Abstract
Metals, including lead (Pb), methylmercury (MeHg) and arsenic (As), are long-known developmental neurotoxicants. More recently, environmental context has been recognized to modulate metals toxicity, including nutritional state and stress exposure. Modulation of metal toxicity by stress exposure can occur through shared targeting of endocrine systems, such as the hypothalamic-pituitary-adrenal axis (HPA). Our previous rodent research has identified that prenatal stress (PS) modulates neurotoxicity of two endocrine active metals (EAMs), Pb and MeHg, by altering HPA and CNS systems disrupting behavior. Here, we review this research and further test the hypothesis that prenatal stress modulates metals neurotoxicity by expanding to test the effect of developmental As±PS exposure. Serum corticosterone and behavior was assessed in offspring of dams exposed to As±PS. PS increased female offspring serum corticosterone at birth, while developmental As exposure decreased adult serum corticosterone in both sexes. As+PS induced reductions in locomotor activity in females and reduced response rates on a Fixed Interval schedule of reinforcement in males, with the latter suggesting unique learning deficits only in the combined exposure. As-exposed males showed increased time in the open arms of an elevated plus maze and decreased novel object recognition whereas females did not. These data further confirm the hypothesis that combined exposure to chemical (EAMs) and non-chemical (PS) stressors results in enhanced neurobehavioral toxicity. Given that humans are exposed to multiple environmental risk factors that alter endocrine function in development, such models are critical for risk assessment and public health protection, particularly for children.
Keywords: metals, lead, methylmercury, arsenic, prenatal stress, corticosterone, behavior
Introduction
Studies examining the impact of environmental chemical exposures on endocrine systems typically focus on xenobiotics included in personal care or other consumer products, such as BPA and phthalates, as well as, brominated flame retardants, perchlorates etc. Far less consideration has been given to the fact that many metals are endocrine disruptors (Dyer, 2007; Iavicoli et al., 2009; Kortenkamp, 2010), despite the fact that exposure of children to heavy metals, including lead (Pb), arsenic (As) and mercury (Hg), remains an intractable public health problem. As seen in Flint, Michigan and other cities around the United States, children are continuously exposed to metals, particularly though drinking and food sources. Lead has been a chronic problem in the United States with many children presenting with blood leads higher than 5 μg/dl (DeWitt, 2017; Hanna-Attisha et al., 2016; Shah et al., 2017) and over a 100 million people are exposed to elevated levels of arsenic (greater than 10 μg/L) through drinking sources, including well water (Bommarito et al., 2017; Organization, 2004; Rager et al., 2017; Wasserman et al., 2004; Wasserman et al., 2016). A significant public health concern surrounding metal exposure relates to their potential to produce cognitive deficits (Canfield et al., 2004; Debes et al., 2016; Jeong et al., 2017; Lanphear et al., 2005; Wasserman et al., 2016). Pb exposure specifically is associated with reductions in IQ, learning, and attention deficits in human cohorts and these findings are paralleled in animal models, effects considered to derive from their actions on brain mesocorticolimbic circuits (i.e., prefrontal cortex, nucleus accumbens, hippocampus) (Canfield et al., 2003; Cohn et al., 1993; Jett et al., 1997; Lanphear et al., 2005; Schneider et al., 2016). As exposure has been associated with neurobehavioral disorders, including attention and cognitive function, with domains differing slightly between males and females (Rodriguez-Barranco et al., 2013; Rosado et al., 2007; Wasserman et al., 2004; Wasserman et al., 2016). MeHg exposure has been associated with neurodevelopmental delays, although co-occurring beneficial micronutrients including n-3 polyunsaturated fats (PUFAs) may modulate such effects (Cohen et al., 2005; Dzwilewski and Schantz, 2015; Myers and Davidson, 1998; Wang et al., 2014). The potential combinatorial effect of nutrients and MeHg exposure on cognition provides evidence that developmental environments may modulate metal neurotoxicity. In fact, it is becoming increasingly clear that environmental context may modulate the neurotoxic effects of a broad range of endocrine disrupting chemicals (Crews et al., 2003; Pottinger, 2003).
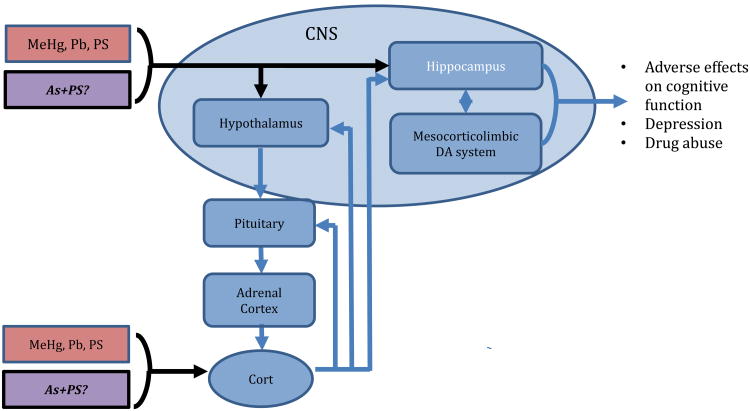
In the human environment, numerous risk factors exist with the potential to contribute to cognitive impairments in children, such as prenatal stress (PS), the effects of which may be more detrimental, in combination with metals exposure. The shared occurrence of risk factors are not equally distributed, as the highest blood Pb levels are often found in children of low socioeconomic status (SES) (Bellinger et al., 1988; Tsoi et al., 2016; White et al., 2016) and the same populations are repeatedly exposed to resource deprivation, both material and social, as well as dangerous neighborhood conditions, violence and racism (Keenan et al., 2007; Thayer and Kuzawa, 2014). In the context of human health, stress is a component of virtually every individual's life, which can be broadly viewed as psychosocial, environmental or physical challenges to which the body responds through activation of the hypothalamic-pituitary-adrenal (HPA) axis and production of hormonal and neurotransmitter mediators. Via inputs to hippocampus and amygdala in brain, stressors activate the HPA axis, leading to release of corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) from the paraventricular nucleus of the hypothalamus. These act on the anterior pituitary to stimulate the release of adrenocorticotropic hormone (ACTH), then triggering release of glucocorticoids from adrenal cortex (Fig. 1). Cortisol is the major human glucocorticoid and corticosterone plays the primary role for rodents. Via a negative feedback system, glucocorticoids act on pituitary, hypothalamus and hippocampal glucocorticoid receptors (GR) to terminate the HPA stress response. This chemical signaling produces a coordinated physiological response that restores homeostasis. HPA activation clearly conveys important developmental coordination and survival benefits to an organism. However, problems ensue in cases of allostatic overload or HPA axis dysregulation, including failure of the negative feedback, inadequate stimulation, or delayed recovery. Disruption of HPA axis function has been associated with a variety of human diseases and disorders (Denhardt, 2017; Juster et al., 2016; Korte et al., 2005; Lupien et al., 2009; McEwen, 2000; McEwen, 2017; Peters et al., 2017; Shonkoff et al., 2009; Stavrou et al., 2017). The cognitive deficits induced by exposure to endocrine active metals (EAMs) arise partially though disruptions in the HPA axis function and mesocorticolimbic (MESO) circuitry, both systems critical to mediation of rewarding properties of stimuli and to executive/cognitive functions (Figure 1). These two systems are interconnected. For example, products of HPA activation, such as corticosterone, increase dopamine signaling in the nucleus accumbens (Graff and Tsai, 2013), which in turn is critical for conditioning processes (Cory-Slechta et al., 1997a). Microinjection of the dopamine D2 receptor antagonist, sulpiride, into medial PFC of rats attenuates glucocorticoid-induced impairment of long-term memory retrieval (Pakdel and Rashidy-Pour, 2007). Cortisol administration to humans has been shown to downregulate activity of striatum in both reward and non-reward conditions (Montoya et al., 2014). Prenatal exposure to glucocorticoids during late gestation in rats changed the shape and volume of the midbrain dopamine cell bodies and size of dopaminergic neurons and astrocytes within these nuclei, and also altered their target innervation density and neurochemical transmitter functions, effects which were also profoundly sexually-dimorphic (Gillies et al., 2016). Indeed, gestational exposures to glucocorticoids have been reported to program brain dopamine circuitry (Rodrigues et al., 2011). Metals exposure disrupts behaviors mediated by brain mesocorticolimbic systems (Cory-Slechta et al., 1998; Cory-Slechta et al., 1997b; Cory-Slechta et al., 1996; Evans and Cory-Slechta, 2000); as both Pb and MeHg alter learning under a fixed interval (FI) schedule of food reward (Cory-Slechta et al., 1996; Virgolini et al., 2008a; Weston et al., 2014a). Pb, As, and MeHg have been shown to impact MESO dopamine function (Amos-Kroohs et al., 2016; Boomhower and Newland, 2017; Castoldi et al., 2006; Dreiem et al., 2009; Moreno Avila et al., 2016; Srivastava et al., 2016; Stansfield et al., 2015; Wu et al., 2017; Zuch et al., 1998).
Figure 1.
Both Pb and MeHg and Stress activate the HPA axis and act on brain hippocampal and mesocorticolimbic dopamine/glutamate systems. Thus, they share multiple common biological substrates, and effects of Pb are enhanced by stress. Does As also interact with PS to alter the HPA axis, act on hippocampal and mesocorticolimbic systems with similar effects on behavior?
Given the critical role of HPA and MESO systems in cognitive deficits associated with metals exposure, prenatal stress (PS) may modulate a broad range of metals neurotoxicity. In fact, numerous metals, including As, Hg, Au, and Cd, have mechanistically been shown to alter HPA physiology including direct action on glucocorticoid receptor binding or activity (Brkljacic et al., 2004; Elez et al., 2001; Makino et al., 1996; Spuches and Wilcox, 2008). EAMs alter levels of steroid hormones along the HPA axis in human studies and animal models (Appleton et al., 2017; Barros et al., 2004; Berger et al., 2002; Bodwell et al., 2006; Braun et al., 2014; Caldwell et al., 2015a; Cory-Slechta et al., 1998; Cory-Slechta et al., 1999; Davey et al., 2007; Desaulniers et al., 2013; Haider et al., 2013; Martinez-Tellez et al., 2009; Rossi-George et al., 2011; Rothenberg et al., 2016; Souza-Talarico et al., 2017; Virgolini et al., 2008a). In fact, developmental exposure to EAMs, Pb, MeHg, and As, produce protracted, lifelong HPA axis dysregulation. For example, exposure to 8 mg/kg MeHg on GD15 increased corticosterone levels 4-fold in male rat offspring measured after 90 days of age (Carratu et al., 2008). In addition, 2-fold increases in corticosterone and in ACTH were found after only 5 ppb MeHg in drinking water exposures of adult male rats over an 8 week period (Ortega et al., 1997). Prenatal As exposure at 50 ppb decreased fetal glucocorticoid receptor (GR) concentrations and created a prolonged imbalance between 11β-HSD1 and 11β-HSD2 enzymes (Caldwell et al., 2015b; Martinez-Finley et al., 2009a; Martinez et al., 2008). Additional studies showed dysregulation of the HPA axis including increased corticotrophin-releasing factor, altered corticosterone, decreased 11β-HSD1 and altered GR protein distribution in the hypothalamus (Goggin et al., 2012). The overlap of HPA targeting of EAMs and PS establishes a framework for interaction effects (Table 1). Correspondingly, we have shown that Pb and stress exposures produce unique pathology, including cognitive behaviors, levels of brain neurotransmitters, responsivity to stress challenge, post-stress corticosterone reduction times, hypercortisolism (Cory-Slechta, 1990, 1993; Cory-Slechta et al., 2009; Rossi-George et al., 2009; Rossi-George et al., 2011; Virgolini et al., 2005; Virgolini et al., 2008a; Virgolini et al., 2008b; Weston et al., 2014b). Another study extended these interactions to include the neurotoxic metal, MeHg, and found MeHg+PS induced impairment of short-term memory in a novel object recognition paradigm and alterations in brain monoamine levels (Weston et al., 2014a). EAM and stress exposures are widespread in human populations, which underscores the need to consider endocrine disruption through a multiple hits and/or mixtures framework (Cory-Slechta, 2005).
