Abstract
Background
Severe acute muscle injury results in massive cell damage, causing the release of actin into extracellular fluids where it complexes with the vitamin D binding protein (DBP). We hypothesized that a systemic DBP deficiency would result in a less pro-inflammatory phenotype.
Methods
C57BL/6 wild-type and DBP deficient (DBP−/−) mice received intramuscular injections of either 50% glycerol or phosphate-buffered saline into thigh muscles. Muscle injury was assessed by histology. Cytokine levels were measured in plasma, muscle, kidney and lung.
Results
All animals survived the procedure but glycerol injection in both strains of mice showed lysis of skeletal myocytes, and inflammatory cell infiltrate. The muscle inflammatory cell infiltrate in DBP deficient mice had remarkably few neutrophils as compared to wild-type mice. The neutrophil chemoattractant CXCL1 was significantly reduced in muscle tissue from DBP−/− mice. However, there were no other significant differences in muscle cytokine levels. In contrast, plasma obtained 48 hours after glycerol injection revealed that DBP deficient mice had significantly lower levels of systemic cytokines IL-6, CCL2, CXCL1 and G-CSF. Lung tissue from DBP−/− mice showed significantly decreased amounts of CCL2 and CXCL1 as compared to glycerol-treated wild-type mice. Several chemokines in kidney homogenates following glycerol-induced injury were significantly reduced in DBP−/− mice: CCL2, CCL5, CXCL1 and CXCL2.
Conclusion
Acute muscle injury triggered a systemic pro-inflammatory response as noted by elevated plasma cytokine levels. However, mice with a systemic DBP deficiency demonstrated a change in their cytokine profile 48 hours after muscle injury to a less pro-inflammatory phenotype.
Keywords: vitamin D binding protein, actin, muscle injury, cytokines, inflammation
INTRODUCTION
Tissue destruction and cell death leads to the release of previously sequestered intracellular molecules into extracellular fluids (1). Several intracellular molecules (e.g., HMGB1, ATP, mitochondrial DNA) released into the extracellular environment as a result of cell damage have been shown to function as “alarmins” to signal the immune system to the presence of tissue injury (2, 3). Alarmins induce recruitment and activation of innate immune cells resulting in the generation of pro-inflammatory cytokines (2–4). In addition to alarmins, large quantities of actin are released into extracellular fluids whereby the protein escapes normal intracellular regulatory mechanisms and spontaneously forms F-actin filaments. In animal models, extracellular F-actin filaments have been shown to trigger angiopathic effects in the microcirculation resulting in small vessel occlusion and promotion of disseminated intravascular coagulation (5, 6). Accordingly, higher organisms have evolved an effective extracellular actin scavenging system to remove actin released from dead or damaged cells. It is composed of two plasma proteins: gelsolin, a protein that severs F-actin into G-actin monomers, and vitamin D binding protein (DBP) that tightly binds G-actin in a 1:1 complex and transports it primarily to the liver for clearance (6, 7).
DBP, also known as Gc-globulin or Gc-protein, is a plasma protein that is part of the albumin gene family and is the primary transport molecule for vitamin D sterols in the blood and extracellular fluids (8, 9). DBP is synthesized by hepatocytes and circulates at 5–8 μM (300–450 μg/ml) concentration with a relatively rapid turnover in blood (10). This protein is ubiquitous in vivo and significant quantities (0.5 to 2 μM range) have been detected in nearly all extracellular fluid compartments (10, 11). The plasma concentration of DBP is generally stable, even during an inflammatory acute phase reaction (8). Several studies have demonstrated that in human sepsis, multiple trauma, and acetaminophen-induced liver failure, substantially reduced actin-free DBP levels correlate with poor prognosis (6, 12–14). The decrease in plasma DBP is thought to result from injury-induced consumption, i.e., formation of DBP-actin complexes that were rapidly cleared. Moreover, decreased plasma levels of DBP have a statistical prognostication capability comparable to the Acute Physiology and Chronic Health Evaluation (APACHE) II score, Trauma Revised Injury Severity Score (TRISS), or King’s College criteria in sepsis, trauma, or liver failure, respectively (6, 12, 13). It has been speculated that the primary physiological role of DBP is as a scavenger of extracellular actin since it binds to actin with high affinity (Kd of 10−9 M) and a large fraction of total circulating DBP (> 50%) can be bound to actin following extensive tissue injury (5, 6). It is not clear whether DBP functions primarily as a rapid clearance mechanism for actin, or whether DBP-actin complexes are bioactive (15,16,17). We hypothesize that a systemic deficiency of DBP would result in a less pro-inflammatory phenotype; the presence of free actin would not result in result in the above listed adverse effects of free actin. To this end, we investigated the role of DBP in a muscle injury model using mice with a systemic deficiency in DBP and their wild-type DBP sufficient counterparts. This model was selected since skeletal muscle contains the largest amount of actin of any tissue, and consequently, it is presumed that injury would generate large amounts of DBP-actin in wild-type mice but no complexes in DBP-deficient mice.
