Abstract
The importance of insula in speech control is acknowledged but poorly understood, partly due to a variety of clinical symptoms resulting from insults to this structure. To clarify its structural organization within the speech network in healthy subjects, we used probabilistic diffusion tractography to examine insular connectivity with three cortical regions responsible for sound processing (Brodmann area (BA) 22), motor preparation (BA 44) and motor execution (laryngeal/orofacial primary motor cortex, BA 4). To assess insular reorganization in a speech disorder, we examined its structural connectivity in patients with spasmodic dysphonia (SD), a neurological condition that selectively affects speech production. We demonstrated structural segregation of insula into three non-overlapping regions, which receive distinct connections from BA 44 (anterior insula), BA 4 (mid-insula) and BA 22 (dorsal and posterior insula). There were no significant differences either in the number of streamlines connecting each insular subdivision to the cortical target or hemispheric lateralization of insular clusters and their projections between healthy subjects and SD patients. However, spatial distribution of the insular subdivisions connected to BA 4 and BA 44 was distinctly organized in healthy controls and SD patients, extending ventro-posteriorly in the former group and anterio-dorsally in the latter group. Our findings point to a structural segregation of the insular sub-regions, which may be associated with the different aspects of sensorimotor and cognitive control of speech production. We suggest that distinct insular involvement may lead to different clinical manifestations when one or the other insular region and/or its connections undergo spatial reorganization.
Keywords: speech production, insular organization, probabilistic tractography
Introduction
Speech production is a complex motor behavior that requires multi-level and orchestrated involvement of cortical, subcortical and cerebellar regions for integration between auditory input, sensorimotor transformations, working memory, emotional processing, and motor output (Guenther 2006; Fuertinger et al. 2015; Hickok and Poeppel 2007). Among these, several lesion studies in stroke patients have consistently pointed to the insula as one of the important cortical regions in speech motor control (Baldo et al. 2011; Dronkers 1996). Damages to the left anterior insula (specifically, its superior precentral gyrus) due to stroke have been reported to lead to apraxia of speech (Dronkers 1996; Ogar et al. 2006; Hickok et al. 2014; Nestor et al. 2003) with reduced fluency (Bates et al. 2003; Borovsky et al. 2007) and rapidly changing articulatory movements (Baldo et al. 2011). This pointed to the critical role of insula in speech articulation. However, this view has been challenged by other lesion studies that argued for a more limited insular involvement in speech control, while attributing most articulatory impairment to the damage of left posterior inferior frontal gyrus (Hillis et al. 2004). Available functional MRI (fMRI) studies in healthy subjects have not brought consensus to this debate. Some studies have favored the importance of the anterior insula in speech coordination (Bohland and Guenther 2006; Murphy et al. 1997). Based on the fMRI meta-analysis of speech production, the insula has been suggested to serve as a relay station between the inferior frontal gyrus (Brodmann area (BA) 44) and motor preparatory areas, including the basal ganglia and cerebellum (Eickhoff et al. 2009). Another imaging study has questioned whether the insula is truly involved in speech articulation or is rather associated with processes necessary for task completion, such as modulation of speech and non-speech oral movements (Fedorenko et al. 2015).
Despite these controversies in the prior literature on the exact role of insula in speech control, its abnormal activity as well as aberrant white matter organization and connectivity continued to be discovered in more recent studies of various other neurological disorders affecting voice and speech production (e.g., (Bianchi et al. 2017; Simonyan and Ludlow 2012; Chang et al. 2009; New et al. 2015)). Among these is spasmodic dysphonia (SD, or laryngeal dystonia), which is a neurological movement disorder selectively impairing speech motor control (but not of other vocalizations) due to involuntary spasms in the laryngeal muscles. Although the causative pathophysiology of SD remains unknown, it is considered a network disorder (Battistella et al. 2017) without an apparent brain damage or lesions. Imaging studies have reported most common functional and microstructural alterations in primary sensorimotor cortex, inferior parietal cortex, basal ganglia and cerebellum (Fuertinger and Simonyan 2017; Haslinger et al. 2005; Simonyan and Ludlow 2010, 2012; Kostic et al. 2016), with abnormal hub representation in the superior temporal gyrus (Battistella et al. 2017) and symptom correlations with altered organization of the inferior frontal gyrus (Simonyan and Ludlow 2010, 2012; Kostic et al. 2016). Imaging studies have further suggested that these sensorimotor cortical alterations in SD may be influenced by the presence of selective microstructural changes in the anterior insula and their correlations with SD symptom severity (Simonyan and Ludlow 2012) across different clinical forms of this disorder (Bianchi et al. 2017). A direct comparison between sporadic and familial SD forms has further shown that alterations in the insula, together with left sensorimotor cortex, right somatosensory cortex and supplementary motor area, underlie the formation of the altered network that characterizes distinct SD genotypes (Battistella et al. 2016a). Finally, along with significant connectivity changes within the sensorimotor and frontoparietal cortices, the insula was shown to form an abnormal hub within the large-scale dystonic functional network, suggesting that internal representations of intended movements may be abnormally enhanced in SD (Battistella et al. 2017).
Based on evidence of wide-ranging involvement of insula in different neurological conditions and yet ambiguous role of this structure in speech control, we postulate that examination of its structural cortical connectivity may help shed light into the understanding of specific function(s) of this region at the different stages of speech processing and production. We hypothesized that, in healthy individuals, the insula establishes multiple parallel, spatially segregated pathways of connectivity with cortical regions that are involved in the control of speech comprehension, motor preparation and motor output, respectively. This insular organization may underlie its diverse functional contributions to the speech network, both in healthy individuals and neurological speech disorders. Based on the assumption that the insula is a multimodal brain region, we further hypothesized that its structural connectivity follows the profile of bilateral hemispheric distribution similar to other cortical regions involved in sensorimotor transformations during speech production (Cogan et al. 2014; Silbert et al. 2014; Simonyan and Fuertinger 2015). Finally, we hypothesize that neurological disorders affecting speech production are associated with spatial reorganization of segregated (i.e., normal) insular pathways, particularly affecting its connectivity with cortical regions responsible for speech motor preparation and output.