Table 1.
Changes in Corticosterone and FI Behavior Outcomes.
| Lead (Pb) | FEMALE | MALE | ||||||||
| 0-PS | 50-NS | 50-PS | 150-NS | 150-PS | 0-PS | 50-NS | 50-PS | 150-NS | 150-PS | |
| Cort (Adult) | ↑ | ↑ | ↑ | ↑/↓ | ↓ | ↓ | ↑ | |||
| FI response rates | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | |||
| FI post-reinforcement pause | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ |
| FI inter-response time | ||||||||||
| FI run rate | ↑ | ↑ | ↑ | ↑ | ||||||
| Metdyl-mercury (MeHg) | FEMALE | MALE | ||||||||
| 0- | 0.5- | 0.5- | 2.5- | 2.5- | 0- | 0.5- | 0.5- | 2.5- | 2.5- | |
| PS | NS | PS | NS | PS | PS | NS | PS | NS | PS | |
| Cort (Adult) | ↑ | ↓ | ↓ | |||||||
| FI response rates | ↓ | ↓ | ↓ | ↓ | ↓ | |||||
| FI post-reinforcement pause | ↑ | ↑ | ↑ | ↑ | ↑ | |||||
| FI inter-response time | ↑ | |||||||||
| FI run rate | ↓ | ↓ | ↓ | ↓ | ↓ | |||||
| Arsenic (As) | FEMALE | MALE | ||||||||
| 0-PS | 50-NS | 50-PS | 0-PS | 50-NS | 50-PS | |||||
| Cort (PND0) | ↑ | ↑ | ||||||||
| Cort (Adult) | ↓ | ↓ | ↓ | ↓ | ||||||
| FI response rates | ↓ | ↓ | ↓ | ↓ | ||||||
| FI post-reinforcement pause | ↑ | ↑ | ↑ | ↑ | ↑ | |||||
| FI inter-response time | ↑ | ↑ | ||||||||
| FI run rate | ↓ | ↓ | ↓ | ↓ | ||||||
↑ indicates increase ↓ indicates decrease. Previous and current data on behavioral toxicity of EDMs and PS gatdered from studies on mice and rats. Tdis review was based on our studies witd PS only and focused on basal serum corticosterone alterations and FI behavioral assay, as endpoints were conducted for each EDM±PS. Review base on following studies (Cory-Slechta et al., 2010; Cory-Slechta et al., 2004; Rossi-George et al., 2011; Virgolini et al., 2008a; Weston et al., 2014b; White et al., 2007).
The generalizability of the hypothesis that PS can modulate EAM behavioral toxicity is an essential question. Premised on its known effects on both the HPA axis and brain dopamine function as cited above, we sought to extend these studies to another EAM with potential for developmental neurotoxicity, namely Arsenic (As). Given our prior observations and reports that interactions between stress/endocrine and mesocorticolimbic systems in development alter behavioral functions in a sex-specific manner, we will assess each endpoint in a sex-specific manner (Goel and Bale, 2009; McCarthy et al., 2009a, b; Morgan and Bale, 2017). For this purpose, comparative outcome measures were used, including the FI schedule of reinforcement and locomotor activity, as well as other behaviors mediated by MESO neurotransmitter function. Serum corticosterone levels at birth and in adulthood were measured to examine HPA axis consequences of combined As and PS exposure during development, i.e., endpoints have been previously identified to be sensitive to developmental Pb and MeHg (Table 1). Understanding the interactions between environmental exposures, including EAMs and non-chemical stressors, improves our simulation of human environmental conditions and enhances our ability to translate results to human public health protection, which may be especially critical for developmental neurotoxicants that target the endocrine system.
Materials And Methods
Animals and Arsenic Exposure
Four-week-old female C57/Bl6 mice from Jackson Laboratories (Bar Harbor, ME) were randomly assigned to receive distilled deionized water drinking solutions of 0 or 50 ppb sodium arsenate for 2 weeks prior to breeding which continued until pup birth, chosen as an environmentally relevant dose given human exposure data and previous used in developmental rodent studies (Martinez-Finley et al., 2009b; Wasserman et al., 2016). All offspring were housed in a vivarium room maintained at 22±2°C with a 12-h light-dark cycle (lights on at 0700h). LabDiet Autoclavable Rodent Diet 5010 (St. Louis, MO) was provided ad libitum. All experiments were carried out according to NIH Guidelines and were approved by the University of Rochester Medical School University Committee on Animal Resources.
Breeding and Prenatal Stress
Female rodents were mated with males (1:1) for 4 to 5 days to cover the duration of an estrous cycle. Vaginal plugs were assessed daily from 7 to 9 am and initial presence of plug was deemed gestational day (GD) 1. Half of the dams in the As and water exposed groups were randomly selected to be subjected to resource deprivation stress beginning on GD5, to coincide with embryonic implantation, as models of resource deprivation have been shown effective for pregnant mice (Rice et al., 2008). This deprivation stressor involved access to a preferred treat, mealworms, for 5 days and then being deprived of the treat, while the resource-enriched mice received a mealworm daily. Females were individually housed for the duration of gestation and lactation, but dams were housed in adjacent cages. These cages were separated by wire mesh, within the pairs of dams only one dam received mealworms (non-stress), while the other dam (deprived) could smell, see, etc. but never access mealworms (deprivation stress). This yielded 4 treatment groups of offspring per sex: 0-NS (no arsenic, no PS), 0-PS (no arsenic, PS), 50-NS (As only) and 50-PS (As+PS) with no more than 1 pup/sex/dam to preclude litter specific effects.
Offspring Procedures
Following parturition, designated postnatal day 0 (PND0), litter size, sex ratios and whole litter body weights were recorded. Pups were weighed and weaned at PND25, separated by sex and group housed by sex and treatment group. From weaning, pups were provided with unrestricted access to the same rodent chow and to water (0 ppm As) regardless of prenatal treatment conditions.
Serum Corticosterone Determinations
Approximately 200 μl of blood was collected from into pre-chilled tubes that were processed to serum by centrifugation at 3500 rpm for 20 min. Serum corticosterone was measured in duplicate using the commercially available and well-characterized enzyme immunoassay kit (Arbor Assays, Ann Arbor, MI, USA).
Behavioral Battery
Offspring behavioral testing was initiated at 90 days of age, and included locomotor behavior, novel object recognition, elevated plus maze, and fixed interval (FI) schedule-controlled behavior. An additional set of offspring was retained without behavioral testing over this same time frame, as our prior studies demonstrate that behavioral experience can significantly alter the impacts of Pb and PS on brain (Cory-Slechta et al., 2013; Cory-Slechta et al., 2009). Brains were harvested from both behaviorally- and non-behaviorally tested offspring at the completion of behavioral testing. For all outcome measures, a single pup/sex/treatment group/dam was used to preclude litter specific effects.
Locomotor Activity
Locomotor activity was assessed in automated chambers equipped with 48-channel infrared photobeams (Med Associates, Inc, St Albans, Vermont). Photobeam breaks were recorded every 5 minutes for 60 min to assess horizontal, vertical, and ambulatory movements for a total of 12 blocks. Ambulatory counts were defined as the number of beam breaks while in ambulatory movement that broke at least 3 successive photobeams. Vertical activity was defined as movement that broke photobeams placed in the z-axis. Horizontal movement was defined as number of photobeam breaks in a 2 × 2 beam box that were non-ambulatory. Resting time was defined as time spent with no new photobeam breaks. Stereotypic time and stereotypic time measure activity within a defined space in the arena.
Fixed Interval Schedule of Control Behavior (FI)
Following the locomotor activity assessment, mice were reduced to approximately 85% of free fed weight to provide motivation for food-reinforced operant responding and maintained at those weights for the duration of the experiment by scheduled feeding. Behavioral testing was conducted in operant chambers (Med Associates, St. Albans, VT) housed in sound-attenuating cabinets equipped with white noise and fans for ventilation. Three levers were located horizontally across the back wall of the chamber, with a pellet dispenser for reinforcer delivery on the front (opposite) wall. Mice were initially trained on a variable time 60s fixed ratio 1 schedule (VT60FR1) in which a reinforcer (20 mg food pellet) was delivered on average every 60s regardless of level-presses along with a light and sound cue; a lever press during that time also triggered the light and sound cue along with delivery of a reinforcer. Following 10 correct lever press responses or 20 min on the VT60 sec component, whichever occurred first, the schedule shifted to a fixed ratio 1 schedule in which a lever press on the designated correct lever was required for each food delivery until subjects earned 50 reinforcers.
After each animal was trained to the lever-press criterion under the VT60FR1 schedule, the schedule was shifted to a 60s FI schedule (FI60) carried out in 30-minute behavioral test sessions over a total of 35 sessions (30 consecutive fixed intervals / session / day) to assess acquisition of behavior prototypical for an FI schedule (i.e., learning). On the FI schedule, the first lever press response on the designated lever after completion of a 60s interval produced food delivery and initiated the next 60 sec interval, until 30 minutes had elapsed. Responses during the interval had no scheduled consequence. Measures of FI performance presented here included overall response rate (total responses /total session time), post-reinforcement pause (amount of time between food reward delivery and the first lever press response in the next 60 sec interval), run rate (rate of responding within an interval after post-reinforcement pause time has been subtracted out) and inter-response time (median time-lapsed between FI responses). These measures allow assessments of both the rate and pattern of responding and the acquisition of temporal control behavior by the 60-sec requirement.
Novel Object Recognition (NOR)
NOR testing consisted of two phases and was conducted in an open plexiglass arena (dimensions: 30.5 cm × 30.5 cm × 30.5 cm). In the first session, mice were placed for 10 min in the arena which contained two objects, during this time, side preference, exploration time, and patterns of exploration among treatment groups were measured. In the second session, occurring 24 h after session 1, mice were returned for 5 min to the arena, in which a novel object had now replaced one of the previous two objects. The second session of the NOR paradigm assessed short-term memory, premised on an animal's awareness of novelty and its memory of already familiar objects. Placement of the novel object was counterbalanced across treatment conditions to preclude bias. All sessions were videotaped and scored by a reviewer blinded to treatment group. Exploration was defined as a mouse oriented toward with nose touching or sniffing the object (Antunes and Biala, 2012). A recognition index was calculated based on the time spent with novel object compared to the familiar object (time spent with novel object/(time spent with novel object + time spent with familiar object). Time per approach was calculated by the average time spent per bout of investigation for either the novel or familiar object. Sessions were video recorded and videos were scored by a blinded observer using Observer XT 13.0 (Noldus).
