Abstract
Purpose
Exercise elicits early adaptation of coronary vessels enabling the coronary circulation to respond adequately to higher flow demands. We hypothesized that short-term daily exercise induces biomechanical and functional remodeling of the coronary resistance arteries related to pressure.
Methods
Male rats were subjected to a progressively increasing 4-week treadmill exercise program (over 60 min/day, 1 mph in the final step). In vitro pressure-diameter measurements were performed on coronary segments (119 ± 5 μm in diameter at 50 mm Hg) with microarteriography. The magnitude of the myogenic response and contribution of endogenous nitric oxide and prostanoid production to the wall mechanics and pressure-diameter relationship were assessed.
Results
Arterioles isolated from exercised animals – compared to the sedentary group – had thicker walls, increased distensibility, and a decreased elastic modulus as a result of reduced wall stress in the low pressure range. The arterioles of exercised rats exhibited a more powerful myogenic response and less endogenous vasoconstrictor prostanoid modulation at higher pressures, while vasodilator nitric oxide modulation of diameter was augmented at low pressures (< 60 mm Hg).
Conclusions
A short-term daily exercise program induces remodeling of rat intramural coronary arterioles, likely resulting in a greater range of coronary autoregulatory function (constrictor and dilator reserves) and more effective protection against great changes in intraluminal pressure, contributing thereby to the optimization of coronary blood flow during exercise.
Keywords: Coronary arterioles, Daily exercise, Elasticity, Contractility, Endothelium, Vascular, Remodeling
Introduction
It has been shown that exercise elicits improvement of all parts of the cardiovascular system [1] and that a sedentary lifestyle is a major but modifiable risk factor for cardiovascular diseases [2–4]. Previous as well as recent findings have demonstrated that even short-term exercise improves the ability of the coronary circulation to accommodate higher flow demands during physical exercise [3, 5, 6] in healthy individuals. Moreover, daily exercise activity has been shown to counteract hypertensive [7, 8], hypercholesterolemic [9], and diabetic [10] pathological vessel wall remodeling, thereby reducing the incidence of coronary heart disease [4, 11–14]. Regular exercise promotes arteriolar growth and increases the lumen of larger coronary arteries [3, 4, 15, 16], enriches the existing microvascular collateral network [3, 4, 14, 15], reduces coronary wall stiffness, and improves coronary endothelial function and the vasodilatory reserve [3, 5, 17–21]. The impact of exercise on vascular remodeling depends upon the training duration and its intensity, and the vascular bed involved [2], but even a short-term exercise program has been shown to elicit beneficial effects [21]. However, many details of exercise-induced remodeling of the coronary vessels are still obscure. Blood supply to ventricular tissue is mostly determined by the resistance of intramural arteries and arterioles. Previous studies showed that important differences exist among the vessel generations regarding their biomechanical and pharmacological properties [3, 22], yet only a few studies have investigated the changes of morphological remodeling, altered wall elasticity, contractility, and endothelium-dependent modulation of coronary resistance arteries and arterioles in response to daily exercise. In vitro pressure myography of intramural coronary small arteries and arterioles [10, 22–25] allows such an investigation to be conducted. Analyzing exercise-induced biomechanical adaptation of intramural coronary arterioles can reveal novel mechanisms underlying the beneficial effects reported in epidemiologic and clinical studies.
Thus, in the present study, we hypothesized that a short-term daily exercise program induces substantial biomechanical remodeling of the coronary arterioles, providing a greater capacity to regulate their resistance and thus control coronary blood flow. In order to test this hypothesis, in isolated coronary arterioles of sedentary and exercised rats, intraluminal pressure-related biomechanical properties and myogenic responses were investigated and the modulatory roles of endothelial nitric oxide (NO) and vasoactive prostanoids were studied.
Methods
Animals and Daily Exercise Protocol
Male Wistar rats weighing 250–300 g were divided into exercised (n = 12) and sedentary (n = 25) groups. Exercised rats were subjected to a 4-week progressively increasing treadmill exercise program, described as follows. During the first week, an initial load of 5 min/day at a speed of 0.5 mph was increased to 20 min/day at 0.8 mph, and on the 5th day the rats ran to exhaustion. Their load reached 40 min/day at 1 mph at the end of the second week and 60 min/day at 1 mph at the end of the last week. The rats ran until exhaustion again on the last day. Rats lagging behind and falling off the treadmill were stimulated by mild electric impulses and helped back onto the lane if necessary. Two days of rest was allowed each week. Control sedentary rats were kept in conventional cages. Food and water were given ad libitum. The protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College and conformed to the current guidelines of the National Institutes of Health and the American Physiological Society for the use and care of laboratory animals.
Chemicals
Indomethacin (INDO; an inhibitor of prostaglandin synthesis) was purchased from Cayman Chemicals (Ann Arbor, MI, USA). Nω-nitro-L-arginine (L-NNA; an inhibitor of NO synthase; NOS) and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Pressure Arteriography of Intramural Coronary Arterioles
After the 4-week exercise program the animals were anesthetized with intraperitoneal injections of sodium pentobarbital (50 mg/kg). The chest was quickly opened and the heart was removed and placed in cold (0–4 ° C) oxygenized Krebs solution. Intramural coronary arterioles, end branches of the left anterior descending coronary artery, located close to the apex, with an outer diameter of approximately 100 μm at preparation were prepared under a microscope (Olympus), as described previously [24]. Arterioles were removed, cannulated at both sides with glass micropipettes (outer diameter approx. 100 μm) and mounted in the glass-bottomed vessel chamber, being carefully extended to their original in situ length. The chamber solution contained (in mM), 110 NaCl, 5 KCl, 2.5 CaCl2, 1 MgSO4, 1 KH2PO4, 24 NaHCO3, and 10 glucose. The solution was bubbled with 21% O2 and 5% CO2 balanced with N2 at 37 ° C. Also, a continuous superfusion of the bath solution was applied at a rate of 15 mL/min.
Mounted coronary arterioles were pressurized in a no-flow condition with a pressure-servo syringe reservoir system (Living Systems, Burlington, VT, USA) and were visualized by video microscopy (Olympus, Lake Sussex, NY, USA). Their inner and outer diameters were measured with a calibrated image sharing monitor (Instrumentation for Physiology and Medicine, San Diego, CA, USA). The intraluminal pressure was measured with a pressure transducer (Gould Statham, Oxnard, CA, USA). Data were recorded on a chart recorder (Omega Engineering, Stamford, CT, USA).
