Abstract
Rationale
In cardiomyocytes, NaV1.5 and Kir2.1 channels interact dynamically as part of membrane bound macromolecular complexes.
Objective
To test whether NaV1.5 and Kir2.1 preassemble during early forward trafficking and travel together to common membrane microdomains.
Methods and Results
In patch-clamp experiments, co-expression of trafficking deficient mutants Kir2.1Δ314–315 or Kir2.1R44A/R46A with wildtype (WT) NaV1.5WT in heterologous cells reduced INa, compared to NaV1.5WT alone or co-expressed with Kir2.1WT. In cell surface biotinylation experiments, expression of Kir2.1Δ314–315 reduced NaV1.5 channel surface expression. Glycosylation analysis suggested that NaV1.5WT and Kir2.1WT channels associate early in their biosynthetic pathway, and fluorescence recovery after photobleaching experiments demonstrated that co-expression with Kir2.1 increased cytoplasmic mobility of NaV1.5WT, and vice versa, whereas co-expression with Kir2.1Δ314–315 reduced mobility of both channels. Viral gene transfer of Kir2.1Δ314–315 in adult rat ventricular myocytes and human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) reduced IK1 and INa, maximum diastolic potential (MDP) and action potential depolarization rate, and increased action potential duration (APD). Upon immunostaining, the adaptor protein complex 1 (AP1) colocalized with NaV1.5WT and Kir2.1WT within areas corresponding to t-tubules and intercalated discs. Like Kir2.1WT, NaV1.5WT co-immunoprecipitated with AP1. Site-directed mutagenesis revealed that NaV1.5WT channels interact with AP1 through the NaV1.5Y1810 residue, suggesting that, like for Kir2.1WT, AP1 can mark NaV1.5 channels for incorporation into clathrin-coated vesicles at the trans-Golgi. Silencing the AP1 ϒ-adaptin subunit in hiPSC-CMs reduced IK1, INa and MPD, and impaired rate dependent APD adaptation.
Conclusions
The NaV1.5-Kir2.1 macromolecular complex preassembles early in the forward trafficking pathway. Therefore, disruption of Kir2.1 trafficking in cardiomyocytes affects trafficking of NaV1.5, which may have important implications in the mechanisms of arrhythmias in inheritable cardiac diseases.
Keywords: Ion channels, trafficking, electrophysiology, arrhythmias (mechanisms), protein trafficking arrhythmia
Subject Terms: Basic Science Research, Cell Signaling/Signal Transduction, Electrophysiology, Ion Channels/Membrane Transport, Physiology
INTRODUCTION
In the human heart, three strong inward rectifying channels (Kir2.1, Kir2.2 and Kir2.3) contribute to the inward rectifier potassium current (IK1)1. IK1 is the major current responsible for maintenance of the maximum diastolic potential (MDP) in cardiomyocytes. It modulates excitability and influences the shape of the action potential (AP)2–5. Kir2.1 is the major channel isoform underlying IK1 in human ventricles, with Kir2.2 expressed to a lesser extent. In human atrial cells Kir2.3 is the main carrier of IK16, 7.
An important aspect of Kir2.x channel regulation is precise forward trafficking to specific membrane subdomains2, 7–9. The Golgi acts as a central biosynthetic sorting station, responsible for directing newly synthesized membrane proteins to their appropriate subcellular destination10, 11. Signal-dependent Golgi export processes control membrane surface density of Kir2.x channels12. Transport of Kir2.1 channels from the Golgi is dictated by residues embedded in the NH2 and COOH domains of the channel. These residues form a recognition site for interaction with adaptor protein complex 1 (AP1), thereby marking Kir2.1 for incorporation into clathrin-coated vesicles at the trans-Golgi12.
Adaptor protein complexes are heterotetrameric protein complexes that mediate intracellular membrane trafficking. Of the five different adaptor protein complexes, AP1, AP2 and AP3 are clathrin-associated, whereas AP4 and AP5 are not13. The adaptor protein complexes bind to sorting signals on cytoplasmic tails of cargo proteins. They also recruit clathrin and other accessory proteins and concentrate cargo proteins into vesicular carriers that transport cargo proteins from donor to target membranes13–15.
The voltage-gated cardiac sodium channel consists of a pore-forming alpha subunit (NaV1.5) and one or more beta subunits that modulate, but are not crucial, for functional NaV1.5 expression16. NaV1.5 channels permeate inward sodium current (INa), which is the main depolarizing current in cardiomyocytes, and thus is critical for normal electrical conduction16, 17. Regulation of NaV1.5 channel transport from Golgi to plasma membrane is poorly understood. Examination of NaV1.5 glycosylation suggests the Na+ channel can reach the plasma membrane via two different transport routes: the classical secretory transport route, or a Golgi-independent pathway18.
Cellular processes that regulate trafficking and expression of Kir2.1 and NaV1.5 channels are of interest as potential mediators of proarrhythmic channelopathies. Kir2.1 and NaV1.5 can interact through common partners such as scaffolding, anchoring/adapter proteins, enzymes, and regulatory proteins19–26. We previously demonstrated that both channels belong to multi-protein complexes, enabling one to regulate the other’s expression22, 26, 27. However, it remains unknown whether Kir2.1 and NaV1.5 pre-assemble or interact during early trafficking steps of their secretory pathways.
Here we investigate whether Kir2.1 and NaV1.5 associate early in their respective biosynthetic pathways, and whether they share a coupled forward trafficking process prior to formation of the NaV1.5-Kir2.1 macromolecular complex at the cell membrane. We use trafficking-deficient mutant channels Kir2.1Δ314–315 and Kir2.1R44A/R46A that disrupt Golgi trafficking of Kir2.1, thus impairing its surface expression12, 28–31. We hypothesize that expression of these trafficking-deficient Kir2.1 mutants will reciprocally disturb trafficking and functional expression of associated NaV1.5 channels, consequently altering cellular excitability and establishing a substrate for arrhythmogenesis.
METHODS
The authors declare that all supporting data are available within the article [and its online supplementary files].
Expanded methodology can be found in Online Supplement.
Isolation and culture of cardiac myocytes
Calcium-tolerant adult rat ventricular myocytes (ARVMs) were isolated from hearts of normal adult male Sprague-Dawley rats. Cells were plated on laminin coated tissue culture glass coverslips/dishes and maintained in M199 for viral/non-viral transfer and patch clamping as previously described32.
Cell culture and transfections
Human Embryonic Kidney (HEK-293T) cells were cultured in DMEM (Gibco) supplemented with fetal bovine serum (FBS), penicillin, and streptomycin at 37°C. HEK-293 cells stably expressing NaV1.5+β1 channels were grown under the same conditions with the addition of Geneticin for selection of cells expressing the resistance marker. For patch-clamp experiments, cells were grown in 60 mm dishes and transfected or co-transfected using Lipofectamine.
iCell hiPSC-CM monolayers
Cryopreserved vials of human iCell® Cardiomyocytes were obtained from Cellular Dynamics International, Inc (Madison, WI). After 48 to 72 h of adenoviral overexpression electrophysiological phenotype analysis was performed.
Biotinylation assay
Forty-eight hours after transfection, HEK-293T cells were washed with phosphate-buffered saline and biotinylated for 60 min then incubated with glycine. Biotinylated proteins were analyzed by Western blot and immunoprecipitation assays.
Deglycosylation assay
Total membrane homogenates were solubilized and incubated with mouse anti-Kir2.1 antibody for immunoprecipitation. Samples were incubated with either PNGase-F, Endo-H or phosphate buffer and used for SDS-PAGE analysis.
INa, IK1 and AP recordings in ARVMs, hiPSC-CMs and HEK-293T cells
Voltage-clamp recordings were performed at room temperature and APs were recorded using current-clamp at 37°C.
Fluorescence recovery after photobleaching (FRAP)
NaV1.5WT and Kir2.1WT channels were bound to mTurquoise (mTQ) and venus (Venus) fluorophores for FRAP experiments in COS-7 cells. Analysis was performed using Fiji/ImageJ and Matlab.
Statistics
All data are expressed as mean±SEM. Two-tail Student’s t-test, One or Two-way ANOVA with Bonferroni’s multiple comparison tests performed with Prism 6 were used. p<0.05 was considered significant.
RESULTS
Trafficking-deficient mutant Kir2.1Δ314–315 impairs functional expression of NaV1.5
We conducted patch-clamp experiments in transiently transfected HEK-293T cells. For Kir2.1WT, voltage steps elicited the expected large inward and small outward currents of a strong inward rectifier channel. In contrast, no potassium current was detected for the trafficking-deficient mutant Kir2.1Δ314–315 (Online Figure I A,B), as previously reported28. In addition, we confirmed previous data showing cooperative interaction between Kir2.1WT and NaV1.5WT ion channel expression22, 26, 27. We observed a synergistic effect on INav1.5 density when Kir2.1WT channels were co-expressed (1:1) with NaV1.5WT channels, resulting in increased INav1.5. Co-expression of Kir2.1WT channels with NaV1.5WT channels resulted in larger peak INav1.5 density at −25 mV compared with NaV1.5WT alone (−830±65 pA/pF versus −562±57 pA/pF, respectively; p=0.001, Online Figure I C,D). We also assessed functional consequences on IKir2.1 of co-expressing NaV1.5WT with the trafficking-deficient Kir2.1Δ314–315 channel. No IKir2.1 density was generated by Kir2.1Δ314–315 channels when co-expressed with NaV1.5WT channels. Co-expression of Kir2.1WT, Kir2.1Δ314–315 and NaV1.5WT (0.5:0.5:1) yielded an IKir2.1 density that was 92% smaller than when Kir2.1WT was expressed alone (Online Figure I A,B).
We then co-expressed NaV1.5WT with Kir2.1Δ314–315 and measured NaV1.5WT channel function. INav1.5 density in cells with NaV1.5WT channels expressed alone was larger than in cells that co-expressed Kir2.1Δ314–315, with peak currents at −25 mV of −562±57 versus −275±38 pA/pF, respectively (p<0.0001, Online Figure I C,D). Correspondingly, INav1.5 density in cells with NaV1.5WT channels expressed alone was larger than in cells that co-expressed Kir2.1WT, Kir2.1Δ314–315 and NaV1.5WT subunits (peak current at −25 mV, −562±57 versus −178±34 pA/pF, respectively, p<0.0001, Online Figure I C,D).
To rule out the possibility that decreased INav1.5 density in the presence of the trafficking-deficient mutant Kir2.1Δ314–315 was due to variability in expression from transfection, we used a stable HEK-293 cell line expressing NaV1.5+β1. Cells transfected with Kir2.1Δ314–315 had a lower INav1.5 than control GFP-transfected cells. Cells transfected with Kir2.1Δ314–315 exhibited smaller INav1.5 density −244±37 pA/pF than control GFP-transfected cells −373±50 pA/pF at −25 mV, p<0.003. On the other hand, cells transfected with Kir2.1WT had a larger mean INa peak density −520±69 pA/pF at −25 mV than control cells, p<0.0001, Online Figure II A,B and Online Table I. We observed no differences in voltage-dependence of activation or inactivation, or recovery from inactivation between groups (Online Figure III A-D and Online Table I).
Thus, trafficking deficiency of one channel component of the NaV1.5-Kir2.1 macromolecular complex (in this case Kir2.1Δ314–315) negatively influenced functional expression of the other channel (NaV1.5), possibly by disturbing channel trafficking to the plasma membrane.
