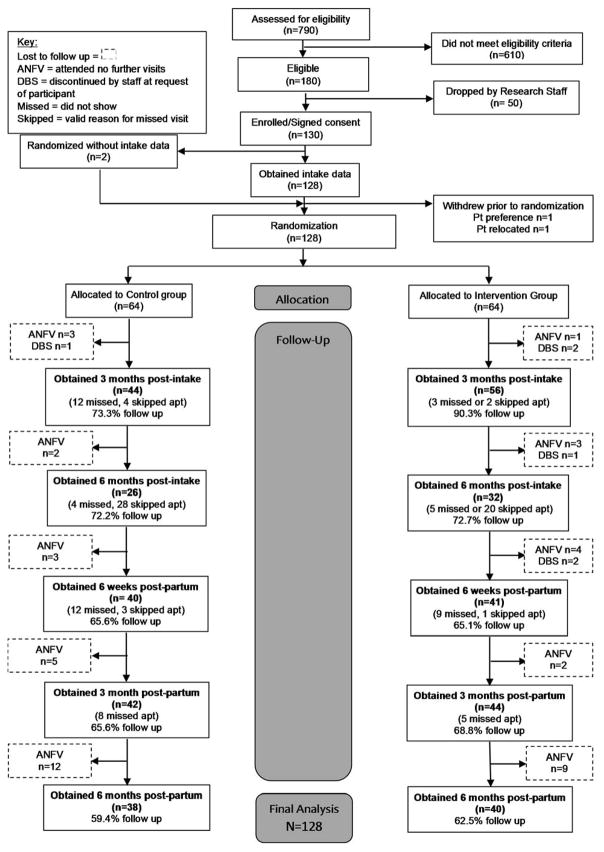
In total, 790 women presenting to their first prenatal visit (i.e., smokers and non-smokers) were assessed for eligibility from March-December 2013, with 180 meeting eligibility criteria. Fifty participants were unable to be reached or expressed disinterest in participating in the study. Of those screened, 130 participants were enrolled of whom 2 individuals withdrew prior to randomization. A total of 128 participants were randomized, 64 into each group. Three participants miscarried while enrolled but prior to randomization. These participants were given the option of continuing or discontinuing with the study, and all chose to continue; two were randomized to the Experimental group and one to the Control group. Sixteen participants (25%) withdrew from the Intervention only (n=13) or from the entire study (n=3). Reasons for withdrawal from the Intervention only were: too busy (n=2), no longer need help (n=3), and no longer interested (n=4). Four participants did not offer a reason for their withdrawal. For withdrawals, data are reported up to the point of study withdrawal (Figure 1).
Figure 1.
CONSORT diagram
Most participants were African American (80.5%), never married (74.8%), high school graduates (62.7%), not currently in the labor force (78.0%), and current smokers (68.5%), with a mean age of 26 (range 18-41). The median number of children per participant was 1 (mean 1.6) with a range of 0 to 7. Thirty-six percent of the women had 0-1 prior pregnancies, 42% had 2-4 prior pregnancies, and 22% had 5 or more prior pregnancies. Of those who had at least one previous pregnancy, approximately 45% had a pregnancy result in miscarriage or stillbirth. Only 17% of participants reported that their current pregnancy was planned. The average age of tobacco initiation was 15.7 years (range 9-29). Among participants reporting having smoked at least 1 day out of the past 90, the average number of tobacco products smoked per day was 8.6, with smoking occurring on average 59 of the past 90 days. Regular weekly alcohol and other drug (AOD) use in the past month (i.e., during pregnancy) was reported by 19%. There were no significant differences in any of these characteristics between groups (Table 1).
Table 1.
