Abstract
Background
Myeloperoxidase (MPO) generates hypochlorous acid (HOCl) during inflammation and infection. We showed that secoisolariciresinol diglucoside (SDG) scavenges radiation-induced HOCl in physiological solutions. However, the action of SDG and its synthetic version, LGM2605, on MPO-catalyzed generation of HOCl is unknown. The present study evaluated the effect of LGM2605 on MPO.
Methods
MPO activity was determined fluorometrically using hypochlorite-specific 3′-(p-aminophenyl) fluorescein (APF). The effect of LGM2605 on (a) the peroxidase cycle of MPO was determined using Amplex Red while the effect on (b) the chlorination cycle was determined using a taurine chloramine assay. Using electron paramagnetic resonance (EPR) spectroscopy we determined the effect of LGM2605 on the EPR signals of MPO. Finally, computational docking of SDG was used to identify energetically favorable docking poses to enzyme's active site.
Results
LGM2605 inhibited human and murine MPO activity. MPO inhibition was observed in the absence and presence of Cl−. EPR confirmed that LGM2605 suppressed the formation of Compound I, an oxoiron (IV) intermediate [Fe(IV)=O] containing a porphyrin π-radical of MPO's catalytic cycle. Computational docking revealed that SDG can act as an inhibitor by binding to the enzyme's active site.
Conclusions
We conclude that LGM2605 inhibits MPO activity by suppressing both the peroxidase and chlorination cycles. EPR analysis demonstrated that LGM2605 inhibits MPO by decreasing the formation of the highly oxidative Compound I. This study identifies a novel mechanism of LGM2605 action as an inhibitor of MPO and indicates that LGM2605 may be a promising attenuator of oxidant-dependent inflammatory tissue damage.
Keywords: Hypochlorous acid, Hypochlorite ion, LGM2605, Macrophages, Myeloperoxidase, SDG
1 Introduction
Myeloperoxidase (MPO), a member of the hemeperoxidase-cyclooxygenase superfamily, is expressed in neutrophils, monocytes and some tissue macrophages, such as microglia. MPO is an essential component in a number of pathological conditions, including cardiovascular, neurodegenerative, inflammatory and immune-mediated diseases [1-5]. The enzyme is involved in the innate immune response as well as in microbial killing by generating MPO-derived oxidants. Considering the involvement of MPO in a large number of human diseases, there is an urgent need for developing a highly selective MPO inhibitor that preserves the MPO activity for host defense, but inhibits the excessive persistent pathophysiological activation of MPO.
MPO catalyzes the reaction between chloride ions and hydrogen peroxide (H2O2) to generate a potent oxidant, hypochlorous acid (HOCl) [5-7]. A mixture of both HOCl and hypochlorite ion (ClO−) are present at physiological pH. HOCl functions as a primary defense mechanism to kill invading microorganisms by oxidative damage [8]. However, excessive HOCl production is known to cause tissue damage. Hypochlorite modifies adenine nucleotides resulting in formation of chloramines that appears to be a major mechanism of neutrophil-mediated toxicity [9-11].
MPO inhibitors would be useful for the treatment of a broad spectrum of human disease conditions, however, the proinflammatory nature of MPO with its strong microcidal activity, makes it a key player in the innate response to foreign invading organisms [5, 12]. This positive aspect of MPO requires critical consideration when developing an MPO inhibitor that can be used as a potential drug, especially in cancer patients who may already be immunocompromised. MPO inhibitors are classified in three categories 1. Agents promoting Compound II (Por-Fe4+-OH, an oxoiron (IV) intermediate) accumulation (Scheme 1), 2. Suicide substrates, and 3. Agents that bind reversibly. The first two categories of agents are unlikely to be effective in vivo due to the presence of high levels of better physiological substrates for the peroxidase cycle such as ascorbate and ureate. The reversible inhibitors bind to the heme binding pocket of the enzyme and compete with the MPO substrates. These include hydroxamic acids and benzoic acid hydrazides. 4-aminobenzoic acid hydrazide is a potent irreversible inhibitor of MPO with an IC50 of 0.3 μM [13]. Irreversible inhibitors do not fulfill the requirement of preserving the positive role of MPO in keeping the innate immune response intact during treatment.
