Abstract
Objectives
Heavy drinking tobacco users are less likely to successfully quit smoking than their moderate drinking counterparts, even when they are prescribed smoking cessation medication. One strategy for improving treatment outcomes in this subgroup of tobacco users may be to combine medication therapies to target both alcohol and tobacco use simultaneously. Adding naltrexone to frontline smoking cessation treatments may improve treatment outcomes in this group.
Method
This double-blind, placebo-controlled human laboratory study examined the effects of varenicline (2 mg/day) and varenicline (2mg/day) combined with a low dose of naltrexone (25mg/day) on alcohol-primed smoking behavior in a laboratory model of smoking relapse in heavy-drinking tobacco users (n = 30). Participants attended a laboratory session and received an alcohol challenge (target BAC = 0.030 g/dL). They completed a smoking delay task that assessed their ability to resist smoking followed by an ad libitum smoking phase (primary outcomes). They also provided ratings of subjective drug effects and craving, and carbon monoxide levels were measured after smoking (secondary outcomes).
Results
Participants receiving varenicline monotherapy delayed smoking longer and smoked fewer cigarettes than those on placebo. Participants receiving varenicline + low-dose naltrexone did not delay smoking longer than those receiving varenicline alone. Participants in both active medication arms smoked fewer cigarettes ad libitum than those receiving placebo.
Conclusions
Varenicline can improve smoking outcomes even after an alcohol prime, supporting its use in heavy drinkers who wish to quit smoking. Findings did not support increased efficacy of combined varenicline + low-dose naltrexone relative to varenicline monotherapy.
Keywords: laboratory study, alcohol-nicotine interactions, naltrexone, varenicline, smoking cessation
Introduction
Heavy drinking is common among tobacco users. Approximately 25% of daily tobacco users endorse frequent binge drinking (McKee et al., 2007). One reason for the high rates of comorbidity is the pharmacological interactions between nicotine and alcohol. Subjectively, alcohol potentiates the positive effects of nicotine—tobacco users who consume alcohol before smoking find the tobacco more satisfying, stimulating, and calming, and will smoke more ad libitum than those receiving placebo beverage (Rose et al., 2004, Mitchell et al., 1995, Verplaetse and McKee, 2016). Tobacco users who also engage in heavy alcohol use are less likely to successfully quit smoking compared to abstinent tobacco users (Toll et al., 2010, DiFranza and Guerrera, 1990).
Given that heavy-drinking tobacco users respond less favorably to frontline smoking cessation medications, it is important to identify treatment strategies for this subgroup of tobacco users. Combining medications intended to treat problem nicotine and alcohol use may improve treatment outcomes in this group. Varenicline (Chantix; Pfizer, New York) is a partial agonist at α4β2 nicotinic acetylcholine receptors (nAChR) that was developed as a smoking cessation aid (Gonzales et al., 2006). Varenicline also has been shown to be effective for reducing alcohol consumption (Litten et al., 2013), although another large clinical trial found no effect of varenicline on rates of heavy drinking (de Bejczy et al., 2015). Another medication that may reduce smoking is naltrexone, which is a μ-opioid receptor antagonist use to treat alcohol use disorder (Krystal et al., 2001). Naltrexone reduces drinking by blocking the pleasurable, rewarding effects of alcohol associated with its reinforcing properties (King et al., 1997, Swift et al., 1994). Clinical trials and meta-analyses have shown that naltrexone is effective at reducing alcohol use among those with alcohol use disorders (Rösner et al., 2010).
Although some studies have shown that naltrexone may reduce smoking behavior (King et al., 2012), the preponderance of evidence does not support the notion that naltrexone reduces smoking (David et al., 2013). Because naltrexone’s primary application in treating alcohol use disorder, it is possible that it may be more effective for reducing smoking among tobacco users who are also heavy drinkers. Along these lines, Ray et al. (2014) found that following a moderate priming dose of alcohol (target BAC = 0.06 g/dL), combination varenicline (2 mg/day) and low-dose naltrexone (25 mg/day) was more effective than either drug alone at reducing cigarette craving both before and after smoking. The varenicline/low-dose naltrexone combination also reduced subjective high following both alcohol and smoking compared to either monotherapy or placebo.
The current study was a placebo-controlled, double-blind human laboratory experiment that compared the effects of varenicline (2 mg/day), combined varenicline (2mg/day) and low-dose naltrexone (25 mg/day), and placebo on alcohol-primed smoking in heavy-drinking daily tobacco users. Participants attended a laboratory session and completed a smoking lapse task that assessed their smoking behavior following a low priming dose of alcohol (target BAC = 0.03 g/dL). The primary outcome variables were time to smoking during the delay phase of the smoking lapse paradigm and number of cigarettes smoked during the ad libitum phase. Secondary outcome variables were self-reported tobacco and alcohol craving and subjective alcohol effects following alcohol prime.