Participant Characteristics
| Categorical Variables | Total | Experimental | Control |
|---|---|---|---|
|
| |||
| % (n) | % (n) | % (n) | |
| Race (n=128) | |||
| African American | 80.5% (103) | 78.1% (50) | 82.8% (53) |
| White | 15.6% (20) | 21.9% (14) | 9.5% (6) |
| Other | 3.9% (5) | 1.6% (1) | 6.3% (4) |
| Marital Status (n=127) | |||
| Married/Living as married | 17.3% (22) | 20.3% (13) | 14.3% (9) |
| Separated/Divorced/Widowed | 7.9% (10) | 10.9% (7) | 4.8% (3) |
| Never married | 74.8% (95) | 68.8% (44) | 81% (51) |
| Education (n=126) | |||
| Less than high school | 37.3% (47) | 35.9% (23) | 37.5% (24) |
| High school grad and beyond | 62.7% (79) | 60.9% (39) | 62.5% (40) |
| Employment Status (n=127) | |||
| Unemployed/Not working/Retired | 78.0% (99) | 46.5% (46) | 53.5% (53) |
| Employed full-time | 11.8% (15) | 14.3% (9) | 9.4% (6) |
| Employed part-time | 10.2% (13) | 12.7% (8) | 7.8% (5) |
| Smoking Status (n=127) | |||
| Current Smoker | 68.5% (87) | 66.7% (42) | 70.3% (45) |
| Recent Quitter | 31.5% (40) | 33.3% (21) | 29.7% (19) |
| Previous Pregnancies (n=127) | |||
| 0-1 | 36.2% (46) | 41.3% (26) | 31.3% (20) |
| 2-4 | 41.7% (53) | 34.9% (22) | 48.4% (31) |
| 5 or more | 22.0 % (28) | 23.8% (15) | 20.3% (13) |
| Previous Miscarriage (n=111) | |||
| Yes | 45.1% (50) | 47.2% (25) | 43.1% (25) |
| No | 55.0% (61) | 52.8% (28) | 56.9% (33) |
| Trimester at intake (n=128) | |||
| 1st | 38.3% (49) | 43.8% (28) | 32.8% (21) |
| 2nd | 61.7% (79) | 56.3% (36) | 67.2% (43) |
| Pregnancy Intention (n=118) | |||
| Intended | 16.9% (20) | 16.7% (10) | 17.2% (10) |
| Did not intend | 83.1% (98) | 83.3% (50) | 82.8% (48) |
| Regular (Weekly) AOD use (n=126) | |||
| Past month | 19.0% (24) | 17.5% (11) | 20.6% (13) |
| 2-12 months | 37.3% (47) | 39.7% (25) | 34.9% (22) |
| 1+ years | 18.3% (23) | 20.6% (13) | 15.9% (10) |
| Never | 25.4% (32) | 22.2% (14) | 28.6% (18) |
|
| |||
| Continuous Variables | Mean (SD) | Mean (SD) | Mean (SD) |
|
| |||
| Age (n=128) | 26.0 (5.0) | 26.3 (5.3) | 25.6 (4.7) |
| Number of children (n=127) | 1.6 (1.6) | 1.4 (1.7) | 1.7 (1.6) |
| Age of tobacco initiation (n=115) | 15.7 (3.5) | 15.3 (3.6) | 16.1 (3.3) |
| Tobacco products per day (n=107) | 8.6 (6.8) | 9.5 (6.3) | 7.7 (7.1) |
| Days smoked of past 90 days (n=107) | 58.5 (36.7) | 65.1 (32.4) | 52.0 (39.7) |
Urinary Cotinine
Cotinine levels were significantly correlated with self-reported tobacco products per day at each time point (r range: 0.26-0.46; p<0.05). There were no significant differences in average cotinine levels between the Experimental and Control group at any of the time points (Table 2).
Table 2.
Urinary Cotinine Levels among Current Smokers
| Group |
Intake Mean (SD) n=87 |
6 Weeks Postpartum Mean (SD) n=41 |
3 Months Postpartum Mean (SD) n=55 |
6 Months Postpartum Mean (SD) n=52 |
|---|---|---|---|---|
|
| ||||
| Experimental | 5.7 (4.0) | 7.5 (3.7) | 5.9 (3.3) | 5.3 (3.4) |
| Control | 5.6 (4.6) | 7.1 (4.8) | 7.0 (2.9) | 5.8 (3.8) |
|
| ||||
| p-value | 0.88 | 0.79 | 0.16 | 0.67 |
Note: The NicCheck I test scale ranges from 0-14 (0: negative; 1-6: low; and 7-14: high nicotine consumer)
Smoking Patterns
Although participants in the Experimental group used tobacco on fewer days in the past 90 days than the Control group at each postpartum time point, there were no statistically significant differences in the number of tobacco products used per day between the Experimental and Control groups (Table 3). More participants in the Experimental group were abstinent at 6 weeks postpartum (39% vs 25%; p=0.18) and 3 months postpartum (25% vs 14%; p=0.21). The percentage of smoking abstinent women was similar for the Experimental and Control groups at 6 months postpartum.
Table 3.
