Abstract
Objective
To test the hypothesis that loss of IL-19 exacerbates atherosclerosis.
Approach and Results
Il19−/− mice were crossed into Ldlr−/− mice. Double knockout (dKO) mice had increased plaque burden in aortic arch and root compared to Ldlr−/− controls after 14 weeks of high fat diet (HFD). dKO mice injected with 10ng/g/day rmIL-19 had significantly less plaque compared to controls. qRT-PCR and western blot analysis revealed dKO mice had increased systemic and intraplaque polarization of T cells and macrophages to pro-inflammatory Th1 and M1 phenotypes, and also significantly increased TNFα expression in spleen and aortic arch compared to Ldlr−/− controls. Bone marrow transplantantion suggests that immune cells participate in IL-19 protection. Bone marrow derived macrophages (BMDM) and vascular smooth muscle cells (VSMC) isolated from dKO mice had significantly greater expression of inflammatory cytokine mRNA and protein compared to controls. Spleen and aortic arch from dKO mice had significantly increased expression of the mRNA stability protein Human antigen R (HuR). BMDM and VSMC isolated from dKO mice also had greater HuR abundance. HuR stabilizes pro-inflammatory transcripts by binding AU-rich elements (AREs) in the 3′ untranslated region (UTR). Cytokine and HuR mRNA stability was increased in dKO BMDM and VSMC, which was rescued by addition of IL-19 to these cells. IL-19 induced expresson of miR133a, which targets and reduced HuR abundance; miR133a levels were lower in dKO mice compared to controls.
Conclusions
These data indicate that IL-19 is an atheroprotective cytokine which decreases abundance of HuR, leading to reduced inflammatory mRNA stability.
Keywords: Interleukin-19, atherosclerosis, TNFα, mRNA stability, HuR
Subject codes: Atherosclerosis, Inflammation, Animal Models of Human Disease, Growth Factors/Cytokines
Introduction
Atherosclerosis and other vascular inflammatory diseases continue to be a significant health and socioeconomic problem in the western and developing world(1). Atherosclerosis is a lipid-driven, chronic vascular inflammatory disease driven and maintained by pro-inflammatory cytokines secreted by both immune and vascular cells, with most studies focusing on the role of pro-inflammatory cytokines in activation of macrophages, endothelial cells, and leukocyte subsets in this disease(2,3). By comparison, a much smaller number of studies focus on the role of endogenous counter-regulatory mechanisms in atherogenesis and their effects on these same cells and impact on atherosclerosis. Potential protective effects of anti-inflammatory cytokines including Th2 interleukins on resident vascular (EC and VSMC) and inflammatory cells remain as a gap in our knowledge. Anti-atherosclerotic interleukins that can target resident vascular cells, in addition to immune cells, have the potential to reduce localized inflammation of the vascular wall as well as polarize global immunity. Identification of such interleukins can lead to potential therapeutic targets.
We have reported a distinctive role for one such anti-inflammatory interleukin, Interleukin-19 (IL-19), in vascular disease(4–6). IL-19 is a member of an IL-10 sub-family which also includes IL-20, IL-22, and IL-24(7,8). IL-19 has unique properties including expression as well as target cells outside of the immune system and NF-κB-independent effects(9). Although expression of Th2 cytokines in atherosclerotic plaque is rare, we previously reported that IL-19 is expressed in multiple cell types in atherosclerotic plaque in both humans and mice(10). We also observed increased IL-19 in symptomatic patients compared to asymptomatic patients. Injection of recombinant IL-19 into atherosclerosis-prone Ldlr−/− mice can reduce the severity of atherosclerosis(10), as well as prevent expansion of existing plaque(11) by multiple effects on adaptive immunity, including polarization of T cells to Th2 and macrophages to M2 phenotypes respectively.
In response to pro-inflammatory stimuli, gene expression in resident vascular and immune cells is regulated by transcriptional and post-transcriptional mechanisms. Factors that control mRNA stability are essential regulators, which allow these cells to rapidly and appropriately respond to environmental cues. The role of mRNA stability proteins in regulation of vascular response to injury, as well as their modification by anti-inflammatory factors represent a second gap in our knowledge on how anti-inflammatory interleukins may be atheroprotective.
IL-19 has inflammation-dampening effects on cultured VSMC and EC, including reduction of expression of pro-inflammatory cytokines and adhesion molecules(5,9,12). Collectively, these studies suggest that IL-19 expression in atherosclerotic plaque may be a compensatory, counter-regulatory mechanism to reduce local inflammation. However, investigation into the absence of IL-19 on development of atherosclerosis has not been tested or reported.
We hypothesized that genetic deletion of IL-19 would augment atherosclerosis in Ldlr−/− mice. To test this hypothesis, we crossed Il19−/− mice with Ldlr−/− to create dKO mice. In this study we report that loss of IL-19 leads to exacerbated atherosclerosis, which can be rescued by treatment with recombinant mouse IL-19 in dKO mice. Further, we report that a potential mechanism for these effects is an increase in pro-inflammatory gene mRNA stability in BMDM and VSMC in dKO mice. IL-19 regulation of mRNA stability may be mediated by miRNA targeting of mRNA stability proteins. From a broader perspective, this study also suggests that mRNA stability proteins may represent targets for novel anti-atherosclerotic therapeutics.
Materials and Methods
A detailed listing of mice and antibodies used are listed in the major resouce table in the Supplementary Material section.
Mouse studies
LDL receptor knock out (Ldlr−/−) mice on the C57BL/6 background purchased from Jackson labs (Catalog #002207) were bred with Il19−/− mice previously described(10,11) to obtain homozygous Il19−/− × Ldlr−/− double knock out mice (dKO). Mice were housed in an ALAC-approved facility and maintained on a standard chow diet until study commencement. Mice of both sexes were entered in the study at 8 weeks of age when normal chow was replaced with an atherogenic diet (42% Fat, 0.2% cholesterol, Harlan atherogenic diet TD.88137). For the rescue study, mice were injected i.p. with 10ng/g/day recombinant mouse IL-19 (BioVision, Catalog #4546) as we have described(10,11) or an equal volume of PBS, 5 days per week for 14 weeks. Fasting lipid content in mouse sera was analyzed by Charles River Research Animal Diagnostic Services. For adaptive bone marrow transplantation, 8 week old male and female Ldlr−/− mice were irradiated (950 rads). Bone marrow was isolated from Ldlr−/− and dKO donors as described below and transplanted via single retro-orbital injection (5 million cells/injection). After 4 weeks, mice were administered atherogenic diet for 14 weeks. All animal procedures adhered to approved Temple University IACUC approved protocols.
Atherosclerotic lesion analysis
Atherosclerotic plaque burden was determined in the aortic intimal surface by en face staining with Sudan IV and in the aortic root by Oil Red O staining as we described(10,11). Lesion size in the aortic arch was determined by quantitative morphometry using the Image Pro Plus program. Aortic root was frozen in OCT medium and sectioned. Four transverse serial sections spaced 70-100μm apart from the aortic sinus to disappearance of valve cusps per aortic root were stained with Oil Red O, and positive stained areas quantitated as a percentage of total area by quantitative morphometry.