The current study addressed two primary goals. First, we sought to determine whether varenicline is effective for reducing smoking following a priming dose of alcohol. Given that varenicline has been shown to reduce both smoking and alcohol use, we hypothesized that varenicline would increase smoking delay and reduce the number of cigarettes smoked compared to placebo. The second goal was to determine whether adding naltrexone to varenicline improved these effects on alcohol and smoking outcomes. We hypothesized that combining varenicline with low-dose naltrexone would further increase smoking delay and reduce the number of cigarettes smoked ad libitum.
Materials and Methods
Participants
Participants were 30 heavy drinking tobacco users age 21 years or older. Five volunteers started the study but were discontinued prior to the laboratory session due to protocol noncompliance. Only data from participants who completed the study are reported. They were required to smoke ≥ 10 cigarettes per day. We required that participants consumed ≥ 7 (women) or 14 (men) drinks per week with at least one weekly binge drinking episode (≥ 4 [women] or 5 [men] drinks during the past 30 days. Exclusion criteria included illicit drug use (except for cannabis use), opioid use (prescribed or illicit), past 30-day use of psychoactive drugs, seeking treatment for alcohol use or smoking, current psychopathology, current suicidal or homicidal ideation, pregnancy or nursing, or medical conditions contraindicating alcohol use (e.g., liver enzymes ≥ 3× normal) or varenicline or naltrexone administration (e.g., known allergy to either drug). Psychopathology was assessed using the Structured Clinical Interview for DSM-IV (SCID-I; First et al., 2002). Volunteers who met criteria for DSM-IV alcohol dependence did not participate, nor did those with any current psychiatric disorder other than DSM-IV alcohol abuse or nicotine dependence.
Procedures
Eligibility screening
The Human Investigation Committee of Yale University approved this study. Following phone screening, participants attended an intake session where they completed a more comprehensive screening and provided written informed consent. They completed a physical examination that included an electrocardiogram, urine toxicology, pregnancy test, and basic blood chemistries.
Medication
Medication condition was double-blind and placebo-controlled. All medications were over-encapsulated with riboflavin to monitor compliance. Randomization to varenicline (2 mg/day), combined varenicline (2 mg/day) with low-dose naltrexone (25 mg/day), or placebo was balanced by sex using urn randomization. The dose of varenicline was selected based on prior research (e.g., McKee et al., 2009) and standard clinical practice. The dose of naltrexone (25 mg/day) is lower than the standard dose of 50 mg/day that is used to treat alcohol use disorder. The low dose was selected based on prior research showing that low-dose naltrexone is effective in combination with other pharmacotherapies in heavy-drinking tobacco users to reduce indices of smoking and drinking behavior (O’Malley et al., 2009; Ray et al., 2014). Varenicline was titrated over 7 days (0.5 mg daily for days 1 and 2, 0.5 mg twice daily for days 3–5, and 1 mg twice daily for days 6 and 7) as was naltrexone (0 mg daily for days 1–3, 12.5 mg/day for day 4, 25 mg/day for days 5–7). Dosing occurred at 8:00 am and 8:00 pm. Medication compliance was monitored with pill count and riboflavin marker on day 8 (Del Boca et al., 1996). This study is registered on Clinicaltrials.gov, NCT00773422.
Laboratory session
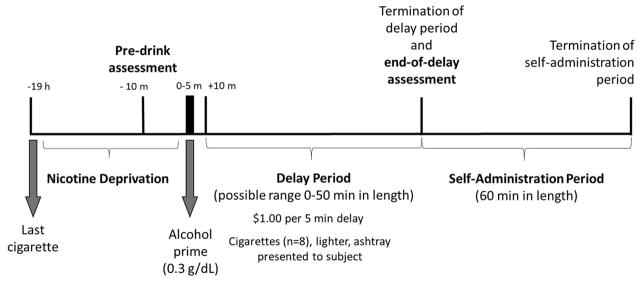
On day 8, participants completed a 14-hour laboratory session conducted at the Yale Center for Clinical Investigation, New Haven, Connecticut (see Figure 1 for a timeline). The laboratory session started at 8:00 am, and baseline assessments of breath alcohol and carbon monoxide, urine drug screen, and urine pregnancy screen were obtained. The final dose of medication was provided at 9:00 am. Participants were instructed not to consume alcohol the night before the session. They also were instructed not to smoke after 9:00 pm the night before the session. Compliance was confirmed verbally and by checking CO levels (< half CO at intake) and zero breath alcohol was confirmed using a breathalyzer. Participants completed an alcohol-primed smoking delay task developed by our group to model alcohol-primed smoking behavior (McKee et al., 2006). Breath alcohol concentrations (BACs) were measured throughout the laboratory session using expired air samples.