Smoking Behaviors – Intervention vs. Control
| Cessation Rate | Past 90-day tobacco use (days) | Number of Tobacco Products per day | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Intervention % | Control % | P | OR 95% CI | Intervention M (SD) [95% CI] |
Control M (SD) [95% CI] |
P | Effect Size η2 | Intervention M (SD) [95% CI] |
Control M (SD) [95% CI] |
P | Effect Size η2 | |
| Intake | 25% | 24% | NS | 1.08 0.4, 2.7 |
65.1 (32) [55,75] |
52.0 (40) [42,62] |
.07 | 0.03 | 9.5 (6) [8,11] |
7.7 (7) [6,10] |
.19 | 0.02 |
| 6 Weeks Postpartum | 39% | 25% | NS | 1.92 0.7, 5.6 |
45.9 (40) [30,62] |
52.1 (36) [39,65] |
.54 | 0.01 | 6.1 (4) [4,8] |
6.9 (6) [5,9] |
.56 | 0.01 |
| 3 Months Postpartum | 25% | 14% | NS | 2 0.6, 7.3 |
57.5 (38) [45,70] |
60.7 (34) [49,72] |
.70 | 0.002 | 7.9 (6) [6,10] |
7.1 (8) [5,9] |
.63 | 0.003 |
| 6 Months Postpartum | 24% | 24% | NS | 1.04 0.3, 3.3 |
55.3 (39) [41,69] |
59.2 (32) [47,71] |
.67 | 0.003 | 6.9 (5) [5,9] |
6.4 (6) [4,8] |
.75 | 0.002 |
Note: Past 90-day tobacco use and number of tobacco products per day reported among participants who smoked at least one day of past 90.
There was a significant decrease in past 90-day tobacco use from intake at each postpartum time point for the Experimental group but not in the Control group. A similar trend was observed for the number of tobacco products used per day. Significantly fewer tobacco products were used at 6 weeks postpartum compared to intake within the Experimental group - this decrease was not evident in the Control group.
Postpartum Behaviors
Very few women reported breast feeding postpartum: 18 at 6 weeks postpartum, 7 at 3 months postpartum, and 6 at 6 months postpartum. There were no differences in postpartum breastfeeding status or number of days smoking while breastfeeding between the Experimental and Control groups. Few women also reported smoking in the same room as their baby: 2 at 6 weeks postpartum, 5 at 3 months postpartum, and 3 at 6 months postpartum.
PPCC Feasibility
PPCC was found to be feasible in terms of recruitment, randomization, and assessment. Recruitment was reached one month earlier than anticipated. Randomization resulted in balanced groups and assessment follow-up rates were higher than 60% at each postpartum measurement point. Specifically, follow-up rates were 69% at 6 weeks postpartum, 70% at 3 months postpartum, and 63% at 6 months postpartum.
Due to drop outs and inability to reach participants, 19 participants did not receive any PPCC intervention sessions. Therefore, 45 participants (70.3%) in the Experimental group received at least one PPCC intervention session, with the average number of sessions delivered being 2.3. The feasibility of implementing PPCC as a set 10-session protocol was not established; however, some women (n=4) completed 10 calls and did not want the calls to end.
DISCUSSION
Interventions to increase smoking reduction and cessation during pregnancy have shown modest results at best and no intervention has yet been shown to significantly improve reduction in relapse postpartum (Reitzel et al., 2010; Hajek et al., 2013; Levine et al., 2016). Our goal was to address limitations noted from previous smoking cessation and relapse prevention studies during postpartum (Hajek et al., 2009) by utilizing a rigorous RCT study design, including pre-pregnancy quitters, extending the intervention to six months postpartum, and addressing known risk factors of relapse (e.g., partner smoking and stress). The PPCC approach focused on monitoring and re-intervention in a way that took into account factors known to be associated with relapse and the chaos of new mothers’ schedules by utilizing proactive telephone counseling. To that end, our study aimed to pilot-test a PPCC protocol and demonstrate its feasibility.
This pilot RCT is one of the first to focus on the postpartum period in a smoking cessation evaluation. Limited benefits of including phone-based continuing care to standard care for smoking cessation during pregnancy were found; however, this was a pilot study that was not adequately powered to find significant effects despite promising trends being observed.