METHODS
Animals
Mice with a systemic deficiency in DBP (DBP−/−) are currently housed at Stony Brook University, the only worldwide source of the DBP−/− mouse strain, which has been fully backcrossed on a wild-type C57BL/6J background for 12 generations (17). The deletion of the DBP gene has been verified by genotyping (Transnetyx, Cordova, TN) and by immunoblotting of DBP−/− plasma. Wild-type DBP+/+ mice were either bred at Stony Brook or obtained from Jackson Labs (Bar Harbor, ME). Mice were housed in a maximum isolation facility and all experiments used 8 to 12-week old mice with equal numbers of males and females. Animal experiments were performed using protocols approved by the Institutional Animal Care and Use Committee at our University
Acute Muscle Injury Model
Briefly, 5 ml/kg (125 μl for a 25g mouse) of sterile 50% glycerol (99.9% pure, Acros Organics, Morris Plains, NJ) was injected along the length of the left thigh muscles of anesthetized mice, as previously described (18, 19). Control mice were injected with 125 μl of sterile phosphate buffered saline (PBS) along the length of the right thigh muscles to serve as the sham-treated group. Different injection sites were used for glycerol versus PBS as a procedural check to verify that mice were either in the sham or experimental groups. There were four treatment groups (2 mouse strains × 2 treatments) that were analyzed 48 hours after treatment to evaluate the start of the post-injury recovery phase. The glycerol-treated group contained 8 animals per group while the PBS injected sham controls had 6 mice per group. The number of mice was chosen based on a power calculation in consultation with our biostatistical core, which indicated that we needed at least 6 mice per group to have a power of 0.8 (i.e. an 80% chance to correctly determine a 10% difference between treatments at P < 0.05). Blood (EDTA plasma), thigh muscles from the injected extremity, kidneys, and lungs were collected 48 hours after injection and immediately frozen at -80°C, samples were thawed and refrozen no more than twice.
Tissue Homogenization Procedure
Frozen tubes containing skeletal muscle, kidneys, or lungs were placed in a 37°C water bath for rapid thawing, followed by placing samples on ice. All procedures used sterile instruments and plasticware in a BSL-2 laminar flow hood. Each tissue was dissected to remove fat and/or connective tissue, cut into small pieces and then added to a sterile 5 ml round bottom tube containing homogenization buffer at a ratio of 0.5 ml/100 mg tissue. Homogenization buffer consisted of PBS, 50 mM Tris-HCl (pH 7.5), 2.5 mM EDTA, 0.5% Triton X-100 with a complete protease inhibitor cocktail (1 mM Pefabloc SC, 0.125 mM E-64 and one Complete mini protease inhibitor tablet; all reagents were purchased from Roche Life Sciences, Indianapolis, IN). Tissue was homogenized on ice using a hand-held electric tissue grinder until it was completely liquefied. The homogenized tissue was next centrifuged at 15,000 × g at 4°C for 10 minutes in a microfuge and the supernatant transferred a new sterile tube. An aliquot was removed from each sample for analysis of total protein using the Bradford protein assay kit (BioRad, Hercules, CA). Samples were then frozen at -80°C both as aliquots from individual mice and pooled samples from each treatment.
Cytokine Analysis
Cytokine levels in total pooled plasma and organ tissue homogenates from each group were first determined by a multiplex ELISA (eBiosciences-Affymetrix/ThermoFisher) analyzing 36 cytokines per sample. Data was analyzed using a BioPlex Luminex plate reader (BioRad) in our Genomics core facility. Pooled samples were used as a cost effective approach to identify to systemic cytokine changes (20–22). This method of screening 36 cytokines allowed us to identify the molecules that differed between wild-type and DBP deficient animals, and subsequently to customize a multiplex ELISA (eBiosciences-Affymetrix/ThermoFisher) for analyzing 12 key cytokines: CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), CXCL1 (GRO-α), CXCL2 (MIP-2), CXCL5 (ENA-78), CXCL10 (IP-10), G-CSF (CSF-3), IL-2, IL-6 and IL-10. These cytokines were measured in samples from both individual mice and three different pools in each of the four experimental groups. To assure the validity of the data, each sample was measured three times: 36-plex of pooled samples as a screen, 12-plex of individual samples, 12-plex of three different pools. Results reported herein are those from a 12-plex of individual samples or pooled samples.
Histology
Organ tissue was evaluated for histological evidence of inflammation and tissue injury in separate groups of mice 48 hours after glycerol or PBS injection, n = 3 mice per group. At the time of dissection, thigh muscle, kidneys and lungs were immersed in 10% neutral buffer formalin for 24 hours and then processed in the Histology Core Lab where H&E stained slides were prepared. H&E stained sections were coded to conceal both the mouse strain and treatment. For the slides of skeletal muscle, the entire tissue section was evaluated by a board-certified pathologist for the degree of inflammation and myonecrosis using a 0 to 4 semi-quantitative grading scale. The degree of tissue inflammation was scored as: no inflammation = 0, minimal = 1, mild = 2, moderate = 3 or severe inflammation = 4. Muscle necrosis was scored as no necrosis = 0, 1–5% = 1, >5–10% = 2, >10–25% = 3 or > 25% = 4. In a separate analysis, the type of inflammatory cell infiltrate in muscle was quantitated by examining three non-overlapping high power (400x) fields. Only H&E slides from glycerol-treated mice were quantitated because PBS-treated mice did not have adequate numbers of inflammatory cells for accurate statistical analysis. The percent neutrophils (identified by size, nuclear morphology and staining pattern) in 200 total inflammatory cells in each of three 400× fields were counted. The data are presented as the mean percent PMN (neutrophils) and MNC (mononuclear cells) of 600 cells counted.
