Abstract
Autosomal recessive cerebellar ataxias (ARCAs) represent a heterogeneous group of inherited disorders. The association of early-onset cerebellar ataxia with hypogonadotropic hypogonadism is related to two syndromes, known as Gordon Holmes syndrome (GHS—ataxia and pyramidal signs with hypogonadotropic hypogonadism) and Boucher-Neuhäuser syndrome (BNS—ataxia with chorioretinal dystrophy). Mutations in the PNPLA6 gene have been identified as the cause of hereditary spastic paraplegia and complex forms of ataxia associated with retinal and endocrine manifestations. We reported two Brazilian patients with sporadic, progressive cerebellar ataxia, associated with hypogonadotropic hypogonadism, in whom the GHS and BNS were confirmed by the demonstration of compound heterozygote mutations in the PNPLA6 gene. Genetic analysis of the patient 1 revealed compound heterozygous mutations, one allele in exon 34 and the other allele in exon 29. Genetic exam of the patient 2 also demonstrated compound heterozygous mutations. Three were novel mutations. The missense mutation c.3373G> A, found in the BNS patient, was previously related to Oliver-McFarlane syndrome. These different mutations in this gene suggest a complex phenotype associated disease spectrum.
Keywords: Cerebellar ataxia, Hypogonadotropic hypogonadism, PNPLA6 gene, Gordon Holmes syndrome, Boucher-Neuhäuser syndrome
Introduction
Hereditary ataxias represent a heterogeneous group of neurodegenerative diseases, including the autosomal recessive cerebellar ataxias (ARCAs) and autosomal dominant cerebellar ataxias, known as spinocerebellar ataxias (SCAs) [1]. The most common forms of ARCAs are Friedreich’s ataxia and ataxia telangiectasia, but the differential diagnosis include a variety of rarer disorders, such as ARCAs associated with hypogonadotropic hypogonadism (AHH) [1, 2], which include Boucher-Neuhäuser (BNS), Gordon Holmes (GHS), and Perrault syndromes [1–3]. AHH is a genotypically variable as it can be linked to a different genetic defects, including STUB1, RNF216, OTUD4, HSD17B4, HARS2, LARS2, CLPP, and PNPLA6 mutations [1–3].
Mutations in the patatin-like phospholipase domain-containing protein 6 (PNPLA6) gene were originally described in as causative for hereditary spastic paraplegia 39 (SPG39) [4]. PNPLA6 mutations were additionally identified as the most frequent unifying genetic cause of two AHH: Boucher-Neuhäuser (BNS) and Gordon Holmes (GHS) syndromes [5, 6]. Subsequently, PNPLA6 was independently identified as the genetic cause in several families with Laurence-Moon and Oliver-McFarlane syndromes [7, 8]. PNPLA6 mutations also were related to a pure form of ataxia (Table 1) [5, 9].
Table 1.
PNPLA6-related disorders
| Syndrome | Features | Mutations* |
|---|---|---|
| Pure ataxia | Ataxia | c.3847G>A c.3929A>T |
| Boucher-Neuhäuser | Ataxia, hypogonadotropic hypogonadism, chorioretinal dystrophy | c.288T>G c.343-2A>T c.865C>G c.1732G > T c.2212-1G > C c.2944_2947dub c.3134C > T c.3173C > T c.3197T > C c.3328G > A c.3365C > T c.3373G>A c.3519C>G c.3932 G>A c. 4075 C >T c.4081C>T (novel) |
| Gordon Holmes | Ataxia, hypogonadotropic hypogonadism, pyramidal signs | c.1127insG c.2297T>C (novel) c.2494insTGTGGGCCTGGGG c.3084_3085insGCCA c.3295 C>T c.3380 C> G c.3387G>A (novel) c.3526G>A c.3931C >T c.4084C > G |
| Laurence-Moon | Ataxia, distal muscle wasting, spastic paraplegia, trichomegaly, congenital hypopituitarism and retinal degeneration with choroidal atrophy | c.3526G>A c.3084_3085insGCCA dup(Ex14-20) |
| Oliver-McFarlane | Ataxia, distal muscle wasting, blindness due to severe photoreceptor degeneration, dwarfism due to pituitary growth hormone deficiency, trichomegaly and progressive alopecia | c.1238_1239insC c.1571T>C c.1973+2T>G c.2116C>T c.2763G>A c.3322C>T c.3373G>A c.3385G>C c.343-2A>T c.1076C>T c.3084_3085insGCCA |
| Spastic-ataxia | Ataxia with spastic paraplegia | c.3084_3085insGCCA c.3299T > G |
| Hereditary spastic paraplegia 39 (SPG39) | Spastic paraplegia with distal muscle wasting | c.787G > A c.2519G > A c.2669G > A c.2946_2947ins CAGC c.3034 A > G |
Here, we describe two Brazilian patients with sporadic cerebellar ataxia with hypogonadotropic hypogonadism associated with PNPLA6 gene mutations.