Figure 1.
Design and timeline of laboratory session (day 8). The session began at 8:00 am and participants abstained from smoking after 9:00 pm on day 7. Pre-drink assessment included QSU and AUQ. End-of-delay assessment included QSU, AUQ, BAES and visual analogue scale.
Alcohol priming dose
The priming dose was administered at 4:00 pm. The alcohol priming drink consisted of 1 part 80 proof liquor of the participant’s choosing to 3 parts mixer chosen from a selection of equicaloric, non-caffeinated, and noncarbonated drinks. The dose of alcohol was calculated to produce blood alcohol levels of approximately 0.03 g/dL using a standardized formula (Watson, 1989).
Delay period
At 4:05 pm, participants were presented with a tray containing eight cigarettes of their preferred brand, a lighter, and an ashtray. Participants were instructed that they could commence smoking at any point over the next 50 min; however, they would earn $1 for each 5-min block of time that they delayed or “resisted” smoking for a maximum of $10 over the next 50 minutes. Participants were informed that they would complete the cigarette self-administration phase after the delay period even if they delayed the full 50 min. BAC was measured every 10 minutes during the delay period.
Cigarette self-administration period
The smoking self-administration period was 60 min long and started once participants decided to end the delay period (or delayed for the full 50 min). Participants were instructed to “smoke as little or as much as you wish.” Participants were further instructed that for each cigarette lit would cost $1 of their $8 “smoking tab.” BAC was measured 30 and 60 minutes into the self-administration period. Money earned for delaying smoking and any unused portion of the “smoking tab” was paid to the subjects at the end of the laboratory session. CO levels were measured immediately after the cigarette self-administration period.
Self-report measures
The alcohol urge questionnaire (AUQ; Bohn et al., 1995) was used to assess alcohol craving. Tobacco craving was assess using the Tiffany Questionnaire of Smoking Urges-- Brief (QSU-B; Cox et al., 2001), which measures desire and intention to smoke due to expected reward (Factor 1; positive reinforcement) and anticipation that smoking would relieve negative affect (Factor 2; negative reinforcement) (range = 1–100). The Biphasic Alcohol Effects Scale (BAES; Martin et al., 1993) measures the subjective stimulating and sedating effects of alcohol (range = 1–10). Subjective intoxication and alcohol liking were assessed using a visual analogue scale (range = 1–100). Treatment emergent adverse events were monitored using the SAFTEE. Symptoms were rated on a 4-point scale (1 = mild, 2 = minimal, 3 = moderate, 4 = severe; Levine and Schooler, 1986). Weekly alcohol use frequency and average quantity per episode over the past 30 days was measured using the Timeline Follow-back (Sobell and Sobell, 1993). A weekly drink quantity variable was calculated that was equivalent to average quantity per episode X weekly frequency.
Timing of Assessments
Adverse events were assessed in person on days 1 and 8 and by phone on day 2. Alcohol and tobacco craving were assessed immediately before participants received their priming dose of alcohol while they were in a sober state (pre-drink assessment) and following the smoking delay period (end-of-delay assessment; see Figure 1). Participants rated their level of craving in both a sober and alcohol-primed state. Subjective alcohol effects were measured following alcohol administration. BAC was measured every 10 minutes after beverage administration during the delay period and every 30 minutes during the cigarette self-administration period.
Data Analysis
The primary outcome variable was time to smoking during the delay phase of the smoking lapse task and number of cigarettes smoked ad libitum. Secondary outcome variables included self-reported craving for alcohol and cigarettes, subjective alcohol effects, and CO levels following smoking. For all analyses, sex, weekly drinking quantity, and family income were included as covariates. Sex was included as a covariate based on prior research confirming gender differences in smoking outcomes (Smith et al., 2015). Weekly alcohol use quantity was included to account for related difference in responses to the low dose alcohol prime. Family income was included because it may be associated with smoking behavior given the role of monetary reinforcement in the protocol. Only significant covariates (i.e., sex, weekly drinking quantity) were retained in the final models presented below. All participants who attended the laboratory session completed the experiment. There were no missing data.