Methods
Experimentally, our first step included the use of probabilistic diffusion tractography in healthy subjects in order to establish the structural connectivity of both normal and disordered insular speech networks with the three cortical target regions responsible for auditory processing (superior temporal gyrus, Brodmann area (BA) 22), motor preparation (inferior frontal gyrus, BA 44), and motor output (laryngeal/orofacial primary motor cortex, ventral portion of BA 4). These insular networks were examined in healthy subjects and SD patients, respectively, who represented a suitable cohort for focused examination of the insular speech network in a neurological disorder without an apparent brain damage. Cortical regions were chosen because of their importance within the speech network (Hickok and Poeppel 2007; Fuertinger et al. 2015). Specifically, in addition to its role in speech comprehension and discrimination of pitch and intensity (Price 2000), BA 22 is the core region for phonological retrieval prior to articulation (Binder 2015). On the other hand, BA 44 is critical for articulatory and semantic preparation to speech production (Price et al. 1996; Schlosser et al. 1998; Silbert et al. 2014) as well as speech timing (Long et al. 2016). Lastly, ventral BA 4 hosts laryngeal and orofacial motor representations and plays a fundamental role in cortical motor output for speech production (Simonyan 2014). Collectively, these regions contribute to and play distinct roles within the large-scale speech network, from speech processing to articulation.
Study participants
Twelve healthy volunteers (7 female/5 male, mean age 55 7.06 years) and 12 patients with SD (7 female/5 male, mean age 54.1 11 years) were enrolled in the study. All participants were monolingual native English speakers and right-handed as determined by the Edinburgh Handedness Inventory (Oldfield 1971). All had normal cognitive performance on the Mini-Mental State Examination. None of the participants had any history of neurological (except for SD in the patient group), psychiatric, or otolaryngological problems. Diagnosis of SD was established based on voice and speech acoustic examination, neurological evaluation, and flexible nasolaryngoscopy in all patients. All SD subjects were fully symptomatic at the time of testing. All participants provided written informed consent, which was approved by the Internal Review Boards of the Icahn School of Medicine at Mount Sinai and Massachusetts Eye and Ear, Harvard Medical School.
MRI acquisition protocol
Data were acquired on a 3T Philips scanner equipped with an 8-channel head coil. The protocol included a T1-weighted gradient-echo sequence (MPRAGE, 172 contiguous slices, 1mm isotropic voxel, TR = 2300 ms, TE = 2.98 ms, FOV = 210 mm). Diffusion-weighted images (DWI) were acquired using a single-shot spin-echo echo-planar imaging (EPI) sequence with 54 contiguous axial slices along 60 non-collinear directions, in addition to one volume without diffusion encoding (b0) at the beginning of the sequence as an anatomical reference for motion and eddy current corrections (TR = 13,000 ms, FOV = 240 mm, matrix = 96 × 96 mm zero-filled to 256 × 256 mm, voxel size 2×2×2 mm, b = 1000s/mm2). To minimize the head movements during scanning, subjects’ head was tightly cushioned and padded. Subjects were instructed to remain motionless throughout the scan; possible movements were monitored online.
Data analysis
Image preprocessing
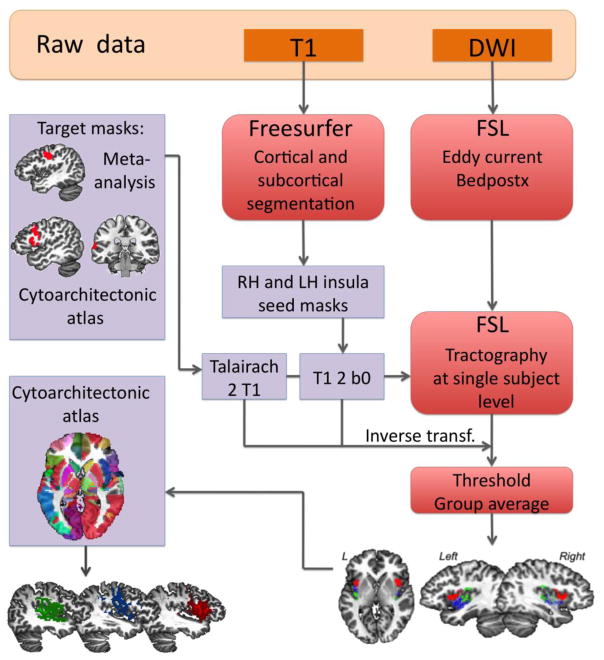
DWI data were analyzed using a combination of FSL, Freesurfer and AFNI software packages (Fig. 1). For each individual, the preprocessing included motion and eddy-current corrections, registration of the DWI to the b0 image using a 12-parameter affine transformation, and computation of the fractional anisotropy (FA) maps in the FSL FDT toolbox. Using the FSL Bedpostx tool, we performed Bayesian estimation of diffusion parameters to model crossing fibers and to build distributions of diffusion parameters within each voxel using the “ball and stick” multi-compartment decomposition model (Behrens et al. 2003). The individual output was used to run probabilistic tractography for connectivity-based segmentation of the insula using the FSL Probtrackx tool (Behrens et al. 2007) with the following settings: 5000 streamlines per voxel; maximal number of steps 2000; step length 0.5 mm; curvature threshold 0.2; 0.01 subsidiary fiber volume fraction threshold; waypoint condition AND, and “loopcheck” to terminate streamlines that loop back on themselves. Probabilistic tractography was performed between each hemispheric insular seed region and each ipsilateral cortical target mask.
Figure 1.