Cell culture
Aorta from Ldlr−/− and dKO mice were excised, endothelial layer removed, and VSMC isolated as we described(6,12,13). VSMC were cultured in DMEM supplemented with 20% fetal bovine serum (FBS). Over 95% of isolated cells were SMC actin positive, and VSMC from passage 2 to 4 were used. For BMDM, femurs and tibiae were flushed with sterile PBS, red blood cells were lysed and removed using ACK lysis buffer (ThermoFisher) and cell strainers. Collected cells were re-suspended and seeded in DMEM + 10% heat inactivated FBS with 100ng/ml M-CSF (Peprotech). Cells were cultured for 6-7 days until confluent. For gene and protein expression analyses, cells were stimulated with 10ng/ml TNFα (Sigma). For RNA stability experiments, cells were treated with 10ng/ml actinomycin D following TNFα stimulation. Untreated samples were used as controls.
RNA extraction and quantitative RT-PCR
RNA from spleen, aortic arch, and cultured cells was isolated using the RNeasy PowerLyzer Tissue & Cells Kit (Qiagen) and Direct-zol™ RNA Miniprep Plus (Zymo Research) for tissue and cells, respectively, according to the manufacturer’s protocol. A full description of RT-PCR is found in the Supplementary Material section.
Western blotting
Protein extracts made as described were separated by SDS-PAGE, transferred to nitrocellulose membrane, incubated with a 1:3000 dilution of primary antibody HuR or TNFα (abcam, Santa Cruz Biotechnology), followed by a 1:4000 dilution of secondary antibody(4,6,9,10,12). A full description of western blotting is found in the Supplementary Material section.
Immunohistochemistry
Aortic roots were frozen in OCT and 5 μm-thick serial sections were cut and placed on microscope slides(4,9,10,12,13). A full description of immunohistochemical methodology is found in the Supplementary Material section.
Intracellular Staining and Flow Cytometry
Flow cytometry analysis was performed on whole blood from mice to detect TNFα in peripheral blood lymphocytes. A full description of intracellular staining and Flow cytometry is found in the Supplementary Material section.
Statistical analysis
Results are expressed as mean ± SEM. A full description of statistical analysis is found in the Supplementary Material section.
Results
Il19−/− × Ldlr−/− dKO mice have increased atherosclerotic plaque
Il19−/− mice were crossed with Ldlr−/− mice. DKO and age and sex-matched Ldlr−/− mice were fed HFD for 14 weeks, and atherosclerotic surface lesion area was determined by en face staining of aortic arch and quantitative morphometry. DKO mice had significantly greater plaque surface area compared to Ldlr−/− control mice (13.41 ± 1.0% in Ldlr−/− vs 21.1 3± 1.2% in dKO, p <0.0001) (Figures 1A, 1B). Lesion area was also assessed in transverse sections of Oil Red O-stained aortic root, which demonstrates significantly increased lesion area in dKO mice compared to Ldlr−/− controls (16.34 ± 2.5% in Ldlr−/− vs 26.11 ± 3.7% in dKO, p <0.05) (Figures 1C, 1D). There was no significant difference in lesion area between sexes in either group. There was also no significant difference in lipid profile (cholesterol: 2155 ± 325.9mg/dl in Ldlr−/− vs 2044 ± 487mg/dl in dKO; triglyceride: 925.4 ± 243mg/dl in Ldlr−/− vs 1022 ± 392.2mg/dl in dKO) (Figure 1E) or weight gain during the course of the study (6.033 ± 1.5g in Ldlr−/− vs 5.346 ± 1.2g in dKO) (Figure 1F, Supplemental Data Figure I). Together these data suggest that absence of IL-19 results in increased severity of atherosclerosis.
Figure 1.
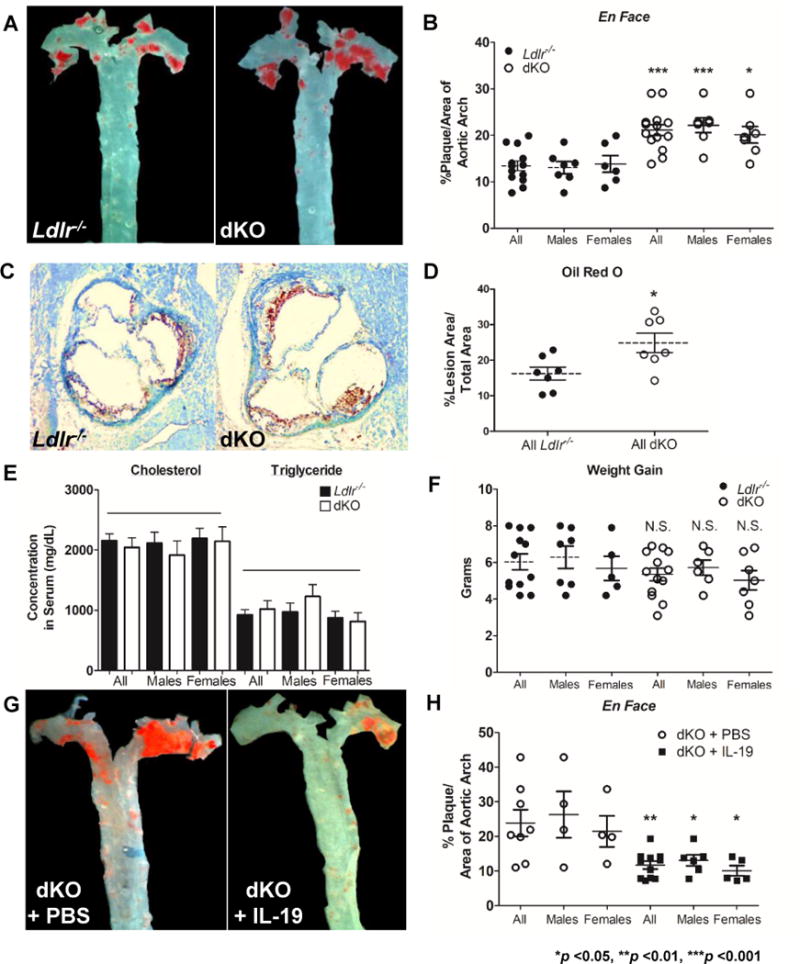
Genetic deletion of IL-19 exacerbates atherosclerosis.
A Representative photomicrograph of aortic arch from Ldlr−/− and dKO mice after consuming HFD for 14 weeks. Surface lesions en face stained with Sudan IV. B Graphic depiction of atherosclerotic lesion size quantitated from en face stained aortic arches as depicted in “A” (n=13 Ldlr−/− vs 14 dKO). C Representative photomicrographs of aortic root stained with Oil Red O from Ldlr−/− and dKO mice after 14 weeks of HFD. D Quantitation of percent positive Oil Red O stained lesion areas from four transverse serial sections of aortic root (n=7 per group).
E Cholesterol and triglyceride levels in circulating plasma do not statistically differ between Ldlr−/− and dKO mice after 14 weeks of HFD (n=8 per group). F Ldlr−/− and dKO mice do not statistically differ in weight gained after 14 weeks of HFD feeding.
Injection of IL-19 rescues dKO phenotype and reduces atherosclerosis. G Representative photomicrograph of aortic arch surface from dKO mice injected with either 10ng/g/day IL-19 or PBS while being fed HFD for 14 weeks; surface lesions en face stained with Sudan IV.
H Graphic depiction of atherosclerotic lesion size quantitated from en face stained aortic arches as depicted in “G” (n=8 dKO + PBS vs 11 dKO + IL-19).
Recombinant IL-19 treatment rescues dKO atherosclerotic phenotype
In order to confirm the role of IL-19 in atherogenesis and to demonstrate specificity of IL-19, dKO mice were administered daily i.p. injections of recombinant mouse IL-19 (10ng/g/day), or an equivalent volume of phosphate buffered saline while simultaneously being fed HFD for 14 weeks. En face staining of aorta shows that dKO mice injected with IL-19 had significantly less plaque burden compared to dKO mice injected with saline (23.89 ± 3.8% in PBS injected vs 11.72 ± 1.1% in IL-19 injected, p <0.01) (Figure 1G, 1H). As in the previous experiment, there were no significant differences in serum lipid profile or weight gain between IL-19 and saline injected mice. Since addition of IL-19 to dKO mice can rescue, or decrease atherosclerosis burden, these data demonstrate IL-19 specificity and suggest that IL-19 can regulate development of atherosclerosis.