Time to smoking was analyzed using hierarchical Cox proportional hazards regression to examine the effect of medication condition on smoking delay. Covariates were entered in the first block and medication condition was entered in a second block. The significance of the change in model fit from the first step tested the overall effect of medication condition. Each medication condition was compared against the reference group (placebo), and a second model compared the varenicline/low-dose naltrexone group against the varenicline only reference group. Hazard ratios (HR; 95% CI) are reported. Cigarettes smoked ad libitum were analyzed using hierarchical ordinal regression to account for the non-normal distribution of the variable. Odds ratios (OR; 95% CI) are reported. Secondary outcome variables (craving, subjective alcohol effects, CO levels after smoking) were analyzed using analyses of covariance (ANCOVAs). Secondary outcomes were examined on an exploratory basis with no adjustment for multiple comparisons. A stepped analytic approach was used for secondary variables. The first ANCOVA compared across all medication conditions (placebo, varenicline, varenicline/low-dose naltrexone), and main effects were probed using a priori t tests. If the omnibus ANCOVA was not significant, then active treatment conditions were combined and compared to placebo. Ratings of alcohol and tobacco craving at each time point (i.e., pre-drink assessment, end-of-delay assessment), were analyzed using mixed-design ANCOVAs. Effect sizes are provided when appropriate, including Cohen’s d for 2 group comparisons and ηp2 for comparisons involving ≥ 3 groups.
Results
Baseline Characteristics, Titration Period, and Breath Alcohol Concentrations
Demographic and baseline information
Participants’ baseline characteristics are reported in Table 1. Groups were well-matched on all baseline characteristics (ps > 0.05) except for family income.
Table 1.
Demographic characteristics and alcohol use and smoking variables assessed at baseline
| Placebo (n = 10) | Varenicline (n = 9) | Varenicline + low dose naltrexone (n = 11) | F | p | |
|---|---|---|---|---|---|
|
|
|||||
| Age (years) | 33.20 (9.73) | 37.67 (10.85) | 29.91 (8.99) | 1.33 | 0.28 |
| Sex (% male) | 70% | 78% | 82% | ||
| Race | |||||
| White | 8 (80%) | 4 (44%) | 6 (55%) | ||
| Other | 2 (20 %) | 5 (56%) | 5 (45%) | ||
| Education | |||||
| ≤ high school | 4 (40%) | 3 (33%) | 6 (55%) | ||
| ≥ college | 6 (60%) | 6 (67%) | 5 (45%) | ||
| Family income | $53,200 ($24,584) | $31,098 ($14,528) | $65,369 ($34,738) | 4.27 | 0.02 |
| Marital status | |||||
| Not married | 10 (100%) | 8 (89%) | 11 (100%) | ||
| Married | 0 (0%) | 1 (11%) | 0 (0%) | ||
| Smoking | |||||
| FTND | 5.44 (1.81) | 6.33 (1.94) | 5.91 (2.77) | 0.35 | 0.71 |
| Cig/day | 12.78 (4.62) | 18.64 (7.29) | 17.16 (8.24) | 0.31 | 0.74 |
| CO level | 29.00 (11.12) | 23.44 (12.27) | 22.36 (10.89) | 0.99 | 0.38 |
| Alcohol use | |||||
| AUDIT | 10.90 (5.82) | 13.11 (5.67) | 12.27 (3.98) | 0.45 | 0.64 |
| Drinking ep/week | 2.96 (2.10) | 4.62 (2.11) | 4.16 (1.68) | 1.84 | 0.18 |
| Drinks per episode | 5.08 (1.76) | 7.00 (3.49) | 6.54 (3.16) | 1.17 | 0.33 |
Note. FTND is the Fagerstrom Test of Nicotine Dependence. Quantity/frequency measures of alcohol use and smoking are based on timeline follow-backs assessing patterns of use four weeks before starting medication. AUDIT is the Alcohol Use Disorder Identification Test. For all ANOVAs, df = 2, 27.
Adverse events
One participant receiving varenicline experienced moderate abdominal pain. All other adverse events were minimal or mild. There were no significant differences between groups in the frequency of adverse events (see Supplementary Table 1).
Changes in Alcohol and Cigarette Use
There was no significant effect of medication condition on self-reported alcohol use or cigarettes per day during the titration week (ps > 0.05).
Medication compliance
All participants were at least 80% medication compliant per pill counts and urine florescence.
Breath alcohol concentrations
BAC measurement differed between participants based on their length of delay, so only peak BAC is reported. Accurate BACs were not recorded for three participants due to equipment malfunction. Mean peak BAC was 0.024 g/dL (SD = 0.012), and was not significantly different among medication groups, F (2, 24) = 1.20, p = 0.32, ηp2 = 0.09. Measured peak BAC was lower than expected given the alcohol dose provided, so our measurement timepoints likely missed true peak BAC.