Double mutant Kir2.1R44A/R46A reduces INav1.5 density in CHO cells
To explore whether other trafficking-deficient Kir2.1 channels can produce similar effects on INav1.5 density, we studied the NH2 double mutant Kir2.1R44A/R46A, which accumulates in the Golgi resulting in decreased membrane expression33. Surprisingly, patch-clamp experiments in HEK-293T and CHO cells showed that the Kir2.1R44A/R46A mutant produced an IKir2.1 density similar to Kir2.1WT channels (Online Figure IV A,B). However, we observed that in transfected CHO cells, INav1.5 density of NaV1.5WT channels expressed alone was larger than in cells that co-expressed Kir2.1R44A/R46A (Online Figure II C,D). Thus, decreased INav1.5 density was not unique to Kir2.1Δ314–315 channels, as other Kir2.1 trafficking-deficient mutants in a different cell line produced similar effects.
Kir2.1Δ314–315 expression reduces INa density in adult rat ventricular myocytes (ARVMs)
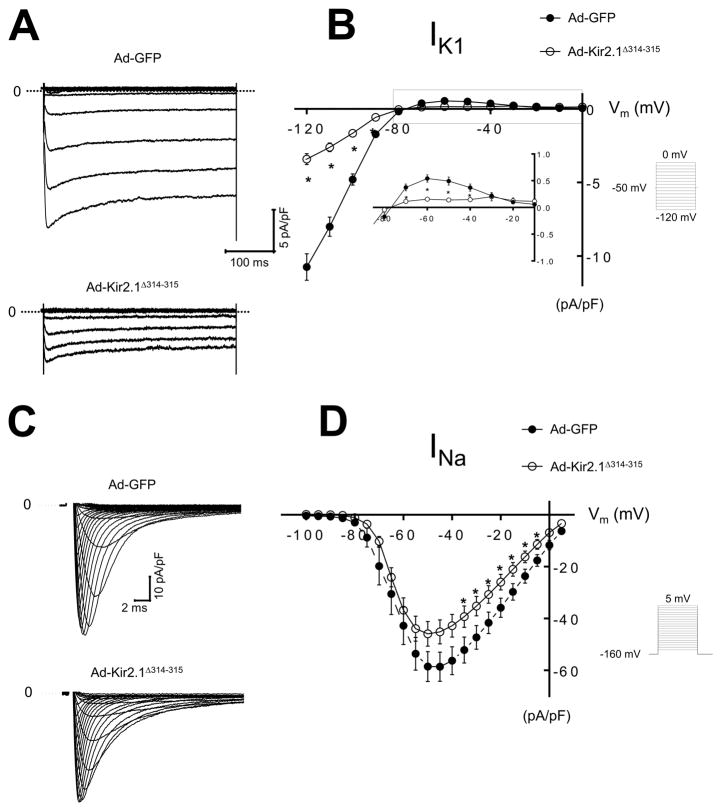
We next studied the functional consequences of expressing Kir2.1Δ314–315 in adult mammalian cardiac cells. Using an adenoviral construct, we overexpressed Ad-Kir2.1Δ314–315 in ARVMs; control myocytes were infected with GFP (Ad-GFP). Cell capacitance was similar in myocytes infected with Ad-Kir2.1Δ314–315 and control Ad-GFP myocytes (100±5 versus 107±2 pF; n=27, n=23; respectively; p=0.4). Representative Ba2+ sensitive IK1 traces from Ad-GFP and Ad-Kir2.1Δ314–315 are shown in Figure 1A. Figure 1B shows the IK1 current-voltage relationship for Ad-GFP and Ad-Kir2.1Δ314–315. As expected, ARVMs transfected with Ad-Kir2.1Δ314–315 generated smaller inward −3±0.4 versus −10±8 pA/pF at −120 mV; p<10−7 and outward 0.15±0.01 versus 0.5±0.06 pA/pF at −60 mV, p<10−5 currents than control. We next investigated functional effects of Ad-Kir2.1Δ314–315 overexpression on INa in myocytes. Representative INa traces from Ad-GFP and Ad-Kir2.1Δ314–315 are in Figure 1C. Overexpression of Kir2.1Δ314–315 decreased INa density compared with Ad-GFP, Figure 1D. Mean peak current density at −35 mV was −39±3 versus −52±4 pA/pF, respectively, p=0.05.
Figure 1. Adenoviral gene overexpression of GFP and Kir2.1Δ314–315 in ARVMs at 48 h.
A. IK1 traces from ARVMs infected with Ad-GFP or Ad-Kir2.1Δ314–315. B. IK1 I/V relationships for Ad-GFP and Ad-Kir2.1Δ314–315 (N=4, n=12 and N=6, n=13, respectively). C. INa traces from ARVMs infected with Ad-GFP or Ad-Kir2.1Δ314–315. D. INa, I/V relationships for Ad- GFP, Ad-Kir2.1Δ314–315 (N=3, n=12 and N=4, n=10, respectively). *p<0.05, mean±SEM, (t-test). N, number of animals; n, number of cells. Insert describes voltage pulse protocols.
To evaluate whether overexpression of Kir2.1Δ314–315 in ARVMs could affect other currents, like transient outward potassium current (Ito), we compared the difference between initial peak and sustained K+ background current density of myocytes infected with Ad-Kir2.1Δ314–315 versus Ad-GFP cells. Peak currents at 30 mV were 8.1±1 versus 7.5±1 pA/pF, respectively, p=0.79. In contrast with the decrease in IK1, Ad-Kir2.1Δ314–315 expression did not modify Ito (Online Figure V).
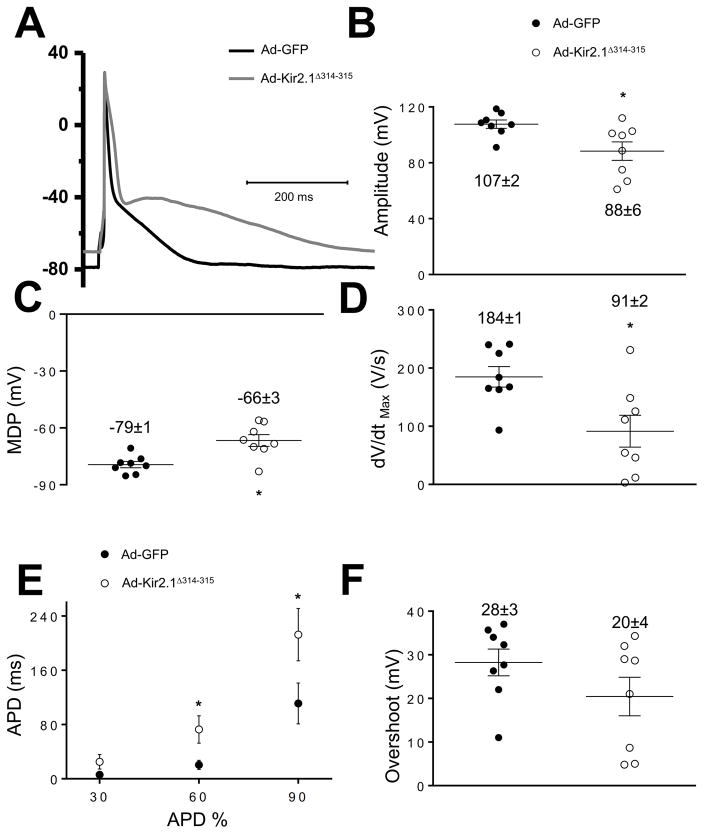
Kir2.1Δ314–315 expression modifies ARVM cardiac action potential
We studied cellular electrophysiological consequences of Kir2.1Δ314–315 expression by recording APs in Ad-Kir2.1Δ314–315-infected ARVMs. As expected, the MDP was reduced compared to control (−66±3 versus −79±1 mV; p=0.0028; Figure 2A,C). MDP decrease was consistent with IK1 reduction (Figure 1A,B). In some Ad-Kir2.1Δ314–315-infected ARVMs, MDP reduction resulted in phase-4 depolarization and generation of spontaneous APs (Online Figure VI). In addition, maximum upstroke velocity (dV/dtmax) was reduced in Ad-Kir2.1Δ314–315 myocytes compared to control 91±2 versus 184±1 V/s, respectively; p=0.012, Figure 2D. These findings were in agreement with reduction in both sodium channel availability during the AP upstroke and INa density in Ad-Kir2.1Δ314–315 myocytes (Figure 1C,D). Moreover, APD was prolonged in Ad-Kir2.1Δ314–315 (Figure 2E). Finally, we observed no difference in AP overshoot between control and Ad-Kir2.1Δ314–315 myocytes (Figure 2F).
Figure 2. Effect Ad-Kir2.1Δ314–315 overexpression on action potential characteristics of ARVMs.
A. Representative action potential recordings from ARVMs infected with adenoviruses encoding Ad-GFP and Ad-Kir2.1Δ314–315 at 1 Hz. B,C,D, E, F Average amplitude (B), maximum diastolic potential (C), maximal dV/dt (D), action potential duration to 30, 60 and 90% of repolarization (E) and overshoot (F) (N=3, n=8 and N=3, n=8, respectively, where N is the number of animals and n is the number of cells) *p<0.05, mean±SEM, (t-test).
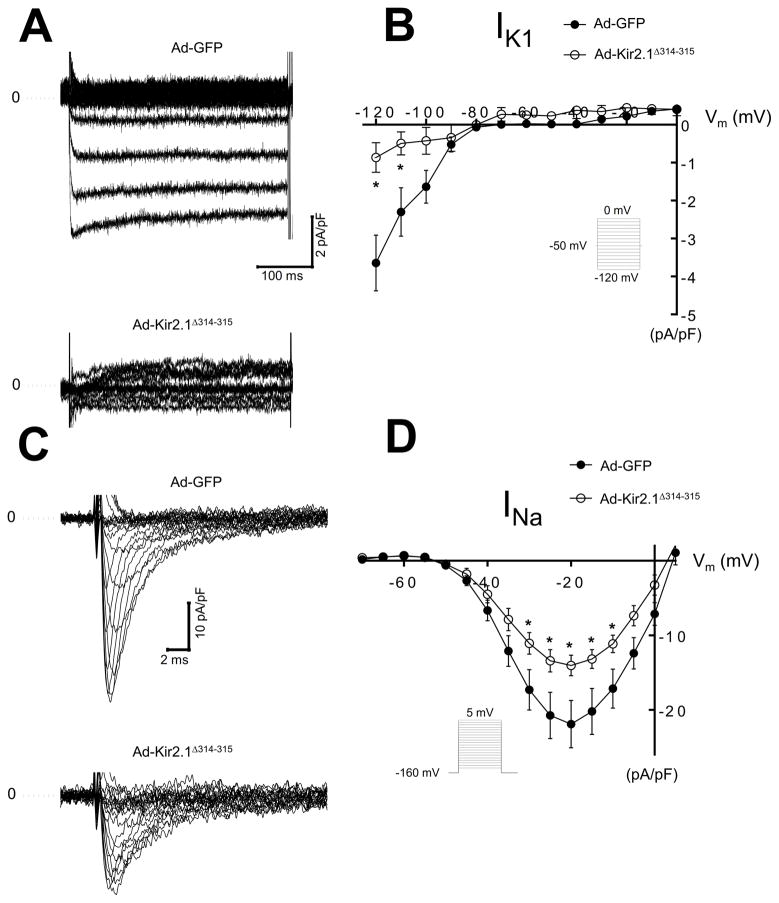
Kir2.1Δ314–315 mutant decreases IK1 and INa in hiPSC-CMs
We determined the consequences of expression of Kir2.1Δ314–315 in hiPSC-CMs by adenoviral infection; control cells were infected with Ad-GFP. Figure 3A shows representative Ba2+ sensitive IK1 traces for Ad-GFP and Ad-Kir2.1Δ314–315. Control IK1 current-voltage relationship is plotted with the Ad-Kir2.1Δ314–315 curve. As expected, hiPSC-CMs infected with Ad-Kir2.1Δ314–315 generated smaller currents than control −0.8±0.3 versus −3.6±0.6 pA/pF at −120 mV, p=0.006, Figure 3B. We next investigated the functional effects of Ad-Kir2.1Δ314–315 overexpression on INa in human myocytes. Representative INa traces are shown in figure 3C. In figure 3D, overexpression of Kir2.1Δ314–315 decreased INa density at several test voltages compared with Ad-GFP. Peak currents at −20 mV were −14±1 versus −21±2 pA/pF, respectively, p=0.014.