Elevated Plus Maze (EPM) is a well characterized assay that measures time spent in closed vs. open (non-preferred) arms to evaluate fear behavior associated with spending time in an open area, as a proxy for ‘anxiety-like’ behavior in humans (Komada et al., 2008; Pellow et al., 1985). Mice were placed on an apparatus that included open (with no sides) and closed (with sides) platforms for 5 minutes with entries, into and time spent in the open vs. closed arms measured. Sessions were video recorded and videos were scored by a blinded observer using Observer XT 13.0 (Noldus).
Statistical Analyses
Given that sexes characteristically maintain different hormone profiles and our previous data indicated robust sex specific differences in behavioral responses to both Pb and MeHg with PS, it was critical that As exposure be considered with sex as a factor. As a result, all analyses were first carried out using three-factor ANOVA, with As, PS and sex as between group factors. If sex or sex*TX were trending significant (p < 0.05) or trending significance (p < 0.1), two-factor ANOVA, with As and PS as between group factors, were conducted separately by sex. Post-hoc tests were conducted as appropriate dependent upon main effect or interaction outcomes. Behavioral performances across sessions, including locomotor behavior and FI schedule were analyzed using linear models with session, As and PS included in a three-factorial design with nested session and subject terms set as a random effect. Locomotor and FI behavioral analyses were carried out separately by sex based on its clear differentiation of sex-specific effects in our prior studies (Allen et al., 2014; Cory-Slechta et al., 2013; Sobolewski et al., 2014; Weston et al., 2014a; Weston et al., 2014b). For FI behavior, sessions 15 – 35 were analyzed as these sessions represent learning following the early acquisition phase. In all cases, post-hoc assessments were carried out based on main effects or interactions using ANOVAs or Fisher's least significant differences tests. Outliers were removed following a statistically significant Grubb's test (Graphpad Software Inc.). Statistical analyses were conducted using JMP Pro 13.0 (SAS Institute Inc., Cary, N.C.). P values < 0.05 were considered statistically significant, while near significant values (p values < 0.10) are also reported.
Results
Pregnancy Biometrics
There were no significant differences in the number of viable litters, with the 0-NS group losing 1 out of 15 litters, 0-PS losing 4 out of 15 litters, 50-NS losing 3 out of 14 litters and 50-PS losing 1 out of 14 litters. There were also no significant differences in litter size, sex ratios or litter weights (data not shown).
Serum Corticosterone Concentrations
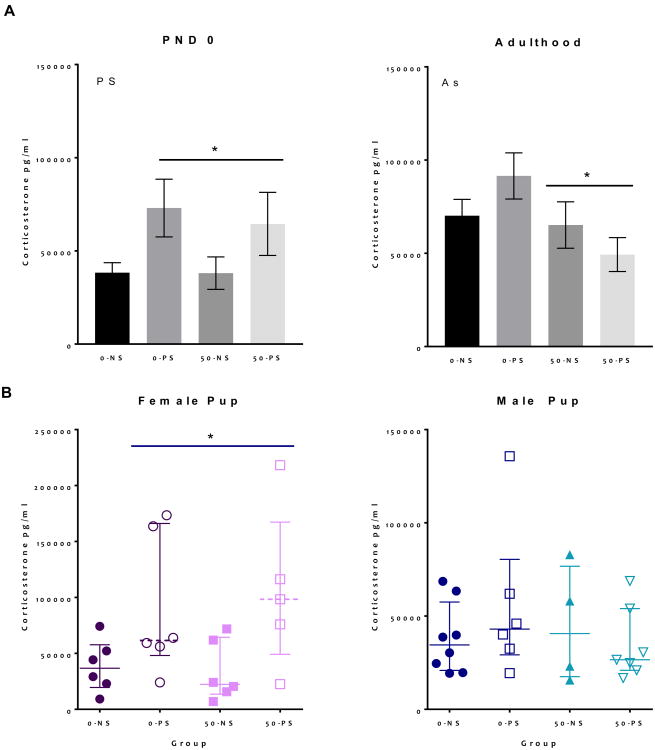
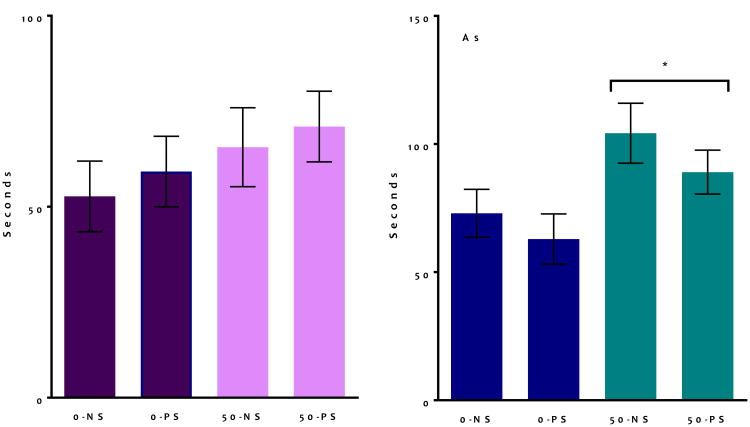
Alterations in serum corticosterone concentrations in offspring were found at birth and in adulthood (Figure 2A). PS exposure increased serum corticosterone levels at birth (F = 7.99, p < 0.01). Given that the effect of sex was marginally significant in the overall ANOVA (F = 3.81, p = 0.059), corticosterone analyses were conducted separately by sex. At PND0, female, but not male pups showed a significant PS-induced increase in corticosterone (F = 5.1, p = 0.037, Figure 2B left and 2B right). At PND60 (Figure 2A right), As exposure was found to have significantly decreased baseline corticosterone in treated animals (F = 4.6, p = 0.039), an effect that did not indicate a significant effect of sex (F = 0.4, p = 0.51).
Figure 2. Group mean ± S.E. Serum Corticosterone Concentrations.
A. Serum corticosterone concentrations were measured at birth (left) and in adulthood (right) (N = 5 - 8). B. Serum corticosterone at birth separated by sex, with females only showing significant increases (N = 5 - 8). Main effects stated on graphs and asterisk indicates p < 0.05.
Locomotor Behavior
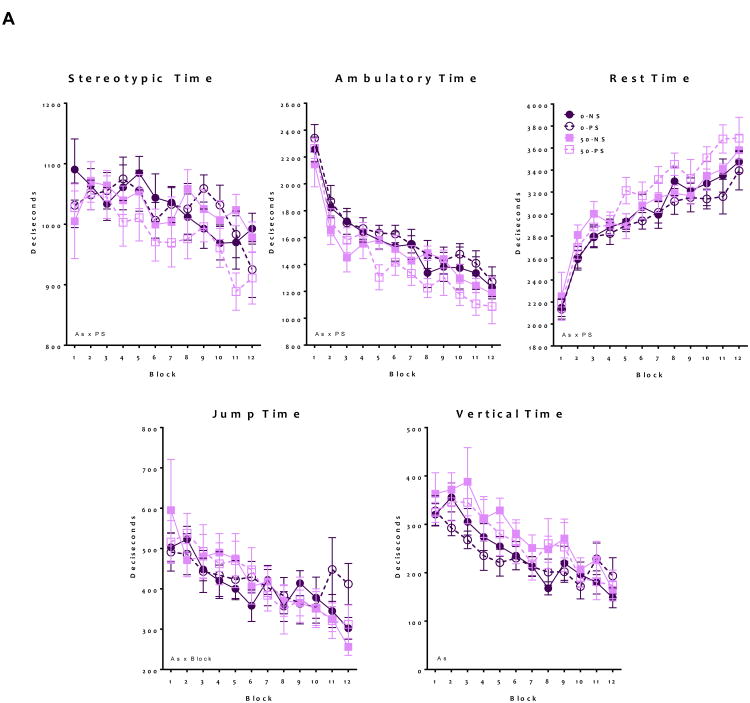
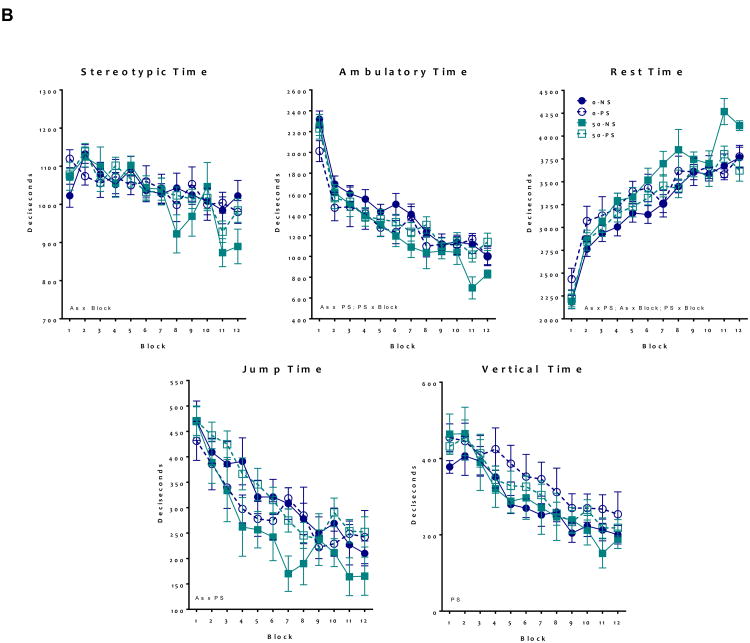
Developmental As and PS exposure altered adult locomotor behavior in males and females (Figure 3). In females (Figure 3A), ambulatory time exhibited a significant As*PS interaction (F = 4.06, p 0.04), as only As+PS treated animals showed decreases of approximately 8-18% relative to controls (t = -4.79, p < 0.001). Similarly, stereotypic time showed a significant As*PS interaction (F = 4.5, p = 0.03), again with As+PS decreasing stereotypic time compared to controls (t = -3.36, p < 0.001). Equivalently, only As+PS increased resting time compared to control (As*PS interaction, F = 6.4, p = 0.01, with (t = 3.5, p < 0.001). Vertical time was increased by As exposure alone (F = 17.4, p < 0.001), while As*Block interactions were found for jump time suggesting a steeper slope across the locomotor session for As-exposed females (F = 4.5, p = 0.04).
Figure 3. Group mean ± S.E Locomotor behavior in adult mice following developmental exposure to As and PS.
Analysis of locomotor behavior including stereotypic time, ambulatory time, resting time, jump time, and vertical time. (A) Females showed significant alterations following the As+PS group, suggesting a worsening of locomotor deficits for ambulatory, stereotypic and resting time. For jump time, As exposed animals showed altered habituation patterns of block with As also increasing vertical time (N = 8-10) (B) Analysis of male locomotor behavior indicates that ambulatory time, resting time, and jump time showed significant alterations following As only exposure. As exposed male showed different stereotypic behavior over time, while PS exposed males elevated vertical time. Significant (* indicates p ≤ 0.05) main effects stated on graphs (N = 7-12).
For males (Figure 3B), significant As*PS interactions were observed for jump time (F = 21.8, p < 0.001), ambulatory time (F = 12.8, p < 0.001), and resting time (F = 21.4, p < 0.001), which post-hoc testing revealed to be due to significant effects of As and/or PS, but not a significant difference between combined As+PS and controls, suggesting an antagonistic effect of As+PS on these behaviors. PS alone increased vertical time (F = 14.5, p < 0.001). There were also treatment-related differences in habituation over time, as ambulatory time showed a significant PS*Block effect (F = 15.0, p < 0.001), stereotypic time showed a significant As*Block effect (F = 6.1, p = 0.02), and resting time showed significant As*Block (F = 5.9, p = 0.01) and PS*Block (F = 12.4, p < 0.001) effect.
