Abstract
Objective
To determine the effects of weight loss and weight loss maintenance (WLM) on weight-specific health-related quality of life (HRQOL) in a 66-week trial.
Methods
Adults with obesity (N=137, 86.1% female, 68.6% black, mean age=46.1 years) who had lost ≥5% of initial weight in a 14-week intensive lifestyle intervention/low-calorie diet (LCD) program were randomized to lorcaserin or placebo for an additional 52-week WLM program. The Impact of Weight on Quality of Life (IWQOL-Lite) scale (including five subscales), Patient Health Questionnaire-9 (depression), and Perceived Stress Scale were administered at the start of the 14-week LCD program, randomization, and week 52 of the randomized controlled trial (RCT; i.e., 66 weeks total).
Results
Significant improvements in all outcomes, except weight-related public distress, were found following the 14-week LCD program (ps<0.05). Improvements were largely maintained during the 52-week RCT, despite weight regain of 2.0–2.5kg across treatment groups. Participants who lost ≥10% of initial weight achieved greater improvements in physical function, self-esteem, sexual life, and the IWQOL-Lite total score than those who lost <5%, and did not differ from those who lost 5–9.9%.
Conclusions
Improvements in weight-specific HRQOL were achieved with moderate weight loss and sustained during WLM.
Keywords: Quality of life, psychosocial, obesity, weight loss
Introduction
High-intensity lifestyle interventions induce losses of 5–10% of initial body weight that are associated with improvements in cardiovascular disease (CVD) risk factors (1,2). These losses also are associated with improvements in health-related quality of life (HRQOL), commonly measured by the Short Form 36 Health Survey (SF-36)(3). On the whole, weight loss is associated with improvements in physical HRQOL but little to no improvement in mental HRQOL, as assessed by the SF-36 (4–7). Relatedly, studies have found small to minimal improvements in depressive symptoms and perceived stress following lifestyle intervention (8–13).
In addition to general HRQOL, aspects of weight-specific HRQOL frequently are assessed by the Impact of Weight on Quality of Life Scale – Lite (IWQOL-Lite), which consists of a total score and five subscales: physical function; self-esteem; sexual life; work; and public distress about one’s weight (14). Greater weight loss is typically associated with increased (i.e., improved) total scores on the IWQOL-Lite (15–19). Results are less consistent for changes on the IWQOL-Lite subscales. Some studies have shown positive effects of weight loss interventions across all subscales (17,20); others have found benefits most consistently for physical functioning (17–21); while some studies also have shown benefits for other subscales such as self-esteem (17,18) and sexual life (15–17,19).
Patients typically do not report additional improvements in HRQOL when acute weight loss ends, after approximately 6 to 9 months, and weight loss maintenance begins. Maintaining weight loss is widely known to be less innately rewarding than losing weight (16,22). Moreover, following completion of a high-intensity lifestyle intervention, individuals with obesity are vulnerable to weight regain, which may be associated with deterioration in HRQOL (and CVD risk factors) (23, 24). Thus, novel interventions are needed to sustain long-term weight loss, in order to maintain improvements in HRQOL and other health outcomes.
The current study examined whether lorcaserin, a serotonin 2C receptor agonist approved for chronic weight management (25,26), would facilitate the maintenance of weight loss and improvements in weight-specific HRQOL and related psychosocial outcomes. Specifically, we examined changes in weight-specific HRQOL, depression, and perceived stress in patients who had lost 5% or more of initial weight during a 14-week intensive lifestyle intervention that included a low-calorie diet (LCD). We anticipated that participants would achieve substantial improvements in these outcomes, particularly in physical health aspects of HRQOL, at the end of this 14-week intervention. Thereafter, we hypothesized that participants randomly assigned to receive lorcaserin for the ensuing 52 weeks would maintain larger weight losses and, thus, significantly greater improvements in weight-specific HRQOL than those assigned to placebo. Consistent with prior studies (7, 15), we also expected that, independent of treatment condition, larger weight losses would be associated with greater improvements in weight-specific HRQOL at the end of the trial.
Methods
Study Design
As described previously (27), this was a two-phase weight loss maintenance trial. Participants attended a 14-week, non-randomized, intensive lifestyle intervention/LCD program (i.e., phase 1), followed by a 52-week double-blind randomized controlled trial (RCT; i.e., phase 2) for participants who lost ≥5% of initial weight during phase 1. The study protocol was approved by the university’s institutional review board, and a data and safety monitoring board provided trial oversight (27).
