Abstract
Background
Viscoelastic measurements of hemostasis indicate that 20% of seriously injured patients exhibit systemic hyperfibrinolysis, with increased early mortality. These patients have normal clot formation with rapid clot lysis. Targeted proteomics was applied to quantify plasma proteins from hyperfibrinolytic (HF) patients to elucidate potential pathophysiology.
Methods
Blood samples were collected in the field or at Emergency Department arrival and thrombelastography (TEG) was used to characterize in vitro clot formation under native and tissue plasminogen activator (tPA)-stimulated conditions. Ten samples were taken from injured patients exhibiting normal lysis time at 30 min (Ly30), “eufibrinolytic” (EF), 10 from HF patients, defined as tPA-stimulated TEG Ly30 >50%, and 10 from healthy controls. Trauma patient samples were analyzed by targeted proteomics and ELISA assays for specific coagulation proteins.
Results
HF patients exhibited increased plasminogen activation. Thirty-three proteins from the HF patients were significantly decreased compared to healthy controls and EF patients; 17 were coagulation proteins with anti-protease consumption (p<0.005). The other 16 decreased proteins indicate activation of the alternate complement pathway, depletion of carrier proteins, and 4 glycoproteins. CXC7 was elevated in all injured patients versus healthy controls (p<0.005), and 35 proteins were unchanged across all groups (p>0.1 and fold change of concentrations of 0.75–1.3).
Conclusion
HF patients had significant decreases in specific proteins and support mechanisms known in trauma-induced hyperfibrinolysis and also unexpected decreases in coagulation factors, factors II, X, and XIII, without changes in clot formation (SP, R times or angle). Decreased clot stability in HF patients was corroborated with tPA-stimulated TEGs.
Level of Evidence
III prognostic.
Keywords: Coagulation, Targeted Proteomics, Fibrinolysis, Traumatic Injury
Background
Systemic hyperfibrinolysis following traumatic injury with hemorrhagic shock occurs in up to 20% of critically injured patients and is associated with a mortality of >50%.1–6 Previous descriptions of trauma-induced coagulopathy (TIC) relied on standard measures of coagulation in fresh plasma, e.g. prothrombin times/International normalized ratios (PT/INR).7–10 A number of coagulation proteins has been assayed to depict this hyperfibrinolytic phenotype with high sensitivity but poor specificity, i.e. minimal capacity to discriminate across many heterogeneous patient samples.10–14 Although delayed clotting may occur due to an apparent decrease in soluble clotting factor levels, this etiology appears to be attributable to a dilutive mechanism from major hemorrhage and crystalloid resuscitation, rather than consumption.3,8,10 Changes in resuscitation practice over the past two decades have significantly ameliorated iatrogenic dilution as a driver of TIC, and attention has shifted to hyperfibrinolysis.
Severe trauma distorts physiological clot remodeling, towards one of two extremes: systemic hyperfibrinolysis (HF) or fibrinolysis shutdown, both of which are associated with increased mortality.5,15–17 The CRASH 2 trial underlines the importance of clinical strategies for inhibiting plasmin-mediated fibrinolysis acutely following injury, and this and other studies suggest the therapeutic utility of the lysine analog tranexamic acid (TXA) in a subset of severely hyperfibrinolytic patients.5,18–20 While the hypothesis that TIC is solely due to diminished clotting factors has been displaced and the key role of deregulated fibrinolysis in TIC recognized, an unbiased survey of the hyperfibrinolytic plasma proteome is lacking.
Thrombelastography (TEG) evaluates not only clot formation times with different initiators but also the kinetics of clot formation, clot strength, and clot remodeling/lysis in whole blood.5,6,21–24 Time course studies establish that many injured patients with systemic hyperfibrinolysis have normal or even rapid clot formation times (R times) and low maximum amplitude (MA, an indicator of clot strength).1,17,18,25,26 While HF presents in many patients as increased lysis measured at 30 minutes after MA (Ly30), HF can also be rapidly detected by observing marked increases in Ly30 following the addition of exogenous tissue plasminogen activator (tPA) to the TEG assay.17,18,23,24,27 The tPA-challenged TEG assay allows the rapid stratification of trauma patients into HF, eufibrinolytic (EF:physiologic), and fibrinolysis shutdown phenotypes and is a better predictor of massive transfusion, a hallmark of systemic hyperfibrinolysis.17,24,27,28 We hypothesize that examination of the plasma proteome of injured patients reveals differences is specific proteins, which further define the fibrinolytic phenotype and provide mechanistic insight into the pathogenesis of trauma-induced coagulopathy (TIC). Application of a controlled mass spectroscopy approach of 142 specific proteins was completed and allowed for quantification of these plasma proteins, which include serpins, coagulation factors, and other proteins known to affect hemostasis, either clotting or fibrinolysis.29,30 Such data may provide a better scientific basis for individualized, goal-directed resuscitation of the critically injured.
Methods
Study Population
Consecutive adult trauma patients (n=130) meeting criteria for the highest level of activation at our Level I trauma center (Denver Health Medical Center) from April 2014 to April 2016 were assigned to the Trauma Activation Protocol approved by the Combined Multi-Institutional Review Board with a waiver of consent. The criteria are patients >18 years of age and traumatic injury with any of the following: (a) blunt trauma with systolic blood pressure SBP <90 mmHg (b) mechanically unstable pelvic injury (open or obvious by physical exam) (c) penetrating neck/torso injuries with (SBP) <90 mmHg (d) gunshot wounds to the neck/torso or stab wounds to the neck/torso that require endotracheal intubation. Patients who did not have blood drawn within 60 minutes of ED arrival, received blood products, being treated with anticoagulants, or transferred from another facility, were excluded. No pre-hospital blood products were administered prior to arrival. Citrated and heparinized whole blood and plasma samples were obtained upon arrival, and 5 different TEG assays were completed: rapid-TEG (rTEG: re-calcified immediately prior to loading into the TEG cup with tissue factor and kaolin), citrated functional fibrinogen (CFF: platelet-blocked, reptilase-initiated + Factor XIII in heparin), CFF + TXA, citrated native (CNTEG, re-calcified native TEG), and CNTEG stimulated with tPA [75 ng/ml]. Fresh plasma samples isolated from whole blood by an initial centrifugation at 5,000g for 7 min followed by a second spin at 12,500g to remove platelets and acellular debris were immediately frozen at −80°C. These samples were used for targeted proteomic analysis and ELISA measurements. The groups were stratified by the Ly30 obtained from tPA (75 ng/ml)-stimulated CNTEG traces: ten consecutive injured patients with HF, defined as Ly30 ≥50% (10 samples), were paired with 10 injured EF patients: injured patients with EF defined as tPA-stimulated CNTEG 20%≥Ly30≥5% over the same time frame as the HF patients, and 10 healthy control subjects.
Plasma depletion of albumin and IgG
Albumin and IgG, were removed from the plasma samples using serum protein immunodepletion resins (Proteome Purify2, R&D Systems, Inc., Minneapolis, MN) as published.29,30 Because of the intentional removal of albumin and immunoglobulins these proteins are obviated from further analysis.
Targeted proteomics
Recombinant isotopically labeled QConCAT proteins, containing a chimeric concatamer of peptides labeled at the lysine and arginine residues (13C6 isotopologues) were mixed with the albumin- and IgG-depleted plasma at 200 or 100 fmol per injection as published.29,30 The QConCAT palette of targeted proteins includes 142 proteins made up of coagulation factors, serpins, carrier proteins, known to affect hemostasis.29,30
Enzyme-linked immunosorbent assays (ELISAs)
ELISA assays were performed in duplicate, with dilutions to ensure proper quantification, per the manufacturer’s instructions. ELISA’s were completed for thrombin (MyBiosource.com, San Diego, CA), antithrombin (AT), thrombin:antithrombin (TAT) complexes, plasminogen (Plg) (AssayPro, St. Charles, MO), α2-antiplasmin, plasmin: α2-antiplasmin (PAP) complexes, tPA, tPA:PAI-1 complexes and PAI-1 (Molecular Innovations, Novi, MI), and thrombin-activated fibrinolysis inhibitor (TAFI) (Sekisui Diagnostics, Stamford, CT).