Analysis of DBP and DBP-actin complexes in plasma
Pooled plasma samples from each experimental group (n = 6 for PBS groups, n = 8 for glycerol-treated groups) were separated by polyacrylamide electrophoresis under non-denaturing conditions (no sodium dodecyl sulfate (SDS)) to preserve DBP-actin complexes. All pooled plasma samples in the four experimental groups had similar total protein levels (± 5%) and the same amount of protein was loaded in each lane. Plasma samples were first diluted 1:1 with non-denaturing sample buffer (50 mM Tris-HCl, pH 6.8, 20% glycerol, 0.1% bromophenol blue tracking dye) and then separated on a 10% native polyacrylamide gel (BioRad) followed by blotting onto a PVDF membrane (Millipore, Bedford, MA). The membrane was probed for immunoreactive DBP using an affinity-purified polyclonal goat anti-human DBP (purchased from DiaSorin and subsequently affinity purified in our lab) followed by HRP-conjugated donkey anti-goat IgG (Santa Cruz Biotechnology, Santa Cruz, CA) as the secondary antibody. Note: DBP is highly conserved in mammals and mouse DBP strongly cross reacts with anti-human DBP. The blot was developed using BioRad ECL reagents and imager. In a separate experiment to validate DBP-actin complex formation in mouse plasma, wild-type C57BL/6J mouse plasma pooled from untreated mice (n = 3) was spiked in vitro with various amounts of purified G-actin (Cytoskeleton, Denver, CO) for 30 minutes at 22°C and then subjected to non-denaturing polyacrylamide electrophoresis and immunoblotting as above. These samples served as a positive control for the position of DBP and DBP-actin bands on the immunoblot. The relative percent difference between DBP band intensity in PBS versus glycerol-treated mouse plasma was estimated by measuring the band pixel density using Adobe Photoshop software (Adobe Systems, San Jose, CA).
Data Analysis and Statistics
Statistical analysis and graphing of quantitative data was performed using GraphPad Prism software version 7.0c (LaJolla, CA). All multiplex ELISA values from tissues homogenates were normalized per mg total tissue protein (pg/mg). Data was analyzed for statistical differences between wild-type and DBP −/− (i.e. DBP KO) mice for each treatment (glycerol or PBS) using an unpaired t-test with the Holm-Sidak correction method. This study was designed to evaluate the difference between the strains (wild-type versus DBP KO) and not between the treatments (PBS versus glycerol), since it was presumed that the glycerol treatment would produce significant injury and inflammation (according to multiple prior publications). Therefore, the student’s t-test was used to evaluate differences between the mouse strains. Nevertheless, data was also evaluated using a two-way ANOVA with Tukey’s multiple comparison method, which showed that statistical differences were maintained but the level of significance was not as great as with the t-test.
RESULTS
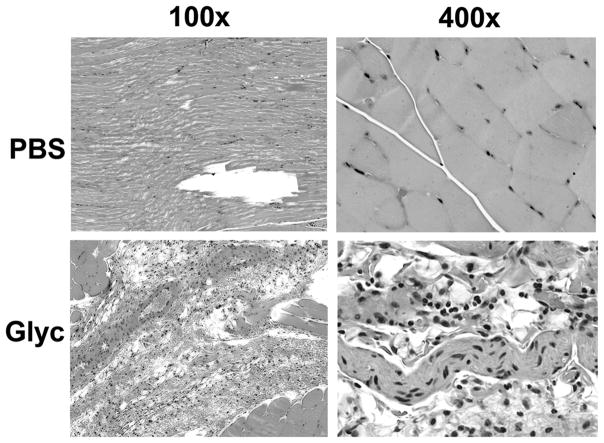
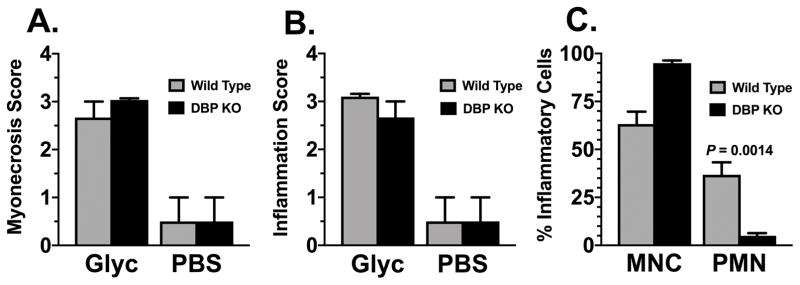
Thigh muscle at the site of glycerol-induced injury demonstrated no morphological difference in tissue damage between DBP-deficient (DBP−/−) and wild-type (DBP+/+) mice (Figure 1). Both strains of mice showed extensive myonecrosis and inflammatory infiltrate 48 hours after glycerol injection that was not observed in the PBS injected sham control groups. Semi-quantitative histopathology scores confirmed that the glycerol injection induced significant necrosis of skeletal myocytes and inflammation (Fig. 2, panels A & B). Moreover, following glycerol injection, wild-type animals had a mixed neutrophil (37%) and mononuclear cell (63%) infiltrate whereas DBP-deficient mice had an almost exclusive mononuclear cell response (95%) with very few neutrophils (Fig. 2, panel C). Analysis of H&E stained sections of kidney and lung tissue by light microscopy showed no evidence of tissue injury in either wild-type or DBP deficient mice (data not shown).
Figure 1.
Histopathology of skeletal muscle injury. Formalin-fixed parafin embedded sections of thigh muscle harvested 48 hours after injection with either 50% glycerol (Glyc) or sham injected with PBS were stained with H&E. Representative sections are a from a wild-type (DBP+/+) mouse. There was no difference in the injury pattern between the WT and KO mice. Images were taken at 100× and 400× magnification. Note: injection site injury and fluid accumulation in sham-treated muscle whereas glycerol treatment induced inflammation and myonecrosis.
Figure 2.