Scheme 1. Proposed Mechanism of MPO Inhibition by LGM2605.
The MPO activity, peroxidase cycle (reactions 1, 5, and 6 with AH2) and halogenation cycle (reactions 1 and 3) can be inhibited with LGM2605 by: 1) blocking the H2O2 binding site of the native MPO Fe (III) (reaction 2 with *AH2); 2) reducing (a two-electron reduction) Compound I, (reaction 4 with *AH2); 2) reducing Compound I to Compound II (reaction 5 with *AH2) and subsequently to the native MPO enzyme (reaction 6 with *AH2), each of reactions 5 and 6 represents a single-electron reduction. A possible reduction of the native (ferric) MPO Fe (III) to the reduced (ferrous) MPO Fe (II) enzyme (reaction 7) was not validated by EPR analysis. AH2 is an oxidizable substrate, *AH2 is SDG (LGM2605).
MPO contains conserved motifs on both the proximal and distal sides of the essential heme prosthetic group, a calcium binding site and at least two covalent bonds linking the heme group to the protein backbone [12, 14]. In addition, MPO contains a sulfonium linkage between the 2-vinyl group and methionine 409. This provides MPO with greater oxidizing potential to oxidize Cl− to Cl+, resulting in generation of HOCl at physiological pH [7, 15]. As a peroxidase, MPO catalyzes one- and two-electron oxidations of both inorganic and organic substrates. During the enzyme reaction, MPO is oxidized by H2O2 from the native enzyme to Compound I (Scheme 1, reaction 1), an oxoiron (IV) intermediate [Fe(IV)=O] containing a porphyrin π-radical via two-electron oxidation. Compound I then reacts with a halide X− (X= Cl−, Br−) in a two-electron oxidation reaction generating HOCl and simultaneously converting the enzyme back to its native state (Scheme 1, halogenation cycle, H2O2 + X− + H+ → HOX + H2O, reactions 1 and 3). In the parallel peroxidase cycle, Compound I is converted to Compound II by a single one-electron reduction. A second one-electron reduction of Compound II converts it back to the native enzyme (peroxidase cycle, Scheme 1, reactions 1, 5 and 6 with AH2, an oxidizible substrate, not secoisolariciresinol diglucoside SDG) [12].
SDG (Supplemental Figure 1A) is a natural bioactive agent, the main lignan found in whole grain flaxseed. We have successfully synthesized SDG and shown that synthetic SDG, LGM2605, has strong antioxidant and free radical (hydroxyl, peroxyl and DPPH) scavenging properties similar to natural SDG [16]. LGM2605 also prevented radiation-induced damage to genomic and plasmid DNA [17]. Until recently, the biological effects of ionizing radiation were associated with oxidative stress caused by the reactive oxygen species (ROS) [18-20]. We have shown that gamma radiation of physiological solutions induces generation of active chlorine species (ACS) formed by interaction of ROS and Cl− and LGM2605 scavenges radiation-induced ACS, both primary ACS radicals such as chlorine atoms and secondary ACS hypochlorite [21]. Most importantly, SDG given in diverse formulations is also non-toxic to animals and humans [22-26].
Due to these characteristics, LGM2605 may potentially interfere with both halogenation and peroxidase cycles of the MPO reactions. Since Compound I contains a porphyrin π cation radical, electron paramagnetic resonance (EPR) has been utilized to study the catalytic mechanism of a variety of peroxidases including MPO [27, 28]. EPR is a very sensitive and powerful tool to detect micro-environmental changes in the electronic nature of radical intermediates and paramagnetic centers formed during catalysis.
The present study was specifically designed to investigate the effect of LGM2605 on MPO in preparations from human leukocytes, elicited mouse macrophages and neutrophils, and RAW 264.7 murine macrophage cells. We investigated the mechanism of LGM2605 action on the peroxidase as well as the halogenation cycles of the MPO and measured their kinetics to determine if LGM2605 interfered with either or both H2O2 and Cl− active sites. To gain further insight into the mechanism of LGM2605 action, we used EPR spectroscopy to directly detect substrate/inhibitor binding to the paramagnetic iron in the heme pocket. Finally, we performed computational docking studies of SDG to identify energetically favorable docking poses to the MPO's active heme site.