Figure 3. ATS1 mutant Kir2.1Δ314–315 decreases IK1 and INa density on human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs).
A. IK1 traces from hiPSC-CMs infected with Ad-GFP or Ad-Kir2.1Δ314–315. B. Superimposed IK1 I/V relationships for Ad-GFP and Ad-Δ314–315 (N=3, n=6 and N=3, n=6, respectively). C. INa traces from hiPSC-CMs infected with Ad-GFP or Ad-Kir2.1Δ314–315. D. INa, I/V relationships for Ad-GFP, Ad-Kir2.1Δ314–315 (N=3, n=7 and N=3, n=17, respectively). *p<0.05, mean±SEM, (t-test). N, number of hiPSC-CMs batches infected, n, number of cells. Insert describes voltage pulse protocols.
Kir2.1Δ314–315 does not alter total NaV1.5 protein levels but impairs NaV1.5 plasma membrane expression
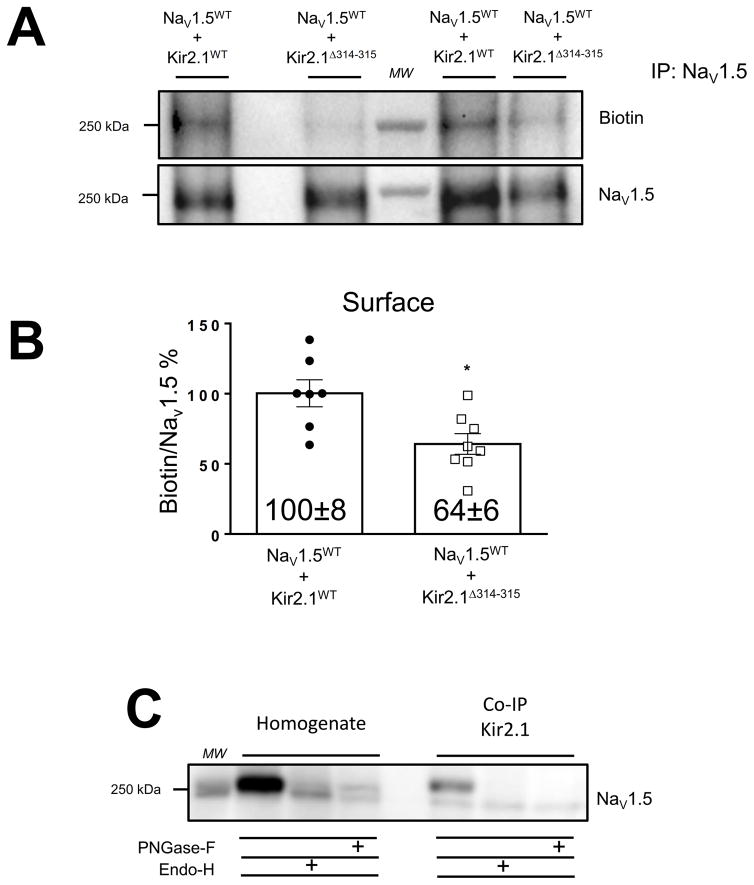
We observed no change in total NaV1.5WT protein expression in whole-cell extracts of HEK-293 (NaV1.5+β1) transfected with either Kir2.1WT or mutant Kir2.1Δ314–315 protein, p=0.14. Similar results were obtained in HEK-293T cells transiently transfected with NaV1.5 alone or co-transfected with either Kir2.1WT or mutant Kir2.1Δ314–315, p=0.25, Online Figure VII B,E. Moreover, as previously, we observed less total protein production of Kir2.1Δ314–315 than Kir2.1WT channel12, 28, 34 (Online Figure VII C,F). Therefore, to determine whether the decrease in INa density in cells co-expressing Kir2.1Δ314–315 was due to a decrease in NaV1.5WT surface membrane expression, we performed cell surface biotinylation experiments in HEK-293T cells after transient co-transfection 1:1 of NaV1.5WT with Kir2.1WT or Kir2.1Δ314–315 channels. Cell lysates were subjected to immunoprecipitation with an antibody against the COOH-domain of NaV1.5 and analyzed by Western blotting. The presence of the trafficking-deficient mutant Kir2.1Δ314–315 resulted in a reduction in NaV1.5WT protein at the surface membrane compared with Kir2.1WT channel, p=0.009, Figure 4A,B.
Figure 4. The trafficking deficient mutant Kir2.1Δ314–315 decreases plasma membrane expression of NaV1.5 channels in HEK-293T cells.
A. Representative Western blots of cell surface biotinylation studies. Samples were immunoprecipitated with rabbit anti-NaV1.5 and detected by immunobloting with mouse anti-NaV1.5 and streptavidin HRP conjugated. B. Graphic of quantification of NaV1.5 cell surface expression. Cell surface membrane expression of NaV1.5 channels was markedly reduced in the presence of Kir2.1Δ314–315 compared with Kir2.1WT. *p<0.05, mean±SEM, (t-test), (NaV1.5WT+Kir2.1WT (n=7) and NaV1.5WT+Kir2.1Δ314–315 (n=8), respectively. C. Whole-cell inputs of HEK-293T co-transfected 1:1 with NaV1.5WT and Kir2.1WT incubated in the absence (control samples) or in the presence of endoglycosidase (Endo-H) or peptide N-Glycosidase-F (PNGase-F). Left, NaV1.5WT has populations that are Endo-H and PNGase-F sensitive. Right, Triton solubilized NaV1.5WT were first immunoprecipitated with Kir2.1WT and then deglycosylated with Endo-H and PNGase-F. Equal amounts of sample were treated and subjected to 7.5% SDS-PAGE gel electrophoresis. Deglycosylation assay experiments were performed in five different batches of cells transfected at different days. MW, molecular weight.
NaV1.5 and Kir2.1 associate early in their biosynthetic pathway
NaV1.5 and Kir2.1 channels are present as complexes together with scaffolding proteins19–26, and co-immunoprecipitate in both forward and reverse co-immunoprecipitation reactions22. However, the spatial and temporal coordination of their association remains unclear regarding NaV1.5 and Kir2.1 coupling before, during or after insertion into the membrane. To determine whether association occurs before the channels reach the plasma membrane we examined their sensitivity to Endoglycosidase-H (Endo-H), which cleaves high mannose carbohydrates and indicates association with the endoplasmic reticulum (ER)-Golgi apparatus35. Whole-cell homogenates and immunoprecipitates from HEK-293T cells co-transfected 1:1 with NaV1.5WT and Kir2.1WT channels were analyzed by Western blot after treatment with Endo-H or Peptide-N-Glycosidase-F (PNGase-F), which enzymatically removed glycans added in the ER or all N-linked carbohydrates, respectively (Figure 4C). NaV1.5WT channels that co-immunoprecipitated with Kir2.1WT channels were partially Endo-H-sensitive, as evidenced by the appearance of a lower molecular weight band after Endo-H treatment. The positive association between Endo-H-sensitive NaV1.5WT and Kir2.1WT channels suggested that the NaV1.5-Kir2.1 complex assembly occurred at least partially when the two channels trafficked through the ER-Golgi. Moreover, in co-immunoprecipitation experiments using an antibody against Nav1.5 in cells co-transfected with NaV1.5WT and Kir2.1WT or Kir2.1Δ314–315, Nav1.5WT was able to pull down both Kir2.1WT and mutant Kir2.1Δ314–315 (Online Figure VIII). Taken together, these data indicate that NaV1.5 and Kir2.1 channels can associate early in their biosynthetic pathway.
Kir2.1WT increases while Kir2.1Δ314–315 impairs NaV1.5 mobility toward plasma membrane
We conducted fluorescence recovery after photobleaching (FRAP) experiments to assess whether Kir2.1 and NaV1.5 traffic together along a common pathway. We subcloned each channel gene with one of two separate fluorophores (SCN5A with mTq2, and KCNJ2 or KCNJ2Δ314–315 with Cp173venus), then used confocal microscopy and FRAP to quantify channel mobility when transfected alone or co-transfected together into COS-7 cells. After photobleaching a small region of interest (ROI = 5 μm diameter), the rate of fluorescence recovery was measured as an index of movement of unbleached channels entering the ROI. Online Figure IX A shows FRAP data for Kir2.1WT Venus alone and for Kir2.1WT co-transfected with NaV1.5WT mTQ. Presence of NaV1.5WT mTQ channels did not modify the fraction of mobile Kir2.1WT Venus channels (Online Figure IX B), but accelerated the fluorescence recovery within the ROI t1/2= 2.3±0.3 min (n=8) versus 4.7±0.6 min (n=7); p=0.002. These changes are better appreciated in Online Figure IX C, which shows Kir2.1WT Venus recovery normalized to the maximum fluorescence recorded after photobleaching in the absence and the presence of NaV1.5WT mTQ. The mobile fraction of NaV1.5WT mTQ channels increased when co-transfected with Kir2.1WT Venus (Online Figure IX D-F), although its t1/2 was not modified t1/2=2.4±0.2 min (n=11) versus 2.0±0.2 min (n=10), respectively; p=0.17. The mobile fraction and t1/2 for both NaV1.5WT mTQ and Kir2.1WT Venus channels were nearly identical when measurements were performed in the presence of the other channel (i.e. NaV1.5WT mTQ and Kir2.1WT Venus, and vice versa; Online Figure IX G-I). Furthermore, co-transfection with Kir2.1Δ314–315Venus decreased the mobile fraction, p<0.005, Online Figure IX E and delayed recovery of NaV1.5WT mTQ2 channels t1/2=3.1±0.4 min, n=9, p=0.11, Online Figure IX D-F. Altogether, the results strongly suggest that Kir2.1WT-NaV1.5WT complexes travel more efficiently than NaV1.5WT mTQ2 channels alone and faster than Kir2.1WT Venus channels alone, and that the presence of Kir2.1Δ314–315Venus reduces both the amount and rate of NaV1.5WT mTQ channel protein trafficking.