Quantitative histopathology score of skeletal muscle injury. Representative H&E sections of skeletal muscle from wild-type (DBP+/+) and DBP deficient (DBP KO) mice harvested 48 hours after injection with either 50% glycerol (Glyc) or sham injected with PBS. Panel A: Myonecrosis score. Panel B: Inflammation score. Panel C: Neutrophils (PMN) and mononuclear cells (MNC) as a % of total inflammatory cells in glycerol-treated mice. Gray bars indicate wild type (DBP+/+) animals whereas black bars represent DBP deficient (DBP KO) mice. Numbers are mean ± SEM of % cells, n=3 per group. Statistical significance is indicated.
Pooled plasma samples were analyzed for circulating levels of DBP and DBP-actin complexes by immunoblotting (Supplemental Figure). As expected, there was no immunoreactive DBP in pooled plasma from either PBS or glycerol-treated DBP-deficient mice. DBP was detected in wild-type mouse plasma, but the band intensity in glycerol-treated animals was decreased by approximately 65% as compared to PBS-treated mice, perhaps reflecting systemic consumption of DBP by the glycerol-induced injury. However, there was no detectable DBP-actin band in either wild-type treatment group, likely because the complexes are cleared. In contrast, as a positive control, pooled plasma from a separate group of untreated wild-type mice was spiked with purified G-actin in vitro for 30 minutes prior to electrophoresis. A distinct band corresponding to the DBP-actin complex was noted migrating just above the main DBP band (Supplemental Figure).
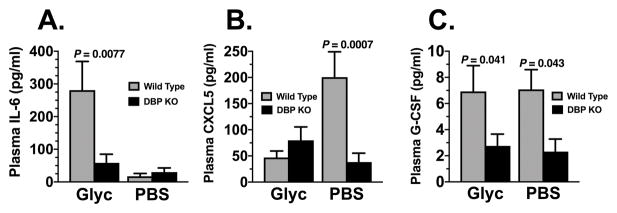
Analysis of 36 cytokines in pooled samples allowed us to customize a multiplex ELISA to measure 12 key cytokines previously mentioned. We subsequently evaluated these cytokine levels in samples from individual animals (plasma, muscle, kidney) or in pooled samples (lung) from wild-type mice (WT) and DBP−/− (KO) mice 48 hours after intramuscular injection of glycerol or PBS. Intramuscular glycerol injection induced a marked systemic inflammatory response in wild-type mice, as evidenced by a significant increase in plasma IL-6 levels (Fig. 3 panel A). However, the amount of plasma IL-6 in DBP KO mice was significantly lower than in wild-type animals (Fig. 3A). Furthermore, as described above, a significant decrease in neutrophil infiltrates in muscle was noted in glycerol-damaged muscle in DBP−/− mice (Fig. 2C). Hence, we examined two additional cytokines that are involved in neutrophil development (G-CSF) and tissue recruitment/homeostasis (CXCL5) (Fig. 3, panels B & C). Plasma levels of CXCL5 and G-CSF are both decreased in DBP deficient mice versus their wild-type counterparts, perhaps providing a partial explanation of why DBP KO mice had significantly fewer neutrophils recruited to the glycerol-damaged muscle. No differences were observed between WT and KO mice in plasma levels of CCL3, CCL4 and CXCL10 following glycerol administration. IL-2 was not detected in any sample.
Figure 3.
IL-6, CXCL5 and G-CSF levels in EDTA plasma analyzed by multiplex ELISA. Gray bars indicate wild type (DBP+/+) animals whereas black bars represent DBP deficient (DBP KO) mice. Numbers are mean ± SEM of pg/ml in plasma, n = 6 mice/group for sham-treated mice and 8 mice/group for glycerol-treated animals. Statistical significance is indicated.
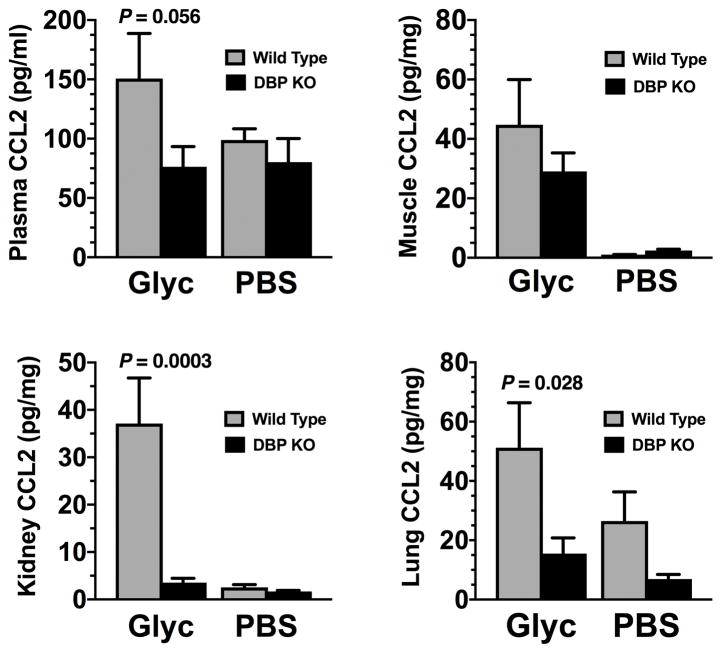
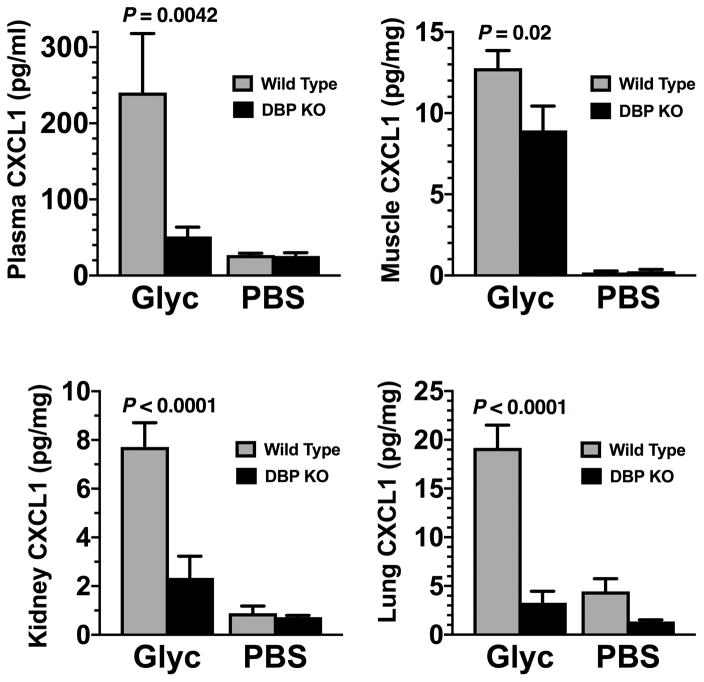