Flowchart illustrates the main steps of data analysis pipeline.
Insular seed and cortical target mask formation
To extract the insula and cortical target masks, we performed a cortical parcellation of each subject’s T1-weighted image using the Freesurfer software combined with the cytoarchitectonic maximum probability and macrolabel atlas in the Talaraich-Tournoux space (Eickhoff et al. 2005) (Fig. 1). The anatomical labels corresponding to the left and right insula were identified in the subjects’ native space, converted to binary masks, and used as seed masks for tractography. The target masks included BA 44, BA 22, and ventral BA 4 regions, the latter encompassing the laryngeal/orofacial representations within the primary motor cortex (Simonyan 2014; Bouchard et al. 2013). As described above, these target regions were chosen for focused examination of the cortical regions involved in auditory processing, sensorimotor preparation, and motor output during speech production, respectively. However, this approach also represented a limitation of the current study due to the fact that the contemporary views of functional anatomy of speech control involve much broader range of cortical and subcortical regions along the dorsal and ventral streams of information processing (Fuertinger et al. 2015; Hickok and Poeppel 2007). The insular and cortical masks were spatially registered to each subject’s diffusion space by applying a nonlinear transformation in the AFNI software. In addition, we created an exclusion mask, two slices wide, along the sagittal plane to exclude spurious tracts passing between the two hemispheres. Cortical target regions functioned as waypoint classification masks for quantifying connections to the insula.
Insular segmentation
To determine the subdivisions of the insula that receive distinct connections from BA 44, BA 22, and BA 4, we computed the number of streamlines reaching each target mask from each voxel of the insular mask in each subject. We used the seeds_to_waypoints files from FSL’s ProbtrackX tool, which at each voxel of the insula show the number of streamlines from the voxel reaching the target mask. After thresholding to the top 10%, the insular masks were binarized to create masks of high intensity cortical projections for each subject. Each of the segmented insular subdivisions was back-registered to the AFNI standard Talairach space using a combination of rigid, affine, and non-linear transformations and averaged within the healthy and patient groups, separately. The overlap between insular subdivisions based on these parcellations was subtracted, producing three independent insular subregions for each hemisphere with unique projections to each cortical target.
Quantitative analysis
To quantitatively assess differences in the spatial organization of each segmented insular subdivision in healthy subjects and their reorganization in SD patients, we calculated the percentage of the cluster volume overlap between the two groups using the Dice’s coefficient (DC) (Dice 1945; Tie et al. 2014; Battistella et al. 2016b). DC measures are calculated as the ratio of twice the number of non-zero voxels common to both images divided by the total number of non-zero voxels in each image. The DC of 1 refers to complete correspondence between the two groups; the DC of 0 refers to no overlap between the two groups.
Because any (random) assignment of a larger sample of subjects into two groups may give rise to a pattern of partial overlap between the two groups, we tested (separately for the three different insular subdivisions per hemisphere) whether the degree of overlap between healthy subjects and SD patients is smaller than would be expected in case of random assignment of participants to two groups. We performed a permutation test for each insular subdivision per hemisphere by generating 100 random sampling distributions of spatial overlap (a total of 600) using a bootstrapping procedure with replacement. The level of statistical significance was set considering the percentage of the permuted values and the total number of permutations per each insular subdivision.
Probabilistic tractography between the insular subdivisions and cortical targets
We quantified the connected fiber tracts between the parcellated insular subdivisions and cortical targets (BA 44, BA 22, and BA 4) by performing a probabilistic tractography with the AFNI FATCAT toolbox, which allows for faster computation time and greater versatility of the quantitative measures extracted from the tractography algorithm (Taylor et al. 2012; Taylor and Saad 2013). Uncertainty of diffusion parameters was calculated using jackknife resampling over multiple iterations. All insular and cortical masks were first trimmed to include only the gray matter and then inflated to include the underlying white matter. The threshold for white matter inclusion was determined using each subject’s FA map. The FATCAT ALGOPTS tractography parameters included 0.2 threshold for DWI FA map; 120 degrees max angle; 0 mm minimum physical length of tracts; 0.1 threshold, i.e., 50 tracts must pass through a voxel for a connection to be included; 5 seeds per voxel per Monte Carlo iteration, and 1000 Monte Carlo iterations. Between-group statistical differences in the normalized number of streamlines (calculated as the ratio of the number of streamlines and the number of voxels in the target mask) originating from each insular sub-region and reaching each cortical target mask were assessed using independent two-sample t-test at p 0.05, corrected for multiple comparisons.
Hemispheric laterality assessment
Based on its connectivity profile, we tested the hemispheric dominance of each insular subdivision i by calculating the laterality index (LI) across subjects within each specific group, separately, using the equation: LIi = (Li −Ri)/(Li + Ri). In each subject, Li represented the number of voxels in region i in the left hemisphere and Ri in the right hemisphere. A positive value represented left lateralization; a negative value represented right lateralization. Within-group statistical significance was tested using an independent t-test at p 0.05, corrected for multiple comparisons.