2. Materials and Methods
2.1. Chemicals
Sodium hypochlorite, myeloperoxidase from human leukocytes (neutrophils) and myeloperoxidase (MPO) fluorometric activity assay kit were purchased from Sigma-Aldrich (St. Louis, MO). Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit was purchased from Life Technologies (Carlsbad, CA). Hydrogen peroxide was purchased from Fisher Scientific. Dulbecco's phosphate buffered saline (DPBS ×1, 21-031-CV) with or without calcium and magnesium was purchased from Mediatech Inc. (Manassas, VA). Commercially available SDG (LGM2605) was synthesized by Chemveda Life Sciences Pvt. Ltd. (Hyderabad, India) based on the procedure developed by our group [16].
2.2. Human Myeloperoxidase Activity
Myeloperoxidase from human leukocytes was used to determine the effect of LGM2605 on MPO activity. MPO activity was assayed using the myeloperoxidase fluorometric activity kit as described by the manufacturer by determining the increase in fluorescence intensity of the fluoroprobe, aminophenyl fluorescein (APF), at 485 nm excitation / 535 nm emission at room temperature using a SpectraMax i3× Multi-Mode microplate reader (Molecular Devices, Sunnyvale, CA). The activity was determined in presence of various concentrations of LGM2605 between 0-100 μM. The data are presented as relative fluorescence units (RFU).
2.3. Derivation of Elicited Mouse Macrophages
Mice (C57Bl/6) were injected intraperitoneally with 2 ml of thioglycollate medium from BD (Franklin Lakes, NJ). 72 hours after injection, abdominal lavage was performed with 10 ml sterile 1× PBS containing Ca2+ and Mg2+. Cells were counted and placed in 12-well plates (1.5 million cells per well). Lipopolysaccharide (LPS) (5 ng/ml) containing 0, 25, 50 and 100 μM LGM2605 was added. The total volume per well was 250 μl. After 1 min, 50 μl supernatant was taken and placed in 96-well clear bottom black plates for determining myeloperoxidase activity.
2.4. Derivation of Elicited Mouse Neutrophils
To obtain mouse neutrophils, mice (C57Bl/6) were injected intraperitoneally with 2 ml of thioglycollate medium. 24 hours after injection, abdominal lavage, cell plating and LPS/LGM2605 treatment were performed as described above. MPO activity was assayed, using the myeloperoxidase fluorometric activity kit. Taurine chlorination was determined using the TMB (3,3′,5,5′-tetra-methyl-benzidine) assay as described below.
2.5. Myeloperoxidase Activity of Mouse-Elicited or RAW 264.7 Macrophages
Elicited mouse macrophages or RAW 264.7 macrophages were plated in RPMI-1640 medium (1 million cells per well) overnight in a 12-well plate. 24 hours later, the medium was changed and cells were washed twice with PBS containing Ca2+ and Mg2+. PBS containing LPS (5 ng/ml) alone or LPS with PMA (100 ng/ml) and LGM2605 (0 or 100 μM) was added. After 1 min, the supernatant (50 μl) was taken and analyzed using the myeloperoxidase fluorometric activity kit. Supernatant aliquots (50 μl) were placed in 96-well black clear bottom plates and mixed with 50 μl solution containing (substrate and probe) hydrogen peroxide and APF. The final concentrations of H2O2 and APF were 50 μM and 25 μM, respectively. MPO activity was continuously monitored by determining the increase in fluorescence at 535 nm. Data are expressed as RFU.
2.6. Determination of Myeloperoxidase Activity (Peroxidase Cycle)
The effect of LGM2605 on MPO activity was determined using the Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit that determines H2O2 concentration based on the oxidation of Amplex Red to the fluorescent product resazurin. The excitation and fluorescence emission wavelengths were 530 nm and 590 nm, respectively. The assay was performed in the absence of chloride with varying concentrations of H2O2, in the presence or absence of LGM2605. Michaelis-Menten enzyme kinetics were determined using GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla, CA, USA (www.graphpad.com). The maximum enzyme velocity (Vmax) and Michaelis-Menten constant (Km) were determined using the model Y = Vmax*X / (Km + X), where X represents the varying concentrations of H2O2.