Glycerol-induced muscle injury in wild-type mice resulted in increased levels of CCL2 (a monocyte chemoattractant) and CXCL1 (a neutrophil chemoattractant), in plasma, muscle, kidney, and lung (Figs. 4 and 5). CCL2 levels in kidney and lung were significantly lower in DBP KO mice than in WT mice whereas plasma CCL2 levels just failed to reach statistical significant difference (p=0.056, Fig. 4). Meanwhile CXCL1 levels were significantly reduced in DBP KO mice as compared to wild-type mice following glycerol induced muscle injury in all tissues examined (Fig. 5). These results demonstrate that, as compared to wild-type mice, DBP deficient mice produce significantly less quantities of two major inducible chemoattractants (CCL2, CXCL1) after tissue injury.
Figure 4.
CCL2 levels in plasma and tissue homogenates (muscle, kidney, lung) analyzed by multiplex ELISA. Gray bars indicate wild type (DBP+/+) animals whereas black bars represent DBP deficient (DBP KO) mice. Data are reported as pg/ml for plasma or pg/mg total tissue protein. Numbers are mean ± SEM, n = 6 mice/group for sham-treated mice and n = 8 mice/group for glycerol-treated animals. Lung tissue values are from pooled individual samples (n = 3 sample pools). Statistical significance is indicated.
Figure 5.
CXCL1 levels in plasma and tissue homogenates (muscle, kidney, lung) analyzed by multiplex ELISA. Gray bars indicate wild type (DBP+/+) animals whereas black bars represent DBP deficient (DBP KO) mice. Data are reported as pg/ml for plasma or pg/mg total tissue protein. Numbers are mean ± SEM, n = 6 mice/group for sham-treated mice and n = 8 mice/group for glycerol-treated animals. Lung tissue values are from pooled individual samples (n = 3 sample pools). Statistical significance is indicated.
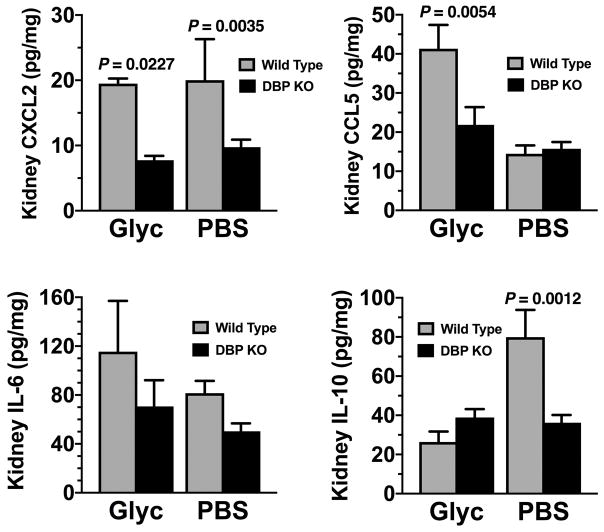
As kidney damage is a serious consequence of acute muscle injury, we further analyzed kidney homogenates following glycerol administration. Besides, the significant decreases in CCL2 and CXCL1 levels in DBP KO mice, kidney homogenates from glycerol-injected DBP−/− mice also had significantly lower levels of the chemokines CXCL2 and CCL5 versus wild-type mice (Fig. 6). IL-10 was significantly elevated in sham-treated wild-type mice versus DBP deficient animals, and these levels decreased with glycerol-induced injury but only in wild-type mice (Fig. 6). Thus, kidney homogenates from DBP KO mice have significantly diminished levels of these proinflammatory cytokine as compared to their wild-type counterparts.
Figure 6.
Levels of CXCL2, CCL5, IL-6 and IL-10 in kidney homogenates analyzed by multiplex ELISA. Gray bars indicate wild type (DBP+/+) animals whereas black bars represent DBP deficient (DBP KO) mice. Numbers represent mean ± SEM of pg/mg total tissue protein, n = 6 mice/group for sham-treated mice and 8 mice/group for glycerol-treated animals. Statistical significance is indicated.
DISCUSSION
To investigate the role of DBP in acute muscle injury, a glycerol-induced acute muscle injury model was utilized. This model, as opposed to a femur fracture model for example, was selected because skeletal muscle has the largest amount of actin of any tissue and injured muscle fibers will release these very large quantities of actin. To our knowledge, this is the first report of a possible role of the effects of DBP deficiency on systemic and tissue cytokines levels following acute muscle injury.