Coronary arterioles were allowed to equilibrate for 1 h in Krebs solution at 60 mm Hg intraluminal pressure, during which all arterioles included in the study developed a substantial active spontaneous tone. Then, the intraluminal pressure was first decreased to 2 mm Hg, which was followed by a stepwise increase up to 150 mm Hg in 10-mm Hg pressure steps. The steady state diameter at each step was measured (allowing approx. 5 min equilibration for each step). The pressure was then set back to 60 mm Hg and either Nω-nitro-L-arginine (L-NNA, 10−5 M, an inhibitor of NOS; Sigma-Aldrich), or INDO (2.8 × 10−5 M, an inhibitor of prostaglandin synthesis; Cayman Chemicals) were administered in the superfusion solution and incubated for 20–30 min. The pressure steps were repeated as above in the presence of the given inhibitor. Finally, a passive pressure-diameter relationship was obtained in Ca2+-free Krebs solution (containing 10−4 M sodium-nitroprusside and 1.0 mM EGTA, Sigma-Aldrich, in addition to the Krebs without CaCl2).
Computations and Statistical Analysis
In coronary arterioles, tangential stress was computed using the Laplace-Frank equation, σ = p · ri/h, where σ is the tangential stress, p is the pressure, ri is the inner radius, and h is the wall thickness. Incremental distensibility was computed as D = ΔV/(VΔp), where ΔV is the change of lumen volume from an initial value of V if a pressure change of Δp is applied. The incremental tangential elastic modulus was computed using the formula Einc = 2(rori2/[ro2 − ri2]) · (Δp/Δro), where ro and ri are the actual values of outer and inner radii, and Δro is the change in outer radius induced by an alteration of intraluminal pressure of Δp [26]. Constrictions of coronary arterioles were characterized as reduction of the inner radius relative to the fully dilated state (passive diameter, relaxed) at the same intraluminal pressure (also referred to as the isobaric active strain). All parameters are expressed as the mean ± standard error of the mean. For statistical comparisons of parameters of sedentary and exercised animals 1- and 2-way ANOVAs were used with the Tukey pairwise comparison test. Significance levels of p < 0.05 were accepted to confirm statistically significant differences.
Results
Body Weight and Heart Weight
The 4-week exercise program elicited a significant reduction in the body weight of rats compared to sedentary rats (382.8 ± 6.7 vs. 405.3 ± 6.7 g, p < 0.05), whereas their heart weights did not differ (1.32 ± 0.04 vs. 1.30 ± 0.03 g).
Daily Exercise-Induced Alterations in Geometry and Elasticity of Coronary Arterioles
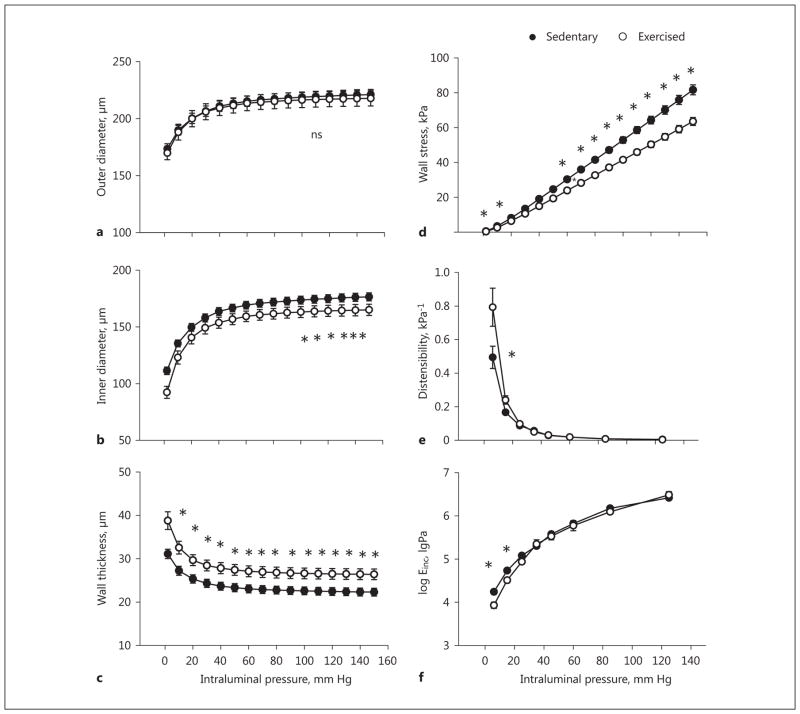
Compared to sedentary coronary arterioles, in the maximally dilated state, the outer diameters were not different (Fig. 1a), whereas the inner diameter and isobaric tangential wall stress were significantly reduced, and wall thickness was significantly increased in the coronary arterioles of exercised rats (Fig. 1b–d). Also, the coronary arterioles of exercised rats showed significantly increased isobaric distensibility and reduced tangential elastic moduli at low pressures (Fig. 1e, f).
Fig. 1.
Exercise-training induced remodeling of rat intramural coronary arterioles. Geometrical and elastic alterations in the passive state compared with sedentary controls. Transmural pressure is plotted against outer diameter (a), inner diameter (b), wall thickness (c), tangential wall stress (d), incremental distensibility (e), and incremental tangential elastic modulus (f). Mean values were used from 2-way ANOVA tests with Tukey paired comparisons: * p < 0.05 between sedentary (n = 25) and exercised (n = 12) groups.