Loss of IL-19 influences global and local inflammatory responses
The development and severity of atherosclerosis is influenced by systemic adaptive immunity as well as local adaptive immunity within the plaque. To determine if loss of IL-19 had systemic and/or local effects on the adaptive immune response, spleen and aorta were harvested from Ldlr−/− and dKO mice after 14 weeks of HFD feeding, at the time of euthanasia. Markers associated with T cell and macrophage polarity were quantitated by qRT-PCR. Figure 2A shows significantly increased levels of mRNA of several pro-inflammatory markers in dKO spleen, including TNFα, TBet, IL-12β, and IL-1β, suggesting a globally polarized Th1, M1 profile in dKO mice. Correspondingly, there was significantly decreased expression of Tregs marker, FOXP3, and anti-inflammatory cytokine, IL-10 in spleen from dKO mice. There were no significant differences in GATA3, Arg2, or Arg1 mRNA in spleen between dKO and control mice, suggesting no differences in Th2 or M2 populations in spleen (Supplemental Data Figure II). Similarly, Figure 2C displays significantly increased TNFα, TBet, Arg2, IL-12β, IL-1β, MCP-1, and RORγ mRNA abundance in the aortic arch from dKO compared to Ldlr−/− mice, indicating an immune profile within the plaque polarized to Th1, Th17, and M1. Interestingly, abundance of Th2 marker, GATA3, and IL-10 mRNA were significantly decreased in aortic arch of dKO mice. Despite differences in macrophage polarization, no significant difference was noted in total number of infiltrating macrophages in the atherosclerotic lesions of dKO mice compared to control mice (Supplemental data Figure IV).
Figure 2.
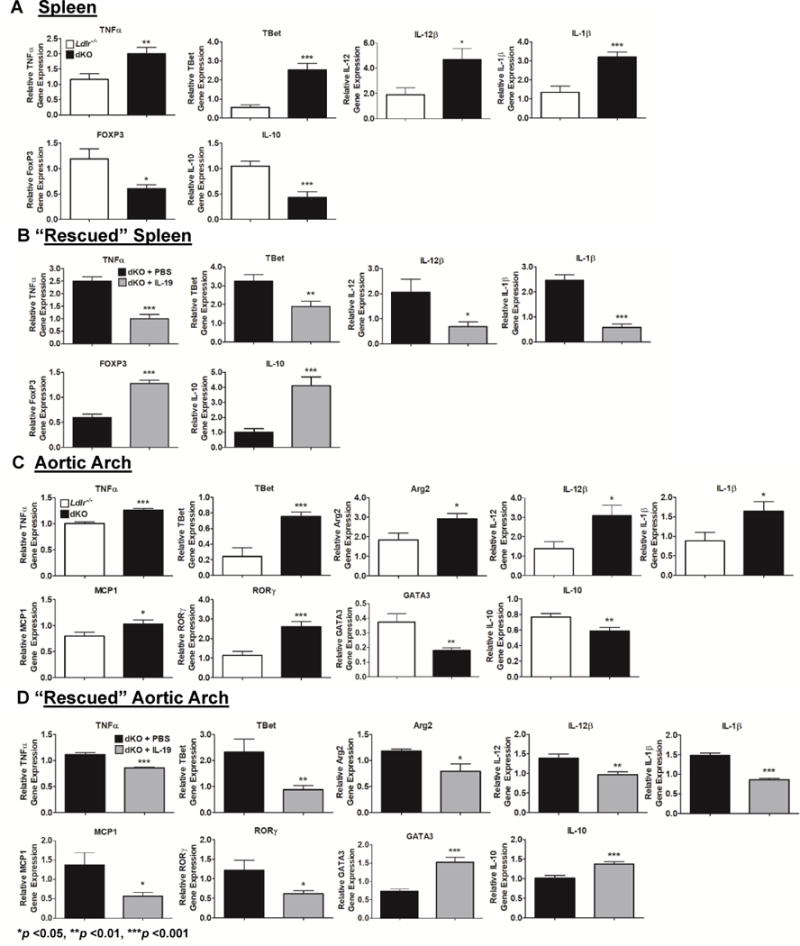
Deletion of IL-19 polarizes the systemic and local immune responses. IL-19 treatment is able to “rescue” phenotype.
Quantitative RT-PCR analysis of spleen and aortic arch harvested from Ldlr−/− and dKO mice after 14 weeks of HFD (A, C) and dKO mice simultaneously injected with IL-19 or PBS during HFD feeding (B, D). At the time of euthanasia RNA was extracted, reverse transcribed, and amplified using primers for the genes shown. (n=8 spleen, aortic arch per group).
Treatment of dKO mice with IL-19 was able to rescue the immune cell profile in spleen, as Figure 2B shows significantly decreased levels of TNFα, TBet, IL-12β and IL-1β mRNA, as well as significantly increased FOXP3 and IL-10 mRNA when compared to dKO mice treated with saline. Figure 2D shows that aortic arch from dKO mice treated with IL-19 have significantly decreased TNF α, TBet, Arg2, IL-12β, MCP-1, RORγ, and IL-1β mRNA, and significantly greater levels of GATA3 and IL-10 mRNA compared to PBS injected control mice. When taken together, this data suggests that mice receiving IL-19 treatment have decreased Th1, Th17, and M1 associated gene expression both systemically and locally, compared to saline treated controls, and suggests that differences in plaque severity in dKO mice compared to Ldlr−/− controls may be mediated by both systemic and intraplaque polarization of adaptive immunity. Raw numbers for all qRT-PCR are shown in Supplementary Data Tables 1 and 2.
The effects of deletion of IL-19 on circulating immune cells was determined by flow cytometry. There is no difference in the number of circulating IFNγ+ or FoxP3+ T cells, nor TNF α+ or CD206+ macrophages in Ldlr−/− and dKO mice after 14 weeks of HFD (Supplemental data Figure IIIA). However, we did observe a significantly greater percentage of TNFα+ CD4 T cells in dKO mice compared to Ldlr−/− controls, consistent with Th1, M1 polarization observed in spleen and aortic arch from dKO mice. We observed no differences in total numbers of circulating leukocytes, including lymphocytes, neutrophils, and monocytes in these mice (Supplemental Data Figure IVB).
Immune cells participate in IL-19 anti-atherosclerotic effects
IL-19 has confirmed immunoregulatory effects, and it was important to determine the major effector cell of IL-19 atheroprotection. To this end, dKO mice were transplanted with bone marrow isolated from dKO or Ldlr−/− control mice and fed HFD for 14 weeks. En face analysis indicates that mice receiving dKO BM had significantly more atherosclerotic lesion area compared to control mice (8.46 ± 0.63% for Ldlr−/− vs 12.62 ± 0.85% for dKO, p <0.01) (Figure 3A and 3B). This suggests that immune cells participate in IL-19 mediated atheroprotection.
Figure 3.