Statistical analyses
The data are reported as the median ± interquartile ranges for all patient demographics, coagulation assays, and targeted proteomics and the mean ± the standard error of the mean for the ELISA data. Because the data was not normally distributed, statistical differences among the 3 patient groups were compared using a non-parametric Kruskal-Wallis test followed by the Dwass, Steel, Critchlow-Fligner multiple comparison procedure with statistical differences at p<0.005 for proteins and p<0.05 for other clinical tests. Z means testing at 1.25 times the standard deviation was employed to determine the proteins that were unchanged among groups. For the normally distributed ELISA data, as determined by the Shapiro-Wilk test for normal distribution, statistical differences (p<0.05) were determined by an independent analysis of variance (ANOVA) followed by a Bonferroni test for multiple comparisons.
Results
Patient Demographics
Patients with HF were the most severely injured cohort and compared to EF patients and had higher injury severity scores (ISS, NISS) and Glasgow coma scales (GCS) (Table 1). The HF patients showed no difference in age or BMI compared to the EF patients. Moreover, HF patients evidenced a lower plasma pH with higher base deficits versus the EF patient group, although there was no difference in initial field fluid administration or in time to blood sample collection after injury: 19/20 samples were acquired within 1 hour (Table 1). The longest interval to sample collection was 3 hours 25 minutes in a stabbed female, who had vital signs in the field and survived without receiving any pre-hospital fluids. Seventy percent of the HF patients died within 12 hours of injury, all required massive transfusions, and emergency thoracotomy were required in 50% (Table 1). The citrated r-TEGs were employed for clinical decisions as published because: 1) tissue factor- and kaolin-activated TEGs provide faster results and 2) citrate prevents sample clotting because some of these patients are paradoxically hypercoagulable soon after injury.6,28
Table 1.
Patient demographics, clinical characteristics, and laboratory values.
| Variables | Controls (C) n=10 | |||||||
|---|---|---|---|---|---|---|---|---|
| Median | Lower quartile | Upper quartile | p-value | |||||
| C vs HF | C vs EF | |||||||
| Total Hematocrit (%) | 46.9 | 45.1 | 47.7 | 0.0147 | 0.0176 | |||
| INR | 1.0 | 1.0 | 1.1 | 0.001 | 0.0069 | |||
| PTT | 29.3 | 28.5 | 29.9 | 0.0016 | 0.0033 | |||
| Fibrinogen | 273 | 242.8 | 292 | 0.0094 | No Data | |||
| Hyperfibrinolysis (HF) n=10 | Eufibrinolysis (EF) n=10 | p-value* HF vs EF | ||||||
| Median | Lower quartile | Upper quartile | Median | Lower quartile | Upper Quartile | |||
| Age (years) | 32.3 | 24.6 | 43.4 | 36.4 | 23.4 | 59.6 | 0.8242 | |
| Men | 80% | 90% | 1.00 | |||||
| Blunt mechanism | 40% | 20% | 0.6285 | |||||
| NISS | 46.5 | 38 | 51.8 | 17 | 12 | 23.8 | 0.0074 | |
| Max AIS | Head/Neck | 0 | 0 | 3.5 | 0 | 0 | 0 | 0.2696 |
| Chest | 3.5 | 0 | 5 | 1.5 | 0 | 3 | 0.0340 | |
| Abdomen/Pelvis | 2.0 | 0 | 3.5 | 1.0 | 0 | 3.3 | 0.5070 | |
| Extremities | 1.0 | 0.8 | 2.3 | 1.0 | 0 | 2.3 | 0.8740 | |
| Time from injury (min) | 42.0 | 26.0 | 58.8 | 35.0 | 28.0 | 42.8 | 0.5418 | |
| 1st Measurement (ED) | SBP (mmHg) | 40.0 | 0.0 | 121.5 | 108.0 | 100.0 | 128.0 | 0.1509 |
| GCS | 3.0 | 3.0 | 3.0 | 15.0 | 12.25 | 15 | 0.0073 | |
| pH | 6.9 | 6.8 | 7.0 | 7.3 | 7.3 | 7.3 | 0.0045 | |
| Base Excess (mEq/L) | −19.5 | −11 | −13.3 | −5.0 | −7.0 | −2.0 | 0.0128 | |
| Hematocrit (%) | 35.9 | 30.7 | 40.5 | 40.3 | 38.9 | 44.0 | 0.1509 | |
| INR | 1.8 | 1.4 | 2.0 | 1.1 | 1.1 | 1.2 | 0.0022 | |
| PTT | 66.1 | 48.7 | 98.9 | 27.5 | 25.3 | 28.2 | 0.0025 | |
| Fibrinogen | 107 | 87.5 | 108.5 | No data | No data | No data | ||
| Crystalloid infused (ml) | 450 | 62.5 | 500 | 400 | 200 | 875 | 0.8582 | |
| Units/6 hrs | Red Blood Cells | 17.5 | 3.8 | 26.3 | 0 | 0 | 0 | 0.0017 |
| Plasma (FFP) | 10 | 2.0 | 12.0 | 0 | 0 | 0 | 0.0017 | |
| Platelets | 1.5 | 0.0 | 2.8 | 0 | 0 | 0 | 0.3143 | |
| Cryoprecipitate | 0.5 | 0 | 1.8 | 0 | 0 | 0 | 0.3143 | |
| Death /24 hrs. | 70% | 0 | 0.0031 | |||||
The data are presented as the median ± the interquartile range.
Statistical differences were determined by Wilcoxon’s, Kruskal-Wallis, or Fisher’s Tests;
NISS: New Injury Severity Score; Max AIS: Maximum Abbreviated Injury Scale score; ED: emergency department (arrival): C = healthy controls. Massive transfusion = 10 RBC units transfused in 24 hours. A unit of apheresis platelets = 3 × 1011 platelets.
TEG Measurement of HF Patients
TEG assays were conducted concomitantly in whole blood samples of all injured patients upon admission. These data included measurements from A) CNTEGs, B) tPA-CNTEGs with 75 ng/ml tPA (tPA-CNTEGs), and C) rTEGs (Table 2), along with CFF-TEGs, which assess platelet independent clotting in whole blood initiated with tissue factor, and CFF-TEG with tranexamic acid (CFF+TXA), to inhibit plasmin activity (Supplementary Data, Table 1.). The HF plasma samples selected for QConCAT proteomic analyses all showed high Ly30 (≥50%) in routinely obtained tPA-CNTEG assays compared to samples from EF patients 5%≥Ly30≤20% and healthy controls (Table 2) (p<0.001). In addition, for all TEG experiments the initial clot formation parameters Split Time (SP) and R were not significantly different between whole blood samples from HF patients vs. samples from EF patients and healthy controls (p>0.05). However, both the angle and the MA, which are dependent upon fibrinogen and platelets, from HF patients were significantly decreased versus EF patients and healthy controls (p<0.01 for both) (Table 2).6,28
Table 2.