A complete systemic deficiency of DBP is the only known difference between DBP-deficient and wild-type C57BL/6J mice, these mice are otherwise phenotypically normal (17). Previous experiments have shown that wild-type DBP+/+ complement-activated serum instilled into the lungs of DBP−/− mice can restore their diminished influx of neutrophils into the airspaces (17), suggesting that repletion of DBP in these deficient mice can reverse the neutrophil recruitment defect. We hypothesized that this effect of DBP is mediated through either the actin or vitamin D binding functions, and since actin binding is immediate and observed during tissue injury we focused on DBP-actin complexes. Indeed, 48 hours after acute muscle injury, an entirely different systemic cytokine profile, favoring an anti-inflammatory/reparative phenotype, is generated in DBP deficient mice compared to their wild-type counterparts. This correlates with the reduced neutrophilic infiltrate in muscle.
Our acute muscle injury model demonstrated extensive myonecrosis and inflammation in the thigh muscles of all mice injected with 50% glycerol. Moreover, glycerol treatment of wild-type mice also resulted in a 65% reduction in total plasma DBP, perhaps reflecting injury induced consumption, that obviously was not seen in DBP-deficient animals. However, except for CXCL1, there was no difference between wild-type and DBP-deficient mice in muscle cytokine levels. Nevertheless, the cell composition of the inflammatory infiltrates was significantly different. It remains to be determined why there were significant differences between several systemic, kidney and lung cytokines but little difference at the site of tissue injury. One possible explanation is that the dose of glycerol used for this model induces mild muscle injury as compared to several other agents such as toxins (cardiotoxin, notexin), chemicals (barium chloride) or freeze injury that produce more extensive damage that peaks 4–6 days after injury (19, 23)
In contrast to the limited local cytokine effects at the site of muscle injury, significant changes in cytokine levels after glycerol-induced injury were observed in the plasma, lung and particularly the kidneys of wild-type mice. Kidney damage is an important consequence of severe muscle injury (24, 25). The glycerol-induced muscle injury model can also induce acute renal failure at twice the glycerol concentration used herein when coupled with water deprivation (26). Although kidneys from wild-type mice had elevated levels of several chemokines (CCL2, CCL5, CXCL1, CXCL2) versus DBP deficient animals, evidence of kidney damage by light microscopy was not observed, as expected. Injury to glomeruli and tubules is often subtle and usually requires examination via electron microscopy to reveal damage.
An interesting finding in this study is that DBP−/−mice had almost no neutrophils at site of muscle injury. This data indicates that a systemic deficiency of DBP results in a reduction in neutrophilic inflammation. Perhaps this is partly mediated by decreased levels of several neutrophil chemoattractants in plasma (ie CXCL1, CXCL2, CXCL5 and G-CSF). Significantly reduced levels of the chemokines CCL2 and CXCL1 in almost all harvested tissues from DBP deficient mice were a key finding in this study. CXCL1 (GRO-α) is a major inducible chemoattractant, generated during inflammation that contributes to neutrophilic tissue infiltrates (27). Indeed, CXCL1 is the mouse homologue of human IL-8 (CXCL8) and has very similar functions and disease associations as IL-8 (28). CCL2 (MCP-1) is also a major inducible chemoattractant; it contributes to monocytic tissue infiltrates (24). In addition to reduced CXCL1 and CCL2 levels, DBP deficient mice also had significantly decreased levels, in other cytokines that induce neutrophil development in the bone marrow (G-CSF), mobilization from the bone marrow to the blood (CXCL2) and recruitment from blood into tissue (CXCL5) (29–31). These cytokine results are consistent with our previously published studies investigating the effects of DBP in acute and chronic lung injury, where DBP deficient mice had significantly fewer neutrophils in lung parenchyma and bronchoalveolar fluid after injury. Moreover, in these prior studies, the diminished neutrophilic inflammation correlated with less tissue injury and better survival (16, 17). Lung tissue was also examined since lungs are a prime target of systemic injury and also a key organ for systemic neutrophil regulation (32). Indeed, DBP−/− mice had significantly lower levels of CCL2 and CXCL1 in lung homogenates.
There are several limitations to the current study. First, this work was performed using inbred mice housed in an aseptic maximum isolation room, so potential extrapolation of these results to traumatic muscle injury in humans would be debatable and premature. Second, the experimental approach in this study examined tissues only at a single time point (48 hours) after acute muscle injury, and consequently do not address the very dynamic spectrum of cellular and molecular changes that occur during the process of host response to tissue injury.
CONCLUSIONS
The results from this study clearly demonstrate that DBP deficient mice had significantly lower amounts of several tissue and plasma cytokines following acute muscle injury, suggesting that DBP deficiency may play an as yet unrecognized role in traumatic injury. As DBP deficiency exposes tissues to large quantities of free actin, our findings refute the concept that free-actin is pro-inflammatory. It is possible that DBP deficiency, and consequently the inability to generate DBP-actin complexes, is the principal reason is the principal reason that DBP KO mice have a less pro-inflammatory phenotype. The experiments reported herein, coupled with our previously published studies using DBP deficient mice, suggest that DBP deficiency would preclude the generation of DBP-actin complexes; these complexes may have alarmin-like functions and are thereby pro-inflammatory (16, 17). However, at present, there is no direct method to quantitate the rapidly cleared DBP-actin complexes in biological fluids. Additional study is needed to understand the role of DBP deficiency and consequently the absence of DBP-actin complexes in the host response to tissue injury. DBP and actin are ubiquitous proteins that are ideal candidates to act as rapid sentinels of tissue injury.