Localization of AP1 ϒ-adaptin subunit in ARVMs
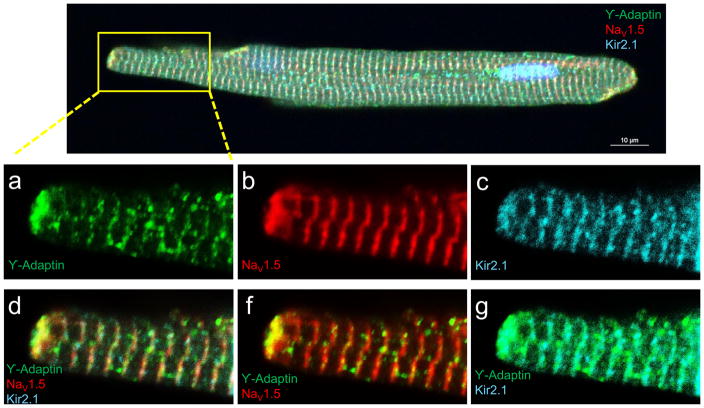
Forward protein trafficking along intracellular compartments is accomplished by transport vesicles that form at sites where coat proteins have been recruited15, 36. Previously it was shown that an interaction with the AP1 ϒ-adaptin subunit marks the incorporation of Kir2.1WT channels into clathrin coated vesicles at the trans Golgi network12. Also previous studies have shown that Kir2.1 and NaV1.5 channels present similar subcellular distributions at t-tubules and the intercalated discs7, 20–22, 26, 37, 38 We therefore sought to determine whether in addition to Kir2.1, AP1 ϒ-adaptin also interacts with NaV1.5 in ARVM. On the top panel of Figure 5, immunostaining with an antibody against AP1 ϒ-adaptin, shows a striated and punctate pattern distributed within regions corresponding to the t-tubules and the intercalated discs. Magnifications of the box region (panels a-g) shows that AP1 ϒ-adaptin colocalizes with NaV1.5 and Kir2.1 channels at both the t-tubular regions and more intensely the intercalated discs (panels d,f and g).
Figure 5. Localization of AP1 ϒ-adaptin, NaV1.5 and Kir2.1 in ARVMs.
Top, Overlapping immunostaining for ϒ-adaptin (green), NaV1.5 (red) and Kir2.1 (cyan)., a,d, ϒ-adaptin expresses in the t-tubular regions and the intercalated discs. Intense punctate signal densities were also found randomly distributed within the cytoplasm. NaV1.5 and Kir2.1 show similar subcellular distribution at the t-tubules and intercalated discs, b and c, respectively. ϒ-adaptin, colocalization with NaV1.5 (f) and Kir2.1 (g). d. Colocalization of the three proteins (Scale bar: 10 μm).
NaV1.5 Golgi export depends on interaction with the AP1 ϒ-adaptin subunit clathrin adaptor complex
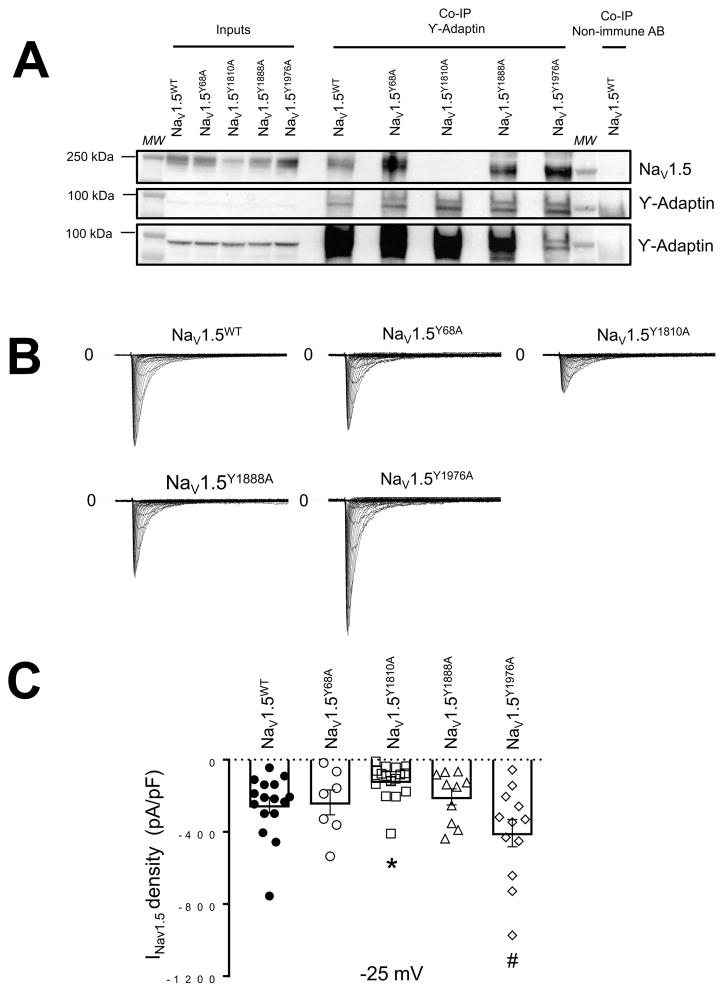
Next, we conducted co-immunoprecipitation experiments to investigate whether, similar to Kir2.1WT, NaV1.5WT protein interacts with the AP1 ϒ-adaptin subunit. We used an antibody against the COOH-domain of NaV1.5 for immunoprecipitation from whole-cell homogenates of HEK-293T cells transfected with NaV1.5WT alone or co-transfected 1:1 with Kir2.1WT or Kir2.1Δ314–315. Immunoprecipitated proteins were prepared for Western blot analysis and probed with anti-AP1 ϒ-adaptin monoclonal antibody. As illustrated in Online Figure X A and B, NaV1.5 channels pulled down AP1 ϒ-adaptin; the reverse interaction demonstrated that AP1 was also able to pull down NaV1.5 in immunoprecipitation experiments. Since selective recruitment of the coat protein is based on sequence information within the cytoplasmic domains of the respective cargo protein33, 39–44, we next investigated whether the NaV1.5 COOH and/or NH2 domains contained the typical consensus motif (YXXΦ; X being any amino acid and Φ being a bulky, hydrophobic residue) for adaptin binding. In the COOH-domain of NaV1.5 channels we found four consensus motifs for AP1 binding, beginning at amino acid positions Y1794, Y1810, Y1888 and Y1976. In the NH2 domain one consensus motif for AP1 begins at amino acid position Y68. We therefore subjected the Tyrosine (Y) residues of the consensus motifs to sequential Alanine (A) replacement mutagenesis and assessed whether the mutant NaV1.5 channels interacted with the AP1 ϒ-adaptin subunit and whether they were functional. Immunoblots of all Alanine-substitutions (NaV1.5Y68A, NaV1.5Y1810A, NaV1.5Y1888A and NaV1.5Y1976A) showed a band at ~250 KDa in all inputs and co-immunoprecipitation samples from NaV1.5 mutants and NaV1.5WT channels (Figure 6A). No change in total protein expression was observed between AP1 binding mutant consensus motifs and the NaV1.5WT channel (Online Figure XI A,B). Moreover, all Alanine-substitutions expressed functional NaV1.5 channels (Figure 6B). However, INa density was reduced for the NaV1.5Y1810A mutant (Figure 6B,C). This result was consistent with the observed loss of interaction between NaV1.5Y1810A and the AP1 ϒ-adaptin subunit (Figure 6A). Taken together, the data suggest that similar to Kir2.1, a significant proportion of NaV1.5 channels interact with AP1 and are selected for export from the Golgi in a signal dependent manner through an AP1 clathrin adaptor interaction.
Figure 6. Alanine-scanning mutagenesis of NaV1.5 channels for typical consensus motif to adaptin binding.
A. Representative Western blot after immunoprecipitation (IP) with ϒ-adaptin specific antibody. Detected by immunoblot were NaV1.5 and ϒ-adaptin proteins on HEK-293T cells transfected with NaV1.5WT, Nav1.5Y68A, Nav1.5Y1810A, Nav1.5Y1888A and Nav1.5Y1976A (n=3). Botton (ϒ-adaptin #) shows a longer exposure of the same immunoblot shown in Middle. B. Current traces from NaV1.5WT, Nav1.5Y68A, Nav1.5Y1810A, Nav1.5Y1888A and Nav1.5Y1976A. C. Peak INav1.5 density at −25 mV of NaV1.5WT, Nav1.5Y68A, Nav1.5Y1810A, Nav1.5Y1888A and Nav1.5Y1976A (n=16, n=7, n=16, n=10 and n=12, respectively). All constructs/combinations were tested at least in three different batches of cells transfected in at least three different days. MW, molecular weight. *p<0.05, NaV1.5WT vs NaV1.5Y1810A, # p<0.05, NaV1.5WT vs NaV1.5Y1976A, mean±SEM, Two-way ANOVA.
Silencing the AP1 ϒ-adaptin subunit reduces INa, IK1 and modifies the action potential characteristics of hiPSC-CMs
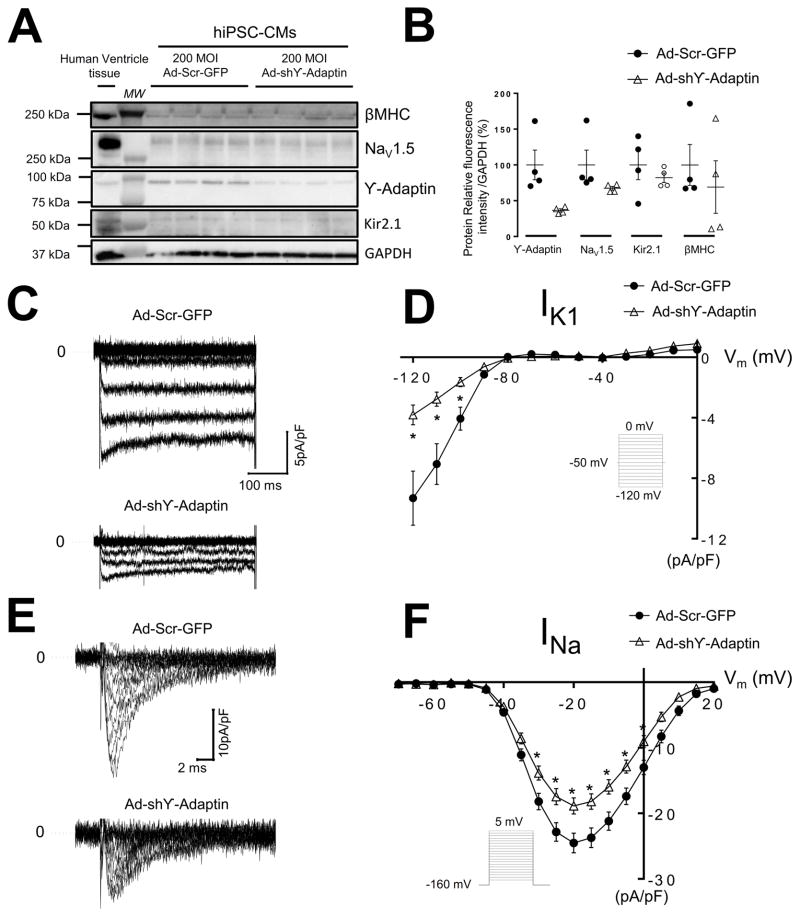
We used adenoviral transfer technology to knock down the expression of AP1 ϒ-adaptin subunit and measured INa and IK1 densities in hiPSC-CMs. We cultured hiPSC-CM monolayers for 7 days on 96-well plates as describe previously.45 hiPSC-CM monolayers were infected with shRNA for AP1 ϒ-adaptin (Ad-shϒ-adaptin) at varying MOIs to determine the level needed for knockdown; samples were collected 3 days postinfection. Separate groups of hiPSC-CM monolayers were infected with control Scramble-GFP virus (Ad-Scr-GFP), Online Figure XII A,B. On Western Blot analysis, 200 MOI reduced relative levels of AP1 ϒ-adaptin protein by ~63 % without suppressing total NaV1.5WT or Kir2.1WT protein levels (Figure 7A,B). IK1 density was smaller in hiPSC-CMs infected with Ad-shϒ-adaptin compared with control GFP infected hiPSC-CMs (−9.3±1.7 versus −3.8±0.5 pA/pF at −120 mV, p=0.01, Figure 7C,D). Similar to IK1, infection with Ad-shϒ-adaptin also reduced INa density compared to Ad-Scr-GFP. Peak currents at −20 mV were −18±1 versus −24±1 pA/pF, respectively, p=0.004, Figure 7E,F.