Thrombelastography of injured patients with systemic hyperfibrinolysis versus injured patients with eufibrinolysis and healthy controls
| TEG | Control (C) | C vs. HF | Hyperfibrinolysis (HF) | HF vs. EF | Eufibrinolysis (EF) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Med | 25th % | 75th % | P-Value | Median | 25th % | 75th % | P-Value | Med | 25th % | 75th % | ||
| CNTEG | SP | 11.4 | 10.8 | 12.5 | 0.1221 | 8.5 | 6.0 | 9.9 | 0.7750 | 7.6 | 6.8 | 8.6 |
| R | 13.2 | 12.2 | 13.6 | 0.1221 | 9.9 | 6.9 | 10.7 | 0.6595 | 8.5 | 7.8 | 9.4 | |
| Angle | 52.9 | 42.4 | 54.4 | 0.5378 | 44.0 | 39.5 | 50.6 | 0.0337 | 60.9 | 55.1 | 65.6 | |
| MA | 57.5 | 54.5 | 60.5 | 0.0054 | 38.2 | 28.1 | 45.5 | 0.0008 | 64.2 | 62.5 | 66.3 | |
| LY30 | 1.8 | 1.1 | 3.2 | 0.0023 | 41.1 | 11.0 | 69.3 | 0.0004 | 0.8 | 0.7 | 0.9 | |
| CNTEG +tPA (75 ng /ml) | SP | 9.1 | 0.9 | 10.2 | 0.8560 | 7.3 | 5.6 | 11.6 | 0.7748 | 6.9 | 5.8 | 7.2 |
| R | 12.4 | 11.3 | 13.3 | 0.3049 | 8.3 | 6.6 | 13.5 | 0.8173 | 7.8 | 6.8 | 8.4 | |
| Angle | 44.3 | 27.5 | 48.3 | 0.8352 | 48.2 | 23.1 | 50.2 | 0.0603 | 60.7 | 57.7 | 62.0 | |
| MA | 50.5 | 48.0 | 56.5 | 0.0035 | 20.3 | 14.0 | 32.0 | 0.0006 | 60.0 | 57.5 | 63.0 | |
| LY30 | 8.8 | 5.9 | 10.3 | 0.0007 | 80.0 | 72.0 | 89.7 | 0.0005 | 7.6 | 7.1 | 8.8 | |
| r-TEG | SP | 0.5 | 0.5 | 0.5 | 0.0381 | 0.7 | 0.6 | 0.8 | 0.6172 | 0.6 | 0.6 | 0.7 |
| R | 0.7 | 0.7 | 0.7 | 0.0379 | 0.8 | 0.8 | 1.5 | 0.6401 | 0.8 | 0.7 | 1.0 | |
| Angle | 70.6 | 67.9 | 72.4 | 0.0071 | 58.6 | 49.4 | 65.5 | 0.0055 | 71.6 | 67.2 | 77.1 | |
| MA | 60.0 | 57.5 | 62.5 | 0.0020 | 45.3 | 31.0 | 52.0 | 0.0007 | 63.5 | 61.5 | 67.5 | |
| LY30 | 2.1 | 1.3 | 3.8 | 0.0569 | 46.1 | 4.1 | 70.1 | 0.2195 | 1.5 | 1.1 | 2.6 | |
Med = median; CN = Citrated Native; r = rapid TEG; SP = Split Time; R = time to beginning of clot formation MA=Maximum Amplitude; Ly30 = lysis measured at 30 minutes after MA.
Increases in Ly30 with tPA for HF patients are 40.4±9.4% to 80.0±3.0% from CNTEG to tPA-CNTEG (p<0.001), with smaller changes in EF patients (0.9±0.2% to 7.9±3.0%) vs. healthy controls (3.0±1.0% to 9.5±1.4%) similar to previous data.17,27,31 Importantly, half (5/10) of the HF cohort exhibited lysis at the cutoff of Ly30≥50% in the CNTEG and are also close to the line of identity suggesting a minimal response to the tPA challenge (Supplementary Fig. 1). The lack of tPA response appears to occur if the Ly30 is already >70%. Also, the HF patients had a higher Ly30 in CFF-TEGs (Supplementary Fig. 2, panel A, 51.2±11.3%) compared to the EF patients (8.32±8.32%) and the healthy controls (0.23±0.1%). Furthermore, the addition of TXA to the CFF-TEGs nullified the Ly30 (the HF and EF patients were 0.0±0.0%, and the controls 0.1±0.1%) in these platelet-independent clots (Supplementary Fig. 2, panel B), confirming overactive plasminolysis as the likely dominant mechanism. Although increased, the rTEG Ly30 did indicate significant increases in Ly30 in the HF patients versus either the EF patients or healthy controls, which is most likely due to the small sample size, although the Ly30 cutoff was ≥3.0% for all HF patients. These patients had Ly30 ≥50% on the tPA-stimulated CN-TEGs further reinforcing the likely requirement for tPA-CNTEGs to define the HF group.
Coagulation Assays and Coagulation Proteins
The HF patients had significantly increased PT/INR’s and activated partial thromboplastin times (aPTT) versus healthy controls (p=0.001 and p=0.0016) and the lower fibrinogen concentration demonstrated significance (p=0.0094) (Table 1). The EF patients had PT/INR and aPTT values that were were significantly decreased (p=0.0069 and p=0.0033, respectively) vs. controls (Table 1). In comparison to the EF patients the HF group also had significantly lower plasma pH (p=0.0045), increased PT/INR and aPTT (p=0.0022 and p=0.0025, respectively). The HF patient group also received increased number of RBC units and plasma versus the EF patients (both p=0.0017).
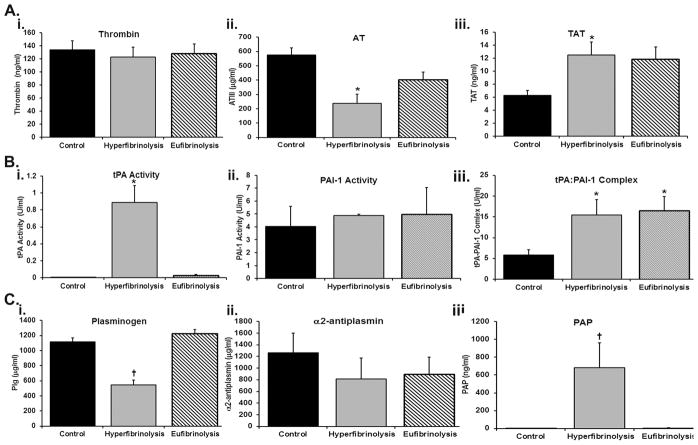
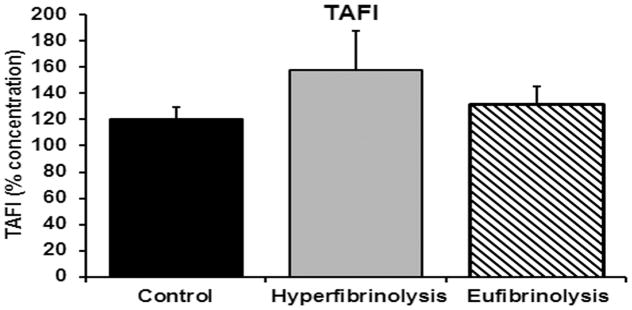
ELISA measurement of coagulation proteins and related serpins revealed that in comparison to normal controls the HF patients demonstrated similar thrombin activity with significantly decreased anti-thrombin concentrations (p<0.05) and significantly increased TAT complexes (p<0.05). As compared to EF patients, the concentrations of anti-thrombin and TAT complexes from HF patients were not different (Fig. 1, panel A). Conversely, plasminogen concentrations in HF patients was significantly less than either the EF patients or the healthy controls with a concomitant increase in PAP complexes with plasminogen concentrations not showing statistical differences across the three groups (Fig. 1, panel C). TAFI concentrations were also not different across the three groups (Fig. 2). Moreover, previous data from these same patient groups indicate that the HF patients had significantly increased tPA activity versus healthy controls and EF patients (p<0.005) (Fig. 1 panel Bi). The HF patients also had decreased but not statistically significant PAI-1 activity (Fig. 1, panel Bii) vs. both healthy controls and EF patients, but there was a significant increase (p<0.005) tPA:PAI-1 complexes in the EF patients and HF patients compared to healthy controls (Fig. 1, panel Biii).
Figure 1.