Supplementary Material
Analysis of plasma DBP and DBP-actin complexes. Aliquots of pooled plasma samples (n = 6 for PBS groups, n = 8 for glycerol-treated groups) were separated on a 10% non-denaturing (no SDS) polyacrlyamide gel and transferred to PVDF membrane for immunoblotting using an affinity purified anti-human DBP. Note: DBP is highly conserved in mammals and mouse DBP strongly cross reacts with anti-human DBP. KO = DBP deficient (DBP−/−) mice, WT = Wild-type (DBP+/+) mice. All pooled plasma samples in the four experimental groups had similar total protein levels (± 5%) and the same amount of protein was loaded in each lane. In a separate experiment, pooled WT plasma from untreated mice (n = 3) was treated with the indicated concentration of purified G-actin for 30 minutes at 22°C and then subjected to electrophoresis and blotting. These samples served as a positive control for DBP-actin complexes, the position of the DBP and DBP-actin bands are indicated on the right side of the figure.
Acknowledgments
Funding: Stony Brook Department of Surgery competitive grant program (RSJ), and National Institutes of Health (RRK), Stony Brook University School of Medicine Office of Scientific Affairs
The authors would like to thank the Stony Brook University School of Medicine Genomics Core Facility for the expert assistance with the analysis of multiplex ELISA plates. In addition, the School of Medicine Office of Scientific Affairs provided financial support for this project.
Footnotes
Level of Evidence: Level III
Conflicts of Interest: None
Presentation:
This work was presented in part at the 75th annual meeting of the American Association for the Surgery of Trauma in Waikoloa, HI in September, 2016.
This work was presented with significant additional data at the 76th annual meeting of the American Association for the Surgery of Trauma in Baltimore, MD in September, 2017.
AUTHOR CONTRIBUTIONS
RK: study design, performed experiments, data analysis and interpretation, manuscript preparation.
TT: performed experiments, data analysis and interpretation
JD: data analysis and interpretation
JV: study design, critical revision
RJ: study design, performed experiments, data analysis and interpretation, manuscript preparation
References
- 1.Stoecklein VM, Osuka A, Lederer JA. Trauma equals danger--damage control by the immune system. J Leukoc Biol. 2012;92(3):539–51. doi: 10.1189/jlb.0212072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papurica M, Rogobete AF, Sandesc D, Cradigati CA, Sarandan M, Dumache R, Horhat FG, Bratu LM, Nitu R, Crisan DC, et al. Using the Expression of Damage-Associated Molecular Pattern (DAMP) for the Evaluation and Monitoring of the Critically Ill Polytrauma Patient. Clin Lab. 2016;62(10):1829–40. doi: 10.7754/Clin.Lab.2016.160226. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itaggaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oppenheim JJ, Tewary P, de la Rosa G, Yang D. Alarmins initiate host defense. Adv Exp Med Biol. 2007;601:185–94. doi: 10.1007/978-0-387-72005-0_19. [DOI] [PubMed] [Google Scholar]
- 5.Haddad JG, Harper KD, Guoth M, Pietra GG, Sanger JW. Angiopathic consequences of saturating the plasma scavenger system for actin. Proc Natl Acad Sci USA. 1990;87(4):1381–5. doi: 10.1073/pnas.87.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meier U, Gressner O, Lammert F, Gressner AM. Gc-globulin: roles in response to injury. Clin Chem. 2006;52(7):1247–53. doi: 10.1373/clinchem.2005.065680. [DOI] [PubMed] [Google Scholar]
- 7.dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, Nosworthy NJ. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83(2):433–73. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- 8.Bouillon R, Van Assche FA, Van Baelen H, Heyns W, De Moor P. Influence of the vitamin D-binding protein on the serum concentration of 1,25-dihydroxyvitamin D3. Significance of the free 1,25-dihydroxyvitamin D3 concentration. J Clin Invest. 1981;67(3):589–96. doi: 10.1172/JCI110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun RF. New perspectives on the vitamin D binding protein. Cell Biochem Funct. 2012;30(6):445–56. doi: 10.1002/cbf.2835. [DOI] [PubMed] [Google Scholar]
- 10.Cooke NE, Haddad JG. Vitamin D binding protein (Gc-globulin) Endocr Rev. 1989;10:294–307. doi: 10.1210/edrv-10-3-294. [DOI] [PubMed] [Google Scholar]
- 11.White P, Cooke N. The multifunctional properties and characteristics of vitamin D-binding protein [Review] Trends Endocrinol Metab. 2000;11(8):320–7. doi: 10.1016/s1043-2760(00)00317-9. [DOI] [PubMed] [Google Scholar]
- 12.Antoniades CG, Berry PA, Bruce M, Cross TJ, Portal AJ, Hussain MJ, Bernal W, Wendon JA, Vergani D. Actin-free Gc globulin: a rapidly assessed biomarker of organ dysfunction in acute liver failure and cirrhosis. Liver Transpl. 2007;13(9):1254–61. doi: 10.1002/lt.21196. [DOI] [PubMed] [Google Scholar]
- 13.Dahl B, Schiodt FV, Ott P, Wians F, Lee WM, Balko J, O’Keefe GE. Plasma concentration of Gc-globulin is associated with organ dysfunction and sepsis after injury. Crit Care Med. 2003;31(1):152–6. doi: 10.1097/00003246-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Goldschmidt-Clermont PJ, Lee WM, Galbraith RM. Proportion of circulating Gc (vitamin D-binding protein) in complexed form: relation to clinical outcome in fulminant hepatic necrosis. Gastroenterology. 1988;94(6):1454–8. doi: 10.1016/0016-5085(88)90686-5. [DOI] [PubMed] [Google Scholar]