2.7. Determination of Myeloperoxidase Activity (Chlorination Cycle) by Taurine Chlorination
MPO activity was measured in 200 μl PBS containing 5 mM taurine, MPO (37.5 ng/assay), and various concentrations of LGM2605. The reaction was started with the addition of 50 μM H2O2. The samples were incubated for 30 min at 37 °C and the reaction stopped by the addition of 25 μl (25 μg) catalase. Tubes were kept on ice for 10 min, centrifuged, and a 200 μl aliquot was placed in 96-well black clear bottom plates and mixed with 50 μl of TMB reagent in the dark. Standards using hypochlorite solutions (1-20 μM) were run in parallel. Plates were put on shaker for 10 min and then read at 645 nm. The activity of MPO was determined as the increase in absorbance and expressed as enzyme units (1 unit = taurine chloramine generated by 1 μM hypochlorite per 30 min).
2.8. Determination of Myeloperoxidase Protein Levels by ELISA
MPO protein levels were determined in cell supernatants using enzyme-linked immunosorbent assays (ELISA). ELISA kits were purchased from Abcam (Cambridge, MA). Samples were run undiluted in duplicate and assays were performed according to manufacturer's instructions. Values for MPO are reported as picograms per milliliter (pg/ml) of cell supernatant.
2.9. EPR Spectroscopy of MPO
EPR samples were freshly prepared. Native freeze-dried MPO enzyme from human leukocytes was dissolved in 50 mM sodium phosphate buffer pH 7.4 to 1 mg/ml (12.34 μM). 230 μl of enzyme solution was transferred into four different tubes for four different conditions: (a) untreated; (b) H2O2; (c) pre-incubation with LGM2605 followed by H2O2; (d) LGM2605. Samples were first incubated with 10 μl of 12.5 mM LGM2605 (to the final concentration at 500 μM) or the buffer for 5 min on ice. Samples were transferred into EPR tubes after the addition of 10 μl of 15 mM H2O2 (to a final concentration at 60 μM). The mixture was immediately frozen at 5 sec in an ethanol/dry ice bath and stored in liquid nitrogen. We used a special mixer for mixing samples quickly in EPR tubes, which was previously described [29]. EPR spectra were recorded by a Bruker Elexsys E500 spectrometer at X-band (9.4 GHz) using an Oxford Instrument ESR900 helium flow cryostat.
2.10. Computational Docking Simulation Studies
Computational docking of SDG on MPO's active heme site was performed as reported by Shiba et al. [30] to determine whether possible interaction of SDG with the enzyme's active site is energetically favorable providing thus, a possible explanation of the inhibitory activity. We used the recently published structure of MPO (PDB code 5MFA) for its superior resolution and coordinates of all loops near the active site. SDG (pubchem ID 9917980) and control quercetin (pubchem ID 5280343) (Supplemental Figure 1B) were docked within a search space of roughly 10 Å surrounding all active site residues, including the heme group. AutoDock Vina [31] was used with the Amber 2003 force field, without long range electrostatics, and with implicit solvent parameters. Analyses of hydrogen bonds and other SDG-protein contacts, as well as figure preparation were performed with Chimera (UCSF) [32].
2.11. Statistical Analysis of the Data
The data obtained are presented as mean ± standard error of the mean (SEM). The data were subjected to one-way analysis of variance (ANOVA) with post-hoc comparisons (Tukey's multiple comparisons tests) using GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla, CA, USA (www.graphpad.com). Differences between treatment groups were determined at alpha=0.05. Asterisks shown in figures indicate significant differences between groups (* = p<0.05, ** = p<0.01, *** = p<0.001 and **** = p<0.0001).
3. Results
In this study we determined the effect of LGM2605 on the activity of human myeloperoxidase, on MPO from elicited and RAW 264.7 murine macrophages and in murine elicited neutrophils. The mechanism of LGM2605 action was investigated on the peroxidase cycle as well as on the chlorination cycle of MPO.