The current study represents a secondary analysis of the RCT, with primary findings reported previously (28). In sum, 137 participants completed the 14-week LCD program, lost >5% of initial weight, and accepted randomization in a 52-week RCT. Participants lost a mean 9.3±2.9% of initial weight (10.7±4.0 kg) during the 14-week LCD program, which was associated with clinically meaningful (≥5%) improvements in cardiometabolic risk factors (e.g., blood pressure and insulin) (28). Participants in the two groups gained 2.0 ± 0.8 and 2.5 ± 0.8 kg, respectively, from randomization to week 52, with no significant differences between groups (28). Thus, lorcaserin did not significantly improve the maintenance of lost weight at the end of the 52-week trial. As measured from the start of the 14-week LCD program, lorcaserin and placebo-treated participants maintained losses at week 52 of the RCT (66 weeks in total) of 7.8±0.8% and 6.6±0.9% (9.4 and 7.5 kg), respectively (p = 0.32) (28). Additionally, the physical health component score of the SF-36 improved significantly from the start of the 14-week LCD program to week 52 (increase from 47.8 to 50.9, p < .001) but the mental health component score did not (increase from 51.0 to 51.3, p = .71) (28).
Participants
Adults with obesity were recruited (27) and were eligible for phase 1 if they were age 21–65 years, and had a body mass index (BMI) ≥33 kg/m2 and ≤55 kg/m2 (or ≥30 kg/m2 and an obesity-related comorbidity). Exclusion criteria included serious medical conditions and current major depression or use of anti-depressants (27). Participants who did not lose ≥5% of initial weight in phase 1 were not eligible for phase 2.
Procedures
During phase 1, participants were provided 14 weekly, 90-minute group lifestyle modification sessions. They were prescribed a structured 1000–1200 kcal/d diet that included the daily consumption of four meal replacement shakes (HMR 800), combined with an evening meal of a frozen-food (or shelf-stable) entrée, a garden salad, and two servings of fruit. Participants were instructed to record their daily food intake and increase their physical activity, as described previously (27).
Participants who completed the 14-week LCD program, lost ≥5% of initial weight, and wished to continue to participate were randomly assigned to lorcaserin or placebo (initiating phase 2). Participants also were provided group weight loss maintenance (WLM) counseling every-other-week for the first 12 weeks (i.e., 6 sessions) and once every 4 weeks for the remainder of the 52 weeks (i.e., 10 sessions). Approximately half of the sessions were conducted in person and the other half by teleconference, given the potentially greater convenience to participants of conference calls. Participants were instructed to consume a calorie-reduced diet of conventional foods based on current dietary guidelines in order to maintain their weight loss (although participants were allowed to continue to lose weight if desired). Participants also were instructed to increase their physical activity to 225 minutes/week by week 40 and to continue to monitor their food intake and weigh themselves at least once per week.
Study Outcomes
Weight-specific HRQOL and related psychosocial outcomes were assessed at baseline (prior to the start of phase 1), randomization (after completion of phase 1), and week 52 of phase 2. Measures included the IWQOL-Lite (14), the Patient Health Questionnaire (PHQ-9) (29), and the Perceived Stress Scale – 14 item version (30). The IWQOL-Lite includes five subscales that assess the effects of weight on physical function, self-esteem, sexual life, work, and public distress. A total score is also computed. Scores are transformed and range from 0–100, with higher scores signifying better QOL. The PHQ-9 is recommended by the American Psychiatric Association as an optimal, brief depression scale (31). Scores range from 0 to 27, with established cutoffs of 0–4 for minimal symptoms of depression, 5–9 for mild, 10–14 for moderate, 15–19 for moderately severe, and 20–27 for severe symptoms of depression (29). The Perceived Stress Scale assesses thoughts and feelings related to stressful events in the past month, with scores ranging from 0–56 (higher scores indicate greater perceived stress) (30). All scales were pro-rated to account for missing items. Height and weight were measured at screening, and weight was measured at all on-site group visits.
Statistical Analyses
Baseline means and changes from baseline to week 14 were calculated for all measures. Analysis of variance and logistic regression were used to test for differences in patient characteristics (demographics, height, and weight) by treatment condition at randomization; any characteristics that differed and were significantly associated with outcome measures were included in subsequent analyses that compared conditions.
Repeated measures analyses of variance (ANOVAs) were used to determine whether changes in weight-specific HRQOL measures from randomization (week 0) to week 52 differed by condition. Analyses were repeated to assess changes from the start of the LCD program (week -14) to week 52. Additionally, hierarchical linear regression models were used to determine the contribution of weight loss to changes in weight-specific HRQOL, depression, and perceived stress, controlling for variables that may be related to weight loss: demographics (age, race, gender); baseline body mass index (BMI); and treatment condition. Continuous predictor variables included in the regression analyses were centered at their means. Analyses of covariance (ANCOVAs) were used to determine the effects of losing <5%, 5–9.9%, and ≥10% of body weight on all weight-specific HRQOL outcomes, controlling for demographics, baseline BMI, and treatment condition. Post-hoc paired comparisons tested for differences in weight-specific HRQOL among the three levels of weight loss.