Quantification of coagulation factors, serpins, and serine protease:inhibitor complexes in injured patietns with HF and EF vs. healthy controls. The figure illustrates the measured protein activity or concentrations by ELISA. Panel A, from left to right, consists of thrombin activity (i), anti-thrombin (ii), thrombin:anti-thrombin complexes (TAT) (iii). Panel B depicts from left to right the concentration of tissue plasminogen activator (tPA) (i), plasminogen activator inhibitor (PAI-1) (ii) and tPA:PAI-1 complexes (iii). Panel C shows from left to right: plasminogen (i), α2-antiplasmin (ii), and the plasmin:α2-antiplasmin (PAP) complexes (iii). All data are expressed as the means ± the standard error of the means. *=p<0.05 versus the healthy controls and †=p<0.05 versus both EF patients and the healthy controls. Significance was measured by an independent analysis of variance followed by Bonferroni’s test for multiple comparisons.
Figure 2.
Thrombin-activated fibrinolysis inhibitor (TAFI) in injured patients with HF, EF and healthy controls. TAFI (% concentration) is illustrated for healthy controls (control) and injured patients with hyperfibrinolysis and eufibrinolysis. All data are expressed as the means ± the standard error of the means. *=p<0.05 versus the healthy controls and †=p<0.05 versus both EF patients and the healthy controls. Significance was measured by an independent analysis of variance followed by Bonferroni’s test for multiple comparisons.
Mass spectroscopy Measurement of Plasma Proteins
Of the 142 proteins analyzed by the targeted mass spectrometry approach with heavy labeled internal standards (QConCAT), 11 were measured against two reporter proteotypic peptides with excellent agreement between the quantification of both parent ions and transition fingerprints: correlation >0.95. The concentrations of 35 plasma proteins did not change amongst the three groups as defined by p>0.1 and fold change of (0.75–1.3), which, included two distinct polypeptides from von Willebrand factor (Supplementary Table 2). These proteins consist of the most abundant soluble plasma proteins including: α2-macroglobulin (A2M), all fibrinogen chains, haptoglobin, apo-lipoprotein E, etc. Structural proteins including fibronectin, vimentin, and filamin were unchanged among groups. Coagulation factors: Factors V and VIII, serpins: A1, E1, and G1 and complement Factor C9 all remained unchanged among all three groups as well as the antiprotease α2-macroglobulin.
Importantly, intracellular biomarker proteins from circulating blood cells were not increased compared to normal plasma. Specifically, myeloperoxidase (MPO), matrix metalloprotease-2 (MMP2), MMP8, MMP9, and neutrophil elastase (ELANE) from neutrophils (PMNs) and other leukocytes, and platelet factor 4 (PLF4) and platelet glycoprotein 5 (GP5) from platelets were not changed vs. the healthy controls, despite severe injuries. Surprisingly, CXCL7, a platelet granule chemokine, was elevated in all injured patients (HF and EF) versus healthy controls (p<0.001) and there were no other statistically different proteins between the EF patients and the healthy controls (p>0.005).32 Seventy-one proteins were not statistically different amongst the three groups and did not make the statistical cutoff of p<0.005 nor the cutoffs for “no change”: p>0.1 and fold change 0.75–1.3 <2 (Supplementary Table 2).
There were a few rare, dramatic increases in plasma proteins. Thus, dramatic changes >two-fold of the mean were documented for fatty acid binding protein-1 (FABP1) and glyceraldehyde 3-phopsphate dehydrogenase (GAPDH) in a few patients; however, most of the injured subjects evidenced similar levels as compared to the healthy controls (p≥0.01). Isolated increases in intracellular proteins may reflect the mechanism of injury because the patient who was crushed in an escalator had a 10-fold increase in muscle myoglobin vs. all other groups.
Coagulation proteins
Thirty-three of the 142 measured proteins were significantly decreased as compared to EF patients with physiologic fibrinolysis and the healthy controls (p<0.005) (Table 3). Fourteen of these 33 plasma proteins were coagulation-related enzymes, including: prothrombin (factor II), thrombin, a serine-protease, and factor XIIIB. The depletion of prothrombin structural fragments (F2a, F2b) in hyperfibrinolytic patients does not appear to affect clot formation (ACT or R, in any TEG experiment, Table 2) or in the ELISA measure of active thrombin, which may be expected in a decrease of only 50%. Three distinct protein epitopes implicate the contact pathway: factor XII, plasma kallikrein (both serine-proteases), together with their co-factor Kinninogen-1 (KNG1), a cystatin, decreased by 50%.33 The probe sequence for KNG1 (starting at aa 67) is within the first cystatin domain of the KNG1 heavy chain and unaffected by possible cleavage by factor XII or kallikrein (aa 379 and 389).34 The plasma ‘anticoagulants’, proteins C and S, were also significantly decreased as was fibrinogen in the HF group which was significantly decreased compared to the healthy controls (Table 1).
Table 3.
Plasma proteins that are decreased in HF patients as compared to EF patients and healthy controls
| Gene | Common Name | Hyperfibrinolysis | Eufibrinolysis | HF vs EF | ||||
|---|---|---|---|---|---|---|---|---|
| Median | Q1 | Q3 | Median | Q1 | Q3 | P value | ||
| APCS | Serum amyloid P-component | 0.22 | 0.15 | 0.27 | 0.46 | 0.45 | 0.55 | 0.0005 |
| F2a | Prothrombin | 0.57 | 0.34 | 0.78 | 1.18 | 1.05 | 1.20 | 0.0005 |
| F2b | Prothrombin | 0.49 | 0.28 | 0.68 | 0.96 | 0.85 | 1.01 | 0.0005 |
| GC | Vitamin D-binding Protein | 1.43 | 0.88 | 1.68 | 2.38 | 2.12 | 2.56 | 0.0005 |
| AZGP1 | Alpha-2-Glycoprotein 1 | 1.23 | 0.65 | 1.33 | 1.84 | 1.72 | 1.98 | 0.0006 |
| C5 | Complement 5 | 0.46 | 0.34 | 0.61 | 0.83 | 0.72 | 0.91 | 0.0006 |
| KLKB1 | Kallikrein B1 (plasma) | 0.29 | 0.22 | 0.40 | 0.56 | 0.51 | 0.61 | 0.0006 |
| ITIH2 | Inter-α-Trypsin Inhibitor Heavy Chain 2 | 1.09 | 0.53 | 1.31 | 1.77 | 1.68 | 1.90 | 0.0008 |
| RBP4 | Retinol Binding Protein 4 | 0.94 | 0.57 | 1.07 | 1.73 | 1.51 | 2.20 | 0.0008 |
| SERPINF2a | Alpha 2-Antiplasmin | 0.54 | 0.39 | 0.92 | 1.41 | 1.27 | 1.48 | 0.0008 |
| APOA4 | Apolipoprotein A4 | 1.92 | 1.17 | 2.86 | 3.29 | 3.20 | 3.76 | 0.0011 |
| TF | Transferrin | 17.67 | 12.29 | 22.16 | 31.52 | 26.98 | 35.98 | 0.0011 |
| APOA2 | Apolipoprotein A2 | 17.04 | 10.65 | 19.54 | 25.92 | 23.17 | 30.84 | 0.0015 |
| C3 | Complement 3 | 3.59 | 2.04 | 4.23 | 6.01 | 5.46 | 6.81 | 0.0015 |
| PROS1a | Protein S alpha | 0.08 | 0.04 | 0.11 | 0.16 | 0.13 | 0.16 | 0.0015 |
| SERPINA4 | Kallikrein Inhibitor | 0.19 | 0.11 | 0.22 | 0.36 | 0.30 | 0.40 | 0.0015 |
| SERPINF2b | Alpha 2-Antiplasmin | 0.95 | 0.50 | 1.33 | 1.77 | 1.57 | 1.89 | 0.0015 |
| AHSG | Alpha 2-HS Glycoprotein | 2.74 | 2.14 | 3.66 | 5.91 | 5.42 | 6.52 | 0.0019 |
| F12 | Coagulation Factor XII | 0.09 | 0.06 | 0.16 | 0.28 | 0.24 | 0.33 | 0.0019 |
| ITIH1 | Inter-α-Trypsin Inhibitor Heavy Chain 1 | 1.21 | 0.57 | 1.37 | 1.83 | 1.61 | 2.02 | 0.0019 |