Results from this study are comparable to other postpartum intervention studies. A randomized controlled trial of postpartum phone based intervention for smoking abstinence was performed by Reitzel and colleagues (2010), who reported a slight improvement in abstinence rates with postpartum phone based counseling in their unadjusted analysis as well as a slightly lower attrition rate compared to our study. However, their intervention included in-person counseling sessions for half of the study group, which makes it difficult to determine if phone based counseling alone was the reason for the identified effect. In addition, the cohort consisted of pregnant women who had quit smoking during pregnancy, some of whom were recruited through advertisements, which likely represents a population with a higher motivation level than those included in the PPCC study. Similar to results reported herein was an apparent increase in effect size in participants who reported heavier smoking (Reitzel et al, 2010). A second study evaluating the feasibility and cost effectiveness of postpartum phone based smoking abstinence counseling found similar rates of adherence to treatment with 86% receiving one phone call and 46% receiving three phone calls (Parker et al., 2007). A more recent study of a postpartum-adapted, behavioral smoking relapse prevention intervention that included content focused on women's postpartum concerns about mood, stress, and weight (Strategies to Avoid Returning to Smoking [STARTS]) or a supportive, time and attention-controlled comparison (SUPPORT) also did not differentially improve rates of sustained tobacco abstinence postpartum compared with a time and attention-controlled comparison (Levine et al., 2016).
Results converge to paint a picture of the immense challenge of addressing postpartum relapse to smoking. This challenge should not serve to hinder further research, but should highlight how dire the need is for more work in this area. The strengths of the PPCC study are its focus on continuing care in the postpartum period when risk of relapse and increased smoking is high, use of a randomized pretest-posttest control design to evaluate PPCC, individualized intervention tailored to a high-risk population of pregnant smokers, and biochemical verification of smoking. Promising trends were noted in this population and the infrastructure is in place for incorporating PPCC into quitlines should a larger trial find significant effects; however, modifications to PPCC would be needed prior to the conduct of a larger trial.
There were several limitations with this study. As this was a pilot study, the sample size was small and there was a low dose of the intervention that was received by participants in the Experimental condition. These factors may explain the lack of statistical significance between the two groups. There was also a trend observed with the Experimental group being heavier smokers than the Control group, which could be indicative of these individuals finding it more challenging to quit or cut down on smoking. There was a significant dropout rate in the Experimental group; several participants were unable to be contacted or had issues with transportation and childcare limiting their ability to follow-up in person and provide urine samples for cotinine testing. NRT and e-cigarette use were not assessed in this study and no distinction was made between tobacco products, all of which may have impacted cotinine levels. Finally, there was a possibility that any effect of PPCC could be explained at least in part by increased contact with participants. Each of these limitations limit the generalizability of pilot findings but would be addressed in a larger clinical trial through modifications to the PPCC protocol. A larger trial may modify PPCC to include contingency management, which has been shown to have the largest benefit to smoking cessation during pregnancy but has only been minimally tested in the postpartum period (Higgins et al., 2010; Higgins et al., 2012).
CONCLUSIONS
This pilot study provides important information for the design of future clinical trials to test postpartum smoking cessation and relapse prevention interventions. A small effect size was observed for PPCC, but power was not sufficient to draw conclusions. Trends were observed that suggest PPCC is a promising intervention, particularly in the first 3 months postpartum. Modifications are needed based on participant feedback, and PPCC may be better suited to a subgroup of pregnant smokers given findings that some found it to be particularly appealing whereas others did not. Those who found it appealing were more likely to complete more calls with a health coach, thus a satisfaction assessment after the second call may be appropriate to gauge interest in PPCC and learn how to best move forward to meet patient need. Based on the results of this study, further modifications of the PPCC protocol are warranted to more thoroughly explore promising trends identified in this pilot RCT. Additional research is imperative to address the continuing trend of high rates of relapse to smoking postpartum.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by a grant from the National Institute on Drug Abuse (NIDA; R34DA032683). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDA or the National Institutes of Health.