Daily Exercise-Induced Alterations in the Myogenic Response of Coronary Arterioles
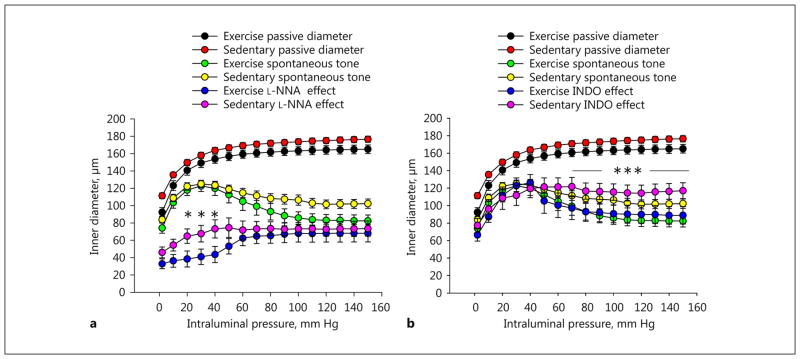
In the lower intraluminal pressure range (up to 40–50 mm Hg), spontaneous tone and diameters were not different between the 2 groups of vessels. At a higher pressure range, however, the arteriolar diameters of exercised rats were significantly smaller compared to those of sedentary rats (Fig. 2, 3a).
Fig. 2.
Exercise training-induced remodeling of rat intramural coronary arterioles. The inner diameter as a function of increasing transmural pressure was raised in a stepwise manner. Spontaneous tone and myogenic constriction in the control condition (green, trained; yellow, sedentary; a, b), in the effect of the NOS inhibitor Nω-nitro-L-arginine (L-NNA, 10−5 M; blue, trained; magenta, sedentary; a), and in the effect of the cyclo-oxygenase inhibitor indomethacin (INDO, 2.8 × 10−5 M; b). Passive pressure-diameter curves are also shown for easy comparison (black, trained; red, sedentary; a, b). Mean values were used from 2-way ANOVA tests with Tukey paired comparisons: * p < 0.05 and *** p < 0.001 between sedentary (n = 10) and exercised (n = 6) groups in the presence of the inhibitors.
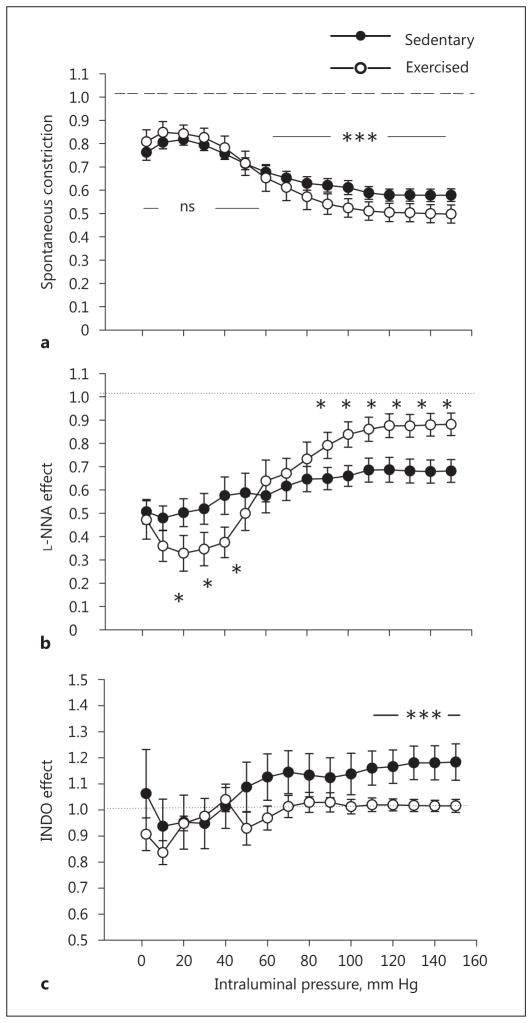
Fig. 3.
Exercise training-induced remodeling of rat intramural coronary arterioles compared with sedentary controls. a Inner diameter in spontaneous tone and myogenic constriction expressed as a ratio of the passive diameter. The dashed line refers to the diameter of arterioles in the passive condition, taken as the reference. Alteration of the spontaneously contracted inner diameter in response to the NOS inhibitor Nω-nitro-L-arginine (L-NNA, 10−5 M; b) and the cyclo-oxygenase inhibitor (INDO, 2.8 × 10−5 M; c). The dotted line (b, c), representing the diameter of arterioles in control myogenic constriction, was taken as a reference at each pressure level. Mean values were used from 2-way ANOVA tests with Tukey paired comparisons: * p < 0.05 and *** p < 0.001 between sedentary (n = 10) and exercised (n = 6) groups.
Daily Exercise Alters the Modulation of Myogenic Response of Coronary Arterioles by Endogenous NO
In the presence of the endothelial NOS (eNOS) inhibitor L-NNA (10 μM), the myogenic response of coronary arterioles was significantly modulated, and was different in the sedentary and exercised groups. At zero pressure, the arteriolar diameters in both groups reduced similarly and significantly. However, in the presence of L-NNA, the coronary arteriolar diameters of exercised rats at lower pressure values were reduced compared to sedentary rats, whereas at higher pressure values arteriolar diameters were raised more in exercised vessels (Fig. 2a, 3b).
Daily Exercise Alters the Modulation of Myogenic Response of Coronary Arterioles by Endogenous Prostanoids
In the presence of INDO, a nonspecific inhibitor of cyclooxygenases (thus the inhibitor of prostanoid production), there were no significant differences in the arteriolar diameters of the 2 groups at the lower range of intraluminal pressure. However, with increases in intraluminal pressure to a higher range (above 70 mm Hg), coronary arteriolar diameters isolated from sedentary rats remained higher compared to those from exercised rats (Fig. 2b, 3c).
Discussion
The salient findings of the present study are that a 4-week daily exercise program induced significant alterations in the morphology, biomechanical characteristics, and myogenic response of intramural arterioles (approx. 120-μm inner diameter at 50 mm Hg) isolated from the left anterior descendent coronary artery, supplying the apical ventricular cardiac muscle of the rat heart. This morphological and functional remodeling of coronary arterioles likely contributes to a wider autoregulatory range and an increased dilator/constrictor reserve, allowing great changes in coronary blood flow during exercise activity.