Results
Insular subdivisions based on cortical connectivity profile
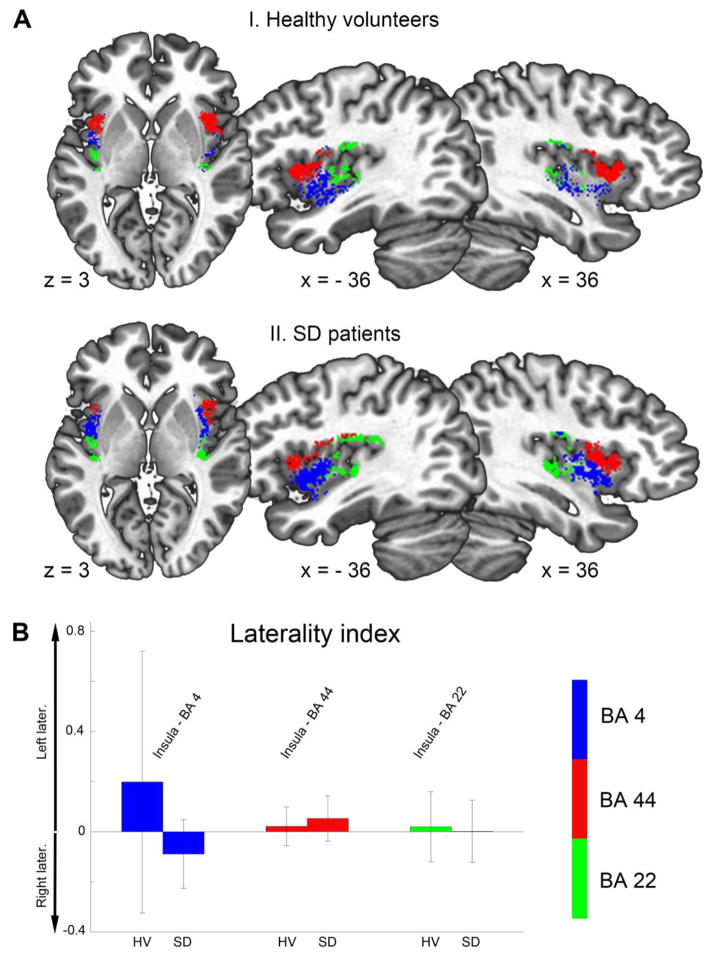
Three distinct insular subdivisions were delineated based on insular connectivity with BA 44, BA 4, and BA 22 in the right and left hemispheres, respectively (Fig. 2A). In healthy subjects, the insular subdivision connected to BA 44 was identified anterior to the central sulcus of the insula, partly overlapping with the dysgranular portion of insula. The insular subdivision connected to BA 4 was located more ventrally and posterior to the central sulcus in dysgranular insula, whereas the insular subdivision connected to BA 22 was the most dorsal and posterior to the central sulcus in the granular portion of insula (Fig. 2A-I and Table 1). There were no significant hemispheric differences in insular connectivity measures in healthy subjects (all p ≥ 0.2) (Fig. 2B). Spatial distribution of the insular subdivision in SD patients showed a parcellation pattern that was visually similar to that of healthy controls (Fig. 2A-II). However, a trend was observed towards left-hemispheric lateralization of insular subdivision connected with BA 4 (uncorrected p = 0.06).
Figure 2.
(A) shows the spatial distribution of the three non-overlapping insular subdivisions with connections to BA 44 (red), BA 4 (blue), and BA 22 (green) in healthy volunteers (upper panel) and SD patients (lower panel). Results are superimposed on the standard Talaraich-Tournoux brain. (B) depicts the laterality index (LI) for insular sub-regions in healthy volunteers (HV) and SD patients.
Table 1.
Center of mass and the number of voxels of the parcellated insula subregions in healthy volunteers and SD patients. Coordinates are given in the standard Talairach-Tournoux space.
| Cortical target regions | Healthy subjects | SD patients | ||
|---|---|---|---|---|
|
| ||||
| Talairach coordinates | # voxels | Talairach coordinates | # voxels | |
|
| ||||
| Left BA 44 | −36 6 10 | 1477 | −32 8 12 | 1176 |
|
| ||||
| Left BA 22 | −32 −26 14 | 494 | −34 −24 13 | 916 |
| −35 −12 5 | 295 | |||
| −35 −15 19 | 152 | |||
|
| ||||
| Left BA 4 | −37 0 −3 | 689 | −35 1 0 | 1367 |
|
| ||||
| Right BA 44 | 35 10 8 | 1575 | 36 11 7 | 1078 |
|
| ||||
| Right BA 22 | 33 −22 11 | 419 | 33 18 9 | 958 |
|
| ||||
| Right BA 4 | 38 −11 1 | 342 | 36 4 2 | 1552 |
| 39 4 −3 | 171 | |||
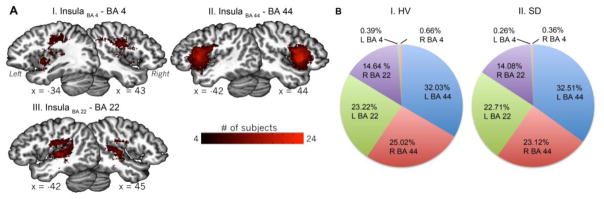
Mean probabilistic streamlines across healthy volunteers and SD patients illustrated white matter fiber pathways connecting each insular subdivision to its corresponding cortical target (Fig. 3A). BA 44 was connected to the corresponding insular cluster through short distance fibers along the superior longitudinal fasciculus, while fibers of the superior corona radiata connected the speech motor cortex (BA 4) to the corresponding insular cluster. Connections from BA 22 to the respective insular subdivision were instead established through the fibers of the lateral branch of superior longitudinal fasciculus. Between-group statistical comparison of the normalized number of streamlines connecting each parcellated insular subdivision with its corresponding target cortical region showed no statistical significance (p ≥ 0.1).
Figure 3.
Panel (A) shows the distribution of white matter tracts between each parcellated insular region and the corresponding cortical target region across all subjects. Panel (B) depicts the normalized proportion (in %) of each insular cluster streamlines reaching the corresponding cortical target region. BA 4 – ventral primary motor cortex; L – left; R – right.
In healthy subjects, examination of the normalized proportion of streamlines from each insular subdivision to the corresponding cortical target (Table 2) showed the prevalence of bilateral insular-BA 44 connectivity (left 32.03%; right 25.02%), followed by insular-BA 22 (left 23.22%; right 14.64%) and insular-BA 4 connectivity (left 0.39%; right 0.66%) (Fig. 3B-I). A similar distribution was also observed in SD patients, although the overall connectivity profile was slightly, but not significantly, reduced (Fig. 3B-II, Table 2).