Footnotes
Clinicaltrials.gov identifier: NCT01684592
References
- American College of Obstetricians and Gynecologists. Committee opinion no. 471: Smoking cessation during pregnancy. Obstet Gynecol. 2010;116:1241–1244. doi: 10.1097/AOG.0b013e3182004fcd. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Collins BN, Juliano LM, Lazev AB. Preventing relapse among former smokers: a comparison of minimal interventions through telephone and mail. J Consult Clin Psychol. 2000;68:103–113. doi: 10.1037//0022-006x.68.1.103. [DOI] [PubMed] [Google Scholar]
- Brandon TH, et al. Self-help booklets for preventing postpartum smoking relapse: a randomized trial. American journal of public health. 2012;102:2109–2115. doi: 10.2105/AJPH.2012.300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin J, Root W. Improving obstetrician-gynecologist implementation of smoking cessation guidelines for pregnant women: an interim report of the American College of Obstetricians and Gynecologists. Nicotine Tob Res. 2004;6(Suppl 2):S253–S257. doi: 10.1080/14622200410001669123. [DOI] [PubMed] [Google Scholar]
- Coleman-Cowger VH. Smoking cessation intervention for pregnant women: a call for extension to the postpartum period. Matern Child Health J. 2012;16:937–940. doi: 10.1007/s10995-011-0837-2. [DOI] [PubMed] [Google Scholar]
- Coleman-Cowger VH, Koszowski B, Rosenberry ZR, Terplan M. Factors associated with early pregnancy smoking status among low-income smokers. Matern Child Health J. 2016;20:1054–1060. doi: 10.1007/s10995-015-1891-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman GJ, Joyce T. Trends in smoking before, during, and after pregnancy in ten states. Am J Prev Med. 2003;24:29–35. doi: 10.1016/s0749-3797(02)00574-3. [DOI] [PubMed] [Google Scholar]
- Curtin SC, Matthews TJ. Smoking Prevalence and Cessation Before and During Pregnancy: Data From the Birth Certificate, 2014. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2016;65(1):1–14. [PubMed] [Google Scholar]
- DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics. 2004;113(4 Suppl):1007–1015. [PubMed] [Google Scholar]
- Fang WL, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Pract. 2004;17:264–275. doi: 10.3122/jabfm.17.4.264. [DOI] [PubMed] [Google Scholar]
- Gadomski A, Adams L, Tallman N, Krupa N, Jenkins P. Effectiveness of a combined prenatal and postpartum smoking cessation program. Matern Child Health J. 2011;15:188–197. doi: 10.1007/s10995-010-0568-9. [DOI] [PubMed] [Google Scholar]
- Hajek P, Stead LF, West R, Jarvis M, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev. 2009;(1):CD003999. doi: 10.1002/14651858.CD003999.pub3. [DOI] [PubMed] [Google Scholar]
- Hajek P, Stead LF, West R, Jarvis M, Hartmann-Boyce J, Lancaster T. Relapse prevention interventions for smoking cessation. The Cochrane Library. 2013 doi: 10.1002/14651858.CD003999.pub4. [DOI] [PubMed] [Google Scholar]
- Hannover W, Thyrian JR, Roske K, et al. Smoking cessation and relapse prevention for postpartum women: results from a randomized controlled trial at 6, 12, 18 and 24 months. Addict Behav. 2009;34:1–8. doi: 10.1016/j.addbeh.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Bernstein IM, Washio Y, et al. Effects of smoking cessation with voucher-based contingency management on birth outcomes. Addiction. 2010;105:2023–2030. doi: 10.1111/j.1360-0443.2010.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Washio Y, Heil SH, Solomon LJ, Gaalema DE, Higgins TM, Bernstein IM. Financial incentives for smoking cessation among pregnant and newly postpartum women. Preventive Medicine. 2012;55:S33–S40. doi: 10.1016/j.ypmed.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DW. Comparison of Niccheck I Test Strip with immunoassay results on urine specimens from pregnant smokers. Therapeutic Drug Monitoring. 1997;19:586. [Google Scholar]
- Hughes J. Reduced smoking: An introduction and review of the evidence. Addiction. 2000;95(Suppl 1):3–7. doi: 10.1080/09652140032008. [DOI] [PubMed] [Google Scholar]
- Levine MD, Cheng Y, Marcus MD, Kalarchian MA, Emery RL. Preventing postpartum smoking relapse: a randomized clinical trial. JAMA internal medicine. 2016;176(4):443–452. doi: 10.1001/jamainternmed.2016.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein E, Hollis JF, Severson HH, et al. Tobacco cessation interventions in health care settings: rationale, model, outcomes. Addict Behav. 1996;21:709–720. doi: 10.1016/0306-4603(96)00030-5. [DOI] [PubMed] [Google Scholar]