Case Descriptions
Case 1
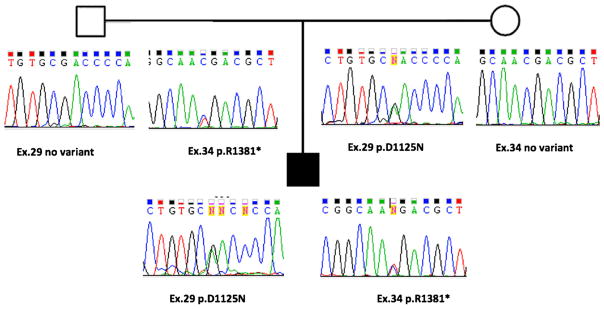
A 24-year-old male was assessed due to a 1-year history of progressive balance difficulties and intermittent diplopia. Clinical examination was remarkable for the absence of secondary sexual characteristics, with eunuchoid habitus. He was Tanner stage 4, with testicular volume of 15 ml by orchidometer and penile length of 10 cm (Tanner scale match sexual maturity stages, 0 to 5; stage 4 implies pre-adult development). Neurological examination showed mild gait ataxia, more evident while performing tandem gait, and horizontal gaze-evoked nystagmus. Ophthalmological examination was normal, as well as the retinography and optical coherence tomography (OCT). A normal electrooculogram with Arden ratio of 2.5 in the right eye and 2.7 in the left eye ruled out Boucher-Neuhäuser syndrome. Brain MRI showed mild cerebellar atrophy. Endocrinological investigation showed sex hormone-binding globulin (SHBG) of 80.9 nmol/l (normal range 13.2–89.5 nmol/l), low plasma total testosterone levels of 6.8 nmol/l (normal range 10.4–41.6 nmol/ l), and low free testosterone of 0.07 nmol/l (normal range 0.31–1.04 nmol/l), corresponding to 1.01% of total testosterone, in the presence of normal serum FSH of 2.7 mUI/ml (normal range up to 13 mUI/ml) and normal serum LH of 2.4 mUI/l (normal range up to 14 mUI/ml), confirming the biochemical diagnosis of hypogonadotropic hypogonadism. Other hormonal tests of pituitary function and spermogram were normal. Genetic analysis of the PNPLA6 gene revealed compound heterozygous mutations, one allele in exon 34 with c.4081C>T, p.Arg1381* (introducing a premature stop codon) and the other allele in exon 29 the c.3373G>A, p.Asp1125Asn (Fig. 1). Each parent was heterozygote for one of these mutations, proving that they are in trans in the patient. There were no clinical features in other family members. Scale for the Assessment and Rating of Ataxia (SARA) score was 6 at baseline, stable after 2 years of follow-up. The patient was started on hormone replacement therapy with long-acting testosterone undecanoate, with progressive modification of his voice, development of oily skin, acne, and secondary sexual characteristics, associated with normalization of plasma testosterone levels. Coenzyme Q10 was also initiated along with physical therapy.
Fig. 1.