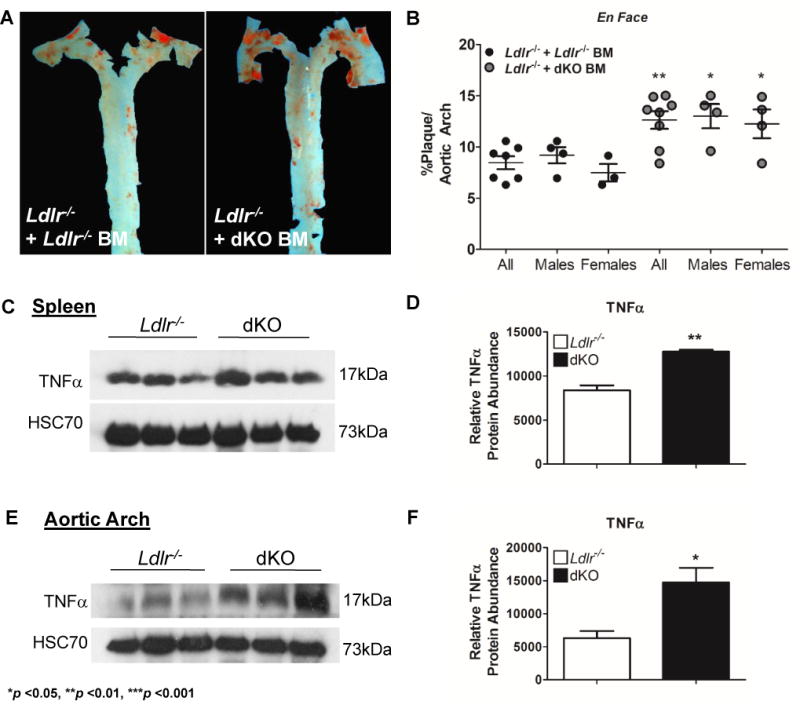
Immune cells participate in IL-19 mediated atheroprotection.
A Representative photomicrograph of aortic arch from Ldlr−/− transplanted with Ldlr−/− or dKO BM, after consuming HFD for 14 weeks. Surface lesions en face stained with Sudan IV. B Graphic depiction of atherosclerotic lesion size quantitated from en face stained aortic arches as depicted in “A” (n=7 Ldlr−/− recipients vs 8 dKO recipients).
Deletion of IL-19 increases TNFa expression.
Increased TNFa protein expression in the spleen (C) and aortic arch (E) of dKO mice compared to Ldlr−/− mice after 14 weeks of HFD, determined by immunoblot and consequent densitometry (D, F). (n=3 spleen, aortic arch per group).
Loss of IL-19 increases TNFα expression and stability in BMDM and VSMC
TNFα is a potent pro-inflammatory cytokine that participates in atherogenesis(14). TNFα protein abundance was significantly elevated in the spleen of dKO mice after 14 weeks of HFD compared to Ldlr−/− control mice (838 ± 56AU in Ldlr−/− vs 1276 ± 20AU in dKO, p <0.001) (Figures 3C, 3D). Similarly, TNFα protein was increased in the aortic arch of dKO mice compared to Ldlr−/− control mice (630.7 ± 107AU in Ldlr−/− vs 1471 ± 221AU in dKO, p <0.05) (Figures 3E, 3F). These data suggest dKO mice have both a global and local increase in pro-inflammatory TNFα expression.
In order to determine the molecular mechanisms that may be responsible for the increased abundance of TNFα in dKO mice, we isolated BMDM and VSMC from Ldlr−/− and dKO mice. BMDM and VSMC were cultured and stimulated with TNFα, a potent pro-inflammatory stimulus. Both BMDM and VSMC from dKO mice expressed significantly increased amounts of TNFα mRNA compared to Ldlr−/− controls (BMDM: 88.10 ± 4.62AU in Ldlr−/− vs 190.5 ± 3.093AU in dKO, p <0.001; VSMC: 5.098 ± 0.775AU in Ldlr−/− vs 10.54 ± 0.309AU in dKO, p <0.01) (Figures 4A, 4G). To further define a mechanism, this experiment was repeated with the addition of the transcription inhibitor actinomycin D after TNFα stimulation. TNFα mRNA was significantly more stable in cells isolated from dKO mice compared to Ldlr−/− control mice (Figures 4B, 4H). In order to confirm the specificity of IL-19 in regulating TNFα mRNA stability, we next attempted to rescue increased TNFα mRNA stability in dKO cells by pre-treating them with IL-19. Pre-treatment with IL-19, prior to TNFα stimulation in dKO BMDM and VSMC significantly decreases TNFα mRNA stability when compared to control dKO cells (Figures 4C, 4I). We examined mRNA transcripts of additional pro-inflammatory cytokines known to participate in atherogenesis and also regulated by IL-19. Similar results were obtained with IL-1β in BMDM, and MCP1 in VSMC (Figures 4E and F, and 4K and L, respectively). These data suggest that IL-19 can control pro-inflammatory cytokine abundance in BMDM and VSMC by regulation of mRNA stability.
Figure 4.
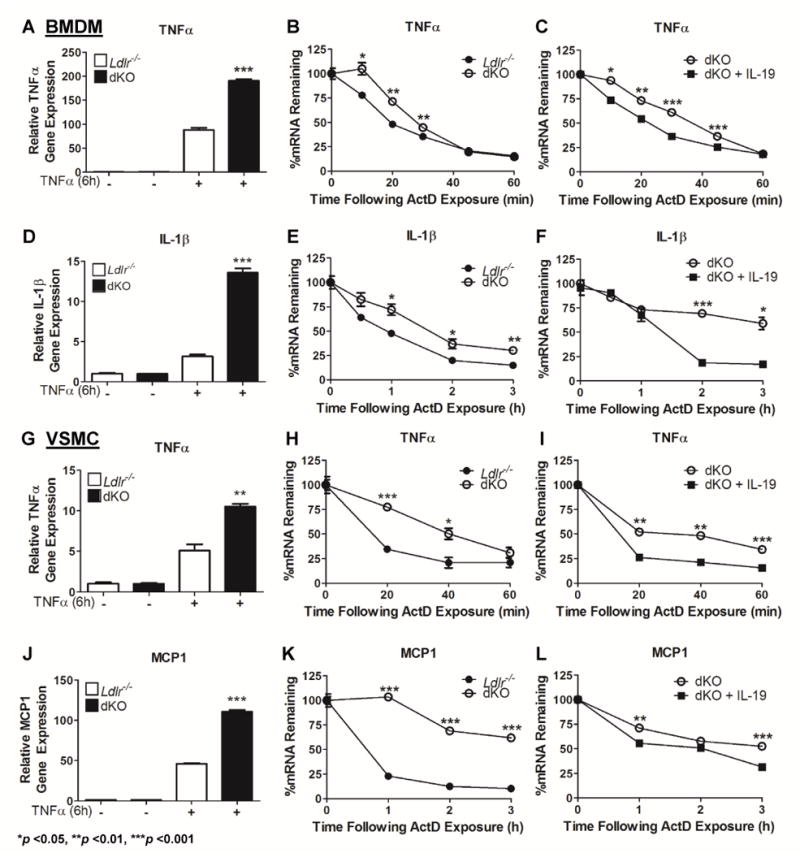
Deletion of IL-19 increases expression and mRNA stability of pro-inflammatory transcripts.
BMDM and VSMC were cultured from Ldlr−/− and dKO mice. Cells were stimulated with TNFa to induce expression of pro-inflammatory cytokines.
A, D, G, J Quantitative RT-PCR was performed on BMDM and VSMC. DKO BMDM and VSMC both demonstrate increased expression of TNFa, as well as IL-1b and MCP1 in each respective cell type, compared to Ldlr−/− controls (n=3 per group).