3.1. LGM2605 Inhibits Human Myeloperoxidase Activity Dose-Dependently in Cell-Free Systems
To establish if LGM2605 inhibited MPO activity, the effect of LGM2605 on human leukocyte MPO was determined. Figure 1A shows a time-course of human MPO activity in the presence of LGM2605. MPO activity was significantly decreased in the presence of LGM2605 at 50 and 100 μM (Figures 1A and 1B) by as much as 75%. At these concentrations, LGM2605 acts as an antioxidant as shown previously by us and other investigators [16, 33, 34]. The autoxidation of the probe APF was similar in the presence of H2O2 alone without MPO and at different concentrations of LGM2605. The data in Figure 1A has been corrected for APF autoxidation. In preliminary experiments, we observed that the fluorescence of the probe did not increase in the presence of MPO alone without H2O2, indicating that either an MPO reaction intermediate or a product is responsible for the increase in fluorescence.
Figure 1. LGM2605 Inhibits Human Myeloperoxidase Activity.

Figure 1A shows the effect of LGM2605 on human MPO activity. Linear time-course of the MPO activity is shown in presence of 25, 50, and 100 μM LGM2605. Figure 1B shows the effect of LGM2605 on MPO activity expressed as % of MPO by control, which was calculated based on the linear part of the curve to determine the inhibitory effect of the compound. All samples were run in duplicates. The data are presented as mean ± SEM. * (p<0.05), *** (p<0.001), and **** (p<0.0001) indicate a statistically significant difference as compared to control.
3.2. LGM2605 Dose-Dependently Inhibits Myeloperoxidase Activity of Elicited Mouse Macrophages
In order to determine the effect of LGM2605 on MPO activity of mouse macrophages, mice were injected with thioglycollate and mouse macrophages were isolated. Figure 2A shows the release of MPO from mouse macrophages as determined by ELISA. Figure 2B shows a time-course of mouse macrophage MPO activity in the presence of LGM2605. LGM2605 at 10, 50, and 100 μM significantly (p<0.05) inhibited MPO activity. The degree of inhibition achieved at 10 μM was similar to that at 50 and 100 μM.
Figure 2. LGM2605 Inhibits Myeloperoxidase Activity of Elicited Mouse Macrophages.

Figure 2A shows the presence of MPO in mouse macrophages as determined by ELISA using mouse MPO antibody. Figure 2B shows the effect of LGM2605 on the activity of MPO from elicited mouse macrophages. The time-course of mouse macrophage MPO activity is shown in the presence of 5, 10, 50 and 100 μM LGM2605. Figure 2C shows the effect of LGM2605 on MPO activity from elicited mouse macrophages as % of control. All samples were run in triplicates. The data are presented as mean ± SEM. *** (p<0.001), and **** (p<0.0001) indicate a statistically significant difference as compared to control.
Figure 2C shows the effect of LGM2605 on MPO activity. LGM2605 at 10 μM significantly (p<0.05) decreased the MPO activity. The % inhibition at 50 and 100 μM LGM2605 was approximately 50% and not significantly higher than at 10 μM.
3.3. LGM2605 Dose-Dependently Inhibits Myeloperoxidase Activity of Elicited Mouse Neutrophils
In order to determine the effect of LGM2605 on MPO activity of mouse neutrophils, mice were injected with thioglycollate and mouse neutrophils were isolated. Supplemental Figure 2A shows the effect of LGM26505 on MPO activity as percent of control, using the fluorescent probe APF. LGM2605 significantly (p<0.01) decreased MPO activity at concentrations greater than 10 μM. Supplemental Figure 2B shows the effect of LGM2605 on MPO activity, using taurine chloramine determination. LGM2605 significantly (p<0.05) decreased MPO activity at 10 and 100 μM by approximately 50%.
3.4. LGM2605 Inhibits Myeloperoxidase Activity of RAW 264.7 Murine Macrophages
We next performed experiments to determine the effect of LGM2605 on MPO obtained from a murine macrophage cell line (RAW 264.7). Macrophages were exposed to LPS ± PMA for 5 min, and the supernatant analyzed using the MPO assay. Figure 3A shows a time-course of MPO activity in LPS-stimulated RAW 264.7 cells in the presence and absence of 100 μM LGM2605. LGM2605 significantly decreased macrophage MPO activity at 50 (p<0.01) and 60 minutes (p<0.001).
Figure 3. LGM2605 Inhibits Myeloperoxidase Activity of RAW 264.7 Murine Macrophages.