Daily Exercise-Induced Alterations in Morphology and Biomechanics of Coronary Arterioles
We found that changes in morphological and geometrical parameters of coronary arterioles were persistent at a wide range of intraluminal pressures (Fig. 1a–c). An increased wall thickness in the fully dilated state without a corresponding change in outer diameter suggests that short-term daily exercise program elicited a hypertrophic wall remodeling. We have found elastic alterations, namely an increased isobaric distensibility and reduced elastic modulus, which could partly be due to geometrical alterations and reduced values of isobaric tangential wall stress (Fig. 1d–f). The few available publications report divergent findings regarding the effects of exercise activity on coronary artery wall elasticity. The stiffness of large subepicardial coronary arteries has been shown to be reduced [17, 27]. The physiological importance is that an elevated isobaric distensibility can contribute to a more effective opening of the coronaries during sudden elevation of transmural pressures, such as occurs during the exercise-modulated cardiac cycle [22].
Daily Exercise-Induced Alterations in the Myogenic Response of Coronary Arterioles
Inner diameters of coronary arterioles in response to the elevation of intraluminal pressure up to 50 mm Hg did not differ significantly between the exercised and sedentary groups (Fig. 2, 3a), i.e., their myogenic response did not differ in this pressure range.
A further stepwise elevation of intraluminal pressure induced an active myogenic response of isolated coronary arterioles [22–25], which was more powerful in the vessels of exercised rats, reducing their diameter below that of coronary arterioles of sedentary rats. Exercise-induced elevated myogenic tones have also been described in previous studies [3, 18, 19, 28, 29]. A potential explanation is that during exercise the dilation of larger proximal arteries [15] accompanied with elevated arterial pressures resulted in pressure elevations in the coronary arterioles investigated in the present study, and this in turn induced morphological thickening of the wall, as well as development of a more powerful myogenic response in the higher pressure range. An increased myogenic tone in exercise-trained vessels is likely to be mediated by calcium-dependent PKC signaling mechanisms [3, 29]. Changes in vascular myogenic responses can also be attributed to calcium oscillations induced by alterations of the activity of voltage-sensitive calcium channels or cGMP-sensitive calcium-dependent chloride channels, as well as voltage-dependent or calcium-dependent potassium channels [8, 30]. One has to take into consideration that a higher myogenic tone means a greater potential range for diameter increase evoked by other vasomotor mechanisms, such as flow-dependent and metabolic responses. For example, at 100 mm Hg, the diameters of coronary arterioles of exercised and sedentary rats were 39 ± 3 and 48 ± 4% of passive diameters, respectively.
Based on the Hagen-Poiseuille law and supposing unaltered blood viscosity, the theoretical possibility to elevate segmental hemodynamic conductance from spontaneous myogenic tone to full dilation would be 7.2-fold in the vessels of sedentary rats, while it would be 13.3-fold in the vessels of exercised rats. Therefore, the present study revealed the underlying mechanism of the elevated dilation reserve, a key factor of exercise-induced coronary remodeling described by earlier studies [6, 14, 16, 19, 31, 32]. In other words, the greater “resting” coronary tone allows a higher dilatory reserve that “can be used” by metabolic vasodilator mechanisms, such as adenosine-induced vasodilation [3, 33], and also by a NO-mediated flow-induced dilator mechanism [34].
Daily Exercise Alters the Modulation of Myogenic Response of Coronary Arterioles by Endogenous NO
The findings with the NOS inhibitor L-NNA (Fig. 2a, 3b) suggest that coronary arterioles of exercised rats would have been almost closed in the range of low pressures (< 50 mm Hg) without the continuous dilatory effect of endogenously produced NO. At higher pressures, however, an opposite condition may exist, in which the limited dilator effect of NO allows the full-strength action of myogenic response in exercised arterioles as compared to that of sedentary coronary arterioles.
We previously showed that L-NNA modulates the basal tone of these coronary arterioles [25], whereas the present work suggests that after the daily exercise program the L-NNA-induced modulation of basal tone greatly depends on the prevailing intraluminal pressure level. In contrast to the sedentary condition, the basal NO production seems to be an important factor keeping the lumen of coronary arterioles of exercised rats open below a pressure of 50 mm Hg. However, when the pressure is further elevated, a less effective NO dilator modulation allows the development of a more forceful pressure-induced myogenic constrictor response. Improvements of coronary endothelial dilations in exercised rats have also been reported, though results are somewhat heterogeneous [2, 3, 17, 18, 20]. Exercise, in addition to eliciting greater changes in the intraluminal pressure, also increases wall shear stress, acting on the endothelial cell surface layer and inducing acute and chronic adaptation mechanisms, such as increased endothelial NO release or decreased endothelin levels, thereby decreasing calcium signals in the vascular smooth muscle cells [3, 4, 20, 21]. It has also been observed in humans that exercise training augments shear-mediated arterial dilation opposing myogenic constriction [35]. Previously, we found that a short-term and chronic exercise program augmented the role of endothelial NO mediation of agonist- and flow-induced dilations in skeletal muscle arterioles [34, 36].
Extrapolating these findings to in vivo conditions, one can propose that a higher level of NO-induced dilation at low pressures may contribute to the opening of the lumen of exercised coronary arterioles (especially those of the left ventricle) during the diastolic phase of the cardiac cycle. On the other hand, the limited NO effect at higher pressures (systolic phase) protects the coronary arterioles form overdistension. Although, the present study was conducted in so-called no-flow conditions, the results still indicate that daily exercise affects the endothelial function of coronary arterioles, as well as indirectly confirming previous findings and demonstrating that the endothelium has an important modulatory effect on their myogenic response.
In the present study, we used inhibitors of NO and prostaglandin synthesis because removal of endothelium from arterioles can sometimes injure smooth muscle, leading to the misinterpretation of findings. Nevertheless, we can safely assume that both NO and prostaglandins were produced and released from the endothelium of these arterioles. In previous studies, we investigated the role of endothelium in the mediation of arteriolar dilation both by the removal and by the inhibition of NO and PG synthesis, and found that in the absence of endothelium these factors are not produced, i.e., dilations are not observed [34, 36]. Thus, our study revealed that the enhanced basal release of endothelium-derived NO and PG is an important modulator of the myogenic mechanism even in no-flow conditions.