Table 2.
Normalized number of streamlines (mean standard deviation) between each parcellated insular sub-region and the corresponding target masks.
| L BA 44 | L BA 22 | L BA 4 | R BA 44 | R BA 22 | R BA 4 | |
|---|---|---|---|---|---|---|
| Healthy participants | 348.8 76.4 | 268.9 120 | 4.5 4.2 | 289.7 24.2 | 169.6 151.9 | 7.6 5.8 |
| SD patients | 445.4 185.3 | 311.1 91.7 | 3.58 5.1 | 316.7 89.5 | 192.9 110 | 4.9 3.5 |
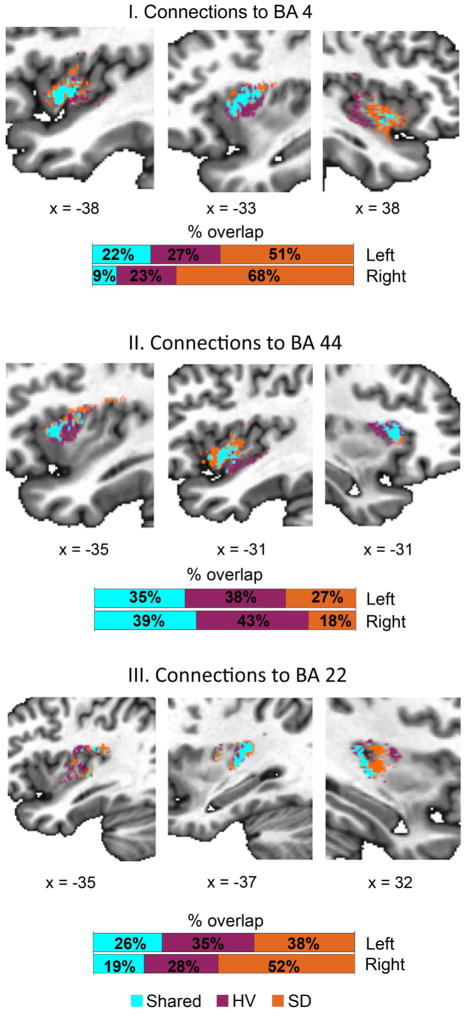
In addition to these qualitative measures, our quantitative analysis revealed different degrees of spatial overlap of the insular subdivisions when comparing SD patients to healthy subjects (Fig. 4, Table 3). This suggested intrinsic reorganization of the insular network in the presence of a speech-related disorder. Specifically, the insular subdivision connected with BA 4 had the smallest overlap between the two groups (left DCinsular-BA 4 = 0.36 [22%] and right DCinsula-BA 4 = 0.17 [9%]) and extended more dorsally and anteriorly in SD patients compared to healthy subjects, who showed connections distributed more ventrally and posteriorly (Fig. 4-I). A similar spatial pattern of dorsal/anterior distribution in SD patients and ventral/posterior distribution in healthy subjects characterized the insular subdivision connected to BA 44, which also showed a greater overlap between the two groups (left DCinsula-BA 44 = 0.51 [35%] and right DCinsula-BA 44 = 0.56 [39%]) (Fig. 4-II). Finally, the insular subdivision connected to BA 22 showed a sparser distribution along the dorsal-ventral and anterior-posterior axes with a low-to-moderate overlap between the two groups (left DCinsula-BA 22 = 0.42 [26%] and right DCinsula-BA 22= 0.32 [19%]) (Fig. 4-III). We further found that the overlap of each insular subdivision between healthy subjects and SD patients was smaller than in case of random assignment of participants to the two groups (9%–39% in empirical data vs. 99.7% in permutations).
Figure 4.
Spatial overlap of parcellated insular subdivisions between healthy volunteers (HV) and SD patients. For each segmented insular cluster, we show the voxels overlapping between HV and SD patients (light blue), specific to HV only (purple), and specific to SD patients only (orange). Color bar indicates the percentage of overlapping and group-specific voxels. BA 4 – ventral primary motor cortex.
Table 3.
Spatial voxelwise overlap (in %) of the insular sub-regions between healthy subjects and SD patients.
| Overlap (%) | HV-specific (%) | SD-specific (%) | |
|---|---|---|---|
| Left insula-BA 4 | 22.3 | 26.7 | 51.0 |
| Left insula-BA 44 | 34.6 | 38.5 | 26.9 |
| Left insula- BA 22 | 26.3 | 35.2 | 38.5 |
| Right insula-BA 4 | 9.3 | 22.8 | 67.9 |
| Right insula-BA 44 | 38.9 | 43.0 | 18.1 |
| Right insula-BA 22 | 19.4 | 28.3 | 52.3 |
Discussion
In this study, we evaluated normal and disordered structural connectivity of the insula with cortical regions involved in speech processing, motor preparation and output. Using probabilistic diffusion tractography in healthy subjects, we identified distinct non-overlapping insular subdivisions that were connected to cortical targets of speech network, namely BA 44 (anterior insula), ventral BA 4 (mid-insula), and BA 22 (posterior insula). Previously, lesion studies argued about which, if any, regions of the insula may play a role in speech control (Fedorenko et al. 2015; Hillis et al. 2004; Dronkers 1996; Ogar et al. 2006; Hickok et al. 2014; Nestor et al. 2003; Bates et al. 2003; Borovsky et al. 2007; Baldo et al. 2011). The results of our study demonstrate that distinct subdivisions of insula establish parallel, largely non-overlapping white matter pathways along its anterior-posterior extent with cortical regions involved in speech processing and production. This spatially segregated organization of insular-cortical connectivity may underlie the differences in functional specializations of insula, ranging from involvement in articulatory modulations to communicative motivation. Furthermore, our findings suggest that damage to the different insular subdivisions may lead to different clinical symptomatology, depending on which branch of insular-cortical connectivity is being altered. As an example, we showed that spatial distribution of insular subdivisions connected to BA 4 and BA 44 is shifted anteriorly and dorsally in SD patients, whose disorder is characterized by selective loss of voluntary control of speech motor output without altered speech processing, compared to more ventral and posterior distribution of the same insular subdivisions in healthy subjects. This explains why past and future lesion studies examining damage to different insular subdivisions as well as imaging studies using different speech-related tasks to elicit insular activity for assessment of its functional contributions to speech control may not necessarily agree in their findings, as they likely capture different aspects of insular processing, depending on underlying distinct structural connectivity with cortical regions of higher-level speech control. Taken together, we conclude that different insular subdivisions adopt different roles within the highly complex and distributed speech network and may distinctly influence clinical symptomatology in patients with different neurological disorders affecting speech control.