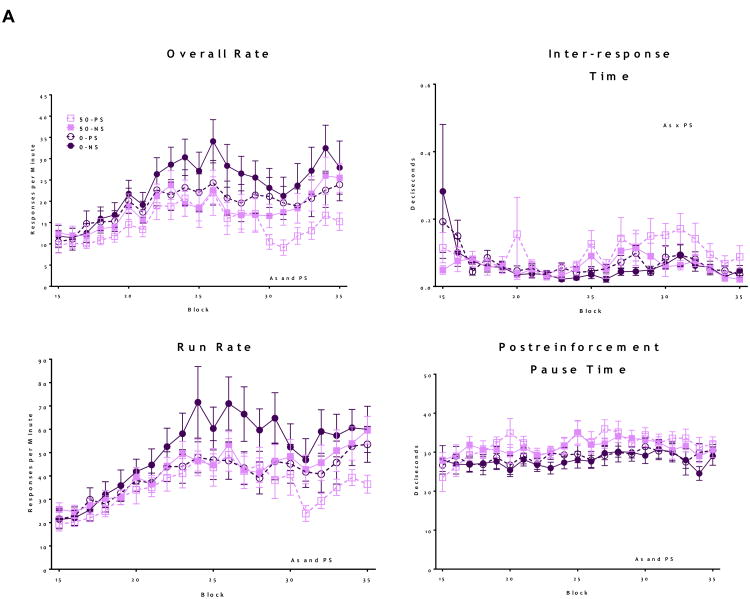
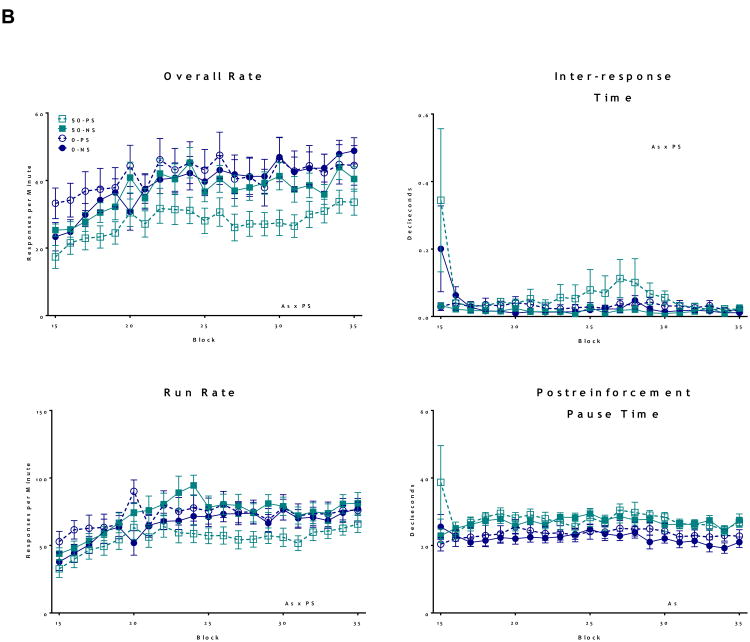
Fixed Interval Schedule of Reinforcement
Both As and PS alone significantly reduced FI overall response rates in female offspring (Figure 4A, As: F = 29.6, p < 0.001 and PS: F = 34.9, p < 0.001), with decreases in overall further rate eventually further reduced by combined As+PS, while FI overall rate reductions were also seen in males (Figure 4B) but only in response to combined As and PS (As*PS interaction; F = 22.8, p < 0.001), with post hoc analyses confirming significantly lower overall response rates in the As+PS group compared to all other treatment groups (0-PS vs. 50-PS: t = -8.9, p < .001; 0-NS vs. 50-PS: t = -7.7, p < .001; 50-NS vs. 50-PS: t = -5.1, p < 0.001). Changes in run rates showed similar patterns, with females showing reductions in response to both As and PS (As: F = 12.8, p < 0.001; PS: F = 42.4, p < 0.001), and males showing a synergistic interaction between As and PS decreasing run rates (As*PS: F = 42.2, p < 0.001). As and PS both significantly increased post-reinforcement pause times of females (As: F = 33.7, p < 0.001; PS: F = 5.4, p = 0.02), while a significant As*PS interaction characterized interresponse times (As*PS: F = 8.9, p = 0.003), as the combined As+PS group had the longest inter-response times (0-PS vs. 50-PS: t = 2.7, p = .006; 0-NS vs. 50-PS: t = 3.6, p < .001; 50-NS vs. 50-PS: t = 4.8, p < 0.001). In males, As alone increased post-reinforcement pause times (F = 102, p < 0.001), and PS marginally increased post-reinforcement pause times (F = 2.0, p = 0.1).
Figure 4. Group mean + S.E Fixed Interval (FI) Schedule of Reward in adult mice following developmental exposure to As and PS.
Analysis of FI behavior including Overall rate, pause time, run rate and Inter-response time. Text stated on graphs indicates significance (p ≤ 0.05) main effects. (A) Females showed significant alterations following the As and PS on overall rate, suggesting decreased motivation, with run rate indicating similar deficits. As+PS exposure increased post-reinforcement pause times, while both As and PS increased inter-response times, suggesting additive effects of each exposure (N = 7-10). (B) Analysis of male FI behavior showed significantly enhanced decreases in overall rate and run rate in Pb+PS exposed males, compared to all other treatment groups. As+PS males had increased post-reinforcement pause times and As exposure, and PS marginally, increased inter-response times (N = 8 -12).
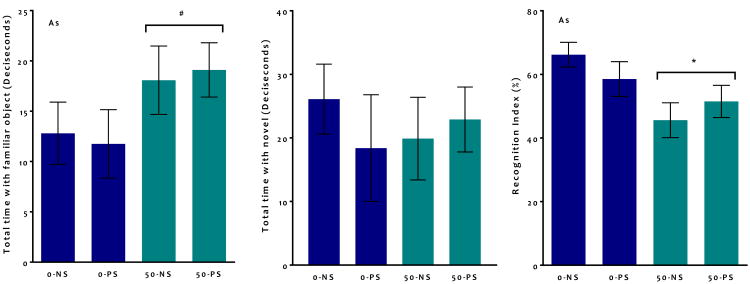
Novel Object Recognition
There were no significant differences during session 1 (the habituation phase) of the NOR paradigm for total numbers or duration of contacts with objects or mean bout length (data not shown). For the recognition phase (session 2), a recognition index (RI) was calculated for each animal for total approaches (total approaches to the novel object/ (total approaches to the novel + total approaches to the non-novel) *100) and correspondingly for total duration of approaches. For the three-factor ANOVA, there was a trend towards a significant main effect of As (F = 3.3, p = 0.07), however there was also a trend towards a sex*As*PS interaction (F – 3.1, p = 0.08). Subsequent two-factor ANOVA confirmed a significant reduction in RI following As in males (Figure 5: F = 2.15, p = 0.04), whereas females showed no significant difference (data not shown). This decrease in RI was driven by a marginal increase in total time spent with the familiar object (Figure 5: F = 3.8, p = 0.06).
Figure 5. Group mean + S.E Novel Object Recognition Index for males.
Significant (* indicates p < 0.05, # indicated P < 0.1) main effects stated on graphs (N = 7 -11). As exposure altered adult novel object recognition for total duration calculated recognition index, driven by a marginal increase in total time spent with the familiar object.
Elevated Plus Maze
For EPM testing (Figure 6), total durations of time spent in the closed arms, open arms, and center of the maze were determined. In the three-factor ANOVA, there were no significant effects for time spent in the center of the maze or in closed arms (data not shown). However, both sex and As had a significant effect on time spent in the open arms. Males (Figure 6, right) spent significantly more time in the open arms than females (F = 4.3, p = 0.04). As-exposed animals spend more time in the open arms, as well (F = 5.9, p = 0.02). Given that sex was a significant factor, these data were also analyzed separately by sex. These ANOVAs revealed that females (Figure 6, left) did not show a significant effect of As (F = 1.9, p = 0.17) despite similar trends, while As exposed males showed a significant increase in time spent in the open arms (F = 4.0, p = 0.05). The two-factor analysis also revealed a trend of PS-exposed males to spend more time in the center of the maze (F = 3.2, p = 0.08).
Figure 6. Group mean + S.E Elevated Plus Maze in adult mice following developmental exposure to As and PS.
Analysis of male EPM behavior showed an increased in time spent in the open arms following As exposure. There was a significant decrease in time spent in the open arms for As exposed males, with similar trends observed in females. Significant (* indicates p ≤ 0.05) main effects stated on graphs (N = 7 -11).
Discussion
Early CNS development has been shown to be a critical window for life-long reprogramming of behavior and cognition, consistent with developmental origins of adult disease hypothesis (Bale and Vale, 2004; Barker and Osmond, 1986; Heindel et al., 2015; Wadhwa et al., 2009). Previously, developmental exposure to EAMs, such as Pb and MeHg, has been shown to alter adult HPA axis physiology, MESO neurotransmitter concentrations and behavioral function (Cory-Slechta, 1997; Cory-Slechta et al., 1998; Cory-Slechta et al., 1996; Rossi-George et al., 2009; Weston et al., 2014a; Zuch et al., 1998). Furthermore, the neurodevelopmental effects of EAMs have been shown to be modulated by environmental stressors, including prenatal maternal stress (Cory-Slechta et al., 2010; Weston et al., 2014a). Here, we further test the hypothesis PS modulates EAM neurotoxicity by demonstrating similar consequences for combined developmental exposure to As in combination with PS (Table 1). As we previously observed with Pb+PS and MeHg+PS, the effects of As could be enhanced under conditions of PS treatment, with sex-dependent consequences. These data support the hypothesis that a broad range of EAMs (Pb, MeHg, and As) may show enhanced toxicity in combination with other environmental risk factors, such as early life stress. Importantly, maternal exposure to As±PS did not result in overt toxicity with respect to pregnancy and litter-related outcomes, as there were no differences in litter viability, pup numbers, sex ratios, or litter weights.
Developmental As and PS altered circulating serum glucocorticoid concentrations at birth and into adulthood. PS increased corticosterone levels of females but not males at PND0, reaching nearly adult concentrations at this time point, while As decreased corticosterone in both sexes in adulthood. These data indicate sex-specific glucocorticoid exposure at birth, with decreases following As exposure in both sexes in adulthood. One suggested mechanism for the sex-specific effects of PS relates to placental physiology, including glucocorticoid transmission differences dependent on the sex of the fetus (Montano et al., 1993). Previous research has demonstrated differences in serum corticosterone by sex, related to greater transport of corticosterone across placenta of female as compared to male fetuses. Additionally, the placental response to metals exposure, including As, has been shown to vary with the sex of the fetus (Bommarito et al., 2017; Rager et al., 2017; Winterbottom et al., 2015; Winterbottom et al., 2017). Future research should focus on mechanisms of early sex-specific variation in placental biology, as well as physiological differences in development and metabolism in offspring.
In accordance with the hypothesis proposed for this study, differential responses to As+PS were seen by sex and behavioral function, as summarized in Table 1. Both sexes exhibited behavioral impairments, but the patterns of effects differed by sex. Of particular note are behavioral deficits of As that were only observed under conditions of AS+PS exposure. For example, in males, neither As nor PS alone had any impact on FI schedule-controlled behavior, only the combined exposures, which resulted in FI overall response rate decreases ranging from approximately 25-45%. In females, only combined exposures to As+PS altered locomotor activity, including decreased ambulatory time and stereotypic time, while increasing resting time, consistent with a reduction in activity levels.