Results
Participant Characteristics
A total of 178 participants began phase 1 of the study. Of these participants, 143 lost ≥5% of initial weight during phase 1, and 137 accepted randomization into phase 2. At baseline, these 137 participants had a mean age of 46.1±10.1 years, and the majority were female (86.1%) and black (68.6%; 24.1% white, 2.9% Asian, and 4.4% multiracial/other). Mean (±SD) BMI at baseline was 40.8±5.9 kg/m2. At randomization, all characteristics were evenly distributed between groups except for height. Height did not correlate significantly with changes in any HRQOL or psychosocial outcome and, thus, was not included as a covariate in between-group analyses of phase 2 data. Overall, 81.8% (112/137) of participants completed the week-52 assessment, including 59 of 69 (85.5%) assigned to lorcaserin and 53 of 68 (77.9%) to placebo (21).
Changes in Weight-Specific HRQOL Outcomes
Phase 1
At the end of the 14-week LCD program, participants reported significant improvements in the IWQOL-Lite total score, as well as the physical function, self-esteem, and sexual life subscales with baseline values increasing by approximately 9 to 13 points (see Table 1). Work scores also improved significantly (by 7.4 points), whereas the 2.4 point increase on the public distress subscale was not statistically significant (p = 0.14). PHQ-9 and Perceived Stress Scale scores decreased significantly (p values < 0.01) but modestly (2.5 and 1.8 points, respectively).
Table 1.
Weight-specific HRQOL and psychosocial characteristics at baseline and after the 14-week low-calorie diet program (i.e., randomization) for participants who qualified for randomization (N = 137).
| Variable | Baseline | Randomization | Change from Baseline to Randomization | Statistical Comparison (p) |
|---|---|---|---|---|
| IWQOL – Lite | ||||
| Total Score | 67.3 ± 19.1 | 76.6 ± 17.7 | 9.3 ± 14.4 | < 0.001 |
| Physical Function | 63.8 ± 22.5 | 76.8 ± 18.0 | 13.0 ± 18.7 | < 0.001 |
| Self-Esteem | 55.6 ± 26.3 | 65.8 ± 26.3 | 10.2 ± 19.4 | < 0.001 |
| Sexual Life | 70.2 ± 29.2 | 79.4 ± 25.7 | 9.2 ± 22.7 | < 0.001 |
| Public Distress | 77.9 ± 24.6 | 80.3 ± 24.3 | 2.4 ± 18.4 | 0.14 |
| Work | 80.3 ± 22.2 | 87.7 ± 18.4 | 7.4 ± 18.7 | < 0.001 |
| PHQ – 9 | 4.9 ± 5.0 | 2.5 ± 3.0 | −2.5 ± 4.5 | < 0.001 |
| Perceived Stress Scale | 20.6 ± 8.5 | 18.8 ± 7.8 | −1.8 ± 6.8 | 0.004 |
Note. Values shown are the mean ± standard deviation. Due to missing data, Ns ranged from 128–134. HRQOL=health-related quality of life; IWQOL=Impact of Weight on Quality of Life; PHQ-9=Patient Health Questionnaire-9.
Phase 2
Randomization values (at the end of the 14-week LCD program) of weight-specific HRQOL and psychosocial measures did not differ significantly between the lorcaserin- and placebo-treated participants with one exception, as shown in Table 2. Public distress scores were significantly higher in the placebo than the lorcaserin group.
Table 2.
Participants’ weight-specific HRQOL and psychosocial characteristics at the time of randomization to lorcaserin plus weight loss maintenance (WLM) counseling or placebo + WLM counseling (N = 137).
| Variable | Lorcaserin + WLM (n = 69) | Placebo + WLM (n = 68) |
|---|---|---|
| IWQOL – Lite | ||
| Total Score | 75.9 ± 19.0 | 77.8 ± 16.1 |
| Physical Function | 75.9 ± 18.1 | 78.1 ± 17.8 |
| Self-Esteem | 67.4 ± 25.7 | 65.1 ± 26.9 |
| Sexual Life | 81.5 ± 25.0 | 78.4 ± 26.1 |
| Public Distress a | 75.9 ± 27.7 | 85.4 ± 18.9 |
| Work | 85.3 ± 22.1 | 90.3 ± 12.4 |
| PHQ – 9 | 2.2 ± 3.2 | 2.7 ± 2.7 |
| Perceived Stress Scale | 19.2 ± 8.0 | 18.2 ± 8.0 |
Note. Values shown are the mean ± standard deviation. HRQOL=health-related quality of life; IWQOL= Impact of Weight on Quality of Life; PHQ-9= Patient Health Questionnaire – 9.
There were no significant differences between treatment conditions, except on the Impact of Weight on Quality of Life Public Distress subscale.
Table 3 shows that, at the end of the 52-week RCT, scores on the IWQOL-Lite scales remained close to their randomization values. For example, on the IWQOL-Lite total score, estimated marginal means increased from randomization by 0.1 points (on a 100-point scale) in lorcaserin-treated participants, and 3.6 points for placebo. Repeated measures ANOVAs revealed that the difference between groups was not significant for changes between randomization and week 52 on the IWQOL-Lite total score or any of the subscales. When examining the main effect of time (thus collapsing across conditions), the public distress score improved significantly from randomization to week 52 (p = 0.04), with no other significant changes over time in any of the other IWQOL-Lite subscales.