Distinct subdivisions of the insular speech network
The anterior insular cluster, which showed strong connections to BA 44, is known to have direct neuroanatomical connections to the inferior frontal and lateral prefrontal regions (Jakab et al. 2012; Deacon 1992), which have been associated with semantic, phonological and syntactic aspects of speech and language production (Bedny et al. 2008; Rodd et al. 2010). More specifically, it has been shown that the ventral portion of left BA 44 is involved in covert articulation (Papoutsi et al. 2009; Zheng et al. 2010) with increased activation for phonological rather than semantic retrieval (Heim et al. 2009). The left dorsal part of BA 44 is instead active during speech processing prior to articulatory recording (Papoutsi et al. 2009). A recent study has also suggested a pre-articulatory function of BA 44 by demonstrating that its activity precedes those of motor and premotor regions (Flinker et al. 2015).
Segregated from this region, the insular subdivision connected to BA 22 was located in the most posterior insula. The latter region establishes extensive connections within the superior temporal gyrus (STG) (Augustine 1996) for the control of auditory and phonological processes for speech and language (Price 2010). The STG region has been associated with auditory feedback (Zheng et al. 2010), exhibiting a trade-off between its activity and that of the secondary somatosensory cortex during object naming (Seghier et al. 2015). Its posterior region appears to respond to both speech perception and production (Buchsbaum et al. 2001), whereas the anterior portion reveals a vowel-specific mismatch response (Leff et al. 2009).
Finally, the mid-insular subdivision was found to establish connections with the laryngeal/orofacial motocortical representation in BA 4. A recent study on neurosurgical patients has discriminated the function of the ventral motor cortex from that of neighboring BA 44 by showing that the former is crucial in maintaining the quality of speaking while the latter is responsible for speech rate (Long et al. 2016). In addition, our previous studies have found that this portion of motor cortex is one of the pivotal regions within the whole-brain functional connectome of speech control and represents the final cortical output region during speech production (Simonyan and Fuertinger 2015; Fuertinger et al. 2015).
Based on probabilistic topography of such regionally segregated representations within the insular subdivisions, we suggest that its most anterior region is involved in preparatory motor processes via its connections with BA 44, whereas the mid-insula is responsible for coordination of the speech motor output. The posterior insula likely plays a greater role in integration of auditory information, where speech recognition and processing first occurs. With these segregated subdivisions, the insula in its entirety represents an important structure that contributes to a wide range of speech processes by being closely integrated at the different stages of cortical speech control.
In addition, the segregated organization of the insular speech network appears to underlie its differential contribution to clinical features of neurological disorders affecting speech control. Particularly, in this study, we found that SD patients were characterized by spatial reorganization of insular subdivisions, such as a more dorsal shift of insular clusters of connectivity with BA 44 and BA 4, compared to a more ventral representation of the same clusters in healthy subjects. This finding attests to abnormal sensorimotor processes during speech preparation and production, which have been a hallmark of SD pathophysiology (Simonyan and Ludlow 2010, 2012; Simonyan et al. 2008; Battistella et al. 2016a). Among these, the anterior and mid-insular regions have been reported to carry structural alterations in SD that are associated with distinct clinical phenotypes of this disorder (Bianchi et al. 2017; Simonyan and Ludlow 2012). Similar insular abnormalities have also been observed in another form of task-specific dystonia that selectively affects writing, i.e., writer’s cramp (Ceballos-Baumann et al. 1997; Lerner et al. 2004), thus leading to an overall conclusion that the anterior/mid-insular sub-regions may be important contributors to the control of complex learned voluntary movements.
Another aspect of the insular speech network observed in our study was a largely bilateral representation of cortical target areas within the insula as well as bilateral structural connectivity with the corresponding cortical regions. These findings are consistent with overall bilateral insular activation during speech processing (Oh et al. 2014) and underscore recently emerging view of the speech control system as a largely bilateral neural network (Cogan et al. 2014; Silbert et al. 2014; Simonyan and Fuertinger 2015; Kumar et al. 2016). Our current study adds the knowledge of bilateral insular connectivity within the speech network that may underlie a functional flexibility in regulating different aspects of speech control.
Conclusion
Our study provides evidence for a structural segregation of the insular subdivisions that are likely associated with different aspects of sensorimotor and cognitive control of speech. Such fine-grained and functionally relevant organization of the insula may unify previous, and at times controversial studies, which argued about non-overlapping roles of this structure in speech control.
Acknowledgments
This study was supported by R01DC01805 and R01DC012545 grants to K.S. from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health.