Primary Outcomes
Smoking delay
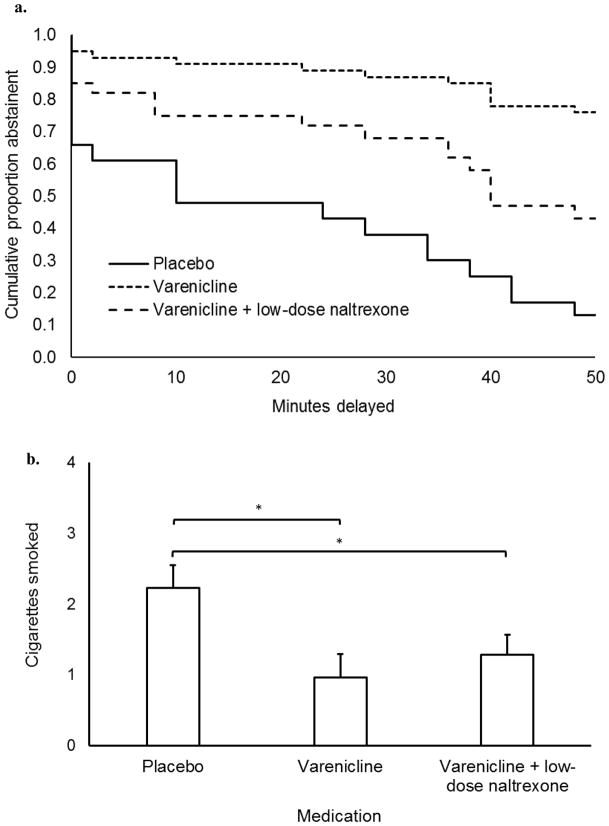
Risk of smoking across the delay period is charted separately by medication group in Figure 2a. Adding medication group to the Cox regression model produced a significant improvement in model fit from the covariate only model, Δχ2 = 6.36, p = .04. Participants receiving varenicline showed a significantly lower hazard ratio compared to those receiving placebo, HR = 0.13 (95% CI = 0.02 – 0.75), p = 0.023. Participants receiving varenicline/low-dose naltrexone did not significantly differ from those receiving placebo, HR = 0.40 (95% CI = 0.12 – 1.37), p = 0.145. Participants receiving varenicline + low dose naltrexone did not differ significantly from those receiving varenicline (ref), HR = 3.03 (95% CI = 0.72 – 12.71), p = 0.129.
Figure 2.
Primary study outcomes. (a) Survival curve describing the length of abstinence during the delay phase of the smoking delay task. (b) Number of cigarettes smoked during the free access phase of the smoking delay task. Brackets correspond to simple comparisons between medication conditions (*p < 0.05). Capped bars are SEMs. Asterisk (*) above brackets indicate that the corresponding comparison is significant (p < .05). Reported values are adjusted for significant covariates. Varenicline dose was 2 mg/day and naltrexone dose was 25 mg/day.
Cigarette self-administration
Medication effects on the number of cigarettes smoked are graphed in Figure 2b. Adding medication group to the ordinal regression model produced a significant improvement in model fit from the covariate only model, Δχ2 = 8.54, p = 0.03. Participants receiving varenicline smoked fewer cigarettes than those on placebo, OR = 0.05 (95% CI = 0.01 – 0.48), p = 0.01, as did those receiving varenicline/low-dose naltrexone, OR = 0.09 (95% CI = 0.01 – 0.63), p = 0.02. Participants receiving varenicline + low dose naltrexone did not differ significantly from those receiving varenicline (ref), OR = 1.76 (95% CI = 0.31 – 9.86), p = 0.52.