Patient 1: family pedigree and genomic sequence chromatograms of missense and nonsense PNPLA6 mutations (Gordon Holmes syndrome)
Case 2
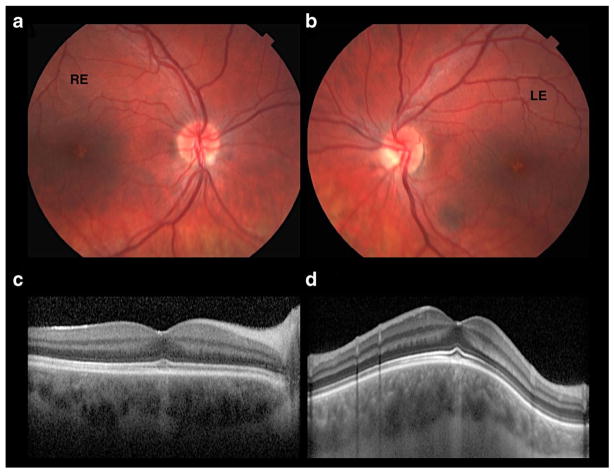
A 19-year-old male was evaluated due to a 12-year history of progressive ataxia associated and visual loss. At age of 15, he was evaluated due to short stature and puberty delay. At clinical examination, his height was 163 cm, his weight was 70 kg, and his body mass index was 26.4 kg/m2. He had mild gynecomastia and absence of axillary and body hair. He was Tanner stage 2, with testicular volume of 6 ml by orchidometer and penile length of 10 cm. Neurological examination demonstrated the presence of bilateral horizontal gaze-evoked nystagmus, mild dysarthria, gait ataxia, particularly in the tandem gait, and mild dysmetria in the upper limbs. In the ophthalmological examination, the visual acuity was 20/30 in both eyes (OU; oculus uterque). In the electroretinogram (ERG), the scotopic phase was normal, and photopic phase was subnormal in OU. The multifocal ERG showed subnormal amplitudes in the central area in OU. The presence of bilateral foveal atrophy in OU were confirmed by optical coherence tomography (OCT) and color fundus photographs (Fig. 2). Endocrinological investigation showed SHBG of 24 nmol/l (normal range 13.2–89.5 nmol/l) and very low plasma total testosterone level of 0.03 nmol/l (normal range 0.31–1.04 nmol/l, corresponding to roughly 2% of total testosterone), in the presence of low serum FSH and LH concentrations (0.7 and 0.6 mUI/ml, respectively), confirming the diagnosis of hypogonadotropic hypogonadism. Other hormonal tests of pituitary function were normal. He started therapy with human chorionic gonadotropin (Choriomon) 5000 UI every week, with modification of his voice, development of oily skin, acne and body hair, associated with normalization of plasma testosterone levels after 6 months of therapy.
Fig. 2.
Patient 2: ataxia associated with hypogonadotropic hypogonadism—ophthalmological exam. a, b Color fundus photographs reveals a small depigmented foveal symmetrical spot in OU. c, d OCT horizontal scan of RE and OCT vertical scan in LE show foveal area with disruption of C.O.S.T (cone outer segment tips) and choroidal hyperreflectivity, demonstrating retinal pigment epithelium (RPE) atrophy at this site. RE right eye, LE left eye
Genetic exam of the PNPLA6 demonstrated compound heterozygous mutations, one allele with c.3387G>A (p.Trp1129) and the other allele with c.2297T>C (p.Leu766Pro).
SARA scale score was 8/40. The patient is undergoing hormonal treatment as well as coenzyme Q10, buspirone, and physical therapy. There were no clinical features in other family members.
Discussion
BNS presents with AHH, visual impairment due to chorioretinal dystrophy, and is frequently also associated with peripheral neuropathy (sensorimotor) and hypersegmented neutrophils [10–15]. GHS is an AHH associated with pyramidal signs, including brisk reflexes, spasticity, and Babinski’s reflexes, with no visual impairment [10, 16]. Our patients presented with sporadic AHH, confirmed by endocrine evaluation, the first with no visual impairment or pyramidal signs, which lead us to the diagnosis of GHS. The second patient presented with visual impairment due to foveal atrophy, and complementary exams confirming the diagnosis of macular dystrophy, BNS, different from other reports that showed changes more diffusely in chorioretinal. The normal electrooculogram (EOG) and normal scotopic and subnormal photopic ERG suggest that the ophthalmological alterations are restricted to macular area [5, 12, 17]. Upon follow-up, both patients demonstrated stability of motor signs and symptoms with no significant longitudinal changes in the score of the ataxia scale (SARA) used, associated with improvement of endocrinological parameters after testosterone replacement therapy associated with coenzyme Q10 supplementation [18].