| CP | Ceruloplasmin | 1.66 | 1.23 | 2.11 | 2.80 | 2.28 | 3.53 | 0.0025 |
| PROS1b | Protein S | 0.11 | 0.07 | 0.15 | 0.20 | 0.18 | 0.22 | 0.0025 |
| F10 | Coagulation Factor X | 0.20 | 0.09 | 0.23 | 0.33 | 0.27 | 0.39 | 0.0033 |
| F13Bb | Factor XIII B Chain | 0.17 | 0.15 | 0.23 | 0.33 | 0.27 | 0.41 | 0.0033 |
| GPLD1 | Glycosylphosphatidyl inositol specific Phospholipase D1 | 0.12 | 0.07 | 0.15 | 0.22 | 0.18 | 0.26 | 0.0033 |
| GSN | Gelsolin | 0.47 | 0.41 | 0.79 | 1.19 | 1.02 | 1.38 | 0.0033 |
| PLGb | Plasminogen | 0.95 | 0.73 | 1.25 | 1.79 | 1.59 | 1.97 | 0.0033 |
| PROCb | Protein C | 0.18 | 0.13 | 0.24 | 0.33 | 0.29 | 0.36 | 0.0033 |
| SERPIND1 | Heparin Cofactor 2 | 0.72 | 0.32 | 0.86 | 1.15 | 1.10 | 1.36 | 0.0033 |
| CFH | Complement Factor H | 1.04 | 0.76 | 1.22 | 1.81 | 1.62 | 2.02 | 0.0043 |
| CLU | Clusterin | 0.84 | 0.43 | 1.04 | 1.49 | 1.36 | 1.67 | 0.0043 |
| HPX | Hemopexin | 7.05 | 4.59 | 8.94 | 11.39 | 11.04 | 12.32 | 0.0043 |
| KNG1 | Kininogen-1 | 0.97 | 0.74 | 1.30 | 1.82 | 1.64 | 2.04 | 0.0043 |
The data are expressed as the median ± interquartiles. All 33 proteins were decreased versus identical QConCAT values from healthy controls as well as the EF patients (p<0.005).
Three fragments characterized the plasminolytic aspect of hyperfibrinolysis. One fragment of plasminogen (another short-lived serine-protease, aa 181–191), along with 2 epitopes of its dedicated serpinF2 (α2-antiplasmin) were all concomitantly reduced (p<0.005). These data coincide with both decreased plasminogen and increased PAP complexes in the HF group by ELISA (Fig. 1, Panel B i & iii). However, fibrinogen was depleted, and the TAFI concentrations were unchanged across groups. In addition specific serpins were decreased, including: serpin F2 (α2-antiplasmin), serpin A4 (kallistatin), serpin D1 (Heparin Cofactor II), and the broadly acting inter α-trypsin inhibitor component, ITIH2 compared to EF patients and healthy controls.
Complement
QConCAT probes of 3 proteins confirm the activation of the complement pathway after severe trauma. These include, depletion of the complement zymogens, C3 (53%, p=0.0015) and C5 (56%, p<0.0006). Notably the suicide inhibitor of C5 activation CFH, is also decreased (61% compared to EF samples, p= 0.0043). These results imply that acute injury activates the alternate pathway since neither C2, C4, or C9 reached significance.35
Proteins involved with stress-induced oligomerization
Serum amyloid-P (component APCS) is an oligomerizing protein that was significantly decreased in HF patients. However, the better known inducible pentraxin, C-reactive protein, which was assessed at two sites (CRPa, CRPb), was unchanged, except dramatically in one patient with a documented infection prior to injury, in whom both fragments were elevated >10-fold higher along with significant increases in both lipopolysaccharide binding protein (LBP) and all three fibrinogen chains in comparison to the other injured patients and healthy controls (p<0.001). Supporting traumatic stress denaturation, clusterin (CLU) and hemopexin were also depleted. Lastly, of the apo-lipoproteins constituting HDL, only APOA 2,4, was depleted.
Carrier proteins
Both retinol-binding protein-4 (RBP4) and vitamin D binding protein (GC) were decreased in HF patients vs. EF patients and the healthy controls (p=0.0008). Both the Fe-binding proteins serotransferrin (TF) and hemopexin (HPX) were also significantly decreased in HF patients as was ceruloplasmin (CP).
Glycoproteins
The abundant cystatin domain protein α2-Heremans Schmid (HS)-glycoprotein, fetuin A, was significantly decreased in the HF group compared to the EF patients and the healthy controls, while another minor cystatin, histidine-rich glycoprotein was not. Moreover, glycosylphosphatidyl-inositol specific phospholipase D, a plasma phospholipase D, cleaving inositol anchored proteoglycans was also decreased in the HF group.36 Zinc α2-glycoprotein-1 is an incompletely understood protein that may be an adipokine, which contains the major histocompatibility complex and immunoglobulin domains was also decreased as was glycosylphosphatidyl-inositol specific phospholipase D.37
Discussion
The reported data from this pilot study has demonstrated that injured HF patients were more seriously injured, had a decreased pH, and increased mortality, NISS, PT/INR and PTT, and required more transfusion support in the first 6 hours with both RBCs and plasma. The HF patients also had increased plasmin activity as documented by significantly increased Ly30 on CNTEG, the further augmentation of Ly30 in the tPA-stimulated lysis, and the concomitant increases in tPA and PAP complexes (600-fold) with decreases in plasminogen, PAI-1, and fibrinogen. Importantly, all patients were bleeding when the samples were obtained and were stratified only by the Ly30 on the tPA-CNTEGs: Ly30≥50% for the HF group and 5%≤Ly30≤20% for the EF group. These patients’ presentation and treatment were over a similar time frame with supportive care and surgical interventions completed by the identical teams. The angle, MA, a measure of clot strength, and fibrinogen were significantly decreased in the HF patients, particularly in those TEG assays that involve thrombin activation (rTEG, CNTEG,) but less likely to be significant in platelet-independent assays, CFF-TEG. Thirty-three proteins were significantly decreased in the HF patient group, versus both the EF patients and the normal controls, while 106 did not demonstrate a statistical difference (p>0.005) with 35 of these demonstrating no change (p>0.1, fold change 0.75–1.3). Other TEG parameters are supportive of this hyperfibrinolytic phenotype, especially the CFF-TEG and tranexamic acid reversed the lysis on TEGs from the HF patients. This HF patient group experienced significantly increased mortality of 70% and consisted of patients with high ISS, hemorrhagic shock, and required massive transfusions. These data are supported by a retrospective study of fibrinolytic phenotype in 2,540 trauma patients with 18% of severely injured patients (ISS>15) who appeared hyperfibrinolytic (Ly30≥3%) and suffered a death rate of 44%, which was exacerbated by shock, irrespective of the mechanism of injury.3–5,31,38
Previous work has detailed that the overwhelming increases in tPA, not degradation of PAI-1, is responsible for the observed hyperfibrinolysis in injured patients.4,17 The reported data are similar with significantly increased tPA activity and tPA:PAI-1 complexes with decreased, but not significantly, levels of PAI-1 in HF patients versus healthy controls and EF patients. Moreover, the HF patients had significant amounts of plasmin activity, documented by both the increased amounts of plasmin:α2-antiplasmin complexes with the significantly decreased amounts of plasminogen versus both control and EF patients. Thus, plasmin has been directly implicated, which is downstream of tPA and is directly responsible for the hyperfibrinolytic phenotype described.