Figure 7. Silence AP1 ϒ-adaptin subunit reduce INa and IK1 on hiPSC-CMs.
A, B Ad-shϒ-Adaptin infection silence ϒ-Adaptin subunit on hiPSC-CMs by day 3 in culture monolayers compared with control Ad-Scr-GFP without changing NaV1.5WT, Kir2.1WT and β-cardiac myosin heavy chain (βMHC). A. Postinfection Western blot of whole cell preparation of hiPSC-CMs monolayers and human ventricle tissue (100 μg) showing immunodetection of βMHC, NaV1.5WT, ϒ-Adaptin, Kir2.1WT and GAPDH (loading control). B. Quantification of ϒ-Adaptin, NaV1.5WT, Kir2.1WT and βMHC, protein expression on whole cell extract from A. C. IK1 traces from hiPSC-CMs infected with Ad-Scr-GFP or Ad-shϒ-Adaptin, respectively. D. Superimposed IK1 I/V relationships for Ad-GFP and Ad-shϒ-Adaptin (N =3, n=13 and N=3, n=10, respectively). E. INa traces from hiPSC-CMs infected with Ad-Scr-GFP or Ad-shϒ-Adaptin. F. INa, I/V relationships for Ad-Scr-GFP, Ad-shϒ-Adaptin (N=3, n=45 and N=3, n=42, respectively). *p<0.05, mean±SEM, (t-test). N, number of hiPSC-CMs batches infected, n, number of cells. Insert describes voltage pulse protocols. MW, molecular weight.
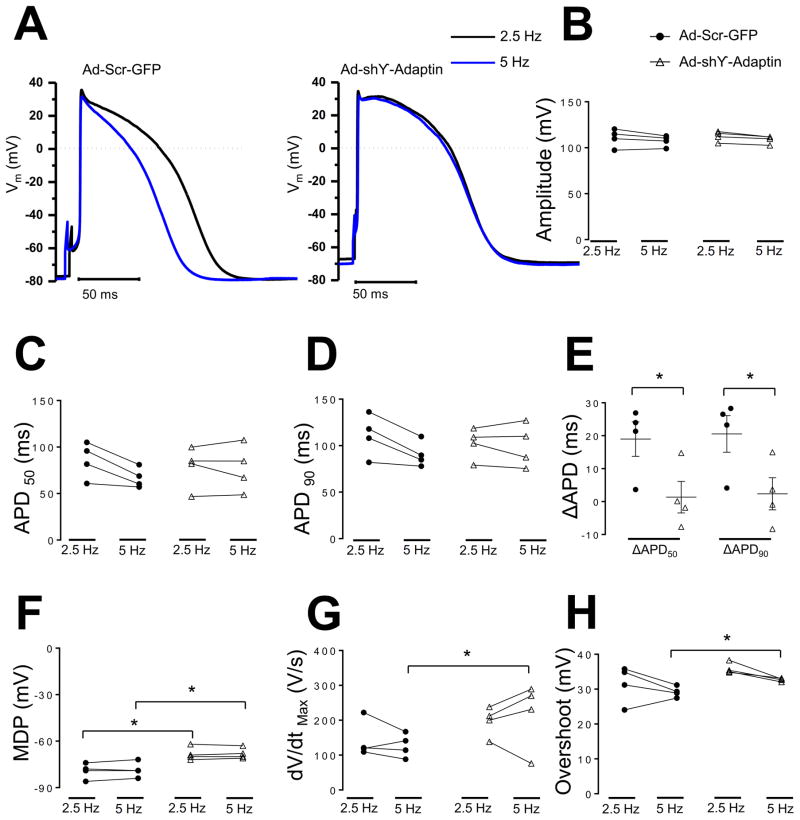
Next, we determined the effect of silencing the AP1 ϒ-adaptin subunit on the action potential characteristics of hiPSC-CMs. In Figure 8F, MDP was reduced in hiPSC-CMs treated with Ad-shϒ-adaptin relative to control hiPSC-CMs infected with Ad-Scr-GFP. All cells were paced at 2.5 and 5 Hz. MDPs were −68±1 (2.5 Hz) and −68±1 mV (5 Hz) in Ad-shϒ-adaptin hiPSC-CMs, versus −79±2 (2.5 Hz) and −78±2 mV (5 Hz) in Ad-Scr-GFP iPSC-CMs (p=0.01 in both cases). Action potential amplitude, APD50, APD90, dV/dtmax and overshoot, were similar in both hiPSC-CMs groups (Figure 8 B,C,D,G,H). Silencing the AP1 ϒ-adaptin subunit impaired the rate dependent APD adaptation of hiPSC-CMs, ΔAPD90, ΔAPD50 were 2±4, 1±4 ms, respectively, in iPSC-CMs Ad-shϒ-adaptin versus 20±4, 18±4 ms in iPSC-CMs Ad-Scr-GFP, respectively, p=0.04 for both ΔAPD, Figure 8E.
Figure 8. Silence AP1 ϒ-adaptin subunit reduce MDP and impairs rate dependent APD adaptation of hiPSC-CMs.
A. Representative action potential recordings from hiPSC-CMs infected with adenoviruses encoding Ad-Scr-GFP and Ad-shϒ-Adaptin at 2.5, 5 Hz (black and blue), respectively. B,C,D, F, G, H Plot individual values, amplitude (B), action potential duration to 50 and 90% of repolarization (C), (D), maximum diastolic potential (F), maximal dV/dt (G), and overshoot (H). Delta APD50 and APD90 repolarization between 2.5 and 5 Hz (ΔAPD550, 90) respectively (N=2, n=4 and N=2, n=4, respectively, N, number of hiPSC-CMs batches infected, n, number of cells. *p<0.05, Ad-Scr-GFP vs Ad-shϒ-Adaptin mean±SEM, (t-test). Action potential amplitude, overshoot, dV/dtmax, APD50 and APD90 were similar in both hiPSC-CMs groups (112±2, 35±0.6 mV, 229±31 V/s, 78±9 and 81±7 ms in Ad-shϒ-adaptin treated iPSC-CMs versus 110±4, 31±2 mV, 142±22 V/s, 85±8 and 88±9 ms in Ad-Scr-GFP treated iPSC-CMs, p>0.05 for Action potential amplitude, overshoot, dV/dtmax, APD50 and APD90 at 2.5 Hz and (108±1, 32±0.2 mV, 197±18 V/s, 77±1 and 79±9 ms in treated Ad-shϒ-adaptin iPSC-CMs versus 107±2, 29±0.6, 127±14 V/s, 66±4 and 72±5 ms, respectively, in Ad-Scr-GFP treated iPSC-CMs, p>0.05 for Action potential amplitude, APD50 and APD90, p=0.04, p=0.004 for dV/dtmax and overshoot, respectively at 5 Hz.
DISCUSSION
We used two trafficking-deficient mutant Kir2.1 channels to provide novel insight into mechanisms that regulate the NaV1.5-Kir2.1 macromolecular complex. Our data reveal that co-expression with Kir2.1Δ314–315 reduces expression of NaV1.5 at the surface membrane without modifying biophysical properties of the channel or total protein production. We show that NaV1.5 and Kir2.1 channels can associate early in their biosynthetic pathway, and that the NaV1.5-Kir2.1 complex traffics more efficiently than NaV1.5 alone, but Kir2.1Δ314–315 impairs NaV1.5 trafficking. Correspondingly, our patch-clamp data demonstrate that reduced NaV1.5 surface membrane expression results in decreased INa density. In both ARVMs and hiPSC-CMs expression of mutant Kir2.1Δ314–315 channel reduced IK1 and INa, MDP and dV/dtmax, and increased APD. In immunostaining experiments AP1 ϒ-adaptin shows a t-tubular distribution and is also concentrated at the intercalated disc colocalizing with both NaV1.5 and Kir2.1. Site-direct mutagenesis revealed that NaV1.5WT interacts with AP1 ϒ-adaptin through the NaV1.5Y1810 residue. Finally, silencing the AP1 ϒ-adaptin subunit in hiPSC-CMs reduced IK1, INa and MDP, and impaired rate dependent APD adaptation. These data suggest strongly that in cardiomyocytes, NaV1.5 and Kir2.1 channels may travel together to their eventual micro-domains, whether at the lateral membrane, t-tubule or intercalated disc; disruption of Kir2.1 trafficking affects trafficking of NaV1.5; and Golgi export of a proportion of NaV1.5WT channels depends on interaction with the AP1 ϒ-adaptin subunit clathrin adaptor complex. The Kir2.1Δ314–315 mutant is expressed and processed into mature channels but at much lower efficiency than Kir2.1WT;12, 28, 34 we observed less total protein production for Kir2.1Δ314–315 than Kir2.1WT (Online Figure VII C,F). Nevertheless, Kir2.1Δ314–315 was sufficient to affect trafficking of NaV1.5 to the cell surface whether it was expressed to form homotetramers, or to form heterotetramers with Kir2.1WT (Figure 1C,D, Figure 3C,D, Online Figure I C,D and Online Figure II B). Further, when we co-transfected Kir2.1WT, Kir2.1Δ314–315 and NaV1.5WT, the IKir2.1 density was 92% smaller than the IKir2.1 density of Kir2.1WT when expressed alone. These results agree with previous reports showing that, while Kir2.1Δ314–315 forms channels that do not traffic to the membrane surface, they retain their ability to co-assemble with, and exert a dominant negative effect on the Kir2.1WT channels.28 Therefore, it is possible that the IKir2.1 density recorded is elicited by Kir2.1WT subunits co-assembled as homotetramers rather than Kir2.1WT or Kir2.1Δ314–315 subunits rescued by the expression by NaV1.5WT channels, Online Figure I A,B.
Expression of the trafficking-deficient mutant Kir2.1Δ314–315 channel significantly reduced INa density in HEK-293T cells, ARVMs and hiPSC-CMs. However, a significant amount of residual current was maintained in all cases, implying that some NaV1.5 channels were able to reach the plasma membrane. Data in the literature suggest that HEK-293 cells, cardiomyocytes and developing neurons contain multiple intracellular storage pools of NaV1.5 channel protein that can be mobilized following a physiologic stimulus46–48. Those reports together with our data suggest that different pools of NaV1.5 channels reach the plasma membrane from distinct origins (Golgi apparatus, unconventional Golgi pathway or storage pools). Based on the report by Mercier and coworkers18, as well as our results, it is likely that the NaV1.5 channels affected by Kir2.1Δ314–315 are nascent NaV1.5 channels that use the classical Golgi secretory route to reach the plasma membrane. The percentage of NaV1.5 channels that use one or the other pathway remains a topic of further experimentation. Even so, the fact that a fraction of NaV1.5WT channels that associate with Kir2.1WT are Endo-H-sensitive, strongly suggests that NaV1.5WT and Kir2.1WT channels can interact early in their biosynthetic pathway.