- Lopez EN, et al. Clinical trials and tribulations: lessons learned from recruiting pregnant ex-smokers for relapse prevention. Nicotine & tobacco research. 2008;10:87–96. doi: 10.1080/14622200701704962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride CM, Curry SJ, Lando HA, Pirie PL, Grothaus LC, Nelson JC. Prevention of relapse in women who quit smoking during pregnancy. Am J Public Health. 1999;89:706–711. doi: 10.2105/ajph.89.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR. Continuing care research: what we have learned and where we are going. J Subst Abuse Treat. 2009;36:131–145. doi: 10.1016/j.jsat.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin R. Recommended cessation counselling for pregnant women who smoke: a review of the evidence. Tob Control. 2000:9. doi: 10.1136/tc.9.suppl_3.iii80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichter M, Nichter M, Adrian S, Goldade K, Tesler L, Muramoto M. Smoking and harm-reduction efforts among postpartum women. Qualitative health research. 2008;18(9):1184–1194. doi: 10.1177/1049732308321738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DR, Windsor RA, Roberts MB, et al. Feasibility, cost, and cost-effectiveness of a telephone-based motivational intervention for underserved pregnant smokers. Nicotine Tob Res. 2007;9:1043–1051. doi: 10.1080/14622200701591617. [DOI] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010;12:647–657. doi: 10.1093/ntr/ntq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prady SL, Kiernan K, Bloor K, Pickett KE. Do Risk Factors for Post-partum Smoking Relapse Vary According to Marital Status? Maternal and Child Health Journal. 2012;16:1364–1373. doi: 10.1007/s10995-011-0899-1. [DOI] [PubMed] [Google Scholar]
- Reitzel LR, Vidrine JI, Businelle MS, et al. Preventing postpartum smoking relapse among diverse low-income women: a randomized clinical trial. Nicotine Tob Res. 2010;12:326–335. doi: 10.1093/ntr/ntq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti NA, Park ER, Regan S, et al. Efficacy of telephone counseling for pregnant smokers: a randomized controlled trial. Obstet Gynecol. 2006;108:83–92. doi: 10.1097/01.AOG.0000218100.05601.f8. [DOI] [PubMed] [Google Scholar]
- Rockhill KM, Tong VT, Farr SL, Robbins CL, D'Angelo DV, England LJ. Postpartum Smoking Relapse After Quitting During Pregnancy: Pregnancy Risk Assessment Monitoring System, 2000–2011. Journal of Women's Health. 2016;25:480–488. doi: 10.1089/jwh.2015.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roske K, Hannover W, Grempler J, et al. Post-partum intention to resume smoking. Health Educ Res. 2006;21:386–392. doi: 10.1093/her/cyh069. [DOI] [PubMed] [Google Scholar]
- Secker-Walker RH, Solomon LJ, Flynn BS, et al. Smoking relapse prevention counseling during prenatal and early postnatal care. Am J Prev Med. 1995;11:86–93. [PubMed] [Google Scholar]
- Severson HH, et al. Predictors of smoking during and after pregnancy: a survey of mothers of newborns. Preventive medicine. 1995;24:23–28. doi: 10.1006/pmed.1995.1004. [DOI] [PubMed] [Google Scholar]
- Solomon LJ, Flynn BS. Telephone support for pregnant smokers who want to stop smoking. Health Promot Pract. 2005;6:105–108. doi: 10.1177/1524839903260642. [DOI] [PubMed] [Google Scholar]
- Stead LF, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2006;3:CD002850. doi: 10.1002/14651858.CD002850.pub2. [DOI] [PubMed] [Google Scholar]
- Steldinger R, Luck W, Nau H. Half lives of nicotine in milk of smoking mothers: implications for nursing. J Perinat Med. 1988;16:261–262. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. The NSDUH report: substance use among women during pregnancy and following childbirth. Rockville, MD: 2009. [Google Scholar]
- Swamy GK, Reddick KL, Brouwer RJ, Pollak KI, Myers ER. Smoking prevalence in early pregnancy: comparison of self-report and anonymous urine cotinine testing. J Matern Fetal Neonatal Med. 2011;24:86–90. doi: 10.3109/14767051003758887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong VT, Jones JR, Dietz PM, D'Angelo D, Bombard JM. Trends in smoking before, during, and after pregnancy - Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000-2005. MMWR Surveill Summ. 2009;58:1–29. [PubMed] [Google Scholar]
- United States Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Center for Behavioral Health Statistics and Quality. National Survey on Drug Use and Health, 2014. Ann Arbor, MI: Inter-university Consortium for Political and Social Research; Mar 22, 2016. [Google Scholar]
- U.S. Preventive Services Task Force. [Accessed on 09/21/2016];Final Update Summary: Tobacco Smoking Cessation in Adults, Including Pregnant Women: Behavioral and Pharmacotherapy Interventions. 2015 https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1?ds=1&s=smoking cessation.
- Wakschlag LS, Pickett KE, Kasza KE, Loeber R. Is prenatal smoking associated with a developmental pattern of conduct problems in young boys? J Am Acad Child Adolesc Psychiatry. 2006;45:461–467. doi: 10.1097/01.chi.0000198597.53572.3e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.