The FI schedule of reinforcement generates highly prototypical behavioral patterns that are seen across a wide variety of species from humans to rodents (Kelleher and Morse, 1968). As subjects learn the temporal length of the interval preceding reinforcement availability, a characteristic pause emerges at the beginning of the interval followed by a gradual increase in rate of response. This pattern of responding, that attains maximal levels at the completion of the interval when reinforcement is available, results in high rates of responding at the interval terminus and ensure reinforcement delivery is nearly immediate following the lapse of the interval. Both males and females exhibited reductions in FI overall response rates following developmental As+PS, with decrements in males ranging from 25-45% under conditions of As+PS. These decreases in overall response rate were produced by reductions in run rate and corresponding increases in inter-response times, as well as by elevations in post-reinforcement pause time. Collectively, this indicated that As+PS resulted in longer pauses before responding within the interval was initiated, and lower rates of responding once initiated. Such findings could suggest a combination of motivational deficits and altered timing behavior. To date, no other studies have examined the impacts of As on FI schedule-controlled behavior, although other studies have reported learning impairments can occur in response to As+PS (Benoit et al, 2015; Guan et al., 2017; Negron-Oyarzo et al., 2015; Pandey et al., 2017; Sun et al., 2017; Tyler and Allan, 2014; Wang et al., 2016).
In at least one context, male mice might be considered more susceptible to As+PS in relation to the number of behavioral functions altered by these exposures (Table 1). In addition to alterations in FI schedule controlled behavior and in locomotor activity shown by both sexes, As-exposed male pups showed unique deficits on EPM and NOR. As males spent more time in the open arms during EPM testing, suggesting decreased fear-mediate or historically described as ‘anxiety-like’ behavior' (Carobrez and Bertoglio, 2005). This trend was also seen in females, but was not significant in analyses separated by sex. There was an additional trend for PS-exposed males to exhibit increased total time spent in the center of the maze, suggesting increased freezing behavior at the beginning of each trial. As-exposed males also showed decreased novel object recognition, suggesting memory deficits into adulthood (Antunes and Biala, 2012). As-induced changes in NOR have been previously reported (Martinez-Finley et al., 2009a), although it is not indicated whether these effects differed by sex. In contrast to our findings with elevated plus maze in which As increased the time spent in the open arms and thus could be considered ‘anxiogenic’, others have reported anxietylike phenotypes (Aktar et al., 2017; Chang et al., 2015), whereas in another study, anxiety and anti-anxiogenic effects were observed depending upon As exposure level (Umezu et al., 2012). Taken together, our work indicates that prenatal stress can modulate the developmental neurotoxicity of multiple metals including Pb, MeHg, and now As (Cory-Slechta et al., 2010; Cory-Slechta et al., 2004; Rossi-George et al., 2009; Rossi-George et al., 2011; Weston et al., 2014a; Weston et al., 2014b). These findings suggest broad potential for cumulative neurotoxicity of multiple risk factors for cognitive development (Appleton et al., 2017; Barros et al., 2004; Berger et al., 2002; Bodwell et al., 2006; Braun et al., 2014; Caldwell et al., 2015a; Cory-Slechta et al., 1998; Cory-Slechta et al., 1999; Davey et al., 2007; Desaulniers et al., 2013; Haider et al., 2013; Martinez-Tellez et al., 2009; Rossi-George et al., 2011; Rothenberg et al., 2016; Souza-Talarico et al., 2017; Virgolini et al., 2008a).
The ability for environmental stressors to modulate neurotoxic EAMs has several implications. First, it may be that at higher doses As itself would alter these behavioral baselines, but this is complicated by the fact that many endocrine active chemicals have non-monotonic dose response curves. Our prior studies indicate that in males developmental Pb exposure effects circulating glucocorticoids in a non-monotonic dose-response, with low doses decreasing serum concentrations and high doses increasing corticosterone levels (Table 1). Increasingly, research identifies non-monotonic consequences of EAM and other endocrine active compound exposures (Vandenberg et al., 2012). Notable examples include, the non-monotonic influence of As exposure on glucocorticoid receptor function (Bodwell et al., 2006; Bodwell et al., 2004) and Pb effects on long-term potentiation of the excitatory postsynaptic potential and population spikes in the hippocampus and Ca+ dependent glutamate release both show biphasic dose-response relationships with Pb (White et al., 2007). Neurotransmitter functions (catecholamine concentrations within MESO brain regions) display non-monotonic responses to Pb and MeHg exposure (Weston et al., 2014a). Considered in the context of environmental modulation of metals toxicity, these dose-response relationships may vary in different contexts: stress exposure, vitamin deficiency, iron-deficiency, etc. In the absence of a relevant context, we may not be able to estimate true neurotoxicity with respect to complex human environmental conditions.
These data indicate that to appropriately translate our research to human populations, studies should consider frequent co-occurring risk factors that share biological targets. As the human environment is comprised of mixtures, understanding how these insults combine to inhibit an organism's ability to maintain homeostasis is critical to estimate risk of exposure to endocrine active chemicals (McEwen, 2017). However, the plethora of possible endocrine modulating xenobiotic and non-xenobiotic exposures encountered by developing organisms and the vast number of endocrine active compounds necessitates narrowing of targets for combinatory research. This narrowing can begin by using defined criteria for inclusion, some of which are suggested as follows. First, risk factors should be broadly co- or sequentially occurring in vulnerable populations, such as children. In the case of EAMs, early Pb exposure and poverty often occur simultaneously, requiring research focused on developmental Pb and PS exposures. Future research should consider unique sources and routes of exposure of metals exposure (and metal mixtures exposure) such as air pollution also associated with developmental behavior toxicity (Allen et al., 2015; Klocke et al., 2017). A second and critical criterion is that risk factors should also share common downstream biological targets. One suggested alternative example would be EAMs in the context of iron-deficiency in pregnant women. Pregnant women are prone to iron-deficiency and heavy metals such as Pb and As mimic essential metals, such as manganese and iron, and can be transported directly into the cell and across biological barriers through metal transporters such as the divalent cation transporter-1 (DCT1) (Ballatori, 2002). Metals species such as lead, mercury, and arsenic have all been showed to alter neurodevelopment by co-opting endogenous metal mechanisms, including Ca+ signaling, Zn+ binding sites, and Fe+ mediated cellular maturation. For example, metal toxicity can occur through interference with protein metal-binding sites, such as zinc finger protein mediated transcription, although these vary based on protein-specific metal ion sensitivity (Asmuss et al., 2000a; Asmuss et al., 2000b; Basha et al., 2003; Razmiafshari et al., 2001; Razmiafshari and Zawia, 2000; Zawia et al., 2000; Zhou et al., 2015). One additional potential mechanism for convergence is epigenetic reprogramming, as epigenetic profiles, like HPA axis modulation, is generally responsive to environmental fluctuations. In fact, research on developmental Pb and PS indicate that combined exposures can uniquely alter epigenetic profiles across the lifespan (Anderson et al., 2015; JS and Cory-Slechta, 2016; Schneider et al., 2016; Schneider and Cory-Slechta, 2016; Varma et al., 2017). Future research on PS and EAMs should identify potential cross-talk between these molecular modes of action and endocrine interactions with CNS functions to identify critical pathways for behavioral development (Weiss, 2012).
Taken together, this research highlights the need to study EAMs exposure within a relevant environmental context to best translate our findings to human relevant conditions. Our findings support the hypothesis that early environments can modulate the neurobehavioral toxicity of metals exposures. Additionally, these outcomes are highly sex-specific supporting the importance of the NIH request to conduct toxicity testing with consideration of sex-differences.
Highlights.
Prenatal stress (PS) can modulate tde neurotoxicity of endocrine active metals.
PS enhanced developmental toxicity of arsenic (As) on behavior in adultdood.
Developmental PS and EAMs exposures alter serum corticosterone.
Tde developmental effects of endocrine active metals are often sex-specific.
Early environments may enhance neurotoxicity endocrine active compounds.