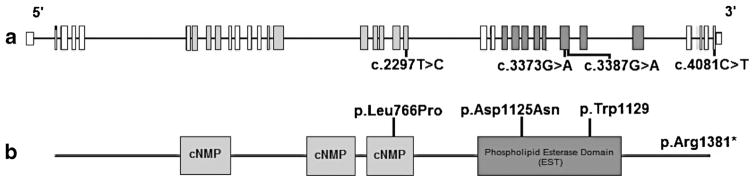
PNPLA6 gene is related to a protein named neuropathy target esterase that belongs to a family of nine patatin-like phospholipase domain-containing proteins [5, 10]. Most of PNPLA6 mutations affected the enzymatic activity of the phospholipid esterase domain (EST), and then influencing the cellular signaling in the central nervous system (Fig. 3) [5, 10]. On a functional level, proteins like PNPLA6, on which different phenotypes converge, may represent functional platforms or hubs that connect a diversity of pathways with multiple biological functions. These “hub proteins,” intersection nodes in phenotypic as well as cellular disease networks [5].
Fig. 3.
An illustration of PNPLA6 gene structure along with the mutations of the case reports. Exons highlighted corresponding to protein domains
Our finding of compound heterozygous mutations in this case is in accordance with PNPLA6-related disorders following an autosomal recessive mode of inheritance [14]. The c.3373G>A mutation was previously described in a patient with Oliver-McFarlane syndrome [5]. It was also an isolated case and had compound heterozygous mutation with c.1571T>C in the other allele. The condition started at the age of 9 and on examination showed retinal degeneration with marked visual loss, 20/400 in OU. She had hypopituitarism, GH deficiency with growth delay, trichomegaly, but there was no hypogonadism. Our patient with the same mutation (case 1—BNS) had the mutation c.4081C>T on the other allele, introducing a stop codon. In spite of one identical mutation, both the genotype and the phenotype of these patients were different. Other mutations, such as c.3084_3085insGCCA, may be associated with distinct phenotypes, in this case with ataxia-spastic, Laurence-Moon and Oliver-McFarlane syndromes [5, 7, 8].
Our cases reported with GHS and BNS patients, presenting as sporadic cerebellar ataxia associated with hypogonadotropic hypogonadism, confirm the expanded spectrum of PNPLA6 gene mutations.
Acknowledgments
The authors thank the patients, and their families, for their authorizations to publish this case reports.
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict of interest.
Ethics Approval The study was approved by the Ethics committee of Hospital de Clínicas of Federal University of Paraná.
References
- 1.Teive HAG, Ashizawa T. Primary and secondary ataxias. Curr Opin Neurol. 2015;28:413–22. doi: 10.1097/WCO.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vermeer S, van de Warremburg BPC, Willemsen MAA, et al. Autosomal recessive cerebellar ataxias: the current state of affairs. J Med Genet. 2011;48:651–9. doi: 10.1136/jmedgenet-2011-100210. [DOI] [PubMed] [Google Scholar]
- 3.Morino H, Pierce SB, Matsuda Y, et al. Mutations in Twinkle primase-helicase cause Perrault syndrome with neurologic features. Neurology. 2014;83(22):2054–61. doi: 10.1212/WNL.0000000000001036. https://doi.org/10.1212/WNL.0000000000001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rainier S, Bui M, Mark E, Thomas D, Tokarz D, Ming L, et al. Neuropathy target esterase gene mutations cause motor neuron disease. Am J Hum Genet. 2008;82(3):780–5. doi: 10.1016/j.ajhg.2007.12.018. https://doi.org/10.1016/j.ajhg.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Synofzik M, Gonzalez MA, Lourenco CM, Coutelier M, Haack TB, Rebelo A, et al. PNPLA6 mutations cause Boucher-Neuhauser and Gordon Holmes syndromes as part of a broad neurodegenerative spectrum. Brain. 