Daily Exercise Reduces Endogenous Prostanoid-Induced Myogenic Constriction in Coronary Arterioles
Inhibition of the synthesis of prostanoids in coronary arterioles elicited the attenuation of a pressure-induced myogenic response, indicating the presence of vasoconstrictor prostanoids in the modulation of coronary tone over 50 mm Hg (Fig. 2b, 3c). One important observation of the present study is that the high pressure-induced vasoconstrictor prostanoid component of the myogenic response – which we have described previously [25] – was not present in the coronary arterioles of exercised rats (Fig. 2b). These observations suggest a complex rearrangement of endogenous prostanoid production/effect by daily exercise in the intramural coronary arterioles. The more powerful myogenic response in the presence of higher pressures seems to develop without the contribution of constrictor prostaglandins in the exercised coronaries. Coronary arteries have been found to synthesize both constrictor and dilator prostanoids [37, 38] competing with each other in different physiological and pathological conditions [27, 37–40]. Indeed, we previously described the differential sensitivity of coronary arterioles from different locations in response to PGF2alfa and in the modulatory role of constrictor prostanoids on the myogenic response [24, 25]. In contrast to coronaries, in skeletal muscle arterioles, we found that a daily exercise program augmented the role of vasodilator prostaglandins mediating flow-induced dilation [34]. The present study shows that short-term daily exercise – in a pressure-dependent manner – alters the production of vasoconstrictor and vasodilator endogenous prostanoids in the wall of coronary arterioles.
Limitations and Interpretation of the Findings
These in vitro findings should be carefully extrapolated to in vivo conditions when transmural pressure changes during the cardiac cycle. Based on a theoretical consideration, the pressure in intramural coronary arterioles in the diameter range of approximately 120 μm can be about 45– 55 mm Hg, depending on the diastolic and systolic cycles [41]. The coronary arterioles included in this study were located in the myocardium of the apex, several hundred micrometers deep from the surface, thus transmural pressure greatly changes during cardiac cycles. Transition between the large distensibility-low pressure and low distensibility-high pressure sections of the pressure-diameter relationship usually refers to levels of physiological in vivo transmural pressures [26]. Analysis of the pressure-passive diameter curves of vessels (Fig. 1a, b, Fig. 2) suggests a working point of around 30 mm Hg in vivo. We have previously shown [25] that at around 50 mm Hg of transmural pressure an important border for the elastic, contractile, and pharmacological properties exists for these vessels. In addition, in the present study we found that with short-term daily exercise-induced remodeling, wall properties below and above this value are differently affected.
Importance of Flow-Induced Vasodilation in Exercise-Induced Vascular Remodeling
During exercise, blood flow/velocity and thus wall shear stress also increases substantially, which elicits dilation [4]. Together with the myogenic constrictor mechanism (systemic blood pressure also increases during exercise), shear stress-induced modulation contributes to the development of the final diameter/tone of coronary arterioles, which depends on the prevailing levels of pressure and wall shear stress, and also on the daily exercise-induced functional and morphological vascular remodeling (investigated in the present study). Indeed, in our previous studies we demonstrated that an identical short-term exercise program augmented flow/shear stress-induced dilations of arterioles mediated by NO and prostaglandins [34, 36, 42, 43]. In this study, we also found an enhanced basal tone of exercised-arterioles compared to sedentary vessels [34]. Alteration of both myogenic and flow/shear stress mechanisms together allow a greater regulatory range of diameter of coronary arterioles serving the needs of increased blood flow to cardiac tissues during exercise. This was discussed in detail in recent review papers [3, 4, 20].
Importance of the Functional Remodeling of Coronary Arterioles to Daily Exercise Activity
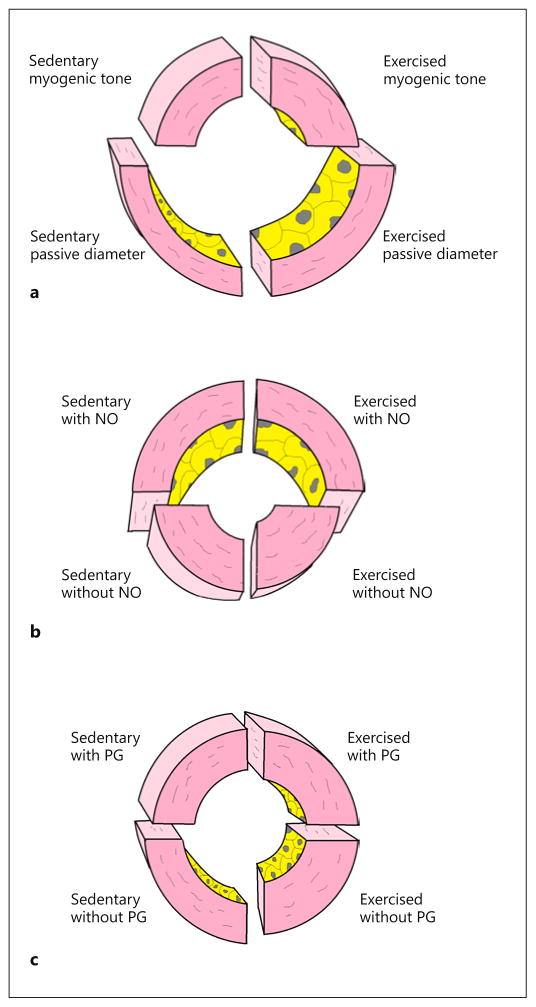
A schematic representation of daily exercise training-induced remodeling of rat intramural coronary arterioles according to the above findings is depicted in Figure 4. Ranges of constriction and dilation are illustrated according to our findings, revealing that in coronary arterioles of exercised rats, ranges of myogenic constriction and NO-dependent dilation are increased at low pressures (illustrated at 30 mm Hg). Interestingly, at high pressures (illustrated at 120 mm Hg), an additional prostanoid-induced constriction modifies the spontaneous myogenic tone, which is attenuated in response to daily exercise training (Fig. 4a–c). Thus, according to our findings the benefits of exercise training are that the coronary NO-dependent dilator range is augmented in parallel with a decreased prostanoid-dependent constrictor control of vascular tone. The augmented myogenic response at a higher pressure range may also be involved in the control of capillary fluid permeability during exercise when the systemic pressure is high. Such functional remodeling provides a possible beneficial effect of exercise training by restoring vascular reserve functions in certain pathological conditions with depressed vascular reactivity and vasodilator reserve [9, 10, 19, 32, 33].
Fig. 4.