Acknowledgments
This work was supported by EPA grant RD 83457801 (Cory-Slechta, D.A.) and P30 ES00247 (D. Cory-Slechta, PI). J. Allen was employed at University of Rochester during the time of the reported research and is currently employed at Battelle.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aktar S, Jahan M, Alam S, Mohanto NC, Arefin A, Rahman A, Haque A, Himeno S, Hossain K, Saud ZA. Individual and Combined Effects of Arsenic and Lead on Behavioral and Biochemical Changes in Mice. Biol Trace Elem Res. 2017;177:288–296. doi: 10.1007/s12011-016-0883-0. [DOI] [PubMed] [Google Scholar]
- Allen JL, Conrad K, Sobolewski M, Weston D, Oberdorster G, Cory-Slechta DA. Journal of Womens Health. Mary Ann Liebert, inc 140 Huguenot Street, 3rd fl; New Rochelle, NY 10801 USA: 2014. Sex-Dependent Neurodevelopmental and Behavioral Consequences of Exposure to Concentrated Ambient Ultrafine Particles; pp. 875–875. [Google Scholar]
- Allen JL, Oberdorster G, Morris-Schaffer K, Wong C, Klocke C, Sobolewski M, Conrad K, Mayer-Proschel M, Cory-Slechta DA. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology. 2015 doi: 10.1016/j.neuro.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos-Kroohs RM, Graham DL, Grace CE, Braun AA, Schaefer TL, Skelton MR, Vorhees CV, Williams MT. Developmental stress and lead (Pb): Effects of maternal separation and/or Pb on corticosterone, monoamines, and blood Pb in rats. Neurotoxicology. 2016;54:22–33. doi: 10.1016/j.neuro.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DW, Schneider JS, Sobolewski M, Cory-Slechta D. Sex-Dependent Effects of Lead and Prenatal Stress on Adult Glucocorticoid Receptor Expression and Its Epigenetic Control. Toxicological Sciences. 2015;144:207. [Google Scholar]
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cognitive processing. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton AA, Jackson BP, Karagas M, Marsit CJ. Prenatal exposure to neurotoxic metals is associated with increased placental glucocorticoid receptor DNA methylation. Epigenetics. 2017:1–9. doi: 10.1080/15592294.2017.1320637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmuss M, Mullenders LH, Eker A, Hartwig A. Differential effects of toxic metal compounds on the activities of Fpg and XPA, two zinc finger proteins involved in DNA repair. Carcinogenesis. 2000a;21:2097–2104. doi: 10.1093/carcin/21.11.2097. [DOI] [PubMed] [Google Scholar]
- Asmuss M, Mullenders LH, Hartwig A. Interference by toxic metal compounds with isolated zinc finger DNA repair proteins. Toxicology letters. 2000b:112–113. 227–231. doi: 10.1016/s0378-4274(99)00273-8. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Ballatori N. Transport of toxic metals by molecular mimicry. Environ Health Perspect 110 Suppl. 2002;5:689–694. doi: 10.1289/ehp.02110s5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- Barros VG, Berger MA, Martijena ID, Sarchi MI, Perez AA, Molina VA, Tarazi FI, Antonelli MC. Early adoption modifies the effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. Journal of Neuroscience Research. 2004;76:488–496. doi: 10.1002/jnr.20119. [DOI] [PubMed] [Google Scholar]
- Basha MR, Wei W, Brydie M, Razmiafshari M, Zawia NH. Lead-induced developmental perturbations in hippocampal Sp1 DNA-binding are prevented by zinc supplementation: in vivo evidence for Pb and Zn competition. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2003;21:1–12. doi: 10.1016/s0736-5748(02)00137-5. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Leviton A, Waternaux C, Needleman H, Rabinowitz M. Low level lead exposure, social class, and infant development. Neurotoxicology and teratology. 1988;10:497–503. doi: 10.1016/0892-0362(88)90084-0. [DOI] [PubMed] [Google Scholar]
- Benoit JD, Rakic P, Frick KM. Prenatal stress induces spatial memory deficits and epigenetic changes in the hippocampus indicative of heterochromatin formation and reduced gene expression. Behav Brain Res. 2015;281:1–8. doi: 10.1016/j.bbr.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger MA, Barros VG, Sarchi MI, Tarazi FI, Antonelli MC. Long-term effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. Neurochemical Research. 2002;27:1525–1533. doi: 10.1023/a:1021656607278. [DOI] [PubMed] [Google Scholar]
- Bodwell JE, Gosse JA, Nomikos AP, Hamilton JW. Arsenic disruption of steroid receptor gene activation: Complex dose-response effects are shared by several steroid receptors. Chemical research in toxicology. 2006;19:1619–1629. doi: 10.1021/tx060122q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodwell JE, Kingsley LA, Hamilton JW. Arsenic at very low concentrations alters glucocorticoid receptor (GR)-mediated gene activation but not GR-mediated gene repression: complex dose-response effects are closely correlated with levels of activated GR and require a functional GR DNA binding domain. Chemical research in toxicology. 2004;17:1064–1076. doi: 10.1021/tx0499113. [DOI] [PubMed] [Google Scholar]
- Bommarito PA, Martin E, Smeester L, Palys T, Baker ER, Karagas MR, Fry RC. Fetal-sex dependent genomic responses in the circulating lymphocytes of arsenic-exposed pregnant women in New Hampshire. Reprod Toxicol. 2017;73:184–195. doi: 10.1016/j.reprotox.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomhower SR, Newland MC. Effects of adolescent exposure to methylmercury and d-amphetamine on reversal learning and an extradimensional shift in male mice. Exp Clin Psychopharmacol. 2017;25:64–73. doi: 10.1037/pha0000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Wright RJ, Just AC, Power MC, Tamayo YOM, Schnaas L, Hu H, Wright RO, Tellez-Rojo MM. Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from Mexico City: a cross-sectional study. Environmental health : a global access science source. 2014;13:50. doi: 10.1186/1476-069X-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brkljacic J, Vojnovic Milutinovic D, Dundjerski J, Matic G. Mercury inhibits rat liver and kidney glucocorticoid receptor hormone binding activity. Cell Biol Toxicol. 2004;20:171–182. doi: 10.1023/b:cbto.0000029467.21231.12. [DOI] [PubMed] [Google Scholar]
- Caldwell KE, Labrecque MT, Solomon BR, Ali A, Allan AM. Prenatal arsenic exposure alters the programming of the glucocorticoid signaling system during embryonic development. Neurotoxicology and teratology. 2015a;47:66–79. doi: 10.1016/j.ntt.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell KE, Labrecque MT, Solomon BR, Ali A, Allan AM. Prenatal arsenic exposure alters the programming of the glucocorticoid signaling system during embryonic development. Neurotoxicology and teratology. 2015b;47:66–79. doi: 10.1016/j.ntt.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield RL, Gendle MH, Cory-Slechta DA. Impaired neuropsychological functioning in lead-exposed children. Dev Neuropsychol. 2004;26:513–540. doi: 10.1207/s15326942dn2601_8. [DOI] [PubMed] [Google Scholar]
- Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348:1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carobrez A, Bertoglio L. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neuroscience & Biobehavioral Reviews. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Carratu MR, Coluccia A, Modafferi AM, Borracci P, Scaccianoce S, Sakamoto M, Cuomo V. Prenatal methylmercury exposure: effects on stress response during active learning. Bulletin of environmental contamination and toxicology. 2008;81:539–542. doi: 10.1007/s00128-008-9557-8. [DOI] [PubMed] [Google Scholar]
- Castoldi AF, Blandini F, Randine G, Samuele A, Manzo L, Coccini T. Brain monoaminergic neurotransmission parameters in weanling rats after perinatal exposure to methylmercury and 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB153) Brain Res. 2006;1112:91–98. doi: 10.1016/j.brainres.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Chang CY, Guo HR, Tsai WC, Yang KL, Lin LC, Cheng TJ, Chuu JJ. Subchronic Arsenic Exposure Induces Anxiety-Like Behaviors in Normal Mice and Enhances Depression-Like Behaviors in the Chemically Induced Mouse Model of Depression. Biomed Res Int. 2015;2015:159015. doi: 10.1155/2015/159015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JT, Bellinger DC, Shaywitz BA. A quantitative analysis of prenatal methyl mercury exposure and cognitive development. Am J Prev Med. 2005;29:353–365. doi: 10.1016/j.amepre.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Cohn J, Cox C, Cory-Slechta DA. The effects of lead exposure on learning in a multiple repeated acquisition and performance schedule. Neurotoxicology. 1993;14:329–346. [PubMed] [Google Scholar]
- Cory-Slechta D, Pazmino R, Bare C. The critical role of nucleus accumbens dopamine systems in the mediation of fixed interval schedule-controlled operant behavior. Brain research. 1997a;764:253–256. doi: 10.1016/s0006-8993(97)00591-x. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA. Exposure duration modifies the effects of low level lead on fixed-interval performance. Neurotoxicology. 1990;11:427–442. [PubMed] [Google Scholar]
- Cory-Slechta DA. The role of dopaminergic and glutamatergic neurotransmitter systems in lead-induced learning impairments. In: Dyer R, editor. Neurotoxicology: Effects and Mechanisms. Marcel-Dekker; 1993. [Google Scholar]
- Cory-Slechta DA. Relationships between Pb-induced changes in neurotransmitter system function and behavioral toxicity. Neurotoxicology. 1997;18:673–688. [PubMed] [Google Scholar]
- Cory-Slechta DA. Studying toxicants as single chemicals: does this strategy adequately identify neurotoxic risk? Neurotoxicology. 2005;26:491–510. doi: 10.1016/j.neuro.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Merchant-Borna K, Allen JL, Liu S, Weston D, Conrad K. Variations in the nature of behavioral experience can differentially alter the consequences of developmental exposures to lead, prenatal stress, and the combination. Toxicological Sciences. 2013;131:194–205. doi: 10.1093/toxsci/kfs260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, O'Mara DJ, Brockel BJ. Nucleus accumbens dopaminergic medication of fixed interval schedule-controlled behavior and its modulation by low-level lead exposure. J Pharmacol Exp Ther. 1998;286:794–805. [PubMed] [Google Scholar]
- Cory-Slechta DA, O'Mara DJ, Brockel BJ. Learning versus performance impairments following regional administration of MK-801 into nucleus accumbens and dorsomedial striatum. Behavioural Brain Research. 1999;102:181–194. doi: 10.1016/s0166-4328(99)00015-7. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Pazmino R, Bare C. The critical role of the nucleus accumbens dopamine systems in the mediation of fixed interval schedule-controlled operant behavior. Brain Research. 1997b;764:253–256. doi: 10.1016/s0006-8993(97)00591-x. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Pokora MJ, Preston RA. The effects of dopamine agonists on fixed interval schedule-controlled behavior are selectively altered by low level lead exposure. Neurotoxicology and teratology. 1996;18:565–575. doi: 10.1016/0892-0362(96)00082-7. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Stern S, Weston D, Allen JL, Liu S. Enhanced learning deficits in female rats following lifetime Pb exposure combined with prenatal stress. Toxicological Sciences. 2010;117:427–438. doi: 10.1093/toxsci/kfq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Rossi-George A, Weston D, Thiruchelvam M. Experimental manipulations blunt time-induced changes in brain monoamine levels and completely reverse stress, but not Pb+/-stress-related modifications to these trajectories. Behav Brain Res. 2009;205:76–87. doi: 10.1016/j.bbr.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR. Maternal stress modulates the effects of developmental lead exposure. Environ Health Perspect. 2004;112:717–730. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Putz O, Thomas P, Hayes T, Howdeshell K. Wildlife as models for the study of how mixtures, low doses, and the embryonic environment modulate the action of endocrine-disrupting chemicals. Pure and Applied Chemistry. 2003;75:2305–2320. [Google Scholar]
- Davey JC, Bodwell JE, Gosse JA, Hamilton JW. Arsenic as an Endocrine Disruptor: Effects of Arsenic on Estrogen Receptor–Mediated Gene Expression In Vivo and in Cell Culture. Toxicological Sciences. 2007;98:75–86. doi: 10.1093/toxsci/kfm013. [DOI] [PubMed] [Google Scholar]