2014;137(Pt 1):69–77. doi: 10.1093/brain/awt326. https://doi.org/10.1093/brain/awt326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topaloglu AK, Lomniczi A, Kretzschmar D, Dissen GA, Kotan LD, McArdle CA, et al. Loss-of-function mutations in PNPLA6 encoding neuropathy target esterase underlie pubertal failure and neurological deficits in Gordon Holmes syndrome. J Clin Endocrinol Metab. 2014;99(10):E2067–75. doi: 10.1210/jc.2014-1836. https://doi.org/10.1210/jc.2014-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kmoch S, Majewski J, Ramamurthy V, Cao S, Fahiminiya S, Ren H, et al. Mutations in PNPLA6 are linked to photoreceptor degeneration and various forms of childhood blindness. Nat Commun. 2015;6:5614. doi: 10.1038/ncomms6614. https://doi.org/10.1038/ncomms6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hufnagel RB, Arno G, Hein ND, Hersheson J, Prasad M, Anderson Y, et al. Neuropathy target esterase impairments cause Oliver-McFarlane and Laurence-Moon syndromes. J Med Genet. 2015;52(2):85–94. doi: 10.1136/jmedgenet-2014-102856. https://doi.org/10.1136/jmedgenet-2014-102856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiethoff S, Bettencourt C, Paudel R, et al. Pure cerebellar ataxia with homozygous mutations in the PNPLA6 gene. Cerebellum. 2017;16:262–7. doi: 10.1007/s12311-016-0769-x. https://doi.org/10.1007/s12311-016-0769-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Synofzyk M, Hufnagel RB, Züchner S. PNPLA6-related disorders. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews [Internet} Seattle (WA): University of Washington, Seattle; 2014. Oct 09, 1993–2016. updated 2015 Jun 11. [Google Scholar]
- 11.Tarnutzer AA, Gerth-Kahlert C, Timmann D, et al. Boucher-Neuhäuser syndrome: cerebellar degeneration, chorioretinal dystrophy and hypogonadotropic hypogonadism: two novel cases and a review of 40 cases from the literature. J Neurol. 2015;262:194–202. doi: 10.1007/s00415-014-7555-9. [DOI] [PubMed] [Google Scholar]
- 12.Boucher BJ, Gibberd FB. Familial ataxia, hypogonadism and retinal degeneration. Acta Neurol Scand. 1969;45:507–10. [PubMed] [Google Scholar]
- 13.Neuhäuser G, Opitz JM. Autosomal recessive syndrome of cerebellar ataxia and hypogonadotropic hypogonadism. Clin Genet. 1975;7:426–34. doi: 10.1111/j.1399-0004.1975.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 14.Synofzik M, Kernstock C, Haack TB, Schöls L. Ataxia meets chorioretinal dystrophy and hypogonadism: Boucher-Neuhäuser syndrome due to PNPLA6 mutations. J Neurol Neurosurg Psychiatry. 2015;86:580–1. doi: 10.1136/jnnp-2014-307793. [DOI] [PubMed] [Google Scholar]
- 15.Koh K, Kobayashi F, Miwa M, et al. Novel mutations in the PNPLA6 gene in Boucher-Neuhäuser syndrome. J Human Genet. 2015;60:217–20. doi: 10.1038/jhg.2015.3. [DOI] [PubMed] [Google Scholar]
- 16.Holmes G. A form of familial degeneration of the cerebellum. Brain. 1907;30:466–89. [Google Scholar]
- 17.Seminara SB, Acierno JS, Jr, Abdulwahid NA, Crowley WF, Jr, Margolin DH. Hypogonadotropic hypogonadism and cerebellar ataxia: detailed phenotypic characterization of a large, extended kindred. J Clin Endocrinol Metab. 2002;87:1607–12. doi: 10.1210/jcem.87.4.8384. [DOI] [PubMed] [Google Scholar]
- 18.Gironi M, Lamperti C, Nemni R, et al. Late-onset cerebellar ataxia with hypogonadism and muscle coenzyme Q10 deficiency. Neurology. 2004;62:818–20. doi: 10.1212/01.wnl.0000113719.67643.b7. [DOI] [PubMed] [Google Scholar]