Exercise training-induced remodeling of rat intramural coronary arterioles: a schematic representation. Wall sections of exercised and sedentary male rat coronary arteriole segments in spontaneous myogenic tone without (a) and with (b, c) inhibitors. a Coronary arterioles in control myogenic tone at 120 mm Hg intraluminal pressure and with the passive diameter. b The effect of the NO inhibitor Nω-nitro-L-arginine (in the presence of prostanoids) reveals an increased (NO-mediated) dilation effect in myogenic tone at 30 mm Hg intraluminal pressure in the coronary arterioles of exercised rats. c The effect of the cyclo-oxygenase inhibitor INDO (in the presence of NO) reveals the presence of endogenous constrictor prostanoids at 120 mm Hg intraluminal pressure in the coronary arterioles of sedentary animals, which is missing in the exercised group.
Mechanical forces, such as shear stress, are importantly involved in the long-term remodeling of vessels, especially in response to regular exercise [3, 4, 20]. It has been shown that regular exercise augments endothelium-dependent dilations not only in the conduit coronary arteries, but also throughout the coronary arteriolar network [3, 20]. In response to a daily exercise program, several enzymes, such as eNOS Mg-SOD, are upregulated, resulting in increased levels of NO, known to prevent unnecessary growth/thickening of the vascular wall. Flow/shear stress-induced endothelium-dependent effects are involved in the remodeling of the vessel wall by optimizing shear stress and elasticity in response to the exercise program [3, 4, 20, 44].
Exercise training has been found to be beneficial in cardiovascular pathologies, such as hypertension, diabetes, or aging-induced cardiovascular diseases. Exercise program-induced vascular remodeling was shown to reverse attenuated NO-dependent vasodilation in diabetes [10], and also reversed hypertension-induced alterations in the expression of K+ channels observed in thoracic aorta smooth muscle cells [8].
Issue of Gender Difference in Exercise-Induced Functional Vascular Remodeling
It is important to note that our study was conducted only in male rats. This was due to the fact that we wanted our findings to be comparable to our previous studies in which flow/shear stress-induced responses were investigated in the vessels of male rats. Nevertheless, in previous studies we found that the presence of estrogen upregulates eNOS and NO production and the dilator capacity of arterioles [45, 46]. Also, we previously found sex differences in functional and biomechanical characteristics of coronary arteries: in female coronaries constrictor responses were decreased, while estrogen replacement therapy after ovariectomy augmented bradykinin-induced NO-mediated dilation in these vessels [47, 48]. In brief, all these findings warrant future studies on how exercise programs affect the vascular function and morphology of female species.
Issue of Aging in Exercise-Induced Functional Vascular Remodeling
Our investigation focused on young animals. However, modulation of the myogenic mechanism by exercise could be important in older age as well. Indeed, animal and human studies revealed that moderate- and long-term exercise activity significantly reduces cardiovascular risk factors, and therefore cardiovascular morbidity and mortality [1, 3, 4, 8, 10, 44]. We have recently shown that from birth to senescence morphological and functional remodeling leads to an increased contractile capacity of arteries [49]. Thus, one can hypothesize that this could be tempered by regular exercise-induced enhanced release of NO and prostaglandins [50].
Conclusions
The present study identified several morphological, biomechanical, and functional adaptations of the intramural coronary arterioles in response to a short-term daily exercise program, which have not been previously reported. Specifically, short-term daily exercise induces adaptation/remodeling of rat intramural coronary arterioles: in the low intraluminal pressure range the distensibility and endothelium-dependent modulation of myogenic tone were augmented, whereas at higher pressures wall thickness increased, wall stress reduced, the myogenic response increased, and the effect of constrictor prostanoids was diminished. Such remodeling of coronary arterioles is likely to result in a greater range of coronary autoregulation, constrictor and dilator reserves, and a more effective protection against great changes in intraluminal pressure, thereby contributing to the optimization of coronary blood flow and capillary permeability function during exercise.
Acknowledgments
This work was supported by the Hungarian Scientific Research Funds (OTKA) K108444, K116954, and FP7 Marie Sklodowska Curie projects – Small Artery Remodeling (SmART and SmArter; A.K.) and grants of NIH PO1 HL-43023 (G. Kaley) and NIH HL-46813 (A.K.). The results leading to this publication could not have been attained without the gracious help of the late Prof. Gabor Kaley (New York Medical College, Valhalla, USA).
Footnotes
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Lavie CJ, Arena R, Swift DL, Johannsen NM, Sui X, Lee DC, Earnest CP, Church TS, O’Keefe JH, Milani RV, Blair SN. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res. 2015;117:207–219. doi: 10.1161/CIRCRESAHA.117.305205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thijssen DH, Maiorana AJ, O’Driscoll G, Cable NT, Hopman MT, Green DJ. Impact of inactivity and exercise on the vasculature in humans. Eur J Appl Physiol. 2010;108:845–875. doi: 10.1007/s00421-009-1260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laughlin MH, Bowles DK, Duncker DJ. The coronary circulation in exercise training. Am J Physiol Heart Circ Physiol. 2012;302:H10–H23. doi: 10.1152/ajpheart.00574.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev. 2017;97:495–528. doi: 10.1152/physrev.00014.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laughlin MH, Muller JM. Vasoconstrictor responses of coronary resistance arteries in exercise-trained pigs. J Appl Physiol. 1998;84:884–889. doi: 10.1152/jappl.1998.84.3.884. [DOI] [PubMed] [Google Scholar]
- 6.Laughlin MH, Oltman CL, Bowles DK. Exercise training-induced adaptations in the coronary circulation. Med Sci Sports Exerc. 1998;30:352–360. doi: 10.1097/00005768-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Rossoni LV, Oliveira RA, Caffaro RR, Miana M, Sanz-Rosa D, Koike MK, Do Amaral SL, Michelini LC, Lahera V, Cachofeiro V. Cardiac benefits of exercise training in aging spontaneously hypertensive rats. J Hypertens. 2011;29:2349–2358. doi: 10.1097/HJH.0b013e32834d2532. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Lu N, Shi L. Exercise training reverses alterations in Kv and BKCa channel molecular expression in thoracic aorta smooth muscle cells from spontaneously hypertensive rats. J Vasc Res. 2014;51:447–457. doi: 10.1159/000369928. [DOI] [PubMed] [Google Scholar]
- 9.Thompson MA, Henderson KK, Woodman CR, Turk JR, Rush JW, Price E, Laughlin MH. Exercise preserves endothelium-dependent relaxation in coronary arteries of hypercholesterolemic male pigs. J Appl Physiol. 2004;96:1114–1126. doi: 10.1152/japplphysiol.00768.2003. [DOI] [PubMed] [Google Scholar]
- 10.Trask AJ, Delbin MA, Katz PS, Zanesco A, Lucchesi PA. Differential coronary resistance microvessel remodeling between type 1 and type 2 diabetic mice: impact of exercise training. Vasc Pharm. 2012;57:187–193. doi: 10.1016/j.vph.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher GF, Balady G, Blair SN, Blumenthal J, Caspersen C, Chaitman B, Epstein S, Sivarajan Froelicher ES, Froelicher VF, Pina IL, Pollock ML. Statement on exercise: benefits and recommendations for physical activity programs for all Americans. A statement for health professionals by the Committee on Exercise and Cardiac Rehabilitation of the Council on Clinical Cardiology, American Heart Association. Circulation. 1996;94:857–862. doi: 10.1161/01.cir.94.4.857. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Balady GJ, Criqui MH. Primary prevention of coronary heart disease: guidance from Framingham: a statement for healthcare professionals from the AHA task force on risk reduction. Circulation. 1998;97:1876–1887. doi: 10.1161/01.cir.97.18.1876. [DOI] [PubMed] [Google Scholar]
- 14.Gielen S, Schuler G, Hambrecht R. Exercise training in coronary artery disease and coronary vasomotion. Circulation. 2001;103:E1–E6. doi: 10.1161/01.cir.103.1.e1. [DOI] [PubMed] [Google Scholar]
- 15.White FC, Bloor CM, McKirnan MD, Carroll SM. Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. J Appl Physiol. 1998;85:1160–1168. doi: 10.1152/jappl.1998.85.3.1160. [DOI] [PubMed] [Google Scholar]
- 16.Windecker S, Allemann Y, Billinger M, Pohl T, Hutter D, Orsucci T, Blaga L, Meier B, Seiler C. Effect of endurance training on coronary artery size and function in healthy men: an invasive follow-up study. Am J Physiol Heart Circ Physiol. 2002;282:H2216–H2223. doi: 10.1152/ajpheart.00977.2001. [DOI] [PubMed] [Google Scholar]
- 17.Stewart JM, Xu X, Ochoa M, Hintze TH. Exercise reduces epicardial coronary artery wall stiffness: roles of cGMP and cAMP. Med Sci Sports Exerc. 1998;30:220–228. doi: 10.1097/00005768-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Heaps CL, Sturek M, Rapps JA, Laughlin MH, Parker JL. Exercise training restores adenosine-induced relaxation in coronary arteries distal to chronic occlusion. Am J Physiol Heart Circ Physiol. 2000;278:H1984–H1992. doi: 10.1152/ajpheart.2000.278.6.H1984. [DOI] [PubMed] [Google Scholar]
- 19.Kozakova M, Galetta F, Gregorini L, Bigalli G, Franzoni F, Giusti C, Palombo C. Coronary vasodilator capacity and epicardial vessel remodeling in physiological and hypertensive hypertrophy. Hypertension. 2000;36:343–349. doi: 10.1161/01.hyp.36.3.343. [DOI] [PubMed] [Google Scholar]
- 20.Duncker DJ, Bache RJ, Merkus D. Regulation of coronary resistance vessel tone in response to exercise. J Mol Cell Cardiol. 2012;52:802–813. doi: 10.1016/j.yjmcc.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Shabeeh H, Seddon M, Brett S, Melikian N, Casadei B, Shah AM, Chowienczyk P. Sympathetic activation increases NO release from eNOS but neither eNOS nor nNOS play an essential role in exercise hyperemia in the human forearm. Am J Physiol Heart Circ Physiol. 2013;304:H1225–H1230. doi: 10.1152/ajpheart.00783.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szekeres M, Nadasy GL, Dézsi L, Orosz M, Tökés A, Monos E. Segmental differences in geometric, elastic and contractile characteristics of small intramural coronary arteries. J Vasc Res. 1998;35:332–344. doi: 10.1159/000025603. [DOI] [PubMed] [Google Scholar]
- 23.Nadasy GL, Szekeres M, Dezsi L, Varbiro S, Szekacs B, Monos E. Brief communication. Preparation of intramural small coronary artery and arteriole segments and resistance artery networks from the rat heart for microarteriography and for in situ perfusion video mapping. Microvasc Res. 2001;61:282–286. doi: 10.1006/mvre.2000.2297. [DOI] [PubMed] [Google Scholar]
- 24.Szekeres M, Dezsi L, Nadasy G, Kaley G, Koller A. Pharmacologic inhomogeneity between the reactivity of intramural coronary arteries and arterioles. J Cardiovasc Pharmacol. 2001;38:584–592. doi: 10.1097/00005344-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Szekeres M, Nadasy GL, Kaley G, Koller A. Nitric oxide and prostaglandins modulate pressure-induced myogenic responses of intramural coronary arterioles. J Cardiovasc Pharmacol. 2004;43:242–249. doi: 10.1097/00005344-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Cox RH. Three dimensional characteristics of arterial smooth muscle activation in vitro. J Appl Physiol. 1974;36:381–384. doi: 10.1152/jappl.1974.36.3.381. [DOI] [PubMed] [Google Scholar]