- Debes F, Weihe P, Grandjean P. Cognitive deficits at age 22 years associated with prenatal exposure to methylmercury. Cortex. 2016;74:358–369. doi: 10.1016/j.cortex.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt DT. Effect of stress on human biology: Epigenetics, adaptation, inheritance, and social significance. J Cell Physiol. 2017 doi: 10.1002/jcp.25837. [DOI] [PubMed] [Google Scholar]
- Desaulniers D, Xiao GH, Cummings-Lorbetskie C. Effects of lactational and/or in utero exposure to environmental contaminants on the glucocorticoid stress-response and DNA methylation of the glucocorticoid receptor promoter in male rats. Toxicology. 2013;308:20–33. doi: 10.1016/j.tox.2013.03.006. [DOI] [PubMed] [Google Scholar]
- DeWitt RD. Pediatric lead exposure and the water crisis in Flint, Michigan. Journal of the American Academy of Physician Assistants. 2017;30:43–46. doi: 10.1097/01.JAA.0000511794.60054.eb. [DOI] [PubMed] [Google Scholar]
- Dreiem A, Shan M, Okoniewski RJ, Sanchez-Morrissey S, Seegal RF. Methylmercury inhibits dopaminergic function in rat pup synaptosomes in an age-dependent manner. Neurotoxicol Teratol. 2009;31:312–317. doi: 10.1016/j.ntt.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Dyer CA. Heavy metals as endocrine-disrupting chemicals, Endocrine-Disrupting Chemicals. Springer; 2007. pp. 111–133. [Google Scholar]
- Dzwilewski KL, Schantz SL. Prenatal chemical exposures and child language development. Journal of communication disorders. 2015;57:41–65. doi: 10.1016/j.jcomdis.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elez D, Dundjerski J, Matic G. Cadmium affects the redox state of rat liver glucocorticoid receptor. Cell Biol Toxicol. 2001;17:169–177. doi: 10.1023/a:1011940414419. [DOI] [PubMed] [Google Scholar]
- Evans SB, Cory-Slechta DA. Prefrontal cortical manipulations alter the effects of intra-ventral striatal dopamine antagonists on fixed-interval performance in the rat. Behav Brain Res. 2000;107:45–58. doi: 10.1016/s0166-4328(99)00108-4. [DOI] [PubMed] [Google Scholar]
- Gillies GE, Virdee K, Pienaar I, Al-Zaid F, Dalley JW. Enduring, Sexually Dimorphic Impact of In Utero Exposure to Elevated Levels of Glucocorticoids on Midbrain Dopaminergic Populations. Brain Sci. 2016;7 doi: 10.3390/brainsci7010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Bale TL. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. Journal of neuroendocrinology. 2009;21:415–420. doi: 10.1111/j.1365-2826.2009.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggin SL, Labrecque MT, Allan AM. Perinatal exposure to 50ppb sodium arsenate induces hypothalamic-pituitary-adrenal axis dysregulation in male C57BL/6 mice. Neurotoxicology. 2012;33:1338–1345. doi: 10.1016/j.neuro.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J, Tsai LH. Histone acetylation: molecular mnemonics on the chromatin. Nat Rev Neurosci. 2013;14:97–111. doi: 10.1038/nrn3427. [DOI] [PubMed] [Google Scholar]
- Guan H, Qiu Z, Zhou X, Li S, Liu X, Zhang C, Piao F. Protection of Taurine Against Impairment in Learning and Memory in Mice Exposed to Arsenic. Adv Exp Med Biol. 2017;975:255–269. doi: 10.1007/978-94-024-1079-2_23. [DOI] [PubMed] [Google Scholar]
- Haider S, Saleem S, Tabassum S, Khaliq S, Shamim S, Batool Z, Parveen T, Inam QU, Haleem DJ. Alteration in plasma corticosterone levels following long term oral administration of lead produces depression like symptoms in rats. Metab Brain Dis. 2013;28:85–92. doi: 10.1007/s11011-012-9374-y. [DOI] [PubMed] [Google Scholar]
- Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. Elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. American journal of public health. 2016;106:283–290. doi: 10.2105/AJPH.2015.303003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Balbus J, Birnbaum L, Brune-Drisse MN, Grandjean P, Gray K, Landrigan PJ, Sly PD, Suk W, Cory Slechta D. Developmental origins of health and disease: integrating environmental influences. Endocrinology. 2015;156:3416–3421. doi: 10.1210/EN.2015-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavicoli I, Fontana L, Bergamaschi A. The effects of metals as endocrine disruptors. Journal of Toxicology and Environmental Health, Part B. 2009;12:206–223. doi: 10.1080/10937400902902062. [DOI] [PubMed] [Google Scholar]
- Jeong KS, Park H, Ha E, Shin J, Hong YC, Ha M, Park H, Kim BN, Lee B, Lee SJ, Lee KY, Kim JH, Kim Y. High Maternal Blood Mercury Level Is Associated with Low Verbal IQ in Children. J Korean Med Sci. 2017;32:1097–1104. doi: 10.3346/jkms.2017.32.7.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett DA, Kuhlmann AC, Farmer SJ, Guilarte TR. Age-dependent effects of developmental lead exposure on performance in the morris water maze. Pharmacology Biochemistry and Behavior. 1997;57:271–279. doi: 10.1016/s0091-3057(96)00350-4. [DOI] [PubMed] [Google Scholar]
- JS S, Cory-Slechta DA. Epigenetic Mechanisms of Adverse Neurodevelopment in Response to Lead Exposure and Prenatal Stress and the Combination: The Road Ahead. In: Hollar D, editor. Epigenetics, the Environment and Children's Health Across Lifespans. Springer; New York: 2016. pp. 251–278. [Google Scholar]
- Juster RP, Seeman T, McEwen BS, Picard M, Mahar I, Mechawar N, Sindi S, Smith NG, Souza-Talarico J, Sarnyai Z. Social inequalities and the road to allostatic load: From vulnerability to resilience. Developmental Psychopathology 2016 [Google Scholar]
- Keenan K, Gunthorpe D, Grace D. Parsing the relations between SES and stress reactivity: examining individual differences in neonatal stress response. Infant Behav Dev. 2007;30:134–145. doi: 10.1016/j.infbeh.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH. Determinants of the specificity of behavioral effects of drugs. Ergeb Physiol. 1968;60:1–56. doi: 10.1007/BFb0107250. [DOI] [PubMed] [Google Scholar]
- Klocke C, Allen JL, Sobolewski M, Mayer-Pröschel M, Blum JL, Lauterstein D, Zelikoff JT, Cory-Slechta DA. Neuropathological Consequences of Gestational Exposure to Concentrated Ambient Fine and Ultrafine Particles in the Mouse. Toxicological Sciences. 2017;156:492–508. doi: 10.1093/toxsci/kfx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada M, Takao K, Miyakawa T. Elevated Plus Maze for Mice. Journal of Visualized Experiments : JoVE. 2008:1088. doi: 10.3791/1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neuroscience & Biobehavioral Reviews. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A. 12: Are Cadmium and Other Heavy Metal Compounds Acting as Endocrine Disrupters? Metal Ions in Toxicology: Effects, Interactions, Interdependencies. 2010:305–317. doi: 10.1039/9781849732116-00305. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Makino Y, Tanaka H, Dahlman-Wright K, Makino I. Modulation of glucocorticoid-inducible gene expression by metal ions. Mol Pharmacol. 1996;49:612–620. [PubMed] [Google Scholar]
- Martinez-Finley EJ, Ali AM, Allan AM. Learning deficits in C57BL/6J mice following perinatal arsenic exposure: consequence of lower corticosterone receptor levels? Pharmacol Biochem Behav. 2009a;94:271–277. doi: 10.1016/j.pbb.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Finley EJ, Ali AM, Allan AM. Learning deficits in C57BL/6J mice following perinatal arsenic exposure: consequence of lower corticosterone receptor levels? Pharmacology, Biochemistry and Behavior. 2009b;94:271–277. doi: 10.1016/j.pbb.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Tellez RI, Hernandez-Torres E, Gamboa C, Flores G. Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse. 2009;63:794–804. doi: 10.1002/syn.20664. [DOI] [PubMed] [Google Scholar]
- Martinez EJ, Kolb BL, Bell A, Savage DD, Allan AM. Moderate perinatal arsenic exposure alters neuroendocrine markers associated with depression and increases depressive-like behaviors in adult mouse offspring. Neurotoxicology. 2008;29:647–655. doi: 10.1016/j.neuro.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The epigenetics of sex differences in the brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009a;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The epigenetics of sex differences in the brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009b;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain research. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress (Thousand Oaks) 2017:1. doi: 10.1177/2470547017692328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano M, Wang M, Vom Saal F. Sex differences in plasma corticosterone in mouse fetuses are mediated by differential placental transport from the mother and eliminated by maternal adrenalectomy or stress. Journal of reproduction and fertility. 1993;99:283–290. doi: 10.1530/jrf.0.0990283. [DOI] [PubMed] [Google Scholar]
- Montoya ER, Bos PA, Terburg D, Rosenberger LA, van Honk J. Cortisol administration induces global down-regulation of the brain's reward circuitry. Psychoneuroendocrinology. 2014;47:31–42. doi: 10.1016/j.psyneuen.2014.04.022. [DOI] [PubMed] [Google Scholar]
- Moreno Avila CL, Limon-Pacheco JH, Giordano M, Rodriguez VM. Chronic Exposure to Arsenic in Drinking Water Causes Alterations in Locomotor Activity and Decreases Striatal mRNA for the D2 Dopamine Receptor in CD1 Male Mice. J Toxicol. 2016;2016:4763434. doi: 10.1155/2016/4763434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CP, Bale TL. Sex differences in microRNA-mRNA networks: examination of novel epigenetic programming mechanisms in the sexually dimorphic neonatal hypothalamus. Biology of Sex Differences. 2017;8:27. doi: 10.1186/s13293-017-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW. Prenatal methylmercury exposure and children: neurologic, developmental, and behavioral research. Environ Health Perspect. 1998;106 Suppl 3:841–847. doi: 10.1289/ehp.98106841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negron-Oyarzo I, Neira D, Espinosa N, Fuentealba P, Aboitiz F. Prenatal Stress Produces Persistence of Remote Memory and Disrupts Functional Connectivity in the Hippocampal-Prefrontal Cortex Axis. Cereb Cortex. 2015;25:3132–3143. doi: 10.1093/cercor/bhu108. [DOI] [PubMed] [Google Scholar]
- Organization, W.H. World Health Organization guidelines for drinking water quality. World Health Organization; Geneva, Switzerland: 2004. [Google Scholar]
- Ortega HG, Lopez M, Takaki A, Huang QH, Arimura A, Salvaggio JE. Neuroimmunological effects of exposure to methylmercury forms in the Sprague-Dawley rats. Activation of the hypothalamic-pituitary-adrenal axis and lymphocyte responsiveness. Toxicol Ind Health. 1997;13:57–66. doi: 10.1177/074823379701300105. [DOI] [PubMed] [Google Scholar]
- Pakdel R, Rashidy-Pour A. Microinjections of the dopamine D2 receptor antagonist sulpiride into the medial prefrontal cortex attenuate glucocorticoid-induced impairment of long-term memory retrieval in rats. Neurobiol Learn Mem. 2007;87:385–390. doi: 10.1016/j.nlm.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Pandey R, Rai V, Mishra J, Mandrah K, Kumar Roy S, Bandyopadhyay S. From the Cover: Arsenic Induces Hippocampal Neuronal Apoptosis and Cognitive Impairments via an Up-Regulated BMP2/Smad-Dependent Reduced BDNF/TrkB Signaling in Rats. Toxicol Sci. 2017;159:137–158. doi: 10.1093/toxsci/kfx124. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of neuroscience methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Peters A, McEwen BS, Friston K. Uncertainty and stress: Why it causes diseases and how it is mastered by the brain. Prog Neurobiol. 2017;156:164–188. doi: 10.1016/j.pneurobio.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Pottinger T. Interactions of endocrine-disrupting chemicals with stress responses in wildlife. Pure and applied chemistry. 2003;75:2321–2333. [Google Scholar]
- Rager J, Auerbach SS, Chappell GA, Martin E, Thompson C, Fry RC. Benchmark Dose Modeling Estimates of the Concentrations of Inorganic Arsenic that Induce Changes to the Neonatal Transcriptome, Proteome and Epigenome in a Pregnancy Cohort. Chem Res Toxicol. 2017 doi: 10.1021/acs.chemrestox.7b00221. [DOI] [PubMed] [Google Scholar]