Table 3.
Changes in weight-specific HRQOL and psychosocial outcomes at week 52, as measured from randomization (week 0) and the start of the low-calorie diet program (week -14).
| Variable | Lorcaserin + WLM | Placebo + WLM | Comparison Between Groups (p) | Change for Total Sample |
|---|---|---|---|---|
| IWQOL – Lite Total | ||||
| From week 0 | +0.1±1.8 | +3.6±2.0 | 0.18 | +1.9±1.3 |
| From week -14 | +10.3±2.0 | +11.6±2.3 | 0.68 | +11.0±1.5*** |
| IWQOL – Lite Physical Function | ||||
| From week 0 | −0.5±2.2 | +5.6±2.5 | 0.08 | +2.5±1.7 |
| From week -14 | +14.1±2.8 | +18.1±3.2 | 0.36 | +16.1±2.1*** |
| IWQOL – Lite Self-Esteem | ||||
| From week 0 | −1.0±2.9 | +4.2±3.2 | 0.23 | +1.6±2.1 |
| From week -14 | +9.6±3.1 | +14.9±3.5 | 0.26 | +12.3±2.3*** |
| IWQOL – Lite Sexual Life | ||||
| From week 0 | −1.6±3.0 | +1.9±3.4 | 0.44 | +0.1±2.2 |
| From week -14 | +8.6±2.9 | +9.2±3.4 | 0.89 | +8.9±2.2*** |
| IWQOL – Lite Public Distress | ||||
| From week 0 | +4.5±2.0 | +1.8±2.2 | 0.35 | +3.1±1.5* |
| From week -14 | +4.7±2.8 | +7.1±3.2 | 0.58 | +5.9±2.1** |
| IWQOL – Lite Work | ||||
| From week 0 | −0.4±1.9 | +0.3±2.2 | 0.81 | <−0.1±1.4 |
| From week -14 | +10.2±2.7 | +2.2±3.2 | 0.06 | +6.2±2.1** |
| PHQ-9 | ||||
| From week 0 | +2.2±0.5 | +0.1±0.6 | 0.01 | +1.1±0.4** |
| From week -14 | −0.9±0.7 | −1.7±0.7 | 0.41 | −1.3±0.5* |
| Perceived Stress Scale | ||||
| From week 0 | +1.5±1.0 | +0.8±1.1 | 0.65 | +1.1±0.8 |
| From week -14 | −0.7±1.2 | −1.1±1.3 | 0.84 | −.9±0.9 |
Note. Values shown are estimated marginal means (for change scores) ± standard error of the completer population. Statistical significance of repeated measures analyses of variance for the total sample (testing for changes in scores over time) is indicated as follows:
p< 0.05
p< 0.01
p<0.001.
Due to missing data, ns for the total sample ranged from 83 to 101. Ns by condition ranged from 45 to 55 for lorcaserin, and 37 to 47 for placebo. HRQOL = health-related quality of life; IWQOL –Lite = Impact of Weight on Quality of Life; PHQ-9 = Patient Health Questionnaire – 9; WLM = weight loss maintenance.
PHQ-9 estimated marginal mean scores increased by 2.2 points from randomization to week 52 in lorcaserin-treated participants, which was significantly (p< 0.01) greater than the 0.1 increase in the placebo group. Marginal mean scores on the Perceived Stress Scale also increased slightly in both groups (1.5 and 0.8 points, respectively), with no significant differences between groups. Examining the main effect of time, the Perceived Stress Scale score did not change significantly from randomization to week 52. Altogether, during the 52-week RCT, weight-specific HRQOL and psychosocial outcomes did not change substantially from randomization values, with the exception of the modest improvement on the public distress subscale and a small increase in symptoms of depression among lorcaserin-treated participants.
Phases 1 and 2 combined
Participants in both the lorcaserin- and placebo-treated groups generally maintained large improvements on the various measures of weight-specific HRQOL, as assessed from the start of the 14-week LCD program (week -14) to the end of the 52-week RCT. Table 3 shows that the two treatment groups did not differ significantly on changes on any of the measures over the total 66-week period. Of note, lorcaserin-treated participants concluded the study with an overall estimated 0.9 point reduction on the PHQ-9, despite the small increase in scores during phase 2. When examining the main effect of time, statistically significant improvements were observed on the IWQOL-Lite total score and all five subscales, as well as on the PHQ-9. The Perceived Stress Scale was the only measure that did not change significantly over the 66 weeks.