Our understanding of how newly synthesized plasma membrane proteins are sorted to the cell membrane is still limited. Critical regulatory steps that control the surface expression of ion channels are likely to be involved starting at the endoplasmic reticulum and Golgi. Considerable evidence indicates that targeting or trafficking of ion channels within the cell is frequently mediated by interaction with other proteins through specific amino acid motifs present in the channel sequence20–23, 26, 38, 49. Moreover, evidence suggests the existence of retention and export trafficking signals that regulate functional expression of transmembrane proteins33, 39–44. Still, the precise export signals that regulate exit of NaV1.5 from the Golgi and transit to the plasma membrane are not fully understood. The AP1 clathrin adaptor is one of the best known molecules to facilitate export of cargo proteins from the trans-Golgi network50. We show the distribution of AP1 protein and its colocalization with NaV1.5 and Kir2.1 in cardiac cells and explored whether NaV1.5 contained the typical amino acid consensus motif sequence (YXXΦ) for adaptin binding. Based on the amino acid sequence of the channel we identified four probable consensus binding motifs for the AP1 complex on the COOH domain (Y1794, Y1810, Y1888, Y1976) and one possible motif on the NH2 domain (Y68). Furthermore, our co-immunoprecipitation experiments demonstrate that NaV1.5 interacts with the AP1 complex. Yet NaV1.5Y1810A did not co-immunoprecipitate with AP1 protein, consistent with a decrease in INa density flowing through NaV1.5Y1810A channels. This suggests that, similar to Kir2.1, some NaV1.5 channels may be exported from the Golgi in a signal-dependent manner through an AP1 clathrin adaptor interaction. We observed that NaV1.5Y1976A channels had an increased INa density relative to control. Interestingly the Y1976 residue locates within the PY motif of the NaV1.5 channel,51, 52 and the NaV1.5Y1976A mutant has been shown to impair the Nedd4-2 binding motif53. Therefore, it is likely that the increase in INa density observed for the NaV1.5Y1976A mutant is the result of decreased ubiquitination followed by internalization and/or degradation of NaV1.5Y1976A channels. Future studies will be necessary to confirm that hypothesis.
It has been proposed that the YXXΦ consensus motif recruits specific coat protein complexes that induce formation of transport vesicles, not only destined for certain subcellular localization, but also preferentially loaded with respective cargo proteins54. Thus, based on our data showing that both channels interact with the AP1 complex, we postulate that Kir2.1 and NaV1.5 channels may be loaded in joint transport vesicles to be delivered to common subdomains at the plasma membrane. These data support previous reports on the existence of different pools of NaV1.5 channel trafficking vesicles18 and suggest that trafficking vesicles may carry varying compositions of load proteins. However, even though our data show NaV1.5 can interact with AP1 for transportation, we cannot exclude participation of GGA1, a protein also known to facilitate export cargo from the trans-Golgi network50.
Moreover, we observed that silencing the AP1 ϒ-adaptin subunit in hiPSC-CMs reduced IK1, INa and the MDP and impaired the rate dependent APD adaptation. However, significant amounts of residual IK1 and INa were maintained, implying that some Kir2.1 and NaV1.5 channels reached the plasma membrane. These data suggest that, like NaV1.5 channels, Kir2.1 channels reach the plasma from distinct origins that may include, in addition to the Golgi apparatus, an unconventional Golgi pathway or storage pools and that other proteins may be involved in the export of Kir2.1 and NaV1.5 channels from the trans-Golgi network. The observation that AP1 ϒ-adaptin silencing impaired the rate dependence of APD adaptation in hiPSC-CMs, suggests that AP1 ϒ protein may regulate the expression of ion channels and proteins involved in calcium homeostasis55–58.
The intracellular COOH terminal of NaV1.5 contains several protein-protein interaction motifs. The most well-characterized interaction sites are the calmodulin-biding IQ motif, the PY motif, and a PDZ domain-biding motif59, 60. In contrast, less is known about proteins that interact with the NH2 domain of NaV1.5. The NaV1.5 NH2 domain is critical for expression density, although the specific mechanism is not clear. A putative NaV1.5 NH2 fragment enhances NaV1.5WT channel trafficking by possibly acting as a decoy, allowing NaV1.5WT channels to bypass a regulatory system and reach the plasma membrane61. More recently, Matamoros et al. demonstrated that the NH2 terminus of NaV1.5 has a PDZ-like binding domain that mediates binding to α1-syntrophin, and can act like a chaperone to increase INa and IK1 through enhanced expression of NaV1.5, Kir2.1, and Kir2.2 but not Kir2.3 proteins26.
Our data demonstrate for the first time that NaV1.5 interact with AP1 involved in the trans-Golgi network of cargo export proteins. Similar to the Kir2.1-AP1 complex12, the NaV1.5-AP1 complex interaction may participate in regulation of functional expression of NaV1.5, and contribute to formation of the NaV1.5-Kir2.1 channelosome22
Clinical relevance
The interplay between IK1 and INa is crucial to normal cardiac electrical function. By controlling resting membrane potential, IK1 modifies sodium channel availability and therefore cell excitability, APD, and conduction velocity. IK1 and INa interactions are key determinants of electrical rotor dynamics which are responsible for abnormal, often lethal, cardiac reentrant activity62. Data presented here demonstrate that expression of the trafficking-deficient mutant Kir2.1Δ314–315 in myocytes not only reduces native IK1, but also INa by disrupting functional expression of both channels at the plasma membrane. Previous simulation studies predicted that a reduction in IK1 might have unique effects on AP configuration and arrhythmia susceptibility30. It was anticipated that a reduction in IK1 would prolong the terminal repolarization phase of the cardiac AP by reducing the amount of repolarizing current during the terminal phase of the AP, resulting in QT prolongation in affected individuals30. It is well known that AP prolongation is a prerequisite for early afterdepolarizations that can trigger spontaneous APs. In ARVMs we observed that expression of Kir2.1Δ314–315 resulted in membrane depolarization, marked APD prolongation and spontaneous APs, validating the aforementioned numerical predictions.
Andersen-Tawil Syndrome type 1 (ATS1) is a rare disorder caused by mutations in the KCNJ2 gene coding Kir2.1. Clinical manifestations of ATS1 include periodic paralysis, cardiac arrhythmias, and dysmorphic features. The cardiac manifestations of ATS1 patients with KCNJ2 mutations include QT prolongation, premature ventricular contractions, complex ventricular ectopy, bigeminy, and polymorphic or bidirectional ventricular tachycardia. It has been difficult to correlate functional consequences of individual Kir2.1 mutation12 s with corresponding clinical phenotypes28–30, 63. Our data provide novel insights into the ionic basis of arrhythmia susceptibility in ATS1 patients. We postulate that in addition to reduced IK1, ATS1 patients also have reduced INa as a consequence of disruption of the Kir2.1-NaV1.5 complex by a trafficking-deficient Kir2.1 mutation. Consequently, co-expression of the trafficking-deficient mutant Kir2.1 channel with NaV1.5WT is expected to contribute to arrhythmogenesis in ATS1 patients by reducing MDP and dV/dtmax via two different mechanisms: the voltage dependent mechanism, and effects of physical intermolecular interactions between the two channels. Our data highlight the importance of considering effects of a given mutation on functional expression of other ion channels or proteins that may be a part of the macromolecular complex within which Kir2.1 interacts.
Our study focused on the interplay between Kir2.1 and NaV1.5. However, it is likely that other proteins, including ion channels, are part of the same macromolecular complex. The presence of a trafficking-deficient mutant channel may have the potential to affect functional expression of another channel within its macromolecular complex. Notably, we selected Kir2.1Δ314–315 for its ability to retain the channel in the Golgi and its strong dominant negative effect. We also explored functional consequences of co-expressing NaV1.5 with the double mutant Kir2.1R44A/R46A, whose surface expression is reduced33. We observed that INav1.5 density of cells that co-expressed Kir2.1R44A/R46A was reduced with respect to cells expressing NaV1.5WT channels alone (Online Figure II C,D). Unexpectedly, our functional analysis showed that mutant Kir2.1R44A/R46A channels generated a current density that was similar to IKir2.1WT. Future studies will be necessary to further elucidate the molecular mechanism involved in the regulation of functional expression of Kir2.1 channels.
These data support our contention that a common early anterograde trafficking mechanism is involved in transport of NaV1.5 and Kir2.1 channels to the plasma membrane. Therefore, it seems reasonable to speculate that Kir2.1 mutants that affect trafficking from the endoplasmic reticulum have similar effects. However, while our data uncovers a common early anterograde trafficking pathway for NaV1.5 and Kir2.1 channels, it is premature to expect that all Kir2.1 trafficking-deficient channels will induce a decrease in INa density. In this regard, an unexpected finding was that NaV1.5WT channel failed to rescue mutant Kir2.1Δ314–315 plasma membrane expression. However, it is possible that for trafficking deficient Kir2.1 mutants with weaker trafficking properties, the presence of NaV1.5 channels might rescue and promote surface expression. It is also possible that trafficking-deficient NaV1.5 mutations affect functional expression of Kir2.1 and other membrane channels. In this regard, it has been shown previously that the α-subunit of IKs can interact with and modify the localization and current-carrying properties of the α-subunit of IKr.64 Clearly additional work is needed to unravel the details of regulation of early trafficking of cardiac ion channels and their incorporation into macromolecular complexes at the cell membrane.
Potential limitations
Our study has potential limitations. First, some experiments were performed in heterologous HEK-293T, COS-7 or CHO cell expression systems, which make interpretation of trafficking studies difficult. However, we validated functional expression results by recording native INa and IK1 as well as AP characteristics in ARVMs and hiPSC-CMs. Second, based on our data we are unable to differentiate whether the observed trafficking disruption in NaV1.5 on Kir2.1Δ314–315 expression was due to a delay in expression kinetics, or whether all newly synthetized NaV1.5 channels were retained in the Golgi. Third, we cannot rule out that accumulation of trafficking-deficient mutant Kir2.1Δ314–315 at the Golgi evokes a stress state, and the response to adjust Golgi capacity to myocyte demand might be compromised. Finally, we did not determine whether preferential reduction of NaV1.5 channel was localized to the lateral membrane, T tubules or intercalated discs.
The above limitations notwithstanding, our results provide strong evidence that the NaV1.5-Kir2.1 macromolecular complex preassembles early in its forward trafficking pathway, and that trafficking deficiency and retention of one channel at the Golgi affects trafficking of both channels. Our data also show that NaV1.5 channels may be selected as cargo into Golgi export carriers in a signal dependent manner through an AP1 clathrin adaptor interaction. Altogether, the data provide novel insights into molecular mechanisms underlying arrhythmogenesis in ATS1 patients.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
The main cardiac sodium channel (NaV1.5) and the strong inward rectifier potassium channel (Kir2.1) reciprocally modulate their functional expression, an interplay that is crucial to normal cardiac electrical function. By controlling resting membrane potential, IK1 modifies sodium channel availability and therefore cell excitability, APD, and conduction velocity.
NaV1.5 and Kir2.1 channels physically interact together with multiple other protein partners from early stages of protein trafficking, targeting through membrane anchoring, recycling and degradation.
What New Information Does This Article Contribute?
A pool of NaV1.5 and Kir2.1 channels preassemble during early forward trafficking and travel together to common membrane microdomains.
Similar to Kir2.1, some NaV1.5 channels can exit the Golgi apparatus by an adaptor protein complex 1 (AP1)-dependent trafficking process.
Trafficking deficiency of Kir2.1 channels affects trafficking of both Kir2.1 and NaV1.5 channels complex components, which may have important implications for the mechanisms of arrhythmias in inheritable and possibly other cardiac diseases.