Secondary Outcomes
Cigarette craving
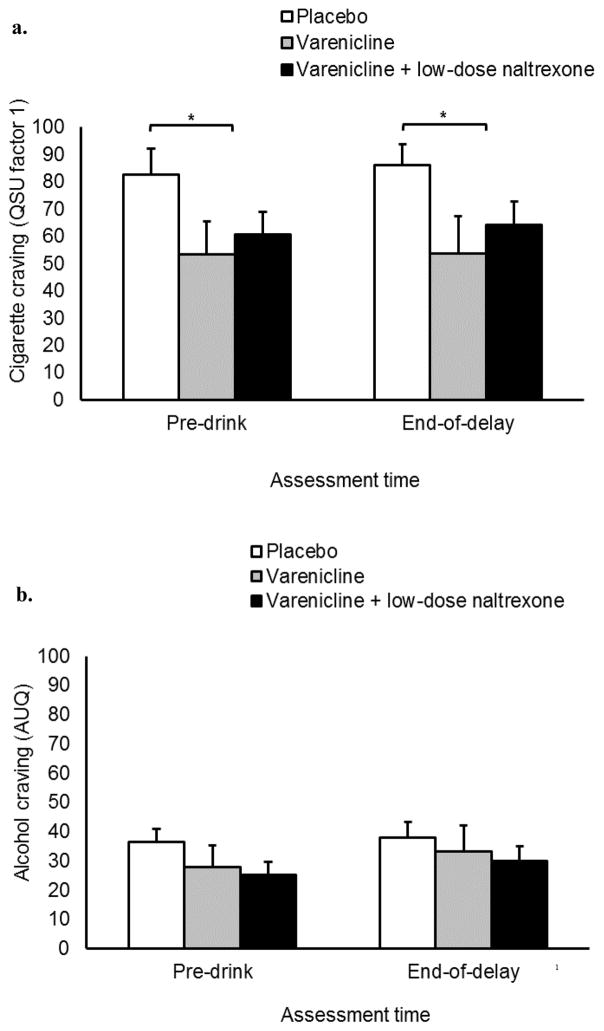
QSU Factor 1 scores are reported in Figure 3a. For the pre-drinking and end-of-delay assessments, the omnibus ANCOVA found no significant main effect of medication condition on QSU Factor 1, F (1, 26) = 2.65, p = 0.11, ηp2 = 0.16. The reduced-model ANCOVA found that participants receiving active medication reported less craving than those receiving placebo, F (1, 27) = 4.53, p = 0.04, d = 0.41. Neither ANCOVA found a significant main effect of time or interaction effect on QSU Factor 1, ps ≥ 0.84.
Figure 3.
Secondary study outcomes. (a) Effects of assessment time and medication condition on QSU Factor 1 scores. (b) Effects of assessment time and medication condition on AUQ scores. Capped bars are SEM. Bracket with * indicate significant differences between placebo and active medication conditions (p < .05). Reported values are adjusted for significant covariates. Varenicline dose was 2 mg/day and naltrexone dose was 25 mg/day.
Collapsed across the entire sample, QSU Factor 2 scores were 18.79 (SD = 22.02; pre-drink assessment), 21.09 (SD = 24.83; end-of-delay assessment). Neither the omnibus nor the reduced model ANCOVA found any significant effect of medication condition or interaction effect, ps ≥ 0.35.
Alcohol craving
Scores on the AUQ are reported in Figure 3b. No ANCOVA found any significant main effect or interaction (ps ≥ 0.11).
CO levels
CO levels are reported in Supplementary Table 2. There was no significant effect of medication on post-smoking CO levels, ps > 0.483.
Subjective alcohol effects
Subjective alcohol effects are reported in Supplementary Table 2. There was no significant effect of medication on BAES or VAS scores with or without covariates included.
Association among Laboratory Smoking Behavior and Craving
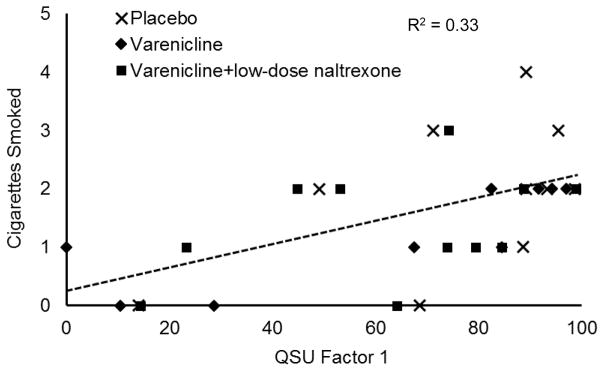
Exploratory correlational analyses were used to test whether self-reported craving was associated with these reductions in smoking behavior. QSU scores were averaged across the pre-drink and end-of-delay assessments. Higher QSU Factor 1 scores were associated with a shorter smoking delay time, r (28) = −0.45, p = 0.01, and more ad libitum smoking, r (28) = 0.57, p < 0.01. This association is plotted in Figure 4. Similarly, higher QSU Factor 2 scores were associated with shorter smoking delay, r (28) = −0.48, p = .01, and more ad libitum smoking, r (28) = 0.44, p = 0.02.
Figure 4.
Relation between craving scores (QSU factor 1) and number of cigarettes smoked during the free access phase of the smoking lapse task. Dashed line is least squares regression line for combined sample.