B, E, H, K The transcription inhibitor Actinomycin D was added at varying times after addition of TNFa. Quantitative RT-PCR was utilized and values were normalized to time point “0” to determine percentages of mRNA remaining over time. DKO BMDM express more stable TNFa and IL-1b mRNA and dKO VSMC express more stable TNFa and MCP1 over time than Ldlr−/− controls (n=3 per group).
C, F, I, L Pre-treatment with IL-19 prior to TNFa stimulation to dKO BMDM and VSMC results in significant reduction of TNFa, IL-1b, and MCP1 RNA stability. (n=3 per group).
HuR is elevated in mice lacking IL-19
Regulation of mRNA stability is an important post-transcriptional mechanism of controlling inflammatory cytokine abundance. Human antigen R, (HuR) is an important regulator of TNFα mRNA stability(15,16) and because IL-19 treatment resulted in decreased pro-inflammatory cytokine mRNA stability, several experiments were performed to draw a relationship between IL-19 expression and HuR abundance. First, we determined if HuR is expressed in murine atherosclerotic plaque. Immunohistochemical localization shows that HuR is expressed more robustly in the area of the plaque, rather than in the media, distal from the plaque. Dual-color immunohistochemistry using lineage-specific antibody shows that HuR expression is similar to IL-19 in that it is expressed in VSMC, macrophages and EC10 (Supplemental data Figure V). Next, HuR mRNA and protein abundance in spleen and aortic arch from Ldlr−/− and dKO mice fed 14 weeks of HFD were quantitated. Figure 5A demonstrates that HuR mRNA is significantly increased in spleen from dKO mice compared to Ldlr−/− controls (1.15 ± 0.2AU in Ldlr−/− vs 2.83 ± 0.4AU in dKO, p <0.01). Figures 5B and 5C show that HuR protein is also significantly greater in dKO spleen (623 ± 22.7AU in Ldlr−/− vs 1081 ± 69.9AU in dKO, p <0.001). Figure 5D-F display that both HuR mRNA and protein are significantly increased in aortic arch of the dKO compared to Ldlr−/− mice (mRNA: 0.77 ± 0.02AU in Ldlr−/− vs 1.17 ± 0.2AU, p <0.001; protein: 326.0 ± 26AU in Ldlr−/− vs 864.7 ± 23AU, p <0.001). Cultured BMDM from dKO mice stimulated with TNFα express significantly increased HuR protein levels compared to BMDM from Ldlr−/− mice (Figures 5G, 5H). Interestingly, unstimulated BMDM from dKO express significantly higher basal levels of TNFα compared to BMDM from Ldlr−/−. Similarly, VSMC explanted from dKO also express significantly greater levels of HuR under basal conditions, as well as following TNFα stimulation when compared to Ldlr−/− VSMC (Figures 5I, 5J). These data suggest that absence of IL-19 is associated with an increase in HuR mRNA and protein abundance.
Figure 5.
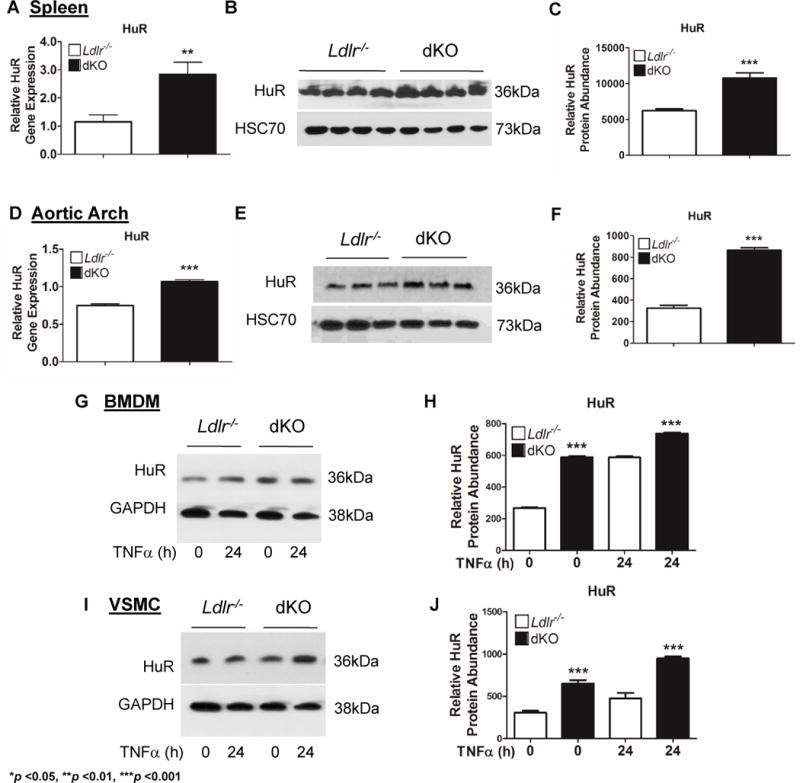
Deletion of IL-19 results in increased HuR abundance.
Spleen and aortic arch were harvested from Ldlr−/− and dKO mice after 14 weeks of HFD, at the time of euthanasia. A, D HuR mRNA expression is significantly increased in the spleen and aortic arch of dKO mice compared to Ldlr−/− controls (n=8 spleen, aortic arch per group). B, E DKO spleen and aortic arch express significantly greater HuR protein levels compared to Ldlr−/− controls determined by immunoblot. C, F Relative HuR protein abundance in “B” and “E” quantitated by densitometry. (n=4 spleen, n=3 aortic arch per group). G – J Protein lysates from BMDM and VSMC stimulated with TNFa for 24 hours and unstimulated controls were immunoblotted with anti-HuR antibody (G, I) and expression quantitated by densitometry (H, J). DKO BMDM and VSMC express significantly greater levels of HuR protein than Ldlr−/− mice (n=4 per group).
IL-19 treatment reduces HuR mRNA stability
We utilized BMDM and VSMC explanted from Ldlr−/− and dKO mice to determine a mechanism for the observed effect of IL-19 on HuR abundance. Using actinomycin D, we observed significantly increased stability of HuR mRNA in both BMDM and VSMC from dKO mice compared to Ldlr−/− controls (Figure 6A and 6D). We next added recombinant mouse IL-19 to dKO cells to rescue HuR mRNA stability. Figures 6B and 6E show that treatment with IL-19 to dKO BMDM and VSMC significantly decreases HuR mRNA stability in the dKO cells, compared to control cells that were not treated with IL-19. Proliferating cell nuclear antigen (PCNA), which lacks AREs in its 3′UTR served as a negative control and Figures 6E and 6F demonstrate no difference in PCNA mRNA stability between Ldlr−/− and dKO BMDM and VSMC. Together, these data strongly suggest that IL-19 can decrease HuR mRNA stability, and the increase in HuR noted in dKO mice and cells isolated from dKO mice is likely due to loss of IL-19.
Figure 6.