References
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22(3):229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Wilkins DP, Ogar J, Willock S, Dronkers NF. Role of the precentral gyrus of the insula in complex articulation. Cortex. 2011;47(7):800–807. doi: 10.1016/j.cortex.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6(5):448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Battistella G, Fuertinger S, Fleysher L, Ozelius LJ, Simonyan K. Cortical sensorimotor alterations classify clinical phenotype and putative genotype of spasmodic dysphonia. Eur J Neurol. 2016a doi: 10.1111/ene.13067. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella G, Najdenovska E, Maeder P, Ghazaleh N, Daducci A, Thiran JP, Jacquemont S, Tuleasca C, Levivier M, Bach Cuadra M, Fornari E. Robust thalamic nuclei segmentation method based on local diffusion magnetic resonance properties. Brain Struct Funct. 2016b doi: 10.1007/s00429-016-1336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella G, Termsarasab P, Ramdhani RA, Fuertinger S, Simonyan K. Isolated Focal Dystonia as a Disorder of Large-Scale Functional Networks. Cerebral Cortex. 2017;27(2):1203–1215. doi: 10.1093/cercor/bhv313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M, Caramazza A, Grossman E, Pascual-Leone A, Saxe R. Concepts are more than percepts: the case of action verbs. J Neurosci. 2008;28(44):11347–11353. doi: 10.1523/JNEUROSCI.3039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Bianchi S, Battistella G, Huddlestone H, Scharf R, Fleysher L, Rumbach AF, Frucht SJ, Blitzer A, Ozelius LJ, Simonyan K. Phenotype- and genotype-specific structural alterations in spasmodic dysphonia. Mov Disord. 2017 doi: 10.1002/mds.26920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR. The Wernicke area: Modern evidence and a reinterpretation. Neurology. 2015;85(24):2170–2175. doi: 10.1212/WNL.0000000000002219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32(2):821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Borovsky A, Saygin AP, Bates E, Dronkers N. Lesion correlates of conversational speech production deficits. Neuropsychologia. 2007;45(11):2525–2533. doi: 10.1016/j.neuropsychologia.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard KE, Mesgarani N, Johnson K, Chang EF. Functional organization of human sensorimotor cortex for speech articulation. Nature. 2013;495(7441):327–332. doi: 10.1038/nature11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum BR, Hickok G, Humphries C. Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cognitive Sci. 2001;25(5):663–678. doi: 10.1207/s15516709cog2505_2. [DOI] [Google Scholar]
- Ceballos-Baumann AO, Sheean G, Passingham RE, Marsden CD, Brooks DJ. Botulinum toxin does not reverse the cortical dysfunction associated with writer’s cramp. A PET study. Brain. 1997;120(Pt 4):571–582. doi: 10.1093/brain/120.4.571. [DOI] [PubMed] [Google Scholar]
- Chang SE, Kenney MK, Loucks TM, Ludlow CL. Brain activation abnormalities during speech and non-speech in stuttering speakers. Neuroimage. 2009;46(1):201–212. doi: 10.1016/j.neuroimage.2009.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan GB, Thesen T, Carlson C, Doyle W, Devinsky O, Pesaran B. Sensory-motor transformations for speech occur bilaterally. Nature. 2014;507(7490):94–98. doi: 10.1038/nature12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon TW. Cortical connections of the inferior arcuate sulcus cortex in the macaque brain. Brain Res. 1992;573(1):8–26. doi: 10.1016/0006-8993(92)90109-m. [DOI] [PubMed] [Google Scholar]
- Dice LR. Measures of the Amount of Ecologic Association between Species. Ecology. 1945;26(3):297–302. doi: 10.2307/1932409. [DOI] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384(6605):159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30(9):2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Fillmore P, Smith K, Bonilha L, Fridriksson J. The superior precentral gyrus of the insula does not appear to be functionally specialized for articulation. J Neurophysiol. 2015;113(7):2376–2382. doi: 10.1152/jn.00214.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinker A, Korzeniewska A, Shestyuk AY, Franaszczuk PJ, Dronkers NF, Knight RT, Crone NE. Redefining the role of Broca’s area in speech. Proc Natl Acad Sci U S A. 2015;112(9):2871–2875. doi: 10.1073/pnas.1414491112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertinger S, Horwitz B, Simonyan K. The Functional Connectome of Speech Control. PLoS Biol. 2015;13(7):e1002209. doi: 10.1371/journal.pbio.1002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertinger S, Simonyan K. Connectome-wide phenotypical and genotypical associations in focal dystonia. J Neurosci. 2017 doi: 10.1523/JNEUROSCI.0384-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther FH. Cortical interactions underlying the production of speech sounds. J Commun Disord. 2006;39(5):350–365. doi: 10.1016/j.jcomdis.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Dresel C, Castrop F, Roettinger M, Ceballos-Baumann AO. “Silent event-related” fMRI reveals reduced sensorimotor activation in laryngeal dystonia. Neurology. 2005;65(10):1562–1569. doi: 10.1212/01.wnl.0000184478.59063.db. [DOI] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, Ischebeck AK, Friederici AD, Stephan KE, Amunts K. Effective connectivity of the left BA 44, BA 45, and inferior temporal gyrus during lexical and phonological decisions identified with DCM. Hum Brain Mapp. 2009;30(2):392–402. doi: 10.1002/hbm.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hickok G, Rogalsky C, Chen R, Herskovits EH, Townsley S, Hillis AE. Partially overlapping sensorimotor networks underlie speech praxis and verbal short-term memory: evidence from apraxia of speech following acute stroke. Front Hum Neurosci. 2014;8:649. doi: 10.3389/fnhum.2014.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127(Pt 7):1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Jakab A, Molnar PP, Bogner P, Beres M, Berenyi EL. Connectivity-based parcellation reveals interhemispheric differences in the insula. Brain Topogr. 2012;25(3):264–271. doi: 10.1007/s10548-011-0205-y. [DOI] [PubMed] [Google Scholar]