Discussion
This study examined the effects of varenicline and combined varenicline/low-dose naltrexone on heavy-drinking tobacco users’ ability to resist smoking following a priming dose of alcohol. Our first goal was to test whether varenicline increased the ability to resist smoking and reduced ad libitum smoking in tobacco users under the effects of alcohol. We found that even following a priming dose of alcohol, participants given varenicline monotherapy showed more favorable outcomes on most study endpoints. Although it is well-established that varenicline reduces smoking, this study is among the first to examine the effects of varenicline on smoking outcomes following alcohol consumption using a laboratory-based smoking lapse paradigm (also see Ray et al., 2014). The varenicline only group showed increased smoking delay and both medication conditions including varenicline showed decreased ad libitum smoking following an alcohol prime. This medication effect is important as nicotine shows increased rewarding effects when co-administered with alcohol (Rose et al., 2004), and alcohol priming can increase tobacco users’ urge to smoke (Epstein et al., 2007). Clinically, this supports the use of varenicline among tobacco users attempting to quit who intend to continue drinking, although additional research is needed to confirm this preliminary finding.
The second goal of this study was to test the effects of a low dose of naltrexone alongside varenicline in terms of effects on smoking. Low-dose naltrexone failed to produce a reduction in craving or smoking behavior beyond varenicline alone. Results of the current study are not consistent with those reported by Ray et al. (2014), which showed that combination varenicline/low-dose naltrexone produced greater reductions in craving and positive subjective drug effects compared to either medication alone. Although this study did not examine smoking behavior in the laboratory, they did find that combination therapy resulted in reduced smoking during the titration week compared to monotherapy or placebo. An important difference between these two studies is the priming dose of alcohol used. In the Ray et al. (2014) study, participants received alcohol prime that produced a target BAC of 0.06 g/dL — we used a smaller dose of alcohol (target BAC = 0.03 g/dL). Naltrexone enhancement of varenicline may be more apparent following larger doses of alcohol. Ray et al. (2014) also included a larger sample, which improved their power to detect medication effects. Nonetheless, our findings are consistent with other recent research suggesting that naltrexone may not improve smoking outcomes among heavy-drinking tobacco users (Kahler et al., 2017).
Neither medication affected subjective ratings of alcohol effects. This was surprising given prior research showing that naltrexone produces robust decreases in self-reported “high” and “stimulation” following alcohol administration (Volpicelli et al., 1995, King et al., 1997). Varenicline also has been shown to attenuate positive subjective alcohol effects (Childs et al., 2012). That neither drug condition reduced positive alcohol effects may be related to the low dose of alcohol used in this study. This dose has been shown to prime smoking behavior (McKee et al., 2006); however, it may not have produced a robust subjective alcohol effect profile. Indeed, many participants reported consuming approximately six beverages during an average drinking session. This may have produced a floor effect that prevented the medications from reducing the subjective effects below what was observed for participants receiving placebo.
Conclusions
Varenicline may reduce smoking even in patients who are likely to consume alcohol during their quit attempt. Findings support using varenicline as a smoking cessation medication among heavy drinkers who are expected to continue drinking. Given evidence that varenicline can reduce rates of drinking in this population (Fucito et al., 2011), varenicline may be particularly useful in the co-management of alcohol use disorder and smoking. Combining low-dose naltrexone with varenicline did not add any benefit beyond varenicline alone to reduce smoking in a group of heavy drinking tobacco users.
Supplementary Material
Acknowledgments
Funding: Supported by NIH grants R01AA017976 (SAM); R01AA022285 (SAM); T32DA007238 (WR); P50AA15632
Footnotes
Drs. Roberts, Shi, and Tetrault have no conflicts of interest. Dr. McKee has consulted to Embera and Cerecor, has had investigator-initiated grants from Pfizer and Cerecor, and has ownership interest in Lumme.