Cardiac NaV1.5 and Kir2.1 function within macromolecular complexes to control cardiac excitability. Cellular processes that regulate trafficking and expression of Kir2.1 and NaV1.5 channels are of interest as potential mediators of proarrhythmic channelopathies and arrhythmogenesis in other cardiac diseases, including heart failure. However, it remains unknown whether Kir2.1 and NaV1.5 pre-assemble or interact during early anterograde trafficking steps of their secretory pathways. Here, we demonstrate that the NaV1.5 and Kir2.1 form a complex that preassembles early in its forward trafficking pathway. Further, we found that NaV1.5 channels may be selected as cargo into Golgi export carriers in a signal dependent manner through an AP1 clathrin adaptor interaction. Moreover, trafficking deficiency and retention of Kir2.1 channel at the Golgi apparatus affects trafficking of NaV1.5 channels. Our data highlight the importance of considering effects of trafficking defective mutations on functional expression of other ion channels or proteins that may be a part of the macromolecular complex within which the proteins interact. Our study offers a novel paradigm regarding the molecular mechanism of arrhythmia susceptibility in monogenic ion channels diseases such as the Andersen Tawil Syndrome and potentially other diseases, including Brugada syndrome.
Acknowledgments
We gratefully acknowledge Nulang Wang for technical excellence in isolation and culture of cardiac myocytes. We gratefully acknowledge Yan Chen for technical excellence in the production of the mutant plasmids.
SOURCES OF FUNDING
This work was supported in part by the National Heart, Lung, and Blood Institute (R01 Grant HL122352) and grants from Transatlantic Networks of Excellence Program from the Leducq Foundation, and Fondos FEDER, Madrid, Spain, to J. Jalife. D. Ponce-Balbuena and B. C. Willis were supported by American Heart Association Postdoctoral Fellowships 14POST17820005 and 12POST12030292, respectively. F.J Alvarado was supported by American Heart Association Predoctoral Fellowship 14PRE19500012 E. Delpón and R. Caballero were funded with Fondos FEDER and Ministerio de Economía y Competividad (SAF2014-58769-P).
Nonstandard Abbreviations and Acronyms
- AP
Action potential
- AP1
Adaptor protein complex 1
- AP2
Adaptor protein complex 2
- AP3
Adaptor protein complex 3
- AP4
Adaptor protein complex 4
- AP5
Adaptor protein complex 5
- APD
Action potential duration
- ARVM
Adult rat ventricular myocytes
- ATS1
Andersen-Tawil Syndrome type 1
- CHO
Chine hamster ovary
- COS-7
CV-1 (simian) in Origin carrying the SV40 genetic material
- EAD
Early afterdepolarizations
- Endo-H
Endoglycosidase-H
- ER
Endoplasmic reticulum
- FBS
Fetal bovine serum
- FRAP
Fluorescence-recovery-after-photobleaching
- HEK
Human embryonic kidney
- hiPSC-CMs
Human induce pluripotent stem cell derived cardiomyocytes
- IK1
Inward rectifier potassium current
- INa
Inward sodium current
- Ito
Transient outward potassium current
- Kir
Inward rectifier potassium channel
- MDP
Maximum diastolic potential
- NaV1.5
Voltage-gate sodium channel
- PBS
Phosphate buffer saline
- PNGase-F
Peptide-N-Glycosidase-F
- PVCs
Premature ventricular contraction
- ROI
Regions of interest
- VT
Ventricular tachycardia
- WT
Wildtype
Footnotes
DISCLOSURES
None.
AUTHOR CONTRIBUTIONS
DP-B, GG-S, CRV, HHV, RC, ED, and JJ conceived experiments, designed research, and discussed results and strategy. DP-B, RC, FJD-G, ENJ-V, AMR, TJH, KFC, BCW, FJA, MZ, RJR, KK, MP-H, and MM performed experiments and collected, analyzed and discussed data; JJ directed the study. DP-B and JJ wrote the manuscript, which was revised and approved by all authors.
References
- 1.Wang Z, Yue L, White M, Pelletier G, Nattel S. Differential distribution of inward rectifier potassium channel transcripts in human atrium versus ventricle. Circulation. 1998;98:2422–8. doi: 10.1161/01.cir.98.22.2422. [DOI] [PubMed] [Google Scholar]
- 2.Anumonwo JM, Lopatin AN. Cardiac strong inward rectifier potassium channels. Journal of molecular and cellular cardiology. 2010;48:45–54. doi: 10.1016/j.yjmcc.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichols CG, Makhina EN, Pearson WL, Sha Q, Lopatin AN. Inward rectification and implications for cardiac excitability. Circulation research. 1996;78:1–7. doi: 10.1161/01.res.78.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Vaquero M, Calvo D, Jalife J. Cardiac fibrillation: from ion channels to rotors in the human heart. Heart rhythm: the official journal of the Heart Rhythm Society. 2008;5:872–9. doi: 10.1016/j.hrthm.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiological reviews. 2004;84:1341–79. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 6.Gaborit N, Le Bouter S, Szuts V, Varro A, Escande D, Nattel S, Demolombe S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. The Journal of physiology. 2007;582:675–93. doi: 10.1113/jphysiol.2006.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melnyk P, Zhang L, Shrier A, Nattel S. Differential distribution of Kir2.1 and Kir2.3 subunits in canine atrium and ventricle. American journal of physiology Heart and circulatory physiology. 2002;283:H1123–33. doi: 10.1152/ajpheart.00934.2001. [DOI] [PubMed] [Google Scholar]
- 8.Christe G. Localization of K(+) channels in the tubules of cardiomyocytes as suggested by the parallel decay of membrane capacitance, IK(1) and IK(ATP) during culture and by delayed IK(1) response to barium. Journal of molecular and cellular cardiology. 1999;31:2207–13. doi: 10.1006/jmcc.1999.1034. [DOI] [PubMed] [Google Scholar]
- 9.Schram G, Pourrier M, Melnyk P, Nattel S. Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function. Circulation research. 2002;90:939–50. doi: 10.1161/01.res.0000018627.89528.6f. [DOI] [PubMed] [Google Scholar]
- 10.Farquhar MG, Palade GE. The Golgi apparatus: 100 years of progress and controversy. Trends in cell biology. 1998;8:2–10. doi: 10.1016/S0962-8924(97)01187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu F, Crump CM, Thomas G. Trans-Golgi network sorting. Cellular and molecular life sciences: CMLS. 2001;58:1067–84. doi: 10.1007/PL00000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma D, Taneja TK, Hagen BM, Kim BY, Ortega B, Lederer WJ, Welling PA. Golgi export of the Kir2.1 channel is driven by a trafficking signal located within its tertiary structure. Cell. 2011;145:1102–15. doi: 10.1016/j.cell.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SY, Guo X. Adaptor protein complexes and intracellular transport. Bioscience reports. 2014:34. doi: 10.1042/BSR20140069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonifacino JS. Adaptor proteins involved in polarized sorting. The Journal of cell biology. 2014;204:7–17. doi: 10.1083/jcb.201310021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paczkowski JE, Richardson BC, Fromme JC. Cargo adaptors: structures illuminate mechanisms regulating vesicle biogenesis. Trends in cell biology. 2015;25:408–16. doi: 10.1016/j.tcb.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catterall WA. Molecular properties of voltage-sensitive sodium channels. Annual review of biochemistry. 1986;55:953–85. doi: 10.1146/annurev.bi.55.070186.004513. [DOI] [PubMed] [Google Scholar]
- 17.Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, Kallen RG, Mandel G, Meisler MH, Netter YB, Noda M, Tamkun MM, Waxman SG, Wood JN, Catterall WA. Nomenclature of voltage-gated sodium channels. Neuron. 2000;28:365–8. doi: 10.1016/s0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 18.Mercier A, Clement R, Harnois T, Bourmeyster N, Bois P, Chatelier A. Nav1.5 channels can reach the plasma membrane through distinct N-glycosylation states. Biochimica et biophysica acta. 2015;1850:1215–23. doi: 10.1016/j.bbagen.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Gavillet B, Rougier JS, Domenighetti AA, Behar R, Boixel C, Ruchat P, Lehr HA, Pedrazzini T, Abriel H. Cardiac sodium channel Nav1.5 is regulated by a multiprotein complex composed of syntrophins and dystrophin. Circulation research. 2006;99:407–14. doi: 10.1161/01.RES.0000237466.13252.5e. [DOI] [PubMed] [Google Scholar]
- 20.Gillet L, Rougier JS, Shy D, Sonntag S, Mougenot N, Essers M, Shmerling D, Balse E, Hatem SN, Abriel H. Cardiac-specific ablation of synapse-associated protein SAP97 in mice decreases potassium currents but not sodium current. Heart rhythm: the official journal of the Heart Rhythm Society. 2015;12:181–92. doi: 10.1016/j.hrthm.2014.09.057. [DOI] [PubMed] [Google Scholar]
- 21.Petitprez S, Zmoos AF, Ogrodnik J, Balse E, Raad N, El-Haou S, Albesa M, Bittihn P, Luther S, Lehnart SE, Hatem SN, Coulombe A, Abriel H. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1.5 in cardiomyocytes. Circulation research. 2011;108:294–304. doi: 10.1161/CIRCRESAHA.110.228312. [DOI] [PubMed] [Google Scholar]
- 22.Milstein ML, Musa H, Balbuena DP, Anumonwo JM, Auerbach DS, Furspan PB, Hou L, Hu B, Schumacher SM, Vaidyanathan R, Martens JR, Jalife J. Dynamic reciprocity of sodium and potassium channel expression in a macromolecular complex controls cardiac excitability and arrhythmia. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2134–43. doi: 10.1073/pnas.1109370109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowe JS, Palygin O, Bhasin N, Hund TJ, Boyden PA, Shibata E, Anderson ME, Mohler PJ. Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway. The Journal of cell biology. 2008;180:173–86. doi: 10.1083/jcb.200710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohler PJ, Splawski I, Napolitano C, Bottelli G, Sharpe L, Timothy K, Priori SG, Keating MT, Bennett V. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9137–42. doi: 10.1073/pnas.0402546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balijepalli RC, Kamp TJ. Caveolae, ion channels and cardiac arrhythmias. Progress in biophysics and molecular biology. 2008;98:149–60. doi: 10.1016/j.pbiomolbio.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matamoros M, Perez-Hernandez M, Guerrero-Serna G, Amoros I, Barana A, Nunez M, Ponce-Balbuena D, Sacristan S, Gomez R, Tamargo J, Caballero R, Jalife J, Delpon E. Nav1.5 N-terminal domain binding to alpha1-syntrophin increases membrane density of human Kir2.1, Kir2.2 and Nav1.5 channels. Cardiovascular research. 2016 doi: 10.1093/cvr/cvw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Utrilla RG, Nieto-Marin P, Alfayate S, Tinaquero D, Matamoros M, Perez-Hernandez M, Sacristan S, Ondo L, de Andres R, Diez-Guerra FJ, Tamargo J, Delpon E, Caballero R. Kir2.1-Nav1.5 Channel Complexes Are Differently Regulated than Kir2.1 and Nav1.5 Channels Alone. Front Physiol. 2017;8:903. doi: 10.3389/fphys.2017.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bendahhou S, Donaldson MR, Plaster NM, Tristani-Firouzi M, Fu YH, Ptacek LJ. Defective potassium channel Kir2.1 trafficking underlies Andersen-Tawil syndrome. The Journal of biological chemistry. 2003;278:51779–85. doi: 10.1074/jbc.M310278200. [DOI] [PubMed] [Google Scholar]