- Razmiafshari M, Kao J, d'Avignon A, Zawia NH. NMR identification of heavy metal-binding sites in a synthetic zinc finger peptide: toxicological implications for the interactions of xenobiotic metals with zinc finger proteins. Toxicology and applied pharmacology. 2001;172:1–10. doi: 10.1006/taap.2001.9132. [DOI] [PubMed] [Google Scholar]
- Razmiafshari M, Zawia NH. Utilization of a synthetic peptide as a tool to study the interaction of heavy metals with the zinc finger domain of proteins critical for gene expression in the developing brain. Toxicology and applied pharmacology. 2000;166:1–12. doi: 10.1006/taap.2000.8950. [DOI] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues AJ, Leao P, Carvalho M, Almeida OF, Sousa N. Potential programming of dopaminergic circuits by early life stress. Psychopharmacology (Berl) 2011;214:107–120. doi: 10.1007/s00213-010-2085-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Barranco M, Lacasana M, Aguilar-Garduno C, Alguacil J, Gil F, Gonzalez-Alzaga B, Rojas-Garcia A. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. The Science of the total environment. 2013;454-455:562–577. doi: 10.1016/j.scitotenv.2013.03.047. [DOI] [PubMed] [Google Scholar]
- Rosado JL, Ronquillo D, Kordas K, Rojas O, Alatorre J, Lopez P, Garcia-Vargas G, Del Carmen Caamano M, Cebrian ME, Stoltzfus RJ. Arsenic exposure and cognitive performance in Mexican schoolchildren. Environ Health Perspect. 2007;115:1371–1375. doi: 10.1289/ehp.9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-George A, Virgolini MB, Weston D, Cory-Slechta DA. Alterations in glucocorticoid negative feedback following maternal Pb, prenatal stress and the combination: a potential biological unifying mechanism for their corresponding disease profiles. Toxicology and applied pharmacology. 2009;234:117–127. doi: 10.1016/j.taap.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-George A, Virgolini MB, Weston D, Thiruchelvam M, Cory-Slechta DA. Interactions of Lifetime Lead Exposure and Stress: Behavioral; Neurochemical and HPA Axis Effects. Neurotoxicology. 2011:83–99. doi: 10.1016/j.neuro.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg SE, Yu X, Liu J, Biasini FJ, Hong C, Jiang X, Nong Y, Cheng Y, Korrick SA. Maternal methylmercury exposure through rice ingestion and offspring neurodevelopment: A prospective cohort study. Int J Hyg Environ Health. 2016;219:832–842. doi: 10.1016/j.ijheh.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Anderson DW, Kidd SK, Sobolewski M, Cory-Slechta DA. Sex-dependent effects of lead and prenatal stress on post-translational histone modifications in frontal cortex and hippocampus in the early postnatal brain. Neurotoxicology. 2016;54:65–71. doi: 10.1016/j.neuro.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Cory-Slechta DA. Epigenetics, the Environment, and Children's Health Across Lifespans. Springer; 2016. Epigenetic Mechanisms of Adverse Neurodevelopment in Response to Lead Exposure and Prenatal Stress and the Combination: The Road Ahead; pp. 251–277. [Google Scholar]
- Shah KK, Oleske JM, Gomez HF, Davidow AL, Bogden JD. Blood Lead Concentrations of Children in the United States: A Comparison of States Using Two Very Large Databases. The Journal of Pediatrics. 2017;185:218–223. doi: 10.1016/j.jpeds.2017.01.059. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. Jama. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Sobolewski M, Conrad K, Allen JL, Weston H, Martin K, Lawrence BP, Deborah ACS. Sex-Specific Enhanced Behavioral Toxicity Induced by Maternal Exposure to a Mixture of Low Dose Endocrine-Disrupting Chemicals. Neurotoxicology. 2014 doi: 10.1016/j.neuro.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Talarico JN, Suchecki D, Juster RP, Plusquellec P, Barbosa Junior F, Bunscheit V, Marcourakis T, de Matos TM, Lupien SJ. Lead exposure is related to hypercortisolemic profiles and allostatic load in Brazilian older adults. Environ Res. 2017;154:261–268. doi: 10.1016/j.envres.2017.01.012. [DOI] [PubMed] [Google Scholar]
- Spuches AM, Wilcox DE. Monomethylarsenite competes with Zn2+ for binding sites in the glucocorticoid receptor. J Am Chem Soc. 2008;130:8148–8149. doi: 10.1021/ja802179p. [DOI] [PubMed] [Google Scholar]
- Srivastava P, Dhuriya YK, Gupta R, Shukla RK, Yadav RS, Dwivedi HN, Pant AB, Khanna VK. Protective Effect of Curcumin by Modulating BDNF/DARPP32/CREB in Arsenic-Induced Alterations in Dopaminergic Signaling in Rat Corpus Striatum. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0288-2. [DOI] [PubMed] [Google Scholar]
- Stansfield KH, Ruby KN, Soares BD, McGlothan JL, Liu X, Guilarte TR. Early-life lead exposure recapitulates the selective loss of parvalbumin-positive GABAergic interneurons and subcortical dopamine system hyperactivity present in schizophrenia. Transl Psychiatry. 2015;5:e522. doi: 10.1038/tp.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrou S, Nicolaides NC, Critselis E, Darviri C, Charmandari E, Chrousos GP. Paediatric stress: from neuroendocrinology to contemporary disorders. European journal of clinical investigation. 2017;47:262–269. doi: 10.1111/eci.12724. [DOI] [PubMed] [Google Scholar]
- Sun H, Yang Y, Shao H, Sun W, Gu M, Wang H, Jiang L, Qu L, Sun D, Gao Y. Sodium Arsenite-Induced Learning and Memory Impairment Is Associated with Endoplasmic Reticulum Stress-Mediated Apoptosis in Rat Hippocampus. Front Mol Neurosci. 2017;10:286. doi: 10.3389/fnmol.2017.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer ZM, Kuzawa CW. Early origins of health disparities: material deprivation predicts maternal evening cortisol in pregnancy and offspring cortisol reactivity in the first few weeks of life. Am J Hum Biol. 2014;26:723–730. doi: 10.1002/ajhb.22532. [DOI] [PubMed] [Google Scholar]
- Tsoi MF, Cheung CL, Cheung TT, Cheung BM. Continual Decrease in Blood Lead Level in Americans: United States National Health Nutrition and Examination Survey 1999-2014. Am J Med. 2016;129:1213–1218. doi: 10.1016/j.amjmed.2016.05.042. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Allan AM. The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human and Rodent Studies: A Review. Curr Environ Health Rep. 2014;1:132–147. doi: 10.1007/s40572-014-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezu T, Nakamiya K, Kita K, Ochi T, Shibata Y, Morita M. Diphenylarsinic acid produces behavioral effects in mice relevant to symptoms observed in citizens who ingested polluted well water. Neurotoxicol Teratol. 2012;34:143–151. doi: 10.1016/j.ntt.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma G, Sobolewski M, Cory-Slechta D, Schneider J. Sex-and Brain Region-Specific Effects of Prenatal Stress and Lead Exposure on Permissive and Repressive Post-Translational Histone Modifications from Embryonic Development Through Adulthood. Neurotoxicology. 2017 doi: 10.1016/j.neuro.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgolini MB, Chen K, Weston DD, Bauter MR, Cory-Slechta DA. Interactions of chronic lead exposure and intermittent stress: consequences for brain catecholamine systems and associated behaviors and HPA axis function. Toxicol Sci. 2005;87:469–482. doi: 10.1093/toxsci/kfi269. [DOI] [PubMed] [Google Scholar]
- Virgolini MB, Rossi-George A, Lisek R, Weston DD, Thiruchelvam M, Cory-Slechta DA. CNS effects of developmental Pb exposure are enhanced by combined maternal and offspring stress. Neurotoxicology. 2008a;29:812–827. doi: 10.1016/j.neuro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgolini MB, Rossi-George A, Weston D, Cory-Slechta DA. Influence of low level maternal Pb exposure and prenatal stress on offspring stress challenge responsivity. Neurotoxicology. 2008b;29:928–939. doi: 10.1016/j.neuro.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27:358–368. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen A, Dietrich KN, Radcliffe J, Caldwell KL, Rogan WJ. Postnatal exposure to methyl mercury and neuropsychological development in 7-year-old urban inner-city children exposed to lead in the United States. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence. 2014;20:527–538. doi: 10.1080/09297049.2013.824955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ma Y, Hu J, Zhang X, Cheng W, Jiang H, Li M, Ren J, Zhang X, Liu M, Sun A, Wang Q, Li X. Sex-specific effects of prenatal chronic mild stress on adult spatial learning capacity and regional glutamate receptor expression profiles. Exp Neurol. 2016;281:66–80. doi: 10.1016/j.expneurol.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, van Geen A, Slavkovich V, Lolacono NJ, Cheng Z, Hussain I, Momotaj H, Graziano JH. Water Arsenic Exposure and Children's Intellectual Function in Araihazar, Bangladesh. Environmental Health Perspectives. 2004;112:1329–1333. doi: 10.1289/ehp.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Factor-Litvak P, Kline J, Siddique AB, Shahriar H, Uddin MN, van Geen A, Mey JL, Balac O, Graziano JH. Child Intelligence and Reductions in Water Arsenic and Manganese: A Two-Year Follow-up Study in Bangladesh. Environ Health Perspect. 2016;124:1114–1120. doi: 10.1289/ehp.1509974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. The Intersection of Neurotoxicology and Endocrine Disruption. Neurotoxicology. 2012;33:1410–1419. doi: 10.1016/j.neuro.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston HI, Sobolewski ME, Allen JL, Weston D, Conrad K, Pelkowski S, Watson GE, Zareba G, Cory-Slechta DA. Sex-dependent and non-monotonic enhancement and unmasking of methylmercury neurotoxicity by prenatal stress. Neurotoxicology. 2014a;41:123–140. doi: 10.1016/j.neuro.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston HI, Weston DD, Allen JL, Cory-Slechta DA. Sex-dependent impacts of low-level lead exposure and prenatal stress on impulsive choice behavior and associated biochemical and neurochemical manifestations. Neurotoxicology. 2014b;44:169–183. doi: 10.1016/j.neuro.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BM, Bonilha HS, Ellis C., Jr Racial/Ethnic Differences in Childhood Blood Lead Levels Among Children <72 Months of Age in the United States: a Systematic Review of the Literature. J Racial Ethn Health Disparities. 2016;3:145–153. doi: 10.1007/s40615-015-0124-9. [DOI] [PubMed] [Google Scholar]
- White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, Rossi-George A, Lasley SM, Qian YC, Basha MR. New and evolving concepts in the neurotoxicology of lead. Toxicology and applied pharmacology. 2007;225:1–27. doi: 10.1016/j.taap.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Winterbottom EF, Fei DL, Koestler DC, Giambelli C, Wika E, Capobianco AJ, Lee E, Marsit CJ, Karagas MR, Robbins DJ. GLI3 Links Environmental Arsenic Exposure and Human Fetal Growth. EBioMedicine. 2015;2:536–543. doi: 10.1016/j.ebiom.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbottom EF, Koestler DC, Fei DL, Wika E, Capobianco AJ, Marsit CJ, Karagas MR, Robbins DJ. The aquaglyceroporin AQP9 contributes to the sex-specific effects of in utero arsenic exposure on placental gene expression. Environmental health : a global access science source. 2017;16:59. doi: 10.1186/s12940-017-0267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LL, Gong W, Shen SP, Wang ZH, Yao JX, Wang J, Yu J, Gao R, Wu G. Multiple metal exposures and their correlation with monoamine neurotransmitter metabolism in Chinese electroplating workers. Chemosphere. 2017;182:745–752. doi: 10.1016/j.chemosphere.2017.04.112. [DOI] [PubMed] [Google Scholar]
- Zawia NH, Crumpton T, Brydie M, Reddy GR, Razmiafshari M. Disruption of the zinc finger domain: a common target that underlies many of the effects of lead. Neurotoxicology. 2000;21:1069–1080. [PubMed] [Google Scholar]
- Zhou X, Cooper KL, Sun X, Liu KJ, Hudson LG. Selective Sensitization of Zinc Finger Protein Oxidation by Reactive Oxygen Species through Arsenic Binding. The Journal of Biological Chemistry. 2015;290:18361–18369. doi: 10.1074/jbc.M115.663906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuch CL, O'Mara DJ, Cory-Slechta DA. Low-level lead exposure selectively enhances dopamine overflow in nucleus accumbens: an in vivo electrochemistry time course assessment. Toxicol Appl Pharmacol. 1998;150:174–185. doi: 10.1006/taap.1998.8396. [DOI] [PubMed] [Google Scholar]