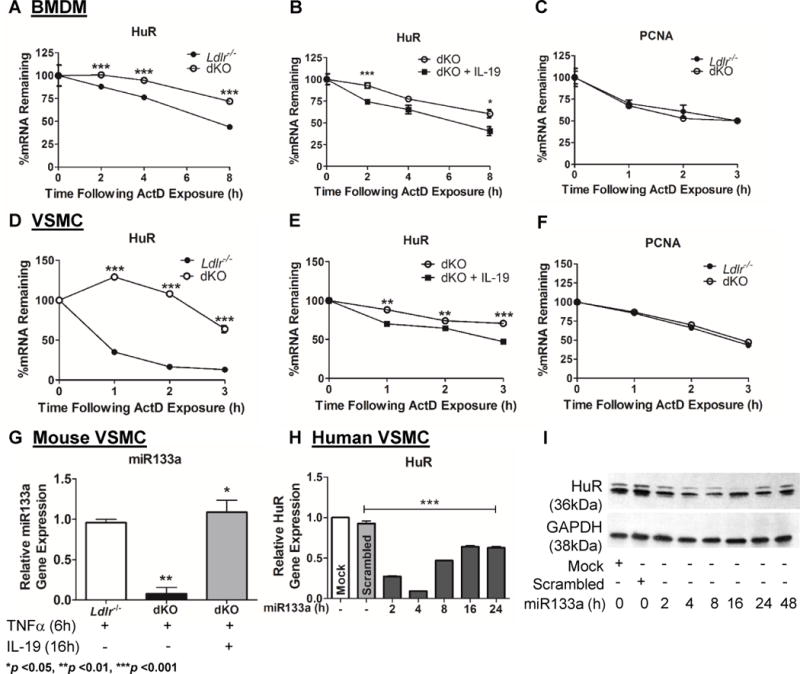
Deletion of IL-19 increases mRNA stability of HuR. BMDM and VSMC were cultured from Ldlr−/− and dKO mice. Cells were stimulated with TNFa and the transcription inhibitor Actinomycin D was added at varying times after stimulation. Quantitative RT-PCR was performed and values were normalized to time point “0” to determine percentages of HuR mRNA remaining over time. A, D Loss of IL-19 results in significantly increased stability of HuR mRNA in dKO BMDM and VSMC. (n=3 per group). B, E Pre-treatment with IL-19 prior to TNFa stimulation of dKO BMDM and VSMC results in significantly decreased stability of HuR mRNA (n=3 per group). C, F qRT-PCR analysis showing stability of PCNA mRNA, which lacks AREs in the 3′UTR, in Ldlr−/− and dKO BMDM and VSMC stimulated with TNFa (n=3 per group). G dKO VSMC stimulated with TNFa express lower levels of muscle specific miRNA, miR133a, compared to Ldlr−/− control VSMC. Pre-treatment with IL-19 is able to “rescue” levels of miR133a in dKO VSMC (n=3 per group). H Time course showing HuR mRNA is significantly decreased after transfection with miR133a mimic in human VSMC. RNA was extracted at the indicated times after transfection, and HuR mRNA abundance detected by qRT-PCR. I Time course showing HuR protein is significantly decreased following transfection with miR133a mimic in human VSMC. Cell lysates were western blotted using the indicated antibodies.
It was important to determine a more detailed mechanism of how IL-19 might decrease HuR mRNA stability. We previously described that IL-19 induced expression of miR133a, a muscle-specific miRNA, in human VSMC(17). In searching a public database [www.targetscan.org] one predicted target for miR133a is HuR 3′UTR (Supplemental Data VIA). To determine if this was a mechanism for differences in HuR abundance, we found that dKO VSMC expressed significantly less miR133a compared to Ldlr−/− control VSMC. miR133a expression trended lower in dKO compared with control aorta (Supplemental Data VIB). Importantly, miR133a expression could be induced or rescued in dKO VSMC by the addition of IL-19 (Figure 6G). Figure 6H shows that transfection of miR133a significantly reduced HuR mRNA abundance in hVSMC, and Figure 6H validates this at the protein level, suggesting that one potential mechanism for IL-19 reduction in HuR abundance is mediated by miR133a expression.
Discussion
The major finding from this study is that the absence of the anti-inflammatory cytokine IL-19 results in increased atherosclerosis, which can be reversed by injection of recombinant mouse IL-19 into the Il19−/− × Ldlr−/− dKO mice. Th1 interleukins are considered to be pro-inflammatory and pro-atherogenic, and in general, Th2 interleukins are proposed to be anti-atherogenic (14,18–20). Thus, alteration in the balance of these interleukins could potentially affect the development and severity of atherosclerosis. Previous studies led us to hypothesize that IL-19 may represent a counter-regulatory mechanism to reduce local inflammation. The present study showed increased systemic and intraplaque expression of the pro-inflammatory cytokine TNFα in Ldlr−/− mice lacking IL-19. Importantly, injection of IL-19 into dKO mice significantly reduced plaque burden to levels comparable to Ldlr−/− mice, confirming atheroprotective effects and validating specificity of IL-19 in this system.
Genetic deletion of the archetypal Th2 interleukin IL-10 also reduces atherosclerosis formation in several studies (21,22), but there are some notable differences from loss of IL-19. In Il10−/− mice crossed into the ApoE background, total cholesterol was higher in the dKO mice, where in our study no difference in cholesterol between Ldlr−/− and dKO was noted (22,23). The anti-inflammatory cytokine, IL-33 also induces Th2 cytokine responses, and injection of IL-33 into ApoE mice has been shown to ameliorate development of atherosclerosis25. In both of these cases, authors concluded that protection was due to adaptive immune modulation.
TNFα is considered to be a master inflammatory cytokine as it induces expression of multiple pro-inflammatory genes(24). A significantly greater amount of circulating TNFα + CD4+ T cells in dKO compared to Ldlr−/− mice following 14 weeks of high fat diet consumption suggested systemic effects of IL-19. This was confirmed as spleen from dKO mice had increased expression of TNFα mRNA and protein, and T-Bet expression was increased in dKO spleen compared to Ldlr−/−. Differences in intraplaque gene expression were more pronounced than systemic differences; in addition to increased TBet expression which was seen in spleen, GATA3 was reduced and Arg2 was increased within the aortic arch, indicating a local reduction in Th2 T cells and an increase in pro-inflammatory M1 macrophages. Importantly, aortic arch from dKO mice had significantly greater expression of TNFα protein compared to controls. The anti-atherosclerotic effects of IL-19 are unique in that they appear to be both indirect and direct by affecting adaptive immune responses and by dampening inflammation in macrophages and vascular smooth muscle cells within the plaque area. It was interesting to note that there was less IL-10 mRNA expression in dKO aortic arch compared to controls. This may be a direct effect of loss of IL-19 and/or an additional cause for exacerbated plaque size; decreased levels of IL-10 can be considered to be a contributing factor, as IL-10 has anti-atherosclerotic effects22,23. Concordant with that observed for Ldlr−/− and dKO mice, the immune cell profile in aortic arch of dKO mice rescued with IL-19 resembled that of Ldlr−/− mice, demonstrating localized anti-inflammatory effects of IL-19.
With this in mind, it was important to determine if IL-19 anti-atherosclerotic effects were mediated by polarization of adaptive immunity or by direct anti-inflammatory effects on resident vascular cells. Transplantation of dKO BM into Ldlr−/− mice results in significantly increased plaque size compared with control BM. This confirms that at least some of IL-19 athero-protective effects are mediated by immune cells. This is not surprising as IL-19 is a Th2 interleukin with confirmed immunomodulatory activity(25–28). When taken together, IL-19 anti-atherosclerotic effects are likely both indirect, by effecting adaptive immune responses, and direct, by dampening inflammation in macrophages and vascular smooth muscle cells within the plaque area.
An additional feature distinguishing IL-19 from IL-10 and IL-33 is that IL-19 is expressed in VSMC as well as immune cells; how the absence of IL-19 affects the response of these cells to inflammatory stimuli has not been reported. We explanted BMDM and VSMC from dKO and Ldlr−/− mice to closely characterize the inflammatory response in each. In both cell types, the cells lacking IL-19 had an increased inflammatory response to TNFα stimulation. More detailed analysis determined that loss of IL-19 resulted in increased pro-inflammatory cytokine mRNA stability, which could be reversed, or “rescued” by addition of IL-19 to dKO BMDM and VSMC. While this does not rule out transcriptional effects, it does reveal that one mechanism for IL-19 anti-inflammatory activity takes place post-transcriptionally. Reduction of pro-inflammatory cytokine mRNA stability by IL-19 is consistent with our hypothesis that IL-19 acts as a compensatory, negative regulatory factor in response to inflammatory stimuli.