- 15.Harper KD, McLeod JF, Kowalski MA, Haddad JG. Vitamin D binding protein sequesters monomeric actin in the circulation of the rat. J Clin Invest. 1987;79:1365. doi: 10.1172/JCI112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge L, Trujillo G, Miller EJ, Kew RR. Circulating complexes of the vitamin D binding protein with G-actin induce lung inflammation by targeting endothelial cells. Immunobiology. 2014;219(3):198–207. doi: 10.1016/j.imbio.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trujillo G, Habiel DM, Ge L, Ramadass M, Cooke NE, Kew RR. Neutrophil Recruitment to the Lung in Both C5a- and CXCL1-Induced Alveolitis Is Impaired in Vitamin D-Binding Protein-Deficient Mice. J Immunol. 2013;191(2):848–56. doi: 10.4049/jimmunol.1202941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komada T, Usui F, Kawashima A, Kimura H, Karasawa T, Inoue Y, Kobayashi M, Mizushina Y, Kasahara T, Taniguchi S, et al. Role of NLRP3 Inflammasomes for Rhabdomyolysis-induced Acute Kidney Injury. Sci Rep. 2015;5:10901. doi: 10.1038/srep10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahdy MA, Lei HY, Wakamatsu J, Hosaka YZ, Nishimura T. Comparative study of muscle regeneration following cardiotoxin and glycerol injury. Ann Anat. 2015;202:18–27. doi: 10.1016/j.aanat.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 20.McNicholas S, Talento AF, O’Gorman J, Hannan MM, Lynch M, Greene CM, Humphreys H, Fitzgerald-Hughes D. Cytokine responses to Staphylococcus aureus bloodstream infection differ between patient cohorts that have different clinical courses of infection. BMC Infect Dis. 2014;14:580. doi: 10.1186/s12879-014-0580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aziz N, Nishanian P, Fahey JL. Levels of cytokines and immune activation markers in plasma in human immunodeficiency virus infection: quality control procedures. Clin Diagn Lab Immunol. 1998;5(6):755–61. doi: 10.1128/cdli.5.6.755-761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Poll T, Coyle SM, Moldawer LL, Lowry SF. Changes in endotoxin-induced cytokine production by whole blood after in vivo exposure of normal humans to endotoxin. J Infect Dis. 1996;174(6):1356–60. doi: 10.1093/infdis/174.6.1356. [DOI] [PubMed] [Google Scholar]
- 23.Hardy D, Besnard A, Latil M, Jouvion G, Briand D, Thepenier C, Pascal Q, Guguin A, Gayraud-Morel B, Cavaillon JM. Comparative Study of Injury Models for Studying Muscle Regeneration in Mice. PloS One. 2016;11(1):e0147198. doi: 10.1371/journal.pone.0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scharman EJ, Troutman WG. Prevention of kidney injury following rhabdomyolysis: a systematic review. Ann Pharmacother. 2013;47(1):90–105. doi: 10.1345/aph.1R215. [DOI] [PubMed] [Google Scholar]
- 25.Petejova N, Martinek A. Acute kidney injury due to rhabdomyolysis and renal replacement therapy: a critical review. Crit Care. 2014;18(3):224. doi: 10.1186/cc13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gattai PP, Mafra FF, Wasinski F, Almeida SS, Cenedeze MA, Malheiros DM, Bacurau RF, Barros CC, Camara NO, Araujo RC. Kinin B2 receptor does not exert renoprotective effects on mice with glycerol-induced rhabdomyolysis. World J Nephrol. 2014;3(3):85–91. doi: 10.5527/wjn.v3.i3.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 28.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705–16. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wirths S, Bugl S, Kopp HG. Neutrophil homeostasis and its regulation by danger signaling. Blood. 2014;123(23):3563–6. doi: 10.1182/blood-2013-11-516260. [DOI] [PubMed] [Google Scholar]
- 30.Wengner AM, Pitchford SC, Furze RC, Rankin SM. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood. 2008;111(1):42–9. doi: 10.1182/blood-2007-07-099648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mei J, Liu Y, Dai N, Hoffmann C, Hudock KM, Zhang P, Guttentag SH, Kolls JK, Oliver PM, Bushman FD. Cxcr2 and Cxcl5 regulate the IL-17/G-CSF axis and neutrophil homeostasis in mice. J Clin Invest. 2012;122(3):974–86. doi: 10.1172/JCI60588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicolas-Avila JA, Adrover JM, Hidalgo A. Neutrophils in Homeostasis, Immunity, and Cancer. Immunity. 2017;46(1):15–28. doi: 10.1016/j.immuni.2016.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analysis of plasma DBP and DBP-actin complexes. Aliquots of pooled plasma samples (n = 6 for PBS groups, n = 8 for glycerol-treated groups) were separated on a 10% non-denaturing (no SDS) polyacrlyamide gel and transferred to PVDF membrane for immunoblotting using an affinity purified anti-human DBP. Note: DBP is highly conserved in mammals and mouse DBP strongly cross reacts with anti-human DBP. KO = DBP deficient (DBP−/−) mice, WT = Wild-type (DBP+/+) mice. All pooled plasma samples in the four experimental groups had similar total protein levels (± 5%) and the same amount of protein was loaded in each lane. In a separate experiment, pooled WT plasma from untreated mice (n = 3) was treated with the indicated concentration of purified G-actin for 30 minutes at 22°C and then subjected to electrophoresis and blotting. These samples served as a positive control for DBP-actin complexes, the position of the DBP and DBP-actin bands are indicated on the right side of the figure.