- 27.Hintze TH, Kaley G. Prostaglandins and the control of blood flow in the canine myocardium. Circ Res. 1977;40:313–320. doi: 10.1161/01.res.40.3.313. [DOI] [PubMed] [Google Scholar]
- 28.Muller JM, Myers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise trained-pigs. Circulation. 1994;89:2308–2314. doi: 10.1161/01.cir.89.5.2308. [DOI] [PubMed] [Google Scholar]
- 29.Korzick DH, Laughlin MH, Bowles DK. Alterations in PKC signaling underlie enhanced myogenic tone in exercise-trained porcine coronary resistance arteries. J Appl Physiol. 2004;96:1425–1432. doi: 10.1152/japplphysiol.01077.2003. [DOI] [PubMed] [Google Scholar]
- 30.Jacobsen JG, Aalkjaer C, Nilsson H, Matchkov VV, Freiberg J, Holstein-Rathlou NH. A model of smooth muscle cell synchronization in the arterial wall. Am J Physiol Heart Circ Physiol. 2007;293:H229–H237. doi: 10.1152/ajpheart.00727.2006. [DOI] [PubMed] [Google Scholar]
- 31.Heaps CL, Mattox ML, Kelly KA, Meininger CJ, Parker JL. Exercise training increases basal tone in arterioles distal to chronic coronary occlusion. Am J Physiol Heart Circ Physiol. 2006;290:H1128–H1135. doi: 10.1152/ajpheart.00973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshinaga K, Beanlands RS, Dekemp RA, Lortie M, Morin J, Aung M, McKelvie R, Davies RF. Effect of exercise training on myocardial blood flow in patients with stable coronary artery disease. Am Heart J Heart Circ Physiol. 2006;151:1324e11–e18. doi: 10.1016/j.ahj.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 34.Koller A, Huang A, Sun D, Kaley G. Exercise training augments flow-dependent dilation in rat skeletal muscle arterioles: role of endothelial nitric oxide and prostaglandins. Circ Res. 1995;76:544–550. doi: 10.1161/01.res.76.4.544. [DOI] [PubMed] [Google Scholar]
- 35.Atkinson CL, Carter HH, Naylor LH, Dawson EA, Marusic P, Hering D, Schlaich MP, Thijssen DH, Green DJ. Opposing effects of shearmediated dilation and myogenic constriction on artery diameter in response to handgrip exercise in humans. J Appl Physiol. 2015;119:858–864. doi: 10.1152/japplphysiol.01086.2014. [DOI] [PubMed] [Google Scholar]
- 36.Sun D, Huang A, Koller A, Kaley G. Enhanced NO-mediated dilations in skeletal muscle arterioles of chronically exercised rats. Microvasc Res. 2002;64:491–496. doi: 10.1006/mvre.2002.2450. [DOI] [PubMed] [Google Scholar]
- 37.Karmazyn M, Dhalla NS. Physiological and pathophysiological aspects of cardiac prostaglandins. Can J Physiol Pharmacol. 1983;61:1207–1225. doi: 10.1139/y83-180. [DOI] [PubMed] [Google Scholar]
- 38.Gerritsen ME, Printz MP. Sites of prostaglandin synthesis in the bovine heart and isolated bovine coronary microvessels. Circ Res. 1981;49:1152–1163. doi: 10.1161/01.res.49.5.1152. [DOI] [PubMed] [Google Scholar]
- 39.Needleman P, Kaley G. Cardiac and coronary prostaglandin synthesis and function. N Engl J Med. 1978;298:1122–1128. doi: 10.1056/NEJM197805182982005. [DOI] [PubMed] [Google Scholar]
- 40.Okada T. Hypoxia-induced change in prostanoids production and coronary flow in isolated rat heart. J Mol Cell Cardiol. 1991;23:939–948. doi: 10.1016/0022-2828(91)90136-a. [DOI] [PubMed] [Google Scholar]
- 41.Vis MA, Sipkema P, Westerhof N. Modeling pressure-flow relations in cardiac muscle in diastole and systole. Am J Physiol Heart Circ Physiol. 1997;272:H1516–H1526. doi: 10.1152/ajpheart.1997.272.3.H1516. [DOI] [PubMed] [Google Scholar]
- 42.Sun D, Huang A, Koller A, Kaley G. Shortterm daily exercise activity enhances endothelial NO synthesis in skeletal muscle arterioles of rats. J Appl Physiol. 1994;76:2241–2247. doi: 10.1152/jappl.1994.76.5.2241. [DOI] [PubMed] [Google Scholar]
- 43.Sun D, Huang A, Koller A, Kaley G. Adaptation of flow-induced dilation of arterioles to daily exercise. Microvasc Res. 1998;56:54–61. doi: 10.1006/mvre.1998.2083. [DOI] [PubMed] [Google Scholar]
- 44.Sarelius I, Pohl U. Control of muscle blood flow during exercise: local factors and integrative mechanisms. Acta Physiol. 2010;199:349–365. doi: 10.1111/j.1748-1716.2010.02129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang A, Sun D, Koller A, Kaley G. Gender difference in flow-induced dilation and regulation of shear stress: role of estrogen and nitric oxide. Am J Physiol. 1998;275:R1571–R1577. doi: 10.1152/ajpregu.1998.275.5.R1571. [DOI] [PubMed] [Google Scholar]
- 46.Wu Y, Huang A, Sun D, Falck JR, Koller A, Kaley G. Gender-specific compensation for the lack of NO in the mediation of flow-induced arteriolar dilation. Am J Physiol Heart Circ Physiol. 2001;280:H2456–H2461. doi: 10.1152/ajpheart.2001.280.6.H2456. [DOI] [PubMed] [Google Scholar]
- 47.Mericli M, Nádasy GL, Szekeres M, Várbíró S, Vajo Z, Mátrai M, Ács N, Monos E, Székács B. Estrogen replacement therapy reverses changes in intramural coronary resistance arteries in rats caused by female sex hormone depletion. Cardiovasc Res. 2004;61:317–324. doi: 10.1016/j.cardiores.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 48.Matrai M, Mericli M, Nadasy GL, Szekeres M, Varbiro S, Banhidy F, Acs N, Monos E, Szekacs B. Gender differences in biomechanical properties of intramural coronary resistance arteries of rats, an in vitro microarteriographic study. J Biomech. 2007;40:1024–1030. doi: 10.1016/j.jbiomech.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Ivic I, Vamos Z, Cseplo P, Koller A. From newborn to senescence morphological and functional remodeling leads to increased contractile capacity of arteries. J Gerontol A Biol Sci Med Sci. 2017;72:481–488. doi: 10.1093/gerona/glw085. [DOI] [PubMed] [Google Scholar]
- 50.Sun D, Huang A, Koller A, Kaley G. Decreased arteriolar sensitivity to shear stress in adult rats is reversed by chronic exercise activity. Microcirculation. 2002;9:91–97. doi: 10.1038/sj/mn/7800124. [DOI] [PubMed] [Google Scholar]