The reported data is focused on HF patients, which differs from other reports on trauma-induced coagulopathy (TIC).8,9,21,25,26 TIC has been postulated to represent a sub-type of disseminated intravascular coagulation (DIC), which should result in decreased platelet count, which did not occur.15,21 The reported data are consistent with the clinical series from Copenhagen, which indicated that TIC was not similar to DIC.14,39 In addition, TIC has been described as a dilutional coagulopathy secondary to overzealous administration of crystalloid without proper reconstitution of hemostatic potential.3,8,10 Importantly, 19/20 samples analyzed were collected within 1 hour of injury with comparable saline volumes infused, and 35 proteins were not different between the injured patient groups would argue against crystalloid dilution as a mechanism for the observed systemic hyperfibrinolysis subset of TIC.
Decreases in specific coagulation proteins, Factors II, X, and XIII, as well as TAFI point towards appropriate intervention by slowing/stopping systemic hyperfibrinolysis with an antifibrinolytic, tranexamic acid, followed by plasma, which is the best source of factors II and X and TAFI.40,41 Factor XIII has an in vivo half-life of ~12 days and thus, there are significant amounts of it in plasma, although cryoprecipitate is the best source.40,41 One must be cautious for intervention with tranexamic acid after the first three hours post-injury correlates with increased adverse events and even mortality.42–45
Systemic increases in activated protein C (APC) have also been postulated to be a mechanism for TIC. If this is the case, then one would expect that the SP- and R-times on CNTEG would be increased with concomitant decreases in fibrinogen, factor V, and factor VIII levels/activities.19 In contrast, the reported data does not demonstrate increased SP-/R-times. The thrombin activity is not different in HF patients vs. EF patients and healthy controls, and the fibrinogen concentrations are unchanged (all three chains), although both factor V and factor VIII remain in the normal range. In addition, APC-induced systemic hyperfibrinolysis has been reported to be due to thrombin binding to thrombomodulin via activation of protein C and consumption of PAI-1 with TAFI inhibition being an important mechanism for “fibrinolysis derepression” with TAFI-induced activation of protein C resulting in APC binding to thrombin-thrombomodulin.2,19 The reported data demonstrate a modest increase in TAFI in HF patients, which was not significantly different from the EF patients or the healthy controls, and such maintenance of normal TAFI concentrations in the HF patients requires more data to better define role of APC in injury-induced hyperfibrinolysis.2,19
HF patients, as defined by TEG measurements, had the highest plasma levels of tPA with a concomitant diminished anti-protease defense, or alternatively, near complete conversion of plasminogen to active plasmin. However, EF patients and healthy controls also showed modest response to tPA in the tPA-stimulated TEGs (vertical increase <20%), which may reflect lower levels of tPA or may be due to insufficient anti-protease tPA “buffering”. The HF patients also had significantly prolonged PT/INR and aPTT, which may be due to multiple factor diminutions and/or interference with the clotting cascade.
The proteomic signature of the HF group is dominated by decreases in 1) coagulation proteins, 2) the complement system, specifically anaphylatoxins, 3) proteins involved in stress-induced oligomerization, 4) carrier proteins, and 5) glycoproteins. The decrease in coagulation proteins: factors II, V, XIII and fibrinogen, attests that both anti-protease regulated clot formation and plasminolysis are ongoing. The clearance of serpins, and activation of the contact pathway: factor XII, Kallikrein B1, and Kininogen-1 (F12, KLKB1 KNG) with and significant decrease of both protein S (ProS1) fragments are notable.
HF patients also have decreased plasma concentrations of proteins involved with stress-induced oligimerization. The depletion of such self-associating soluble zymogens that form large oligomers, may lead to unexpected consequences including immunodeficiency.46–48 In this regard serum amyloid P is a pentraxin, self-associates in 5 and 10 units, provides a mechanism for recognizing DAMPs and PAMPs and accompanies amyloid deposits.49 Clusterin (CLU), hemopexin (HPX), and α2-macroglobulin (A2M), also form a class of chaperones oligomerizing with mis-folded proteins in plasma, which often arise during stress.50 However, CLU can stabilize up to 10 equivalents of certain mis-folded plasma proteins, especially at pH ≤7.1.51,52
Traumatic injuries have not been previously linked to decreased plasma concentration of carrier proteins, and the significantly decreased concentrations of retinol binding protein-4 (RBP4), ceruloplasmin (CP), vitamin D binding protein (GC), serotransferrin (TF), and hemopexin (HPX) were unexpected. In plasma, the RBP-retinol complex interacts with transthyretin (TTR), which prevents its loss by filtration through the kidney glomeruli.53 TTR mis-folding leading to amyloids is well known.54 The deficiency of GC has not been linked to trauma before, and may be involved in coagulation.55,56 Similar to RBP4 and TTR, both TF and CP contain beta sheets amenable to common amyloid formation.57 In contrast HPX (like CLU), could stabilize certain mis-folded plasma proteins.
The presented data have a number of limitations. First, viscoelastic measurements of hemostasis and the fibrinolytic system are low shear assays and the effects of factor XIII depletion and other proteins, including fibrinogen, may not be as “functionally represented” as coagulation in vivo. Second, the employed targeted mass spectrometry-based proteomics approach relies heavily on the presence of unique prototypic peptides derived from the concomitant tryptic digestion of the proteins in the sample and the spiked QConCAT standards. Therefore, the approach is limited to the measurement of such peptides and cannot measure the activity of serine-proteases and other enzymes that cut at different residues than arginine or lysine. Future generations of QConCAT peptides could be designed to address this issue by including sequences that can be targeted by proteases in the coagulation and other cascades. Differential clearance of pro-domains and other cleaved domains and the presence of endopeptidases could also confound the results by changing the peptide sequences from those monitored in the QConCAT. With the present set of internal standards, the levels and activation status of tPA and PAI-1 could not be accurately determined. Lastly, plasma samples are filtered prior to mass spectroscopy analysis via QConCAT and there may be some proteins that are non-specifically retained by these columns. This non-specific retention of proteins by these columns is currently being investigated.
In conclusion, HF patients exhibited significant decreases in specific proteins and buffering mechanisms, which were expected in TIC, as well as unexpected decreases in Factors II, X, XII and XIII. Notably, these changes are well correlated to both hyperfibrinolysis with decreased clot strength (MA) with little impact on clot initiation, both the SP and R-times. Thus, the hyperfibrinolytic component of TIC appears mechanistically distinct from derangements of clot formation related to soluble factor dilution/consumption. The unique set of proteome alterations in this subset of severely HF patients may better explain the mechanistic underpinnings of the onset of systemic hyperfibrinolysis after severe injury.
Supplementary Material
Acknowledgments
This work was supported by grants P50 GM049222, NIGMS, NIH, T32 GM008315, NIGMS, NIH, and UM1-HL120877, NHLBI, NIH and the Department of Surgery and the Colorado Clinical and Translational Sciences Institute, School of Medicine, University of Colorado Denver, and Bonfils Blood Center.
Footnotes
The authors have no conflict of interest with the submitted work.
Author Contributions
AB and CCS wrote the manuscript and had major inputs into data analyses and interpretation. EEM, AS, AD’A, EP, helped write the manuscript and were key in data analyses. MD completed all of the QConCATs and wrote portions of the manuscript. MK performed all ELISA data and constructed the tables and Figure 3. KJ added valuable statistical advice and helped to write the manuscript. MPC, EG, HBM, BEH, PE helped to accrue data and aided in data analyses and manuscript preparation. JC and AG were in charge of all sample accrual, supervised the running of all TEGs, and maintained the subject databases and integrity of the data. KH supervised all mass spectrometry studies and ensured that the QConCAT was performed properly and helped in data analysis and writing the manuscript.
References
- 1.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007 May;245(5):812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, Pittet JF. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008 May;64(5):1211–1217. doi: 10.1097/TA.0b013e318169cd3c. [DOI] [PubMed] [Google Scholar]
- 3.Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, Schochl H, Wade CE, Holcomb JB, Matijevic N. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012 Aug;73(2):365–370. doi: 10.1097/TA.0b013e31825c1234. [DOI] [PubMed] [Google Scholar]
- 4.Chapman MP, Moore EE, Ramos CR, Ghasabyan A, Harr JN, Chin TL, Stringham JR, Sauaia A, Silliman CC, Banerjee A. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg. 2013 Dec;75(6):961–967. doi: 10.1097/TA.0b013e3182aa9c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, Banerjee A, Sauaia A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014 Dec;77(6):811–817. doi: 10.1097/TA.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Ann Surg. 2016 Jun;263(6):1051–1059. doi: 10.1097/SLA.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLoughery TG. Coagulation defects in trauma patients: etiology, recognition, and therapy. Crit Care Clin. 2004 Jan;20(1):13–24. doi: 10.1016/s0749-0704(03)00089-7. [DOI] [PubMed] [Google Scholar]
- 8.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, Mackway-Jones K, Parr MJ, Rizoli SB, Yukioka T, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008 Oct;65(4):748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 9.MacLeod JB, Winkler AM, McCoy CC, Hillyer CD, Shaz BH. Early trauma induced coagulopathy (ETIC): prevalence across the injury spectrum. Injury. 2014 May;45(5):910–915. doi: 10.1016/j.injury.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Shaz BH, Winkler AM, James AB, Hillyer CD, MacLeod JB. Pathophysiology of early trauma-induced coagulopathy: emerging evidence for hemodilution and coagulation factor depletion. J Trauma. 2011 Jun;70(6):1401–1407. doi: 10.1097/TA.0b013e31821266e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen MJ, Call M, Nelson M, Calfee CS, Esmon CT, Brohi K, Pittet JF. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg. 2012 Feb;255(2):379–385. doi: 10.1097/SLA.0b013e318235d9e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunbar NM, Chandler WL. Thrombin generation in trauma patients. Transfusion. 2009 Dec;49(12):2652–2660. doi: 10.1111/j.1537-2995.2009.02335.x. [DOI] [PubMed] [Google Scholar]
- 13.Maegele M, Schochl H, Cohen MJ. An update on the coagulopathy of trauma. Shock. 2014 May;41(Suppl):121–25. doi: 10.1097/SHK.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 14.Ostrowski SR, Sorensen AM, Larsen CF, Johansson PI. Thrombelastography and biomarker profiles in acute coagulopathy of trauma: a prospective study. Scand J Trauma Resusc Emerg Med. 2011 Oct;:1964. doi: 10.1186/1757-7241-19-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gando S. Hemostasis and thrombosis in trauma patients. Semin Thromb Hemost. 2015 Feb;41(1):26–34. doi: 10.1055/s-0034-1398378. [DOI] [PubMed] [Google Scholar]
- 16.Moore HB, Moore EE, Morton AP, Gonzalez E, Fragoso M, Chapman MP, Dzieciatkowska M, Hansen KC, Banerjee A, Sauaia A, et al. Shock-induced systemic hyperfibrinolysis is attenuated by plasma-first resuscitation. J Trauma Acute Care Surg. 2015 Dec;79(6):897–904. doi: 10.1097/TA.0000000000000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman MP, Moore EE, Moore HB, Gonzalez E, Gamboni F, Chandler JG, Mitra S, Ghasabyan A, Chin TL, Sauaia A, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J Trauma Acute Care Surg. 2016 Jan;80(1):16–23. doi: 10.1097/TA.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman MP, Moore EE, Moore HB, Gonzalez E, Morton AP, Chandler J, Fleming CD, Ghasabyan A, Silliman CC, Banerjee A, et al. The “Death Diamond”: Rapid thrombelastography identifies lethal hyperfibrinolysis. J Trauma Acute Care Surg. 2015 Dec;79(6):925–929. doi: 10.1097/TA.0000000000000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davenport RA, Guerreiro M, Frith D, Rourke C, Platton S, Cohen M, Pearse R, Thiemermann C, Brohi K. Activated Protein C Drives the Hyperfibrinolysis of Acute Traumatic Coagulopathy. Anesthesiology. 2017 Jan;126(1):115–127. doi: 10.1097/ALN.0000000000001428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, Herrera J, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010 Jul;376(9734):23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 21.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007 Dec;13(6):680–685. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 22.Kashuk JL, Moore EE, Sawyer M, Le T, Johnson J, Biffl WL, Cothren CC, Barnett C, Stahel P, Sillman CC, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg. 2010 Apr;251(4):604–614. doi: 10.1097/SLA.0b013e3181d3599c. [DOI] [PubMed] [Google Scholar]
- 23.Moore HB, Moore EE, Chapman MP, Gonzalez E, Slaughter AL, Morton AP, D’Alessandro A, Hansen KC, Sauaia A, Banerjee A, et al. Viscoelastic measurements of platelet function, not fibrinogen function, predicts sensitivity to tissue-type plasminogen activator in trauma patients. J Thromb Haemost. 2015 Oct;13(10):1878–1887. doi: 10.1111/jth.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore HB, Moore EE, Chapman MP, Huebner BR, Einersen PM, Oushy S, Silliman CC, Banerjee A, Sauaia A. Viscoelastic Tissue Plasminogen Activator Challenge Predicts Massive Transfusion in 15 Minutes. J Am Coll Surg. 2017 Jul;225(1):138–147. doi: 10.1016/j.jamcollsurg.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davenport R, Manson J, De’Ath H, Platton S, Coates A, Allard S, Hart D, Pearse R, Pasi KJ, MacCallum P, et al. Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med. 2011 Dec;39(12):2652–2658. doi: 10.1097/CCM.0b013e3182281af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagemo JS, Christiaans SC, Stanworth SJ, Brohi K, Johansson PI, Goslings JC, Naess PA, Gaarder C. Detection of acute traumatic coagulopathy and massive transfusion requirements by means of rotational thromboelastometry: an international prospective validation study. Crit Care. 2015 Mar;:1997. doi: 10.1186/s13054-015-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore HB, Moore EE, Gonzalez E, Hansen KC, Dzieciatkowska M, Chapman MP, Sauaia A, West B, Banerjee A, Silliman CC. Hemolysis exacerbates hyperfibrinolysis, whereas platelolysis shuts down fibrinolysis: evolving concepts of the spectrum of fibrinolysis in response to severe injury. Shock. 2015 Jan;43(1):39–46. doi: 10.1097/SHK.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Einersen PM, Moore EE, Chapman MP, Moore HB, Gonzalez E, Silliman CC, Banerjee A, Sauaia A. Rapid thrombelastography thresholds for goal-directed resuscitation of patients at risk for massive transfusion. J Trauma Acute Care Surg. 2017 Jan;82(1):114–119. doi: 10.1097/TA.0000000000001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Alessandro A, Dzieciatkowska M, Hill RC, Hansen KC. Supernatant protein biomarkers of red blood cell storage hemolysis as determined through an absolute quantification proteomics technology. Transfusion. 2016 Jun;56(6):1329–1339. doi: 10.1111/trf.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dzieciatkowska M, D’Alessandro A, Hill RC, Hansen KC. Plasma QconCATs reveal a gender-specific proteomic signature in apheresis platelet plasma supernatants. J Proteomics. 2015 Apr;:1201–6. doi: 10.1016/j.jprot.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore HB, Moore EE, Liras IN, Gonzalez E, Harvin JA, Holcomb JB, Sauaia A, Cotton BA. Acute Fibrinolysis Shutdown after Injury Occurs Frequently and Increases Mortality: A Multicenter Evaluation of 2,540 Severely Injured Patients. J Am Coll Surg. 2016 Apr;222(4):347–355. doi: 10.1016/j.jamcollsurg.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von HP, Petersen F, Brandt E. Platelet-derived chemokines in vascular biology. Thromb Haemost. 2007 May;97(5):704–713. doi: 10.1160/th07-01-0066. [DOI] [PubMed] [Google Scholar]