TNFα is an example of a potent, rapid-response pro-inflammatory cytokine whose mRNA stability is post-transcriptionally regulated by mRNA binding proteins (RBPs), allowing the cell to tightly-regulate induction of other cytokines(29). Many pro-inflammatory cytokines, especially TNFα, have unstable mRNAs, often due to the presence of cis-acting AU-rich elements (AREs) in the 3′UTR which promote degradation(30). Trans-acting factors including RBPs bind the AREs and regulate mRNA fate. Human antigen R (HuR) has been shown to recognize and bind the AREs of many cytokine transcripts, including TNFα(30,31), and appears to be the only identified RBP confirmed to stabilize, rather than destabilize, TNFα transcripts.
HuR expression is increased in neointimal hyperplasia(32), and induces VSMC constriction(33). One report suggests that HuR is targeted by IL-10(34). This study showed that HuR is expressed in EC, VSMC, and macrophage in atherosclerotic plaque. We have previously reported that IL-19 affects HuR activity in cultured human VSMC(9). However, a role for HuR in mediation of IL-19 anti-atherosclerotic activity in vivo are lacking. The present study extends those reports and demonstrates that compared to control mice, absence of IL-19 significantly increases HuR mRNA and protein abundance systemically, in spleen, and locally in aortic arch. Further, cultured BMDM and VSMC isolated from dKO mice demonstrate increased amounts of HuR protein relative to cells cultured from Ldlr−/− mice. It was important to determine how IL-19 modifies HuR abundance. HuR mRNA contains conserved and semi-conserved ARE in its 3′UTR, and is reported to regulate its own mRNA stability in a feedback mechanism(35). We used cultured BMDM and VSMC treated with actinomycin D to determine that the absence of IL-19 results in an increase in HuR mRNA stability in both cell types. Notably, treatment with IL-19 in dKO cells rescued the phenotype, significantly reducing HuR mRNA stability below knock out levels, which may explain the increased HuR protein abundance in spleen and aortic arch in dKO mice. A consistent increase in HuR levels over the 14 week course of our study may lead to greater stability of pro-inflammatory transcripts and thus, exacerbated inflammation and plaque formation. It is important to note that there were no differences in the stability of PCNA mRNA, which lacks AREs in its 3′UTR. This further supports the notion that increased stability of TNFα mRNA in dKO cells is due to ARE-dependent stabilization of transcripts by excess HuR.
One mechanism of miRNA function is reduction of mRNA abundance and subsequent reduction in protein. Stimulation with IL-19 induces expression of miRNA133a, a muscle cell-specific miRNA(36). miR133a is predicted to target HuR 3′UTR, and this study showed that miR133a could reduce HuR mRNA and protein abundance. Importantly, miR133a expression was barely detectible in dKO VSMC, but could be rescued to Ldlr−/− control levels with the addition of IL-19, providing a potential mechanism for IL-19 reduction of HuR. This may also explain the increased IL-19 sensitivity of VSMC compared with BMDM in terms of reduction of HuR mRNA stability (Figure 6D). One limitation of this study is that we cannot experimentally determine if mRNA stability is modified in dKO mice because RNA transcription inhibitors including actinomycin D are toxic and cannot be used in vivo. However, considering the well documented role of HuR as an mRNA stability protein together with its increased expression in spleen, aortic arch, and cells explanted from dKO mice, we feel confident that one major mechanism for IL-19 mediated atheroprotective effects is its ability to decrease HuR mRNA abundance, leading to decreases in mRNA stability of pro-inflammatory cytokines.
The regulation of mRNA stability and translation are two levels of post-transcriptional regulation that permit cells to rapidly respond to inflammatory stimuli(31). Although modulation of mRNA stability has been posited as a possible therapeutic strategy(37), surprisingly, there is very little literature exploring the concept of RBPs and mRNA stability being directly regulated by inflammatory stimuli. In conclusion, this manuscript demonstrates that lack of IL-19 leads to increased atherosclerosis. This is likely due to increased mRNA stability of pro-inflammatory cytokines due to increased abundance of the mRNA stability protein, HuR. In the larger picture, it suggests that RBPs may represent a novel class of targets for modalities to attenuate vascular inflammation.
Supplementary Material
Highlights.
Il19−/− × Ldlr−/− double knockout (dKO) mice have significantly more atherosclerotic plaque compared to Ldlr−/− controls
dKO mice have increased pro-inflammatory cytokine expression in aorta and spleen and increased pro-inflammatory cytokine expression and mRNA stability in BMDMs and VSMCs
dKO mice have increased expression of the mRNA stability protein HuR in aorta, spleen and increased expression and mRNA stability of HuR in BMDM and VSMC
All of the above phenotypes can be rescued by the addition of mouse IL-19 in vivo and in vitro
These data indicate that IL-19 is an atheroprotective cytokine that decreases abundance of HuR, leading to reduced pro-inflammatory mRNA stability
Acknowledgments
Acknowledgements: The authors would like to acknowledge the technical expertise of Lauren R. Beckett and Toby P. Thomas in performing these studies.
Sources of Funding: This work was supported by grants HL141108 and HL117724 from the National Heart Lung, and Blood Institute of the National Institutes of Health, and grant 13GRNT1685003 from the American Heart Association to MVA. MR and ABH were supported by American Heart Association pre-doctoral fellowships 16PRE31220005 and 17PRE33670798, and grant 1F31HL137344-01 to ABH.
Abbreviations
- BMDM
bone marrow derived macrophage
- dKO
double knockout
- HuR
Human antigen R
- IL-19
Interleukin-19
- Ldlr
low density lipoprotein receptor
- TNFα
Tumor Necrosis Factor alpha
- UTR
Untranslated region
Footnotes
Disclosure: The author(s) declare no competing financial interests or any other conflict of interest.