- 29.Plaster NM, Tawil R, Tristani-Firouzi M, Canun S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL, Jr, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptacek LJ. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen’s syndrome. Cell. 2001;105:511–9. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 30.Tristani-Firouzi M, Jensen JL, Donaldson MR, Sansone V, Meola G, Hahn A, Bendahhou S, Kwiecinski H, Fidzianska A, Plaster N, Fu YH, Ptacek LJ, Tawil R. Functional and clinical characterization of KCNJ2 mutations associated with LQT7 (Andersen syndrome) The Journal of clinical investigation. 2002;110:381–8. doi: 10.1172/JCI15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donaldson MR, Yoon G, Fu YH, Ptacek LJ. Andersen-Tawil syndrome: a model of clinical variability, pleiotropy, and genetic heterogeneity. Annals of medicine. 2004;36(Suppl 1):92–7. doi: 10.1080/17431380410032490. [DOI] [PubMed] [Google Scholar]
- 32.Kaur K, Zarzoso M, Ponce-Balbuena D, Guerrero-Serna G, Hou L, Musa H, Jalife J. TGF-beta1, released by myofibroblasts, differentially regulates transcription and function of sodium and potassium channels in adult rat ventricular myocytes. PloS one. 2013;8:e55391. doi: 10.1371/journal.pone.0055391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stockklausner C, Klocker N. Surface expression of inward rectifier potassium channels is controlled by selective Golgi export. The Journal of biological chemistry. 2003;278:17000–5. doi: 10.1074/jbc.M212243200. [DOI] [PubMed] [Google Scholar]
- 34.Kolb AR, Needham PG, Rothenberg C, Guerriero CJ, Welling PA, Brodsky JL. ESCRT regulates surface expression of the Kir2.1 potassium channel. Molecular biology of the cell. 2014;25:276–89. doi: 10.1091/mbc.E13-07-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annual review of biochemistry. 1985;54:631–64. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 36.Caster AH, Sztul E, Kahn RA. A role for cargo in Arf-dependent adaptor recruitment. The Journal of biological chemistry. 2013;288:14788–804. doi: 10.1074/jbc.M113.453621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eichel CA, Beuriot A, Chevalier MY, Rougier JS, Louault F, Dilanian G, Amour J, Coulombe A, Abriel H, Hatem SN, Balse E. Lateral Membrane-Specific MAGUK CASK Down-Regulates NaV1.5 Channel in Cardiac Myocytes. Circulation research. 2016;119:544–56. doi: 10.1161/CIRCRESAHA.116.309254. [DOI] [PubMed] [Google Scholar]
- 38.Shy D, Gillet L, Ogrodnik J, Albesa M, Verkerk AO, Wolswinkel R, Rougier JS, Barc J, Essers MC, Syam N, Marsman RF, van Mil AM, Rotman S, Redon R, Bezzina CR, Remme CA, Abriel H. PDZ domain-binding motif regulates cardiomyocyte compartment-specific NaV1.5 channel expression and function. Circulation. 2014;130:147–60. doi: 10.1161/CIRCULATIONAHA.113.007852. [DOI] [PubMed] [Google Scholar]
- 39.Zhou J, Shin HG, Yi J, Shen W, Williams CP, Murray KT. Phosphorylation and putative ER retention signals are required for protein kinase A-mediated potentiation of cardiac sodium current. Circulation research. 2002;91:540–6. doi: 10.1161/01.res.0000033598.00903.27. [DOI] [PubMed] [Google Scholar]
- 40.Zhou J, Yi J, Hu N, George AL, Jr, Murray KT. Activation of protein kinase A modulates trafficking of the human cardiac sodium channel in Xenopus oocytes. Circulation research. 2000;87:33–8. doi: 10.1161/01.res.87.1.33. [DOI] [PubMed] [Google Scholar]
- 41.Ma D, Zerangue N, Lin YF, Collins A, Yu M, Jan YN, Jan LY. Role of ER export signals in controlling surface potassium channel numbers. Science. 2001;291:316–9. doi: 10.1126/science.291.5502.316. [DOI] [PubMed] [Google Scholar]
- 42.Stockklausner C, Ludwig J, Ruppersberg JP, Klocker N. A sequence motif responsible for ER export and surface expression of Kir2.0 inward rectifier K(+) channels. FEBS letters. 2001;493:129–33. doi: 10.1016/s0014-5793(01)02286-4. [DOI] [PubMed] [Google Scholar]
- 43.Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 1999;22:537–48. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 44.Hofherr A, Fakler B, Klocker N. Selective Golgi export of Kir2.1 controls the stoichiometry of functional Kir2.x channel heteromers. Journal of cell science. 2005;118:1935–43. doi: 10.1242/jcs.02322. [DOI] [PubMed] [Google Scholar]
- 45.Herron TJ, Rocha AM, Campbell KF, Ponce-Balbuena D, Willis BC, Guerrero-Serna G, Liu Q, Klos M, Musa H, Zarzoso M, Bizy A, Furness J, Anumonwo J, Mironov S, Jalife J. Extracellular Matrix-Mediated Maturation of Human Pluripotent Stem Cell-Derived Cardiac Monolayer Structure and Electrophysiological Function. Circulation Arrhythmia and electrophysiology. 2016;9:e003638. doi: 10.1161/CIRCEP.113.003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmer T, Biskup C, Bollensdorff C, Benndorf K. The beta1 subunit but not the beta2 subunit colocalizes with the human heart Na+ channel (hH1) already within the endoplasmic reticulum. The Journal of membrane biology. 2002;186:13–21. doi: 10.1007/s00232-001-0131-0. [DOI] [PubMed] [Google Scholar]
- 47.Zimmer T, Biskup C, Dugarmaa S, Vogel F, Steinbis M, Bohle T, Wu YS, Dumaine R, Benndorf K. Functional expression of GFP-linked human heart sodium channel (hH1) and subcellular localization of the a subunit in HEK293 cells and dog cardiac myocytes. The Journal of membrane biology. 2002;186:1–12. doi: 10.1007/s00232-001-0130-1. [DOI] [PubMed] [Google Scholar]
- 48.Yarbrough TL, Lu T, Lee HC, Shibata EF. Localization of cardiac sodium channels in caveolin-rich membrane domains: regulation of sodium current amplitude. Circulation research. 2002;90:443–9. doi: 10.1161/hh0402.105177. [DOI] [PubMed] [Google Scholar]
- 49.Makara MA, Curran J, Little SC, Musa H, Polina I, Smith SA, Wright PJ, Unudurthi SD, Snyder J, Bennett V, Hund TJ, Mohler PJ. Ankyrin-G coordinates intercalated disc signaling platform to regulate cardiac excitability in vivo. Circulation research. 2014;115:929–38. doi: 10.1161/CIRCRESAHA.115.305154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annual review of biochemistry. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 51.van Bemmelen MX, Rougier JS, Gavillet B, Apotheloz F, Daidie D, Tateyama M, Rivolta I, Thomas MA, Kass RS, Staub O, Abriel H. Cardiac voltage-gated sodium channel Nav1.5 is regulated by Nedd4–2 mediated ubiquitination. Circulation research. 2004;95:284–91. doi: 10.1161/01.RES.0000136816.05109.89. [DOI] [PubMed] [Google Scholar]
- 52.Rougier JS, van Bemmelen MX, Bruce MC, Jespersen T, Gavillet B, Apotheloz F, Cordonier S, Staub O, Rotin D, Abriel H. Molecular determinants of voltage-gated sodium channel regulation by the Nedd4/Nedd4-like proteins. Am J Physiol Cell Physiol. 2005;288:C692–701. doi: 10.1152/ajpcell.00460.2004. [DOI] [PubMed] [Google Scholar]
- 53.Luo L, Ning F, Du Y, Song B, Yang D, Salvage SC, Wang Y, Fraser JA, Zhang S, Ma A, Wang T. Calcium-dependent Nedd4–2 upregulation mediates degradation of the cardiac sodium channel Nav1.5: implications for heart failure. Acta Physiol (Oxf) 2017;221:44–58. doi: 10.1111/apha.12872. [DOI] [PubMed] [Google Scholar]
- 54.Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–66. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 55.Boyett MR, Jewell BR. A study of the factors responsible for rate-dependent shortening of the action potential in mammalian ventricular muscle. The Journal of physiology. 1978;285:359–80. doi: 10.1113/jphysiol.1978.sp012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boyett MR. An analysis of the effect of the rate of stimulation and adrenaline on the duration of the cardiac action potential. Pflugers Arch. 1978;377:155–66. doi: 10.1007/BF00582846. [DOI] [PubMed] [Google Scholar]
- 57.Li GR, Yang B, Feng J, Bosch RF, Carrier M, Nattel S. Transmembrane ICa contributes to rate-dependent changes of action potentials in human ventricular myocytes. Am J Physiol. 1999;276:H98–H106. doi: 10.1152/ajpheart.1999.276.1.H98. [DOI] [PubMed] [Google Scholar]
- 58.Lee YS, Hwang M, Song JS, Li C, Joung B, Sobie EA, Pak HN. The Contribution of Ionic Currents to Rate-Dependent Action Potential Duration and Pattern of Reentry in a Mathematical Model of Human Atrial Fibrillation. PloS one. 2016;11:e0150779. doi: 10.1371/journal.pone.0150779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shy D, Gillet L, Abriel H. Cardiac sodium channel NaV1.5 distribution in myocytes via interacting proteins: the multiple pool model. Biochimica et biophysica acta. 2013;1833:886–94. doi: 10.1016/j.bbamcr.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 60.Abriel H. Cardiac sodium channel Na(v)1.5 and interacting proteins: Physiology and pathophysiology. Journal of molecular and cellular cardiology. 2010;48:2–11. doi: 10.1016/j.yjmcc.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 61.Clatot J, Ziyadeh-Isleem A, Maugenre S, Denjoy I, Liu H, Dilanian G, Hatem SN, Deschenes I, Coulombe A, Guicheney P, Neyroud N. Dominant-negative effect of SCN5A N-terminal mutations through the interaction of Na(v)1.5 alpha-subunits. Cardiovascular research. 2012;96:53–63. doi: 10.1093/cvr/cvs211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noujaim SF, Pandit SV, Berenfeld O, Vikstrom K, Cerrone M, Mironov S, Zugermayr M, Lopatin AN, Jalife J. Up-regulation of the inward rectifier K+ current (I K1) in the mouse heart accelerates and stabilizes rotors. The Journal of physiology. 2007;578:315–26. doi: 10.1113/jphysiol.2006.121475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donaldson MR, Jensen JL, Tristani-Firouzi M, Tawil R, Bendahhou S, Suarez WA, Cobo AM, Poza JJ, Behr E, Wagstaff J, Szepetowski P, Pereira S, Mozaffar T, Escolar DM, Fu YH, Ptacek LJ. PIP2 binding residues of Kir2.1 are common targets of mutations causing Andersen syndrome. Neurology. 2003;60:1811–6. doi: 10.1212/01.wnl.0000072261.14060.47. [DOI] [PubMed] [Google Scholar]
- 64.Ehrlich JR, Pourrier M, Weerapura M, Ethier N, Marmabachi AM, Hebert TE, Nattel S. KvLQT1 modulates the distribution and biophysical properties of HERG. A novel alpha-subunit interaction between delayed rectifier currents. The Journal of biological chemistry. 2004;279:1233–41. doi: 10.1074/jbc.M309087200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.