Weight Loss as a Determinant of Changes in Weight-Specific HRQOL
Phase 1
Linear step-wise regression analyses, which included demographic variables and baseline BMI, showed that weight loss did not account for a significant amount of the variance in changes in the weight-specific HRQOL and psychosocial variables during phase 1.1 For example, it accounted for only an additional 0.6% of the variance (p = 0.38) in the improvement in the IWQOL-Lite total score at week 14, with a total of 2.3% of the variance explained by all variables.
Phase 2
In contrast to phase 1, weight change from randomization to week 52 was significantly associated with changes in the IWQOL-Lite total score and three of the five subscales. Controlling for demographic variables, BMI, and treatment condition, weight loss accounted for an additional 15.5% of the variance in the change in IWQOL total score (b = 0.76, SE = 0.18, p < 0.01) and for 10–13% of the variance in changes in physical function (b = 0.90, SE = 0.24, p < 0.01), self-esteem (b = 0.98, SE = 0.31, p < 0.01), and work (b = 0.68, SE = 0.20, p < .001). Weight loss in phase 2 was not significantly associated with changes in sexual life (b = 0.60, SE = 0.33 p = 0.07), public distress (b = 0.29, SE = 0.23, p = 0.21), depression (b = −0.07, SE = 0.06, p = 0.26), or perceived stress (b = 0.04, SE = 0.12, p = 0.73).
Phases 1 and 2 combined
Table 4 shows the regression results for the effects of weight loss on weight-specific HRQOL and psychosocial outcomes, as measured from the start of the 14-week LCD program (week -14) to week 52 of the RCT (i.e., 66 weeks in total), controlling for all covariates. Cumulative weight loss accounted for 9.1% of the variance in the change in the IWQOL-Lite total score at week 52 (p < 0.01). Among the subscales, the strongest association was between weight loss and improvements in physical function (R2 = 0.10, p < 0.01). Weight loss was also associated with improvements in self-esteem (R2 = 0.05 p = 0.04) and was marginally associated with improvements in sexual life (R2 = 0.04, p = 0.06). Cumulative weight loss at week 52 was not associated with changes in public distress, work, depression, or perceived stress.
Table 4.
Effects of weight loss on changes in weight-specific HRQOL and psychosocial outcomes from the start of the low-calorie diet program to week 52 (66 weeks total)
| Dependent Variable | Total R2a | ΔR2 for Weight Loss | b | SE | β | p value |
|---|---|---|---|---|---|---|
| IWQOL-Lite | ||||||
| Total Score | 0.11 | 0.09 | 0.54 | 0.19 | 0.33 | 0.005 |
| Physical Function | 0.13 | 0.10 | 0.79 | 0.26 | 0.35 | 0.003 |
| Self Esteem | 0.08 | 0.05 | 0.60 | 0.28 | 0.24 | 0.037 |
| Sexual Life | 0.08 | 0.04 | 0.53 | 0.28 | 0.23 | 0.057 |
| Public Distress | 0.04 | 0.01 | 0.20 | 0.27 | 0.09 | 0.467 |
| Work | 0.11 | 0.02 | 0.37 | 0.26 | 0.16 | 0.159 |
| PHQ-9 | 0.03 | <0.01 | 0.04 | 0.06 | 0.07 | 0.555 |
| Perceived Stress Scale | 0.01 | <0.001 | 0.01 | 0.11 | 0.01 | 0.925 |
Note.
Regression model included baseline age, race, gender, body mass index, treatment condition, and weight loss from baseline to week 52.
HRQOL = health-related quality of life; IWQOL = Impact of Weight on Quality of Life; PHQ-9 = Patient Health Questionnaire – 9.
Categorical Weight Loss
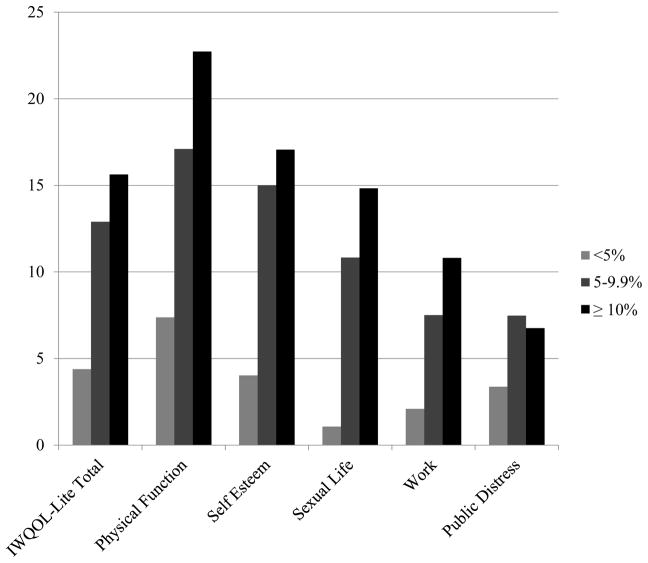
Figure 1 displays the effects of losing <5%, 5–9.9%, and ≥ 10% weight on all weight-specific HRQOL outcomes, as measured from the start of the 14-week LCD program to the end of the 52-week RCT. Significant effects of categorical weight loss (e.g., ≥ 10%) were found for the IWQOL-Lite total score, as well as the physical function, self-esteem, and sexual life subscales. ANCOVA results showed that the main effect of categorical weight loss was significant for these four variables (p values ranged from 0.01 to 0.04, values ranged from 0.08 to 0.12). Pair-wise comparisons showed that for all four variables, weight losses of ≥10% were associated with significantly greater improvements in the weight-specific HRQOL scores than were losses <5% (p values < 0.05). Losses of 5–9.9% were associated with significantly greater improvements on the IWQOL-Lite total score than were losses of < 5% (p = 0.04). However, improvements in physical function, self-esteem, and sexual life were only marginally greater for losses of 5–9.9% than for those <5% (0.05 < p values < 0.10). Losses of ≥10% of initial weight were not associated with significantly greater improvements on any of the four variables than were losses of 5.0–9.9%. No significant effects of categorical weight loss were found for the work and public distress subscales, PHQ-9 scores, or perceived stress.
Figure 1.
Changes in IWQOL – Lite scores by percent weight loss
Note. Analyses of covariance showed significant differences for the IWQOL-Lite total score, as well as the physical function, self-esteem, and sexual life subscales, controlling for all covariates (p values < 0.04; p values > 0.05 for work and public distress subscales). Change scores were significantly smaller for participants who lost < 5% of their weight (n = 32) in comparison to those who lost ≥ 10% (n = 34). IWQOL-Lite total change scores were significantly greater for patients who lost 5–9.9% (n = 23) than for those who lost <5% of their weight (p = 0.04). Scores did not differ between participants who lost ≥ 5% vs. ≥ 10% of their starting weight. IWQOL = Impact of Weight on Quality of Life.
Discussion
This study observed distinctly different changes in weight-specific HRQOL and related psychosocial outcomes during the weight loss and weight loss maintenance phases of the trial. During the initial 14-week LCD program that produced a mean loss of 9.3% of initial weight, participants achieved statistically significant improvements in total IWQOL-Lite scores, as well as the physical function, sexual life, self-esteem, and work subscales. Additionally, mean symptoms of depression, which were low to begin with (due to the exclusion of participants with major depressive disorder or on anti-depressants), declined significantly, though modestly. The present findings are consistent with prior reports of the favorable effects of initial weight loss on multiple components of weight-specific HRQOL and related psychosocial outcomes (7,11,17).
Weight loss, however, was not linearly related to improvements in weight-specific HRQOL and psychosocial outcomes during the 14-week LCD program. It accounted, for example, for only 0.6% of the variance in the change in IWQOL-Lite total scores. The lack of a relationship is likely due to the requirement that all participants had to have lost at least 5% of initial weight to be randomized into the 52-week trial, which limited variability in the sample’s weight losses.
In contrast to the improvements in weight-specific HRQOL during the initial weight loss phase, scores on these measures did not change appreciably during the 52-week RCT, during which weight increased, on average, by 2.0–2.5 kg. As reported previously (28), lorcaserin did not significantly improve weight loss at week 52, as compared with placebo, and we observed no differences between groups in the maintenance of improvements in weight-specific HRQOL scores achieved in the first 14 weeks. PHQ-9 depression scores increased significantly more in lorcaserin- than placebo-treated patients from randomization to week 52 (+2.2 vs. +0.1, respectively). However, mean scores for both groups were within normal limits at both times, and lorcaserin-treated participants completed the trial with a mean PHQ-9 score 0.9 points less than their value at the start of the 14-weeek LCD program.
Examining the treatment groups together, weight-specific HRQOL scores generally did not deteriorate during the 52-week RCT, despite the mean weight regain of 2.0–2.5 kg. Preservation of improvements may have been attributable to the relatively small amount of regain and to the social and cognitive support provided by the 16-session WLM intervention. We were surprised to find that public distress about one’s weight, which did not change with weight loss during phase 1, improved significantly during phase 2 (with weight stability). Distress related to weight stigma may take more time to improve, due to the pervasiveness of such prejudice and some individuals’ internalization of negative weight-based stereotypes (32,33). It also may have improved with the additional social, cognitive, and behavioral support provided by the WLM intervention.
Collapsing across treatment groups, all measures of weight-specific HRQOL and depressive symptoms (though not perceived stress) improved significantly from the start of the 14-week LCD program to the end of the 52-week RCT (i.e., 66 total weeks in total). The magnitude of improvement was greatest for physical function, and larger weight losses were associated with greater improvements in the total IWQOL-Lite score, as well as the physical function and self-esteem subscales. Additionally, losses of 10% or more of initial weight conferred significantly greater benefits than the loss of <5%. This clear effect of ≥ 10% weight loss on weight-specific HRQOL is consistent with prior findings suggesting that large magnitudes of weight loss are needed to substantially improve HRQOL (34). Further studies are needed to clarify the psychosocial effects of 5–9.9% weight loss.
Conclusion
Adults with obesity who achieved an average of 9.3% weight loss during a 14-week LCD program achieved improvements in several measures of weight-specific HRQOL, particularly physical function, as assessed by the IWQOL-Lite. These improvements were generally maintained during a 52-week RCT which provided 16 group WLM sessions, combined with lorcaserin or placebo. Larger weight losses were associated with greater overall improvements in physical function, self-esteem, and total IWQOL-Lite scores, and we found evidence of particularly robust benefits with a loss of ≥10% of initial weight. Adjunctive interventions that target weight-related public distress, depression, and perceived stress may be needed to improve these aspects of psychosocial well-being in patients who report these or related problems.
What is already known
Weight loss is often, but not always, associated with improvements in health-related quality of life (HRQOL).
Such improvements are typically greater for physical versus mental HRQOL.
Weight regain is associated with deteriorations in HRQOL.
What this study adds
Adults with obesity, who lost an average of 9.3% of initial weight in a 14-week intensive lifestyle intervention (with a low-calorie diet), reported significant improvements in weight-specific HRQOL and related psychosocial outcomes, with the greatest improvements in physical functioning.
Improvements were sustained during a 52-week weight loss maintenance program that included behavioral and pharmacological treatment, despite mean weight regain of 2.0–2.5 kg.
Long-term weight loss was linearly related to some but not all aspects of weight-specific HRQOL and psychosocial outcomes. A weight loss of ≥10% was associated with significant improvements in HRQOL.
Acknowledgments
Funding: This study was supported by an investigator-initiated grant from Eisai Pharmaceutical Co (TAW). RLP is supported by a grant from the NHLBI/NIH (#K23HL140176). AMC was supported by an NRSA postdoctoral fellowship from the NINR/NIH (#T32NR007100).
Footnotes
Data available upon request.
Clinical Trial Registration: NCT02388568
Disclosure: RLP discloses serving as a consultant for Weight Watchers. TAW discloses serving on advisory boards for Novo Nordisk and Weight Watchers, as well as receiving grant support, on behalf of the University of Pennsylvania, from Eisai Co and Novo Nordisk. RIB discloses serving as a consultant to Eisai Co. JST discloses serving as a consultant for Novo Nordisk. AMC discloses receiving grant support from Shire Pharmaceuticals, outside the current work. None of the other authors declares any conflicts of interest.
References
- 1.The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–54. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen MD, Ryan DH, Apovian CM, et al. AHA/ACC/TOS guidelines for the management of overweight and obesity in adults. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;129(S2):S102–S38. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Ware JE. SF-36 Health Survey Update. Spine. 2000;25(24):3130–9. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 4.Rejeski WJ, Williamson D. Effects of lifestyle interventions on health related quality of life and physical functioning. In: Wadden TA, Bray GA, editors. Handbook of Obesity Treatment. 2. New York: Guilford Press; In press. [Google Scholar]
- 5.Warkentin LM, Das D, Majumdar SE, Johnson J, Padwal RS. The effect of weight loss on health-related quality of life: Systematic review and meta-analysis of randomized trials. Obes Rev. 2014;15:169–82. doi: 10.1111/obr.12113. [DOI] [PubMed] [Google Scholar]
- 6.Rubin RR, Peyrot M, Wang NY, et al. Patient-reported outcomes in the practice-based opportunities for weight reduction (POWER) trial. Qual Life Res. 2013;22(9):2389–98. doi: 10.1007/s11136-013-0363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroes M, Osei-Assibey G, Baker-Searle R, Huang J. Impact of weight change on quality of life in adults with overweight/obesity in the United States: A systematic review. Curr Med Res Opin. 2016;32(3):485–508. doi: 10.1185/03007995.2015.1128403. [DOI] [PubMed] [Google Scholar]
- 8.Maciejewski ML, Patrick DL, Williamson DF. A structured review of randomized controlled trials of weight loss showed little improvement in health-related quality of life. J Clin Epidemiol. 2005;58(6):568–78. doi: 10.1016/j.jclinepi.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Fabricatore AN, Wadden TA, Higginbothan AJ, et al. Intentional weight loss and changes in symptoms of depression: A systematic review and meta-analysis. Int J Obes. 2011;35(11):1363–76. doi: 10.1038/ijo.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson DA, Rejeski J, Lang W, et al. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med. 2009;169:163–171. doi: 10.1001/archinternmed.2008.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Look AHEAD Research Group. Impact of intensive lifestyle intervention on depression and health-related quality of life in type 2 diabetes: The Look AHEAD Trial. Diabetes Care. 2014;37:1544–53. doi: 10.2337/dc13-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webber KH, Casey EM, Mayes L, Katsumata Y, Mellin L. A comparison of a behavioral weight loss program to a stress management program: A pilot randomized controlled trial. Nutrition. 2016;32:904–909. doi: 10.1016/j.nut.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elder CR, Gullion CM, Funk KL, DeBar LL, Lindberg NM, Stevens VJ. Impact of sleep, screen time, depression and stress on weight change in the intensive weight loss phase of the LIFE study. Int J Obes. 2012;36:86–92. doi: 10.1038/ijo.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life. Obes Res. 2001;9:102–111. doi: 10.1038/oby.2001.13. [DOI] [PubMed] [Google Scholar]