- Kostic VS, Agosta F, Sarro L, Tomic A, Kresojevic N, Galantucci S, Svetel M, Valsasina P, Filippi M. Brain structural changes in spasmodic dysphonia: A multimodal magnetic resonance imaging study. Parkinsonism Relat Disord. 2016;25:78–84. doi: 10.1016/j.parkreldis.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Kumar V, Croxson PL, Simonyan K. Structural Organization of the Laryngeal Motor Cortical Network and Its Implication for Evolution of Speech Production. J Neurosci. 2016;36(15):4170–4181. doi: 10.1523/JNEUROSCI.3914-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff AP, Iverson P, Schofield TM, Kilner JM, Crinion JT, Friston KJ, Price CJ. Vowel-specific mismatch responses in the anterior superior temporal gyrus: an fMRI study. Cortex. 2009;45(4):517–526. doi: 10.1016/j.cortex.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A, Shill H, Hanakawa T, Bushara K, Goldfine A, Hallett M. Regional cerebral blood flow correlates of the severity of writer’s cramp symptoms. Neuroimage. 2004;21(3):904–913. doi: 10.1016/j.neuroimage.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Long MA, Katlowitz KA, Svirsky MA, Clary RC, Byun TM, Majaj N, Oya H, Howard MA, 3rd, Greenlee JD. Functional Segregation of Cortical Regions Underlying Speech Timing and Articulation. Neuron. 2016 doi: 10.1016/j.neuron.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Corfield DR, Guz A, Fink GR, Wise RJ, Harrison J, Adams L. Cerebral areas associated with motor control of speech in humans. J Appl Physiol (1985) 1997;83(5):1438–1447. doi: 10.1152/jappl.1997.83.5.1438. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126(Pt 11):2406–2418. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- New AB, Robin DA, Parkinson AL, Eickhoff CR, Reetz K, Hoffstaedter F, Mathys C, Sudmeyer M, Grefkes C, Larson CR, Ramig LO, Fox PT, Eickhoff SB. The intrinsic resting state voice network in Parkinson’s disease. Hum Brain Mapp. 2015;36(5):1951–1962. doi: 10.1002/hbm.22748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogar J, Willock S, Baldo J, Wilkins D, Ludy C, Dronkers N. Clinical and anatomical correlates of apraxia of speech. Brain Lang. 2006;97(3):343–350. doi: 10.1016/j.bandl.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Oh A, Duerden EG, Pang EW. The role of the insula in speech and language processing. Brain Lang. 2014;135:96–103. doi: 10.1016/j.bandl.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Papoutsi M, de Zwart JA, Jansma JM, Pickering MJ, Bednar JA, Horwitz B. From phonemes to articulatory codes: an fMRI study of the role of Broca’s area in speech production. Cereb Cortex. 2009;19(9):2156–2165. doi: 10.1093/cercor/bhn239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat. 2000;197(Pt 3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Frackowiak RS, Friston KJ. The neural regions sustaining object recognition and naming. Proc Biol Sci. 1996;263(1376):1501–1507. doi: 10.1098/rspb.1996.0219. [DOI] [PubMed] [Google Scholar]
- Rodd JM, Johnsrude IS, Davis MH. The role of domain-general frontal systems in language comprehension: evidence from dual-task interference and semantic ambiguity. Brain Lang. 2010;115(3):182–188. doi: 10.1016/j.bandl.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Schlosser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, Dewey SL, Brodie JD. Functional magnetic resonance imaging of human brain activity in a verbal fluency task. J Neurol Neurosurg Psychiatry. 1998;64(4):492–498. doi: 10.1136/jnnp.64.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Hope TM, Prejawa S, Parker J, Vitkovitch M, Price CJ. A trade-off between somatosensory and auditory related brain activity during object naming but not reading. J Neurosci. 2015;35(11):4751–4759. doi: 10.1523/JNEUROSCI.2292-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert LJ, Honey CJ, Simony E, Poeppel D, Hasson U. Coupled neural systems underlie the production and comprehension of naturalistic narrative speech. Proc Natl Acad Sci U S A. 2014;111(43):E4687–4696. doi: 10.1073/pnas.1323812111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K. The laryngeal motor cortex: its organization and connectivity. Curr Opin Neurobiol. 2014;28:15–21. doi: 10.1016/j.conb.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Fuertinger S. Speech networks at rest and in action: interactions between functional brain networks controlling speech production. J Neurophysiol. 2015;113(7):2967–2978. doi: 10.1152/jn.00964.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Ludlow CL. Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: an fMRI study. Cereb Cortex. 2010;20(11):2749–2759. doi: 10.1093/cercor/bhq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Ludlow CL. Abnormal structure-function relationship in spasmodic dysphonia. Cereb Cortex. 2012;22(2):417–425. doi: 10.1093/cercor/bhr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Tovar-Moll F, Ostuni J, Hallett M, Kalasinsky VF, Lewin-Smith MR, Rushing EJ, Vortmeyer AO, Ludlow CL. Focal white matter changes in spasmodic dysphonia: a combined diffusion tensor imaging and neuropathological study. Brain. 2008;131(Pt 2):447–459. doi: 10.1093/brain/awm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PA, Cho KH, Lin CP, Biswal BB. Improving DTI tractography by including diagonal tract propagation. PLoS One. 2012;7(9):e43415. doi: 10.1371/journal.pone.0043415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PA, Saad ZS. FATCAT: (an efficient) Functional and Tractographic Connectivity Analysis Toolbox. Brain connectivity. 2013;3(5):523–535. doi: 10.1089/brain.2013.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie Y, Rigolo L, Norton IH, Huang RY, Wu W, Orringer D, Mukundan S, Jr, Golby AJ. Defining language networks from resting-state fMRI for surgical planning--a feasibility study. Hum Brain Mapp. 2014;35(3):1018–1030. doi: 10.1002/hbm.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng ZZ, Munhall KG, Johnsrude IS. Functional overlap between regions involved in speech perception and in monitoring one’s own voice during speech production. J Cogn Neurosci. 2010;22(8):1770–1781. doi: 10.1162/jocn.2009.21324. [DOI] [PMC free article] [PubMed] [Google Scholar]