References
- 1.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.von der Thüsen JH, Kuiper J, van Berkel TJC, Biessen EAL. Interleukins in atherosclerosis: molecular pathways and therapeutic potential. Pharmacol Rev. 2003;55:133–166. doi: 10.1124/pr.55.1.5. [DOI] [PubMed] [Google Scholar]
- 4.Gabunia K, Jain S, England RN, Autieri MV. Anti-inflammatory cytokine interleukin-19 inhibits smooth muscle cell migration and activation of cytoskeletal regulators of VSMC motility. AJP Cell Physiol. 2011;300:C896–906. doi: 10.1152/ajpcell.00439.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian Y, Sommerville LJ, Cuneo A, Kelemen SE, Autieri MV. Expression and suppressive effects of interleukin-19 on vascular smooth muscle cell pathophysiology and development of intimal hyperplasia. Am J Pathol. 2008;73:901–909. doi: 10.2353/ajpath.2008.080163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellison S, Gabunia K, Richards JM, Kelemen SE, England RN, Rudic D, et al. IL-19 Reduces Ligation-Mediated Neointimal Hyperplasia by Reducing Vascular Smooth Muscle Cell Activation. Am J Pathol. 2014;184:2134–2143. doi: 10.1016/j.ajpath.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oral HB, Kotenko SV, Yilmaz M, Mani O, Zumkehr J, Blaser K, et al. Regulation of T cells and cytokines by the interleukin-10 (IL-10)-family cytokines IL-19, IL-20, IL-22, IL-24 and IL-26. Eur J Immunol. 2006;36:380–3888. doi: 10.1002/eji.200425523. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher G, Dickensheets H, Eskdale J, Izotova LS, Mirochnitchenko OV, Peat JD, et al. Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10) Genes Immun. 2000;1:442–450. doi: 10.1038/sj.gene.6363714. [DOI] [PubMed] [Google Scholar]
- 9.Cuneo AA, Herrick D, Autieri MV. Il-19 reduces VSMC activation by regulation of mRNA regulatory factor HuR and reduction of mRNA stability. J Mol Cell Cardiol. 2010;49:647–654. doi: 10.1016/j.yjmcc.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellison S, Gabunia K, Kelemen SE, England RN, Scalia R, Richards JM, et al. Attenuation of experimental atherosclerosis by interleukin-19. Arterioscler Thromb Vasc Biol. 2013;33:2316–2324. doi: 10.1161/ATVBAHA.113.301521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabunia K, Ellison S, Kelemen S, Kako F, Cornwell WD, Rogers TJ, et al. IL-19 Halts Progression of Atherosclerotic Plaque, Polarizes, and Increases Cholesterol Uptake and Efflux in Macrophages. Am J Pathol. 2016;186:1361–1374. doi: 10.1016/j.ajpath.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.England RN, Preston KJ, Scalia R, Autieri MV. Interleukin-19 decreases leukocyte-endothelial cell interactions by reduction in endothelial cell adhesion molecule mRNA stability. AJP Cell Physiol. 2013;305:C255–265. doi: 10.1152/ajpcell.00069.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommerville LJ, Kelemen SE, Ellison SP, England RN, Autieri MV. Increased atherosclerosis and vascular smooth muscle cell activation in AIF-1 transgenic mice fed a high-fat diet. Atherosclerosis. 2012;220:45–52. doi: 10.1016/j.atherosclerosis.2011.07.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 15.Fan XC. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the invivo stability of ARE-containing mRNAs. EMBO J. 1998;17:3448–3460. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenberg DR, Maquat LE. Regulation of cytoplasmic mRNA decay. Nat Rev Genet [Internet] 2012 doi: 10.1038/nrg3160. Available from: http://www.nature.com/doifinder/10.1038/nrg3160. [DOI] [PMC free article] [PubMed]
- 17.Gabunia K, Herman AB, Ray M, Kelemen SE, England RN, DeLa Cadena R, et al. Induction of MiR133a expression by IL-19 targets LDLRAP1 and reduces oxLDL uptake in VSMC. J Mol Cell Cardiol. 2017;105:38–48. doi: 10.1016/j.yjmcc.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulte S, Sukhova GK, Libby P. Genetically Programmed Biases in Th1 and Th2 Immune Responses Modulate Atherogenesis. Am J Pathol. 2008;172:1500–1508. doi: 10.2353/ajpath.2008.070776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallat Z, Ait-Oufella H, Tedgui A. Regulatory T cell responses: potential role in the control of atherosclerosis. Curr Opin Lipidol. 2005t;16:518–524. doi: 10.1097/01.mol.0000182532.11512.90. [DOI] [PubMed] [Google Scholar]
- 20.Frostegård J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 21.Potteaux S. Leukocyte-Derived Interleukin 10 Is Required for Protection Against Atherosclerosis in Low-Density Lipoprotein Receptor Knockout Mice. Arterioscler Thromb Vasc Biol. 2004;24:1474–1478. doi: 10.1161/01.ATV.0000134378.86443.cd. [DOI] [PubMed] [Google Scholar]
- 22.Caligiuri G, Rudling M, Ollivier V, Jacob M-P, Michel J-B, Hansson GK, et al. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9:10–17. [PMC free article] [PubMed] [Google Scholar]
- 23.Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 24.Tedgui A. Cytokines in Atherosclerosis: Pathogenic and Regulatory Pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher G, Eskdale J, Jordan W, Peat J, Campbell J, Boniotto M, et al. Human interleukin-19 and its receptor: a potential role in the induction of Th2 responses. Int Immunopharmacol. 2004;4:615–626. doi: 10.1016/j.intimp.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher G. Interleukin-19: multiple roles in immune regulation and disease. Cytokine Growth Factor Rev. 2010;21:345–352. doi: 10.1016/j.cytogfr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Wegenka UM. IL-20: biological functions mediated through two types of receptor complexes. Cytokine Growth Factor Rev. 2010;21:353–363. doi: 10.1016/j.cytogfr.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Yeh C-H, Cheng B-C, Hsu C-C, Chen H-W, Wang J-J, Chang M-S, et al. Induced interleukin-19 contributes to cell-mediated immunosuppression in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. Ann Thorac Surg. 2011;92:1252–1259. doi: 10.1016/j.athoracsur.2011.04.061. [DOI] [PubMed] [Google Scholar]
- 29.Anderson P. Post-transcriptional control of cytokine production. Nat Immunol. 2008;9:353–359. doi: 10.1038/ni1584. [DOI] [PubMed] [Google Scholar]
- 30.Khera TK, Dick AD, Nicholson LB. Mechanisms of TNFα regulation in uveitis: Focus on RNA-binding proteins. Prog Retin Eye Res. 2010;29:610–621. doi: 10.1016/j.preteyeres.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Palanisamy V, Jakymiw A, Van Tubergen EA, D’Silva NJ, Kirkwood KL. Control of Cytokine mRNA Expression by RNA-binding Proteins and microRNAs. J Dent Res. 2012;91:651–658. doi: 10.1177/0022034512437372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pullmann R, Juhaszova M, de Silanes IL, Kawai T, Mazan-Mamczarz K, Halushka MK, et al. Enhanced Proliferation of Cultured Human Vascular Smooth Muscle Cells Linked to Increased Function of RNA-binding Protein HuR. J Biol Chem. 2005;280:22819–22826. doi: 10.1074/jbc.M501106200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pende A, Contini L, Sallo R, Passalacqua M, Tanveer R, Port JD, et al. Characterization of RNA-binding proteins possibly involved in modulating human AT1 receptor mRNA stability. Cell Biochem Funct. 2008;26:493–501. doi: 10.1002/cbf.1472. [DOI] [PubMed] [Google Scholar]
- 34.Rajasingh J. IL-10-induced TNF-alpha mRNA destabilization is mediated via IL-10 suppression of p38 MAP kinase activation and inhibition of HuR expression. FASEB J. 2006;20:2112–2124. doi: 10.1096/fj.06-6084fje. [DOI] [PubMed] [Google Scholar]
- 35.Dai W, Zhang G, Makeyev EV. RNA-binding protein HuR autoregulates its expression by promoting alternative polyadenylation site usage. Nucleic Acids Res. 2012;40:787–800. doi: 10.1093/nar/gkr783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams AH, Liu N, van Rooij E, Olson EN. MicroRNA control of muscle development and disease. Curr Opin Cell Biol. 2009;21:461–469. doi: 10.1016/j.ceb.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eberhardt W, Doller A, Akool E-S, Pfeilschifter J. Modulation of mRNA stability as a novel therapeutic approach. Pharmacol Ther. 2007;114:56–73. doi: 10.1016/j.pharmthera.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Sommerville LJ, Kelemen SE, Ellison SP, England RN, Autieri MV. Increased atherosclerosis and vascular smooth muscle cell activation in AIF-1 transgenic mice fed a high-fat diet. Atherosclerosis. 2012;220:45–52. doi: 10.1016/j.atherosclerosis.2011.07.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