Figure 3A shows the effect of LGM2605 on activity of MPO from RAW murine macrophages treated with LPS. The time-course of RAW 264.7 macrophage MPO activity is shown in the presence of 100 μM LGM2605. Figure 3B shows the effect of LGM2605 on MPO activity from RAW macrophages as % of control. All samples were run in triplicates. Similarly, Figures 3C and 3D show the effect of LGM2605 on MPO activity from RAW murine macrophages treated with both LPS and PMA. The data are presented as mean ± SEM. * (p<0.05), ** (p<0.01), *** (p<0.001), and **** (p<0.0001) indicate a statistically significant difference as compared to control.
Figure 3B shows the effect of LGM2605 on MPO. LGM2605 significantly (p<0.05) decreased the MPO activity to approximately 50%. Similarly, Figures 3C and 3D show the inhibitory effect of LGM2605 on MPO activity of RAW264.7 macrophages treated with both LPS and PMA.
3.5. LGM2605 Inhibits Myeloperoxidase Activity of Peroxidase and Chlorination Cycles (Inhibition of Activity in the Absence and Presence of Cl−)
In order to elucidate the mechanism of inhibition of LGM2605 on MPO activity, the effect of LGM2605 on human leukocyte MPO was performed in the absence of Cl−. This is required for only the peroxidase cycle to run without chlorination. Figure 4A shows a LGM2605 concentration–dependent decrease in MPO activity in presence of 10, 25, 50 and 100 μM LGM2605. LGM2605 significantly (p<0.0001) decreased human MPO activity at 25, 50 and 100 μM. Figure 4A also shows the effect of LGM2605 on MPO activity as % of control. The maximum inhibition was achieved at 50 and 100 μM. These results demonstrate that LGM2605 inhibits the peroxidase cycle of the MPO reaction by as much as 70-75%.
Figure 4. LGM2605 Inhibits Myeloperoxidase Activity of Both Peroxidase and Chlorination Cycles.

Figure 4A shows the absolute and relative effect of LGM2605 on peroxidase cycle of human MPO. The assay was performed in the absence of chloride. Figure 4B shows the absolute and relative effect of LGM2605 on chlorination cycle of MPO by determining chlorination of taurine. All samples were run in triplicates. The data are presented as mean value ± SEM. ** (p<0.01) and **** (p<0.0001) indicate a statistically significant difference as compared to control.
The effect of LGM2605 on human leukocyte MPO was also performed in the presence of Cl− (required for the chlorination cycle to run) and the formation of taurine chloramine was determined. Figure 4B shows that LGM2605 significantly (p<0.01) inhibits the MPO activity in the presence of 100 μM LGM2605 as determined by chlorination of taurine. The inhibition is approximately 50% in this range of concentrations (Figure 4B) and indicates that LGM2605 inhibits the chlorination cycle of the MPO reaction. These results demonstrate that LGM2605 inhibits both the peroxidase and chlorination cycles of MPO reactions.
3.6. LGM2605 Interferes with H2O2 Binding Sites of Myeloperoxidase
In order to elucidate the mechanism of inhibition of LGM2605 on MPO active sites for H2O2 and Cl− the kinetics of the effect of LGM2605 on human leukocyte MPO was performed in the absence of Cl− so that the reaction does not proceed to the chlorination cycle. Figures 5A and 5B show that LGM2605 (25 μM) significantly (p<0.05) inhibits MPO activity at various time points during the time-course and at various H2O2 concentration (2.5-50 μM). In addition, in Supplemental Figure 4, we show how the concentration-dependent inhibition of MPO by 10, 25, 50 and 100 μM LGM2605 led to an average decrease of MPO activity by 21.2%, 47.5%, 59.4%, and 62.0%, respectively. In Figure 5B, we observe a sudden increase of MPO activity beyond 10 μM H2O2 which could be due to several possible reasons: 1) in the absence of Cl−, H2O2-driven reactions become purely peroxidation reactions and the kinetics of that response (such as the sigmoidal curve we observed) are different from classical kinetics for enzymes such as kinases, phosphatases and proteases; 2) the apparent sudden increase of MPO activation at >10 μM H2O2 may reflect the cooperativity effect whereby binding of one molecule may facilitate the binding of the next; 3) the fluorogen Amplex Red, a small molecule, whose structure resembles to some extent quercetin, an MPO inhibitor (Supplemental Figