Abstract
Rationale
Circulating progenitor cells (CPCs) mobilize in response to ischemic injury, but their predictive value remains unknown in acute coronary syndrome (ACS).
Objective
We aimed to investigate the number of CPCs in ACS compared to those with stable coronary artery disease (CAD), relationship between bone marrow PCs and CPCs, and whether CPC counts predict mortality in patients with ACS.
Methods and Results
In 2028 patients, 346 had unstable angina, 183 had an acute myocardial infarction (AMI) and the remaining 1499 patients had stable CAD. Patients with ACS were followed for the primary end point of all-cause death. CPCs were enumerated by flow cytometry as mononuclear cells expressing a combination of CD34+, CD133+, VEGFR2+, or CXCR4+. CPC counts were higher in subjects with AMI compared those with stable CAD even after adjustment for age, sex, race, body mass index, renal function, hypertension, diabetes, hyperlipidemia, and smoking; CD34+, CD34+/CD133+, CD34+/CXCR4+ and CD34+/VEGFR2+ CPC counts were 19%, 25%, 28%, and 142% higher in those with AMI, respectively, compared to stable CAD. There were strong correlations between the concentrations of CPCs and the PC counts in bone marrow aspirates in 20 patients with AMI. During a 2 (IQR 1.31–2.86)-year follow-up period of 529 ACS patients, 12.4% died. In Cox regression models adjusted for age, gender, body mass index, heart failure history, estimated GFR, and AMI, subjects with low CD34+ cell counts had a 2.46-fold (95% CI 1.18–5.13) increase in all-cause mortality, P=0.01. CD34+/CD133+ and CD34+/CXCR4+, but not CD34+/VEGFR2+ PC counts had similar associations with mortality. Results were validated in a separate cohort of 238 patients with ACS.
Conclusions
CPC levels are significantly higher in patients after an AMI compared to those with stable CAD and reflect bone marrow PC content. Among patients with ACS, a lower number of hematopoietic-enriched CPCs are associated with a higher mortality.
Keywords: Acute coronary syndrome, CD34+, CD133+, VEGFR2+, CXCR4+, myocardial infarction, ST-elevation myocardial infarction, non-ST-elevation myocardial infarction, cardiovascular outcomes, progenitor cell, prognosis, outcome
INTRODUCTION
Acute coronary syndromes (ACS) are unpredictable events that occur in patients with coronary artery disease (CAD) as a result of intravascular thrombosis on an acutely disrupted atherosclerotic plaque. There is extensive experimental evidence to demonstrate that circulating progenitor cells (CPCs) and resident stem cells contribute to ventricular recovery and remodeling following an acute ischemic event1, 2. CPCs are mononuclear cells, largely derived from the bone marrow that can be quantified in the peripheral blood using flow cytometry. CD34-expressing mononuclear cells have the potential to differentiate into diverse phenotypes including hematopoietic, endothelial, and non-hematopoietic (mesenchymal, lacking CD45 expression) lineages and participate in vascular and myocardial regeneration3–8. CD133 is a 5-transmembrane antigen of primitive stem cells that is lost during maturation and dual expression of these markers (CD34+/CD133+) identifies a PC-enriched subpopulation9, 10. Co-expression of vascular endothelial growth factor receptor-2 (VEGFR2) appears to identify a rarer subpopulation of CPCs further enriched for endothelial progenitors11, 12. Lastly, co-expression of the chemokine (C-X-C Motif) receptor 4 (CXCR4) that promotes homing of CPCs to stromal-derived factor-rich hypoxic environments, characterize CD34+ CPCs with capacity for tissue repair13. We have previously demonstrated that the number of CPCs are decreased with aging and in women and are predictive of adverse outcomes in patients with CAD, peripheral artery disease and heart failure14–18.
Acute myocardial infarction (AMI) is accompanied by a transient increase in the numbers of CPCs19–21, attributed largely to the increased production of chemo-attractants such as vascular endothelial growth factor (VEGF) in the ischemic myocardium that stimulates PC mobilization from the bone marrow into the circulation in order to effect neovascularization and repair22, 23. A previous small study found a significant correlation between CPCs at one year post-AMI and left ventricular remodeling, suggesting that a robust pool of CPCs is necessary for adequate repair24. Whether the magnitude of CPC response predicts longer term outcomes in AMI remains unknown. We aimed to investigate (1) the numbers of CPCs in subjects with ACS in comparison to those with stable CAD, (2) the relationship between bone marrow PCs and CPCs in AMI, and (3) whether CPC counts predict clinical outcomes. Our hypothesis was that CPC counts will be significantly higher in patients with AMI compared to those with stable CAD and lower levels will be associated with adverse long0term outcomes.
METHODS
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure
Study design
We identified 1446 adults from the Emory Cardiovascular Biobank, a prospective registry of adult patients undergoing cardiac catheterization for suspected or confirmed CAD, and 582 subjects from the MIPS (Mental Stress Ischemia Prognosis Study), a prospective study that recruited patients with stable CAD between June 2011 and August 2014, as previously described25, 26. All subjects had angiographic evidence of CAD. We excluded patients with history of cardiac transplantation on immunosuppressive agents. CPC counts were measured from peripheral blood samples at the time of enrollment at the time of the angiogram which is often within <48 hours of presentation. Demographic characteristics, medical history, medication use, and behavioral habits were documented as previously described25, 26. Both studies were approved by the Institutional Review Board at Emory University (Atlanta, GA). All subjects provided written informed consent.
ACS was diagnosed according to the ACC/AHA guidelines; unstable angina (UA) was defined as worsening of angina class or ischemic chest pain at rest of >30 min, necessitating hospital admission with either ST-segment deviation of at least 0.1 mV or new T-wave inversion in two contiguous leads without creatine kinase or troponin rise. AMI was defined by the presence of self-reported chest pain and evidence of cardiac ischemia on 12-lead EKG or elevated cardiac enzymes greater than twice the upper limit of normal with angiographic findings consistent with acute MI diagnosis. In addition, patients with AMI were classified into ST-elevation MI (STEMI) or Non-ST elevation MI (NSTEMI) based on EKG finding (ST elevation of 0.1 mV or more in at least 2 contiguous leads for STEMI and ischemic changes like new ST-segment deviation or T-wave inversion for NSTEMI).
Validation cohort
To validate the association between CPCS and mortality, we performed a separate analysis in an independent cohort of 238 subjects with ACS enrolled between 2006 and 2008 at Emory University affiliated hospitals (Online Table I). Both cohorts were enrolled under the same protocol, with identical sampling strategies and collection methods but separated in time and by a modified cell quantification methodology.
Progenitor cells assays
Peripheral blood was collected in EDTA tubes and incubated with fluorochrome-labeled monoclonal antihuman mouse antibodies within 4 hours. Cell populations enriched for PCs were enumerated using flow cytometry as CD45dim cells co-expressing hematopoietic-enriched markers (CD34+, CD133+, or CXCR4+) and endothelial-enriched subsets (VEGFR2+). We incubated 300 µL of peripheral blood with 7 µL of FITC-CD34 (BD Biosciences), PerCP-CD45 (BD Biosciences), PE-VEGFR2 (R&D system) and 5 µL APC-CD133 (Miltenyi), and 3ul PE-Cy7-conjugated anti-CXCR4 (EBioscience, clone 12G5) in the dark for 15 minutes. Then 1.5 mL ammonium chloride lysing buffer was added to lyse red blood cells. 1.5 mL staining medium (PBS with 3% heat-inactivated serum and 0.1% sodium azide) was added to stop the lysing reaction. Prior to flow cytometry, 100 µL of AccuCheck Counting Beads (Invitrogen, Cazt#: PCB100) were added to act as an internal standard for direct estimation of the concentration of target cell subsets. At least 2.5 million events were acquired from the cytometer. Flow data were analyzed with Flowjo software (Treestar, Inc.). Absolute mononuclear cell count was estimated as the sum of lymphocytes and monocytes using a Coulter ACT/Diff cell counter (Beckman Coulter). CPC populations are reported as cell counts/mL. In 20 samples that were repeatedly analyzed on two occasions by the same technician, the coefficients of variation of the cell types were: CD34+ 2.9%; CD34+/CD133+ 4.8%; CD34+/CXCR4+ 6.5% and CD34+/CD133+/CXCR4+ 7.5%, CD34+/VEGFR2+ cells 21.6%. There were significant correlations between the CPC subtypes, with moderate-strong correlations between CD34+, CD34+/CD133+, CD34+/CXCR4+ (r range 0.82–0.91, P<0.001), and weak correlations (r range 0.08–0.29, P<0.001) between CD34+/VEGFR2+ subtypes and the aforementioned CPCs.
In the validation cohort, similar methods were used to enumerate CPCs but without the addition PE-Cy7-conjugated anti-CXCR4 or AccuCheck Counting Beads.
Progenitor cells assays in the bone marrow aspirate
To compare PC counts in the bone marrow with CPCs, we studied 20 subjects enrolled in the PreSERVE-AMI study27, a Phase II randomized double-blind placebo-controlled trial (ClinicalTrials.gov identifier: NCT01495364) that enrolled patients with an acute STEMI and ejection fraction≤48%. Between 4 and 9 days post STEMI, we simultaneously obtained bone marrow aspirates and peripheral blood to investigate the relationship between PC subsets in the bone marrow and circulation.
Follow-up and outcomes
Patients were followed for determination of the primary outcome of all-cause death and the secondary outcomes of cardiovascular death. Cardiovascular death was defined as death attributable to an ischemic cardiovascular cause including fatal MI, ischemic stroke or sudden death secondary to presumed cardiovascular cause. Follow-up data was obtained by phone contact, electronic medical record review, and data from the social security death index and state records. Adjudication was conducted by personnel blinded to the data. One-year follow-up data was available for 95% of patients with ACS.
Statistical analysis
Subject characteristics were reported as descriptive statistics with means, medians, standard deviations and ranges. Differences between groups were assessed using the t-test for continuous variables, and chi-square or Fischer exact tests for categorical variables where appropriate. Spearman rank correlation coefficients were used for examining associations between CPCs and continuous variables. Two-sided P-value < 0.05 were considered statistically significant. For non-normally distributed variables such as CPCs counts, Mann-Whitney U test was used to compare groups in unadjusted analyses. For multivariable analyses, CPC counts were examined as continuous variables after log-transformation to achieve normality. Using the discovery cohort, we identified an optimal cut-off value using receiver operating characteristic (ROC) analyses and Youden's index (Sensitivity + Specificity −1) and applied this threshold (as a percentile equivalent of the absolute value) in the validation cohort (Online Figure I). The following cutoffs were used: 2200 cells/ml for CD34+ cells (67th percentile) and 1188 cells/ ml for CD34+/CD133+ cells (70th percentile). Characteristics incorporated in multivariable analyses included variables that correlated in bivariate analysis with the outcome studied. In a separate analysis, we also used stepwise backwards regression to reach a final set of variables for each outcome with P<0.1 as the threshold to retain a variable and obtained similar results. The association between CPC counts and outcomes was examined in Kaplan-Meier and Cox regression models. For outcomes including all-cause death and cardiovascular death, we used competing risk analysis by treating non-cardiovascular death as competing risk event. Analyses were performed using IBM SPSS Statistics Version 22, (Armonk, NY, USA).
RESULTS
Out of 2028 patients enrolled in this study, 346 patients had UA, 160 had NSTEMI, 23 had STEMI and the remaining 1499 patients had stable CAD. Baseline characteristics of the cohort aged 65±12 years are included in Table 1.
Table 1.
Baseline characteristics among subjects with CAD groups
| Stable CAD (n=1499) | Unstable Angina (n=346) | Non-ST elevation Myocardial Infarction (n=160) | ST-elevation Myocardial Infarction (n=23) | P-Value* | |
|---|---|---|---|---|---|
| Age, years mean (SD) | 65.2 (11.6) | 65.5 (12) | 66.7 (11.6) | 60.8 (11.4) | 0.09 |
| Male n (%) | 1040 (69.38) | 203 (58.67) | 97 (60.63) | 17 (73.91) | <0.001 |
| Black n (%) | 353 (23.55) | 62 (17.92) | 51 (31.88) | 8 (34.78) | 0.003 |
| Body Mass Index kg/m2 mean (SD) | 29.3 (6) | 29.9 (6) | 29.6 (6.4) | 29.6 (3.9) | 0.23 |
| Estimated GFR mL/min/1.73 m2 mean (SD) | 71.1 (24.8) | 72.3 (23.8) | 68.2 (25) | 80.5 (19.9) | 0.18 |
| Smoking n (%) | 962 (64.26) | 237 (68.5) | 106 (66.25) | 12 (52.17) | 0.26 |
| Diabetes n (%) | 577 (38.57) | 161 (46.67) | 75 (47.47) | 5 (22.73) | 0.003 |
| Hypertension n (%) | 1265 (84.5) | 325 (93.93) | 150 (93.75) | 19 (86.36) | <0.001 |
| Hypercholesterolemia n (%) | 1182 (78.91) | 285 (82.37) | 126 (80.25) | 20 (90.91) | 0.27 |
| History of heart failure n (%) | 401 (26.75) | 125 (36.13) | 68 (42.5) | 12 (52.17) | <0.001 |
| Ejection fraction % mean (SD) | 52.4 (13.4) | 52.9 (12.6) | 47.6 (14.6) | 42.4 (13.8) | <0.001 |
| History of stroke n (%) | 106 (11.83) | 43 (12.5) | 29 (18.35) | 2 (9.09) | 0.14 |
| History of myocardial infarction n (%) | 405 (27.09) | 100 (29.07) | 88 (63.77) | 17 (80.95) | <0.001 |
| History of PCI n (%) | 741 (49.43) | 201 (58.09) | 93 (58.13) | 15 (65.22) | 0.004 |
| History of CABG n (%) | 440 (29.35) | 96 (27.75) | 48 (30) | 4 (17.39) | 0.58 |
| ACE/ARB use n (%) | 481 (52.45) | 208 (60.12) | 105 (65.63) | 15 (65.22) | 0.003 |
| Aspirin use n (%) | 1234 (82.38) | 303 (87.57) | 145 (90.63) | 21 (91.3) | 0.006 |
| Clopidogrel use n (%) | 561 (37.45) | 187 (54.05) | 104 (65) | 13 (56.52) | <0.001 |
| Statin use n (%) | 1150 (76.87) | 277 (80.06) | 130 (81.25) | 18 (78.26) | 0.41 |
| Beta blocker use n (%) | 1118 (74.63) | 285 (82.37) | 147 (91.88) | 21 (91.3) | <0.001 |
|
| |||||
| CD34+ cell/mL median (IQR) | 1624.97 (1032.96–2447.25) | 1574.93 (1039.89–2466.99) | 1887.68 (1248.71–2810.31) | 1890.62 (1178.71–3087.85) | 0.007 |
| CD34+/CD133+ cell/mL median (IQR) | 746.29 (445.71–1164.71) | 746.54 (467.53–1226.07) | 953.34 (579.09–1418.94) | 853.11 (304.2–1900.51) | 0.001 |
| CD34+/VEGF2R+ cell/mL median (IQR) | 20.02 (0–80.79) | 20.5 (7.58–71.87) | 29.82 (8.52–129.27) | 108.74 (8.46–260.07) | 0.001 |
| CD34+/CXCR4+ cell/mL median (IQR) | 760.68 (448.17–1246.08) | 722.4 (422.47–1258.69) | 963.48 (646.48–1666.94) | 914.52 (616.19–1377.87) | <0.001 |
P value for overall comparison between stable CAD vs. unstable angina vs. non-ST elevation myocardial infarction vs. ST-elevation myocardial infarction.
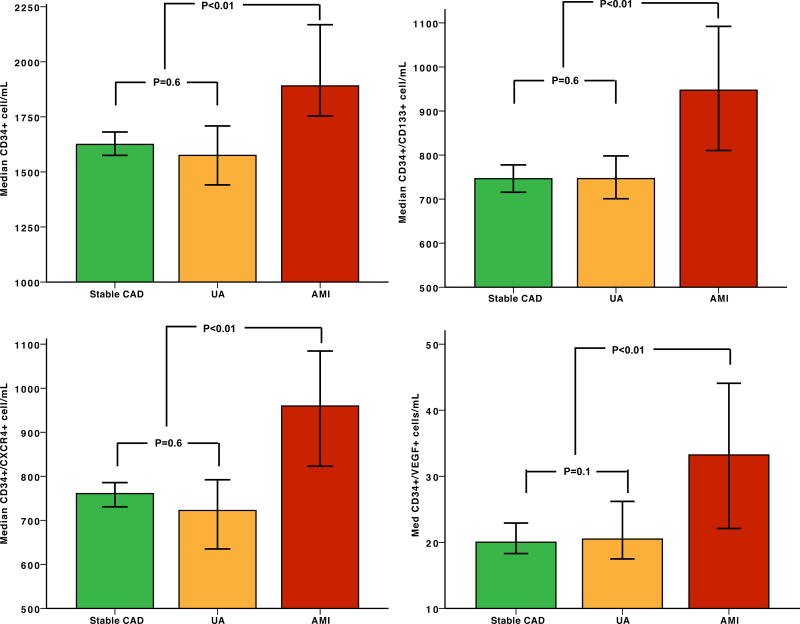
Progenitor cells and ACS
CPC counts were not significantly different in those with UA compared to stable CAD. Compared to subjects with stable CAD or UA, all subsets of CPC counts were significantly higher in those with AMI (Figure 1). All CPC counts were similar in those with NSTEMI and STEMI, except for CD34+/VEGFR2+ PCs that were higher in patients with STEMI (Table 1). After adjustment for clinical characteristics including age, sex, race, body mass index, renal function, hypertension, diabetes, hyperlipidemia, and smoking, presence of AMI was independently associated with a 19%, 25%, 28%, and 142% higher CD34+, CD34+/CD133+, CD34+/CXCR4+ and CD34+/VEGFR2+ CPC counts, respectively (Table 2). These associations remained significant after adjustment for mononuclear cell count such that AMI was associated with 20%, 22%, 30%, and 189% higher CD34+, CD34+/CD133+, CD34+/CXCR4+ and CD34+/VEGFR2+ CPC counts
Figure 1.
Boxplots demonstrating the number of circulating progenitor cells (cell/mL) in patients with stable CAD, unstable angina (UA), and acute MI (AMI).
Table 2.
Linear Regression Model for Predictors of CPC Subsets
| Variable | CD34+ | CD34+/CD133+ | CD34+/CXCR4+ | CD34+/VEGF+ | ||||
|---|---|---|---|---|---|---|---|---|
| % Change (95% CI) | P Value | % Change (95% CI) | P Value | % Change (95% CI) | P Value | % Change (95% CI) | P Value | |
| Age (per 10-y increase) | −9.8 (−12.3 to −7.1) | <0.001 | −11.9 (−14.7 to −9.1) | <0.001 | −8.2 (−11.2 to −5.2) | <0.001 | −0.5 (−11.7 to 12.1) | 0.94 |
| Body mass index (per 5-U increase) | 6.1 (3.4 to 8.9) | <0.001 | 8 (4.9 to 11.2) | <0.001 | 3.6 (0.5 to 6.7) | 0.02 | −13 (−22 to −3.1) | 0.01 |
| GFR (per 10-U increase) | 0.1 (−1.1 to 1.4) | 0.85 | −0.5 (−1.9 to 0.9) | 0.50 | 0 (−1.4 to 1.5) | 0.95 | −6.9 (−11.8 to −1.7) | 0.01 |
| Male | 12.2 (5.2 to 19.5) | <0.001 | 9.3 (1.7 to 17.5) | 0.02 | 11.4 (3.5 to 20) | 0.004 | 3.3 (−21.1 to 35) | 0.82 |
| Black | −11.7 (−18 to −5) | 0.001 | −9.1 (−16.3 to −1.1) | 0.03 | −6.9 (−14.5 to 1.4) | 0.10 | −15 (−37.7 to 16.1) | 0.31 |
| Hypertension | 5.4 (−3.9 to 15.7) | 0.26 | 3.8 (−6.6 to 15.3) | 0.49 | 3.8 (−6.9 to 15.6) | 0.50 | 82.2 (23.1 to 169.9) | 0.003 |
| Former or current smoking | 3.8 (−2.5 to 10.4) | 0.24 | 6.3 (−0.9 to 14) | 0.09 | 7.7 (0.2 to 15.7) | 0.04 | −11.6 (−31.9 to 14.9) | 0.36 |
| Diabetes mellitus | 2 (−4.2 to 8.7) | 0.54 | 4.5 (−2.7 to 12.2) | 0.23 | 4.1 (−3.2 to 12) | 0.28 | 3.5 (−20.6 to 34.7) | 0.80 |
| Hyperlipidemia | 3.7 (−3.9 to 11.9) | 0.35 | 3.8 (−4.8 to 13.1) | 0.40 | 6.2 (−2.8 to 16) | 0.18 | −9.1 (−34 to 25.4) | 0.56 |
| Acute myocardial infarction | 19.2 (7.5 to 32.2) | 0.001 | 24.6 (10.7 to 40.2) | <0.001 | 27.8 (13.2 to 44.2) | <0.001 | 141.6 (55.7 to274.3) | <0.001 |
CI indicates confidence interval; CXCR4, chemokine (C-X-C motif) receptor 4; GFR, glomerular filtration rate; and VEGFR, vascular endothelial growth factor receptor.
Predictors of progenitor cells in AMI
In subjects with AMI presentation, younger age was the only independent predictor of higher numbers of CD34+ and CD34+/CXCR4+ cells; for each decade of younger age, CD34+ counts were 11% higher (95%CI, 0.6%, 22.5%, P=0.04) and the CD34+/CXCR4+ counts 12.4% higher (95%CI 0.6, 25.6%, P=0.04). Estimated GFR was a predictor of CD34+/VEGFR2+ PC counts, such that a 10 mL/min/1.73 m2 higher GFR was associated with an 18.3% (95%CI 4.4%,34.0%, P=0.009) higher CD34+/VEGFR2+ PCs. CPCs did not correlate with the extent of AMI measured as peak troponin concentration (P>0.3).
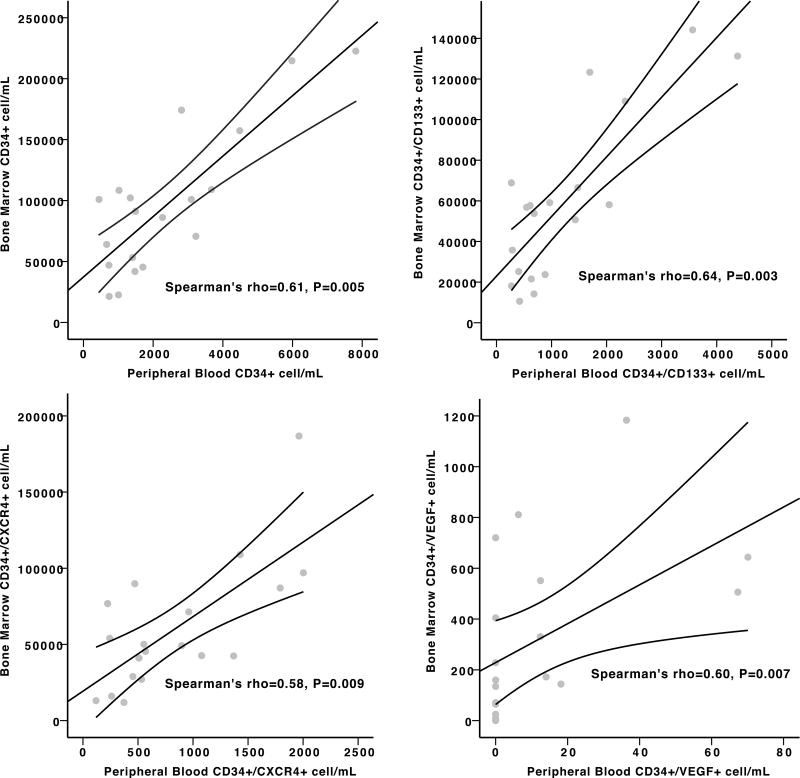
Correlation between peripheral and bone marrow progenitor cells post-AMI
In 20 subjects with STEMI enrolled in the PRESERVE-AMI trial, PC subsets were measured in the bone marrow and peripheral blood concomitantly. Figure 2 illustrates the moderate to strong positive correlations between the concentrations of CD34+, CD34+/CD133+, CD34+/CXCR4+, and CD34+/VEGFR2+ subsets in the bone marrow and the peripheral circulation.
Figure 2.
Scatterplots demonstrating the correlation between (A) CD34+ (B) CD34+/CD133+ (C) CD34+CXCR4+ (D) CD34+/VEGFR2+ progenitor cell subsets in the peripheral blood and bone marrow in patients post-STEMI (20 subjects).
Progenitor cell counts and Outcomes in ACS
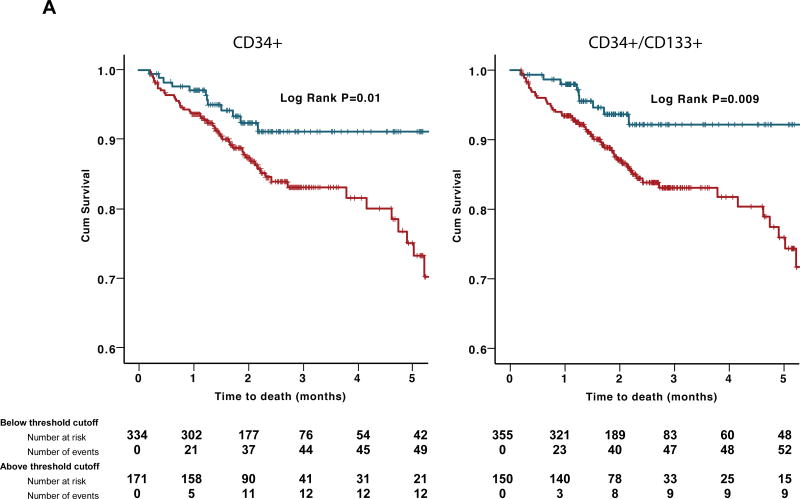
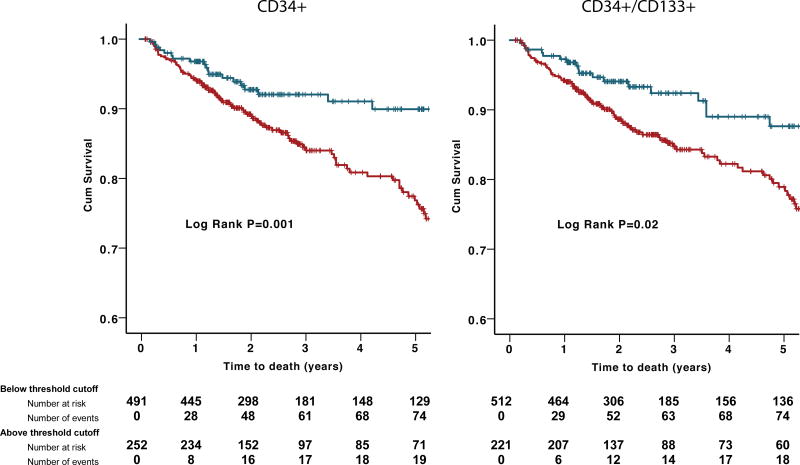
Among 529 patients presenting with an ACS, during a median follow-up period of 2.0 (IQR 1.3–2.9) years, there were 63 (12.4%) deaths from all causes, 41 (8.1%) of which were from cardiovascular causes. CPC were enumerated at the time of the angiogram. In univariate analyses, subjects with low hematopoietic-enriched CPC subsets (CD34+, CD34+/CD133+, and CD34+/CXCR4+) had higher all-cause mortality rates (Figure 3A). In Cox regression models, these results remained significant both as continuous and categorical variables after adjusting for clinical variables that correlated with CPC counts or the outcomes studied (age, gender, body mass index, heart failure history, estimated GFR, and AMI). Thus, subjects with CD34+ cell counts below vs. above the optimal cutoff identified using ROC curve had a 2.55-fold increase in the risk of death compared (95% CI 1.26–5.17, P=0.009). CD34+/CD133+ and CD34+/CXCR4+ cells had similar associations with all-cause mortality (Table 3). The results remained significant even after adjusting for peak troponin and ejection fraction values. However, no association was found between CD34+/VEGFR2+ cells and all-cause mortality. In sensitivity analyses, the predictive value of CPCs was not different in those presenting with UA vs. AMI, interaction P=0.73. Similarly, in an adjusted model, CD34+ and CD34+/CXCR4+ subsets were predictive of cardiovascular death (Online Table II). Thus, subjects with CD34+/CXCR4+ cell counts within the lowest tertile had a 2.61-fold increase (95%CI 1.05–6.47, P=0.04) in the risk of cardiovascular death compared to those in the highest tertile.
Figure 3.
(A)Kaplan–Meier curves for association between hematopoietic-enriched CPC subsets and all-cause death in discovery cohort. Blue lines represent subjects with CPCs above threshold cutoff and red lines represent those below threshold cutoff. (B) Kaplan–Meier curves for association between hematopoietic-enriched CPC subsets and all-cause death in validation cohort. Blue lines represent subjects with CPCs above threshold cutoff and red lines represent those below threshold cutoff.
Table 3.
Association between CPC counts and all-cause death in subjects with ACS.
| Discovery cohort (n=507) | |||||
|---|---|---|---|---|---|
|
| |||||
| Unadjusted HR (95% CI) |
P-Value | Adjusted HR (95% CI) |
P-Value | ||
| CD34+ | Log transformed | 1.41 (1.14–1.74) | 0.001 | 1.45 (1.13–1.85) | 0.003 |
| Threshold cut-off | 2.55 (1.26–5.17) | 0.009 | 2.46 (1.18–5.13) | 0.01 | |
|
| |||||
| CD34+/CD133+ | Log transformed | 1.23 (1.04–1.46) | 0.02 | 1.25 (1.03–1.53) | 0.02 |
| Threshold cut-off | 2.79 (1.33–5.86) | 0.01 | 2.59 (1.20–5.61) | 0.02 | |
|
| |||||
| CD34+/CXCR4+ | Log transformed | 1.33 (1.07–1.64) | 0.01 | 1.31 (1.04–1.66) | 0.02 |
| Threshold cut-off | 2.17 (1.11–4.23) | 0.02 | 2.09 (1.04–4.2) | 0.03 | |
|
| |||||
| CD34+/VEGF+ | Log transformed | 1.07 (0.97–1.18) | 0.16 | 1.06 (0.96–1.17) | 0.27 |
Adjusted for age, gender, body mass index, heart failure history, estimated GFR, and acute MI
Validation analysis
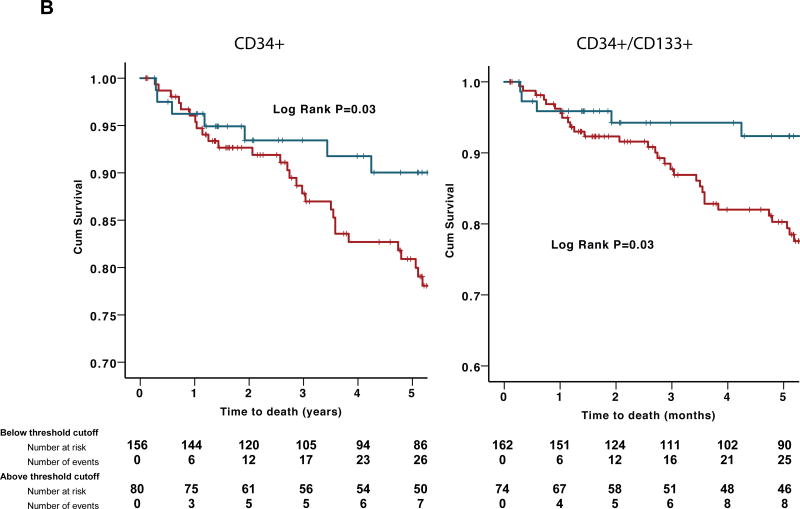
The associations between CPC subsets and mortality were validated in an independent cohort of 238 subjects with acute coronary syndrome (83% unstable angina, 15% NSTEMI and 2% STEMI). Over a median 5.3 (IQR 2.1–7.1) year follow-up period, 41 (17%) patients died. Baseline characteristics are shown in Online Table I. The results re-affirmed a similar association between low CPC counts analyzed as continuous variables and higher mortality (Table 4). Additionally, we applied the optimal cut-off values from the discovery cohort to the validation cohort and demonstrated a significant correlation between lower CPC counts and a higher mortality rate (Table 4, Figure 3B) after adjustment for the abovementioned clinical variables. The associations between CPCS and mortality in the pooled cohorts (745 subjects) are shown in Figure 4.
Table 4.
Association between CPC counts and all-cause death in subjects with ACS
| Validation cohort (n=238) | |||||
|---|---|---|---|---|---|
|
| |||||
| Unadjusted HR (95% CI) |
P-Value | Adjusted HR (95% CI) |
P-Value | ||
| CD34+ | Log transformed | 1.53 (1.16–2) | 0.002 | 1.63 (1.21–2.19) | 0.001 |
| Threshold cut-off | 2.24 (1.03–4.85) | 0.04 | 2.42 (1.1–5.3) | 0.03 | |
|
| |||||
| CD34+/CD133+ | Log transformed | 1.62 (1.24–2.13) | 0.0004 | 1.5 (1.13–1.98) | 0.005 |
| Threshold cut-off | 2.38 (1.05–5.37) | 0.04 | 2.51 (1.1–5.74) | 0.03 | |
|
| |||||
| CD34+/VEGF+ | Log transformed | 1 (0.87–1.14) | 0.94 | 0.95 (0.82–1.09) | 0.46 |
Adjusted for age, gender, body mass index, heart failure history, estimated GFR, and acute MI
Figure 4.
Kaplan–Meier curves for association between hematopoietic-enriched CPC subsets and all-cause death in the pooled cohort. Blue lines represent subjects with CPCs above threshold cutoff and red lines represent those below threshold cutoff.
DISCUSSION
Our study shows that CPC levels are significantly higher in patients after an AMI compared to those with stable CAD. In these patients, the numbers of CPCs closely reflect their content within the bone marrow. More importantly, our study is the first to demonstrate that among patients with ACS, a lower number of CPCs, particularly those enriched for hematopoietic PCs, is associated with a higher mortality. Our results were validated in two independent cohorts totaling over 700 subjects.
Our findings confirm previous data demonstrating an increased time-dependent mobilization of PCs to the peripheral circulation in those with AMI21, 24. Mobilization of PCs starts within a few minutes of an AMI, peaks after several days and normalizes within 60 days20. The myocellular necrosis triggers an acute inflammatory response with hypoxia-dependent upregulation of HIF-1α (Hypoxia-inducible factor 1-alpha), that in turn stimulates expression of stromal cell derived factor-1 alpha (SDF-1α) which constitutes a homing signal for recruitment of CPCs to ischemic tissues28, 29. This homing of CPCs to the areas of ischemia promotes local repair and regeneration30 by preventing apoptosis of cardiomyocytes, secretion of insulin growth factor-1, stimulation of angiogenesis by paracrine secretion of angiogenic growth factors, and recruitment of local stem cells31. HIF-1α activation also promotes synthesis and release of VEGF that circulates in higher concentrations and stimulates nitric oxide-dependent increase in matrix metallopeptidase 9 (MMP-9) in the bone marrow, that in turn leads to release of PCs from their bone marrow niches32. Variations in PC mobilization may also be due to differences in endogenous release of granulocyte-colony stimulating factor (G-CSF) that also promotes mobilization of CPCs from the bone marrow33. Combined, these processes contribute to PC mobilization after AMI and to ultimate repair and regeneration of cardiac and vascular tissue.
To date, it has been unclear whether low numbers of CPCs in the setting of AMI is a consequence of depletion of the PC pool in the bone marrow or due to impairment in the release of PCs from the bone marrow. One small study showed moderate correlation between peripheral blood and bone marrow CD34+CD133+ cell subsets.34 Another study demonstrated weak correlation between circulating CD34+/CD133+ PCs and functional capacity of bone marrow-derived mononuclear cells in patients with heart failure.31 Our data in patients with STEMI showed a robust correlation between bone marrow and peripheral PCs, suggesting that low CPC counts are largely due to exhaustion of PCs in the bone marrow. Reduced CPC counts may also be a marker of advanced illness due to several risk factors and advanced age leading to bone marrow suppression and cell senescence14, 35.
Although the higher number of CPCs in patients with AMI could be partly related to the physiologic increase in the number of white blood cells or mononuclear cells, the increase of CPCs remained significant after adjusting for the mononuclear cell count.
We found no differences in CPC levels between patients presenting with unstable angina compared to those with stable CAD suggesting that myocardial necrosis and not ischemia is the more potent stimulus for mobilization of PCs from the bone marrow. Our findings differ from a previous study that reported significantly higher CPC levels in unstable compared with stable angina patients36. However, that study included relatively small number of patients and used culture assays to quantify PCs, an assay that remains controversial as a measure of circulating PCs. We recently reported that exercise-induced myocardial ischemia was associated with a significant and transient decrease in CPC counts, likely due to SDF-1α –mediated homing to the ischemic myocardium37. As previously reported, we also did not observe a relationship between the infarct size, evaluated by peak troponin levels and CPC counts24. It is likely that cytokines such as VEGF and SDF-1α are better measures of PC mobilization, and as discussed above, the bone marrow reserve of PCs appears to be a major determinant of the magnitude of PC mobilization observed in AMI.
In our study, age was the only predictor of CPC levels following AMI. Several studies have reported a negative impact of advanced age on PC mobilization in response to AMI or cytokine administration14, 38, 39. Additionally, experimental studies in mice models showed that the protective effect of the bone marrow mononuclear cells against atherosclerosis is diminished in advanced compared to young age40.
In spite of the growing number of early-phase clinical trials demonstrating the beneficial effects of intracoronary administration of PCs after AMI, to date, little is known about the importance of the physiologic release of PCs after AMI and its impact on survival41, 42. Adverse myocardial remodeling after AMI is largely unpredictable, but is associated with an unfavorable prognosis1. In rodents, improvement in adverse myocardial remodeling and survival post-AMI was observed after administration of bone-marrow derived PCs24, 43, 44. Whereas one small clinical study found an association between CD34+/CD133+ PCs and preservation of left ventricular function after an anterior AMI, this was not supported by another study45, 46. The angiogenic capacity of CPCs was reported in one study to correlate with myocardial recovery47. One pooled analysis from 3 clinical trials showed that increased bone marrow CD34+ percentage following AMI correlated with a greater improvement in left ventricular function34. In one of the largest study to date, herein we demonstrate a strong and independently predictive value of CPC counts in patients presenting with an ACS, suggesting that the mobilization of CPCs plays a critical role in remodeling, preventing LV dilatation and promoting functional recovery.
Another interesting finding from our study is that hematopoietic but not endothelial-enriched CPC counts were predictive of adverse outcomes. In experimental animals, endothelial-enriched CPCs were shown to promote neoangiogenesis and hematopoietic-enriched CPCs contributed to myocardial regeneration48. We have previously shown no association between endothelial-enriched CPC subset and cardiovascular outcomes in patients with stable CAD, which could be in part because of their very low frequency and poor reproducibility of measurement compared to the more frequent hematopoietic-enriched CPC subsets.49
Strengths of our study include the large numbers investigated, long-term follow up, and the detailed exploration of PC phenotypes in various ACS subsets. Limitations include lack of functional PC assays and single sampling at varying time points after the AMI that depended on when patients’ underwent cardiac catheterization. However, previous studies have clearly demonstrated the time course of CPC elevation after AMI to last several days.
CONCLUSION
Our study demonstrates for the first time that CPC counts are higher in patients after an AMI compared to those with unstable angina or stable CAD. Greater CPC counts are a reflection of a similarly higher PC number in the bone marrow, and is associated with lower mortality in patients with an ACS, suggesting the potential role of CPCs in myocardial repair processes. Our results contribute to a better understanding of the nature and potential consequences of the endogenous regenerative responses after AMI and will likely stimulate development of strategies for novel cell-based therapies, especially in those with a poor capacity for PC mobilization.
NOVELTY AND SIGNIFICANCE.
What Is Known?
Progenitor cells are mobilized from the bone marrow into the circulation in response to tissue injury and contribute to repair and regeneration.
Low levels of circulating progenitor cells are linked to adverse cardiovascular outcomes in patients with stable coronary artery disease. Whether impaired mobilization of progenitor cells during an acute coronary syndrome is associated with worse long-term prognosis is unknown.
Autologous bone marrow derived CD34+ progenitor cell therapies after myocardial infarction have been associated with improved outcomes in early clinical trials.
What New Information Does This Article Contribute?
Acute myocardial infarction, but not unstable angina is associated with mobilization of progenitor cells.
The numbers of progenitor cells mobilized are proportional to their bone marrow content.
Low circulating progenitor cell counts, reflective of reduced regenerative capacity, are predictive of higher mortality in individuals presenting with an acute coronary syndrome.
Progenitor cells are mobilized from the bone marrow into the circulation after tissue injury. Compared to patients with stable coronary artery disease, those with an acute myocardial infarction had significantly higher circulating levels of hematopoietic and endothelial progenitor cells. Circulating progenitor cell counts closely reflected their counts in the bone marrow. Decreased regenerative capacity, measured as lower levels of circulating progenitor cells is an independent predictor of higher mortality and recurrent myocardial infarction rate. Progenitor cell counts thus identify high-risk individuals after myocardial infarction. Novel therapies aimed at enhancing progenitor cell activity, including cell therapies may be beneficial in patients with impaired endogenous regenerative capacity.
Acknowledgments
SOURCES OF FUNDING
AAQ is supported by 5P01HL101398-02, 1P20HL113451-01, 1R56HL126558-01, 1RF1AG051633-01, R01 NS064162-01, R01 HL89650-01, HL095479-01, 1U10HL110302-01, 1DP3DK094346-01, 2P01HL086773-06A1. AST is supported by the Abraham J. & Phyllis Katz Foundation (Atlanta, GA) and NIH/NIA grant AG051633.
We also acknowledge Caladrius, sponsors of PreSERVE-AMI study that enabled bone marrow sampling from patients enrolled in the trial.
Nonstandard Abbreviations and Acronyms
- CPCs
Circulating progenitor cells
- CAD
Coronary artery disease
- ACS
Acute coronary syndrome
- AMI
Acute myocardial infarction
- VEGF
Vascular endothelial growth factor
- CXCR4
Chemokine (C-X-C Motif) receptor 4
- UA
Unstable angina
- STEMI
ST-elevation myocardial infarction
- NSTEMI
Non-ST elevation myocardial infarction
Footnotes
DISCLOSURES
None of the authors have conflicts of interest to disclose.
References
- 1.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 2.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Bone marrow stem cells regenerate infarcted myocardium. Pediatr Transplant. 2003;7(Suppl 3):86–88. doi: 10.1034/j.1399-3046.7.s3.13.x. [DOI] [PubMed] [Google Scholar]
- 3.Hayek SS, MacNamara J, Tahhan AS, Awad M, Yadalam A, Ko YA, Healy S, Ghaini I, Ahmed H, Gray B, Sher S, Ghasemzadeh N, Patel RS, Kim J, Waller EK, Quyyumi AA. Circulating progenitor cells identify peripheral arterial disease in patients with coronary artery disease. Circulation research. 2016 doi: 10.1161/CIRCRESAHA.116.308802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circulation research. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 5.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. The Journal of clinical investigation. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 7.Urbich C, Dimmeler S. Endothelial progenitor cells: Characterization and role in vascular biology. Circulation research. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 8.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. Cd34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 9.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from ac133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 10.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. Ac133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 11.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circulation research. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alaiti MA, Ishikawa M, Costa MA. Bone marrow and circulating stem/progenitor cells for regenerative cardiovascular therapy. Transl Res. 2010;156:112–129. doi: 10.1016/j.trsl.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Seeger FH, Rasper T, Koyanagi M, Fox H, Zeiher AM, Dimmeler S. Cxcr4 expression determines functional activity of bone marrow-derived mononuclear cells for therapeutic neovascularization in acute ischemia. Arterioscler Thromb Vasc Biol. 2009;29:1802–1809. doi: 10.1161/ATVBAHA.109.194688. [DOI] [PubMed] [Google Scholar]
- 14.Al Mheid I, Hayek SS, Ko YA, Akbik F, Li Q, Ghasemzadeh N, Martin GS, Long Q, Hammadah M, Maziar Zafari A, Vaccarino V, Waller EK, Quyyumi AA. Age and human regenerative capacity impact of cardiovascular risk factors. Circ Res. 2016;119:801–809. doi: 10.1161/CIRCRESAHA.116.308461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayek SS, MacNamara J, Tahhan AS, Awad M, Yadalam A, Ko YA, Healy S, Hesaroieh I, Ahmed H, Gray B, Sher SS, Ghasemzadeh N, Patel R, Kim J, Waller EK, Quyyumi AA. Circulating progenitor cells identify peripheral arterial disease in patients with coronary artery disease. Circ Res. 2016;119:564–571. doi: 10.1161/CIRCRESAHA.116.308802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samman Tahhan A, Hammadah M, Sandesara PB, Hayek SS, Kalogeropoulos AP, Alkhoder A, Mohamed Kelli H, Topel M, Ghasemzadeh N, Chivukula K, Ko YA, Aida H, Hesaroieh I, Mahar E, Kim JH, Wilson P, Shaw L, Vaccarino V, Waller EK, Quyyumi AA. Progenitor cells and clinical outcomes in patients with heart failure. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.117.004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topel ML, Hayek SS, Ko YA, Sandesara PB, Samman Tahhan A, Hesaroieh I, Mahar E, Martin GS, Waller EK, Quyyumi AA. Sex differences in circulating progenitor cells. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Abdelhadi N, Alkhoder A, Obideen M, Pimple PM, Levantsevych O, Kelli HM, Shah A, Sun YV, Pearce B, Kutner M, Long Q, Ward L, Ko YA, Hosny Mohammed K, Lin J, Zhao J, Bremner JD, Kim J, Waller EK, Raggi P, Sheps D, Quyyumi AA, Vaccarino V. Telomere shortening, regenerative capacity, and cardiovascular outcomes. Circ Res. 2017;120:1130–1138. doi: 10.1161/CIRCRESAHA.116.309421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaspardone A, Menghini F, Mazzuca V, Skossyreva O, Barbato G, de Fabritiis P. Progenitor cell mobilisation in patients with acute and chronic coronary artery disease. Heart. 2006;92:253–254. doi: 10.1136/hrt.2004.058636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R, De Ferrari GM, Ferlini M, Goffredo L, Bertoletti A, Klersy C, Pecci A, Moratti R, Tavazzi L. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105:199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 21.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 22.Fortunato O, Spinetti G, Specchia C, Cangiano E, Valgimigli M, Madeddu P. Migratory activity of circulating progenitor cells and serum sdf-1alpha predict adverse events in patients with myocardial infarction. Cardiovasc Res. 2013;100:192–200. doi: 10.1093/cvr/cvt153. [DOI] [PubMed] [Google Scholar]