References
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Childs E, Roche DJ, King AC, de Wit H. Varenicline potentiates alcohol-induced negative subjective responses and offsets impaired eye movements. Alcohol Clin Exp Res. 2012;36:906–914. doi: 10.1111/j.1530-0277.2011.01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-Brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;6:CD003086. doi: 10.1002/14651858.CD003086. [DOI] [PubMed] [Google Scholar]
- De Bejczy A, Löf E, Walther L, et al. Varenicline for treatment of alcohol dependence: A randomized, placebo-controlled trial. Alcohol Clin Exp Res. 2015;39:2189–2199. doi: 10.1111/acer.12854. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Guerrera MP. Alcoholism and smoking. J Stud Alcohol. 1990;51:130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- Epstein AM, Sher TG, Young MA, King AC. Tobacco chippers show robust increases in smoking urge after alcohol consumption. Psychopharmacology (Berl) 2007;190:321–329. doi: 10.1007/s00213-006-0438-8. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Williams J, Gibbon M. Structured clinical interview for DSM-IV-TR (SCID-I)-research version. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fucito LM, Toll BA, Wu R, et al. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl) 2011;215:655–663. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha 4 beta 2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. JAMA-J Am Med Assoc. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Cioe PA, Tzilos GK, et al. A double-blind randomized placebo-controlled trial of oral naltrexone for heavy-drinking smokers seeking smoking cessation treatment. Alcohol Clin Exp Res. 2017;41:1201–1211. doi: 10.1111/acer.13396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Cao D, O’Malley SS, et al. Effects of naltrexone on smoking cessation outcomes and weight gain in nicotine-dependent men and women. J Clin Psychopharmacol. 2012;32:630–636. doi: 10.1097/JCP.0b013e3182676956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Meyer PJ. Naltrexone alteration of acute smoking response in nicotine-dependent subjects. Pharmacol Biochem Behav. 2000;66:563–572. doi: 10.1016/s0091-3057(00)00258-6. [DOI] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O’Brien CP. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology (Berl) 1997;129:15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, et al. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Levine J, Schooler NR. AFTEE: A technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22:343–381. [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, et al. A double-bind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med. 2013;7:277–286. doi: 10.1097/ADM.0b013e31829623f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, et al. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addict Biol. 2009;14:99–107. doi: 10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Falba T, O’Malley SS, et al. Smoking status as a clinical indicator for alcohol misuse in US adults. Archives of Internal Medicine. 2007;167:716–721. doi: 10.1001/archinte.167.7.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison ELR, O’Malley SS, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiat. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Krishnan-Sarin S, Shi J, et al. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology (Berl) 2006;189:201–210. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH, de Wit H, Zacny JP. Effects of varying ethanol dose on cigarette consumption in healthy normal volunteers. Behav Pharmacol. 1995;6:359–365. [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, McKee SA, et al. Dose-dependent reduction of hazardous alcohol use in a placebo-controlled trial of naltrexone for smoking cessation. Int J Neuropsychopharmacol. 2009;12:589–597. doi: 10.1017/S146114570800936X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED. Varenicline, low dose naltrexone, and their combination for heavy-drinking smokers: Human laboratory findings. Psychopharmacology (Berl) 2014;231:3843–3853. doi: 10.1007/s00213-014-3519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Brauer LH, Behm FM, et al. Psychopharmacological interactions between nicotine and ethanol. Nicotine Tob Res. 2004;6:133–144. doi: 10.1080/14622200310001656957. [DOI] [PubMed] [Google Scholar]
- Rösner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2010;8:CD001867. doi: 10.1002/14651858.CD001867.pub3. [DOI] [PubMed] [Google Scholar]
- Smith PH, Kasza KA, Hyland A, et al. Gender differences in medication use and cigarette smoking cessation: Results from the International Tobacco Control Four Country Survey. Nicotine Tob Res. 2015;17:463–472. doi: 10.1093/ntr/ntu212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Followback: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten R, editors. Techniques to Assess Alcohol Consumption. Totowa, NJ: Humana Press; 1993. pp. 41–72. [Google Scholar]
- Swift RM, Whelihan W, Kuznetsov O, et al. Naltrexone-induced alterations in human ethanol intoxication. Am J Psychiatry. 1994;151:1463–1467. doi: 10.1176/ajp.151.10.1463. [DOI] [PubMed] [Google Scholar]
- Toll BA, White M, Wu R, et al. Low-dose naltrexone augmentation of nicotine replacement for smoking cessation with reduced weight gain: a randomized trial. Drug Alcohol Depend. 2010;111:200–206. doi: 10.1016/j.drugalcdep.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, McKee SA. An overview of alcohol and tobacco/nicotine interactions in the human laboratory. Am J Drug Alcohol Abuse. 2016;43:186–196. doi: 10.1080/00952990.2016.1189927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Watson NT, King AC, Sherman CE, O’Brien CP. Effect of naltrexone on alcohol “high” in alcoholics. Am J Psychiatry. 1995;152:613–615. doi: 10.1176/ajp.152.4.613. [DOI] [PubMed] [Google Scholar]
- Watson PE. Total body water and blood alcohol levels: updating the fundamentals. In: Crow KE, Batt RD, editors. Human Metabolism of Alcohol. Vol. 1. Boca Raton, FL: CRC Press; 1989. pp. 31–56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.