- 33.Schapira M, Scott CF, Colman RW. Protection of human plasma kallikrein from inactivation by C1 inhibitor and other protease inhibitors. The role of high molecular weight kininogen. Biochemistry. 1981 May;20(10):2738–2743. doi: 10.1021/bi00513a006. [DOI] [PubMed] [Google Scholar]
- 34.Haberland GL. The role of kininogenases, kinin formation and kininogenase inhibition in post traumatic shock and related conditions. Klin Wochenschr. 1978 Apr;56(7):325–331. doi: 10.1007/BF01477391. [DOI] [PubMed] [Google Scholar]
- 35.Ganter MT, Brohi K, Cohen MJ, Shaffer LA, Walsh MC, Stahl GL, Pittet JF. Role of the alternative pathway in the early complement activation following major trauma. Shock. 2007 Jul;28(1):29–34. doi: 10.1097/shk.0b013e3180342439. [DOI] [PubMed] [Google Scholar]
- 36.Huang KS, Li S, Fung WJ, Hulmes JD, Reik L, Pan YC, Low MG. Purification and characterization of glycosyl-phosphatidylinositol-specific phospholipase D. J Biol Chem. 1990 Oct;265(29):17738–17745. [PubMed] [Google Scholar]
- 37.Xu MY, Chen R, Yu JX, Liu T, Qu Y, Lu LG. AZGP1 suppresses epithelial-to-mesenchymal transition and hepatic carcinogenesis by blocking TGFbeta1-ERK2 pathways. Cancer Lett. 2016 May;374(2):241–249. doi: 10.1016/j.canlet.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 38.Schochl H, Frietsch T, Pavelka M, Jambor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma. 2009 Jul;67(1):125–131. doi: 10.1097/TA.0b013e31818b2483. [DOI] [PubMed] [Google Scholar]
- 39.Johansson PI, Stissing T, Bochsen L, Ostrowski SR. Thrombelastography and tromboelastometry in assessing coagulopathy in trauma. Scand J Trauma Resusc Emerg Med. 2009 Sep;:1745. doi: 10.1186/1757-7241-17-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dumont LJ, Papari M, Aronson CA, Dumont DF. Whole Blood collection and component processing. In: Fung MK, Grossman BJ, Hillyer CD, Westhoff CM, editors. Technical Manual. Bethesda: AABB; 2014. pp. 135–165. [Google Scholar]
- 41.Nester T, Jain S, Poisson J. Hemothrapy decisions and their outcomes. In: Fung MK, Grossman BJ, Hillyer CD, Westhoff CM, editors. Technical Manual. Bethesda: AABB; 2014. pp. 499–543. [Google Scholar]
- 42.Johnston LR, Rodriguez CJ, Elster EA, Bradley MJ. Evaluation of Military Use of Tranexamic Acid and Associated Thromboembolic Events. JAMA Surg. 2017 Oct; doi: 10.1001/jamasurg.2017.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore EE, Moore HB, Gonzalez E, Sauaia A, Banerjee A, Silliman CC. Rationale for the selective administration of tranexamic acid to inhibit fibrinolysis in the severely injured patient. Transfusion. 2016 Apr;56(Suppl 2):S110–S114. doi: 10.1111/trf.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt BJ, Morales C, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011 Mar;377(9771):1096–101. 1101. doi: 10.1016/S0140-6736(11)60278-X. [DOI] [PubMed] [Google Scholar]
- 45.Roberts I, Edwards P, Prieto D, Joshi M, Mahmood A, Ker K, Shakur H. Tranexamic acid in bleeding trauma patients: an exploration of benefits and harms. Trials. 2017 Jan;18(1):48. doi: 10.1186/s13063-016-1750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Angelo C, De LA, Zelante T, Bonifazi P, Moretti S, Giovannini G, Iannitti RG, Zagarella S, Bozza S, Campo S, et al. Exogenous pentraxin 3 restores antifungal resistance and restrains inflammation in murine chronic granulomatous disease. J Immunol. 2009 Oct;183(7):4609–4618. doi: 10.4049/jimmunol.0900345. [DOI] [PubMed] [Google Scholar]
- 47.Johnson EE, Wessling-Resnick M. Iron metabolism and the innate immune response to infection. Microbes Infect. 2012 Mar;14(3):207–216. doi: 10.1016/j.micinf.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spadaro G, D’Orio C, Genovese A, Galeotafiore A, D’Ambrosio C, Di GS, Vitale M, Capasso M, Lamberti V, Scaloni A, et al. Proteomic analysis of sera from common variable immunodeficiency patients undergoing replacement intravenous immunoglobulin therapy. J Biomed Biotechnol. 2011 doi: 10.1155/2011/706746. 2011706746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Konttinen YT, Pajarinen J, Takakubo Y, Gallo J, Nich C, Takagi M, Goodman SB. Macrophage polarization and activation in response to implant debris: influence by “particle disease” and “ion disease”. J Long Term Eff Med Implants. 2014;24(4):267–281. doi: 10.1615/jlongtermeffmedimplants.2014011355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yerbury JJ, Poon S, Meehan S, Thompson B, Kumita JR, Dobson CM, Wilson MR. The extracellular chaperone clusterin influences amyloid formation and toxicity by interacting with prefibrillar structures. FASEB J. 2007 Aug;21(10):2312–2322. doi: 10.1096/fj.06-7986com. [DOI] [PubMed] [Google Scholar]
- 51.Poon S, Easterbrook-Smith SB, Rybchyn MS, Carver JA, Wilson MR. Clusterin is an ATP-independent chaperone with very broad substrate specificity that stabilizes stressed proteins in a folding-competent state. Biochemistry. 2000 Dec;39(51):15953–15960. doi: 10.1021/bi002189x. [DOI] [PubMed] [Google Scholar]
- 52.Wilson MR, Yerbury JJ, Poon S. Potential roles of abundant extracellular chaperones in the control of amyloid formation and toxicity. Mol Biosyst. 2008 Jan;4(1):42–52. doi: 10.1039/b712728f. [DOI] [PubMed] [Google Scholar]
- 53.Coward P, Conn M, Tang J, Xiong F, Menjares A, Reagan JD. Application of an allosteric model to describe the interactions among retinol binding protein 4, transthyretin, and small molecule retinol binding protein 4 ligands. Anal Biochem. 2009 Jan;384(2):312–320. doi: 10.1016/j.ab.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 54.Colon W, Lai Z, McCutchen SL, Miroy GJ, Strang C, Kelly JW. FAP mutations destabilize transthyretin facilitating conformational changes required for amyloid formation. Ciba Found Symp. 1996:199228–238. doi: 10.1002/9780470514924.ch14. [DOI] [PubMed] [Google Scholar]
- 55.Janmey PA, Lamb JA, Ezzell RM, Hvidt S, Lind SE. Effects of actin filaments on fibrin clot structure and lysis. Blood. 1992 Aug;80(4):928–936. [PubMed] [Google Scholar]
- 56.Perez-Segura P, Zamorano-Leon JJ, Acosta D, Santos-Sancho JM, Modrego J, Caldes T, de la Hoya M, Diaz-Rubio E, Diaz-Millan I, de Las HN, et al. BRCA2 gene mutations and coagulation-associated biomarkers. Thromb Haemost. 2016 Jan;115(2):415–423. doi: 10.1160/TH15-06-0520. [DOI] [PubMed] [Google Scholar]
- 57.Bielli P, Calabrese L. Structure to function relationships in ceruloplasmin: a ‘moonlighting’ protein. Cell Mol Life Sci. 2002 Sep;59(9):1413–1427. doi: 10.1007/s00018-002-8519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.