- 23.Wojakowski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslankiewicz K, Wyderka R, Ochala A, Ratajczak MZ. Mobilization of cd34/cxcr4+, cd34/cd117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–3220. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 24.Leone AM, Rutella S, Bonanno G, Abbate A, Rebuzzi AG, Giovannini S, Lombardi M, Galiuto L, Liuzzo G, Andreotti F, Lanza GA, Contemi AM, Leone G, Crea F. Mobilization of bone marrow-derived stem cells after myocardial infarction and left ventricular function. Eur Heart J. 2005;26:1196–1204. doi: 10.1093/eurheartj/ehi164. [DOI] [PubMed] [Google Scholar]
- 25.Patel RS, Li Q, Ghasemzadeh N, Eapen DJ, Moss LD, Janjua AU, Manocha P, Al Kassem H, Veledar E, Samady H, Taylor WR, Zafari AM, Sperling L, Vaccarino V, Waller EK, Quyyumi AA. Circulating cd34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circulation research. 2015;116:289–297. doi: 10.1161/CIRCRESAHA.116.304187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Shah AJ, Sun Y, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Raggi P, Sheps DS, Vaccarino V, Quyyumi AA. The mental stress ischemia prognosis study: Objectives, study design, and prevalence of inducible ischemia. Psychosom Med. 2017;79:311–317. doi: 10.1097/PSY.0000000000000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quyyumi AA, Vasquez A, Kereiakes DJ, Klapholz M, Schaer GL, Abdel-Latif A, Frohwein S, Henry TD, Schatz RA, Dib N, Toma C, Davidson CJ, Barsness GW, Shavelle DM, Cohen M, Poole J, Moss T, Hyde P, Kanakaraj AM, Druker V, Chung A, Junge C, Preti RA, Smith RL, Mazzo DJ, Pecora A, Losordo DW. Preserve-ami: A randomized, double-blind, placebo-controlled clinical trial of intracoronary administration of autologous cd34+ cells in patients with left ventricular dysfunction post stemi. Circ Res. 2017;120:324–331. doi: 10.1161/CIRCRESAHA.115.308165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through hif-1 induction of sdf-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 29.Ceradini DJ, Gurtner GC. Homing to hypoxia: Hif-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc Med. 2005;15:57–63. doi: 10.1016/j.tcm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 31.Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer-Rasokat U, Heeschen C, Spyridopoulos I, Dimmeler S, Zeiher AM. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J Am Coll Cardiol. 2007;49:2341–2349. doi: 10.1016/j.jacc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 32.Ling L, Shen Y, Wang K, Jiang C, Fang C, Ferro A, Kang L, Xu B. Worse clinical outcomes in acute myocardial infarction patients with type 2 diabetes mellitus: Relevance to impaired endothelial progenitor cells mobilization. PLoS One. 2012;7:e50739. doi: 10.1371/journal.pone.0050739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leone AM, Rutella S, Bonanno G, Contemi AM, de Ritis DG, Giannico MB, Rebuzzi AG, Leone G, Crea F. Endogenous g-csf and cd34+ cell mobilization after acute myocardial infarction. Int J Cardiol. 2006;111:202–208. doi: 10.1016/j.ijcard.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 34.Cogle CR, Wise E, Meacham AM, Zierold C, Traverse JH, Henry TD, Perin EC, Willerson JT, Ellis SG, Carlson M, Zhao DX, Bolli R, Cooke JP, Anwaruddin S, Bhatnagar A, da Graca Cabreira-Hansen M, Grant MB, Lai D, Moye L, Ebert RF, Olson RE, Sayre SL, Schulman IH, Bosse RC, Scott EW, Simari RD, Pepine CJ, Taylor DA. Cardiovascular Cell Therapy Research N. Detailed analysis of bone marrow from patients with ischemic heart disease and left ventricular dysfunction: Bm cd34, cd11b, and clonogenic capacity as biomarkers for clinical outcomes. Circ Res. 2014;115:867–874. doi: 10.1161/CIRCRESAHA.115.304353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49:741–752. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 36.George J, Goldstein E, Abashidze S, Deutsch V, Shmilovich H, Finkelstein A, Herz I, Miller H, Keren G. Circulating endothelial progenitor cells in patients with unstable angina: Association with systemic inflammation. Eur Heart J. 2004;25:1003–1008. doi: 10.1016/j.ehj.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 37.Hammadah M, Tahhan AS, Al Mheid I, Wilmot K, Ramadan R, Kindya BR, Kelli HM, O'Neal WT, Sandesara P, Sullivan S. Myocardial ischemia and mobilization of circulating progenitor cells. Journal of the American Heart Association. 2018;7:e007504. doi: 10.1161/JAHA.117.007504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 39.Li TS, Kubo M, Ueda K, Murakami M, Mikamo A, Hamano K. Impaired angiogenic potency of bone marrow cells from patients with advanced age, anemia, and renal failure. J Thorac Cardiovasc Surg. 2010;139:459–465. doi: 10.1016/j.jtcvs.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 40.Zhu S, Liu X, Li Y, Goldschmidt-Clermont PJ, Dong C. Aging in the atherosclerosis milieu may accelerate the consumption of bone marrow endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2007;27:113–119. doi: 10.1161/01.ATV.0000252035.12881.d0. [DOI] [PubMed] [Google Scholar]
- 41.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of vegfr-2 and ac133 by circulating human cd34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 42.Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S. Vascular trauma induces rapid but transient mobilization of vegfr2(+)ac133(+) endothelial precursor cells. Circ Res. 2001;88:167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 43.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 45.Kuliczkowski W, Derzhko R, Prajs I, Podolak-Dawidziak M, Serebruany VL. Endothelial progenitor cells and left ventricle function in patients with acute myocardial infarction: Potential therapeutic considertions. Am J Ther. 2012;19:44–50. doi: 10.1097/MJT.0b013e3181e0cab3. [DOI] [PubMed] [Google Scholar]
- 46.Leone AM, Rutella S, Giannico MB, Perfetti M, Zaccone V, Brugaletta S, Garramone B, Niccoli G, Porto I, Liuzzo G, Biasucci LM, Bellesi S, Galiuto L, Leone G, Rebuzzi AG, Crea F. Effect of intensive vs standard statin therapy on endothelial progenitor cells and left ventricular function in patients with acute myocardial infarction: Statins for regeneration after acute myocardial infarction and pci (strap) trial. Int J Cardiol. 2008;130:457–462. doi: 10.1016/j.ijcard.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 47.Numaguchi Y, Sone T, Okumura K, Ishii M, Morita Y, Kubota R, Yokouchi K, Imai H, Harada M, Osanai H, Kondo T, Murohara T. The impact of the capability of circulating progenitor cell to differentiate on myocardial salvage in patients with primary acute myocardial infarction. Circulation. 2006;114:I114–119. doi: 10.1161/CIRCULATIONAHA.105.000588. [DOI] [PubMed] [Google Scholar]
- 48.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 49.Patel RS, Li Q, Ghasemzadeh N, Eapen DJ, Moss LD, Janjua AU, Manocha P, Kassem HA, Veledar E, Samady H, Taylor WR, Zafari AM, Sperling L, Vaccarino V, Waller EK, Quyyumi AA. Circulating cd34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circ Res. 2015;116:289–297. doi: 10.1161/CIRCRESAHA.116.304187. [DOI] [PMC free article] [PubMed] [Google Scholar]