- 15.Kolotkin RL, Crosby RD, Williams GR, Hartley CG, Nicol S. The relationship between health-related quality of life and weight loss. Obes Res. 2001;9:564–571. doi: 10.1038/oby.2001.73. [DOI] [PubMed] [Google Scholar]
- 16.Sarwer DB, Moore RH, Diewald LK, et al. The impact of a primary care-based weight loss intervention on quality of life. Int J Obes. 2013;37:S25–S30. doi: 10.1038/ijo.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolotkin RL, Fujioka K, Wolden ML, Brett JH, Bjorner JB. Improvements in health-related quality of life with liraglutide 3. 0 mg compared with placebo in weight management. Clin Obes. 2016;6:233–242. doi: 10.1111/cob.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolotkin RL, Norquist JM, Crosby RD, et al. One-year health-related quality of life outcomes in weight loss trial participants: Comparison of three measures. Health Qual Life Outcomes. 2009;7:53. doi: 10.1186/1477-7525-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holland-Carter L, Tuerk PW, Wadden TA, et al. Impact on psychosocial outcomes of a nationally available weight management program tailored for individuals with type 2 diabetes: Results of a randomized controlled trial. J Diabetes Complications. 2017;31:891–7. doi: 10.1016/j.jdiacomp.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Napoli N, Shah K, Waters DL, Sinacore DR, Qualls C, Villareal DT. Effects of weight loss, exercise, or both on cognition and quality of life in older adults. Am J Clin Nutr. 2014;100:189–98. doi: 10.3945/ajcn.113.082883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies M, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: The SCALE Diabetes randomized clinical trial. JAM. 2015;314(7):687–99. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 22.Tsai AG, Felton S, Wadden TA, Hosokawa PW, Hill JO. A randomized clinical trial of a weight loss maintenance intervention in a primary care population. Obesity. 2015;23:2015–2021. doi: 10.1002/oby.21224. [DOI] [PubMed] [Google Scholar]
- 23.Foster GD, Wadden TA, Kendall PC, Stunkard AJ, Vogt RA. Psychological effects of weight loss and regain: A prospective evaluation. J Consult Clin Psychol. 1996;64:752–757. doi: 10.1037//0022-006x.64.4.752. [DOI] [PubMed] [Google Scholar]
- 24.Engel SG, Crosby RD, Kolotkin RL, et al. Impact of weight loss and regain on quality of life: Mirror image or differential effect? Obes Res. 2003;11:1207–1213. doi: 10.1038/oby.2003.166. [DOI] [PubMed] [Google Scholar]
- 25.Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 26.O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: The BLOOM-DM study. Obesity. 2012;20:1426–1436. doi: 10.1038/oby.2012.66. [DOI] [PubMed] [Google Scholar]
- 27.Tronieri JS, Alfaris N, Chao AM, et al. Lorcaserin plus lifestyle modification for weight loss maintenance: Rationale and design for a randomized controlled trial. Contemp Clin Trials. 2017;59:105–112. doi: 10.1016/j.cct.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Tronieri JS, Wadden TA, Berkowitz RI, et al. A randomized trial of lorcaserin and lifestyle counseling for maintaining weight loss achieved with a low-calorie diet. Obesity. 2018;26:299–309. doi: 10.1002/oby.22081. [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic severity measures. Psychiatr Ann. 2002;32:1–7. [Google Scholar]
- 30.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 31.American Psychiatric Association. Online Assessment Measures [Web page] American Psychiatric Association; http://www.psychiatry.org/psychiatrists/practice/dsm/dsm-5/online-assessment-measures. Online Assessment Measures. [Web page] http://www.psychiatry.org/psychiatrists/practice/dsm/dsm-5/online-assessment-measures. [Google Scholar]
- 32.Pearl RL, Puhl RM. The distinct effects of internalizing weight bias: An experimental study. Body Image. 2016;17:38–42. doi: 10.1016/j.bodyim.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Puhl RM, Himmelstein MS, Quinn DM. Internalizing weight sitgma: Prevalence and sociodemographic considerations in US adults. Obesity. 2018;26:167–75. doi: 10.1002/oby.22029. [DOI] [PubMed] [Google Scholar]
- 34.Warkentin LM, Majumdar SR, Johnson JA, et al. Weight loss required by the severely obese to achieve clinically important differences in health-related quality of life: Two-year prospective cohort study. BMC Med. 2014;12:175. doi: 10.1186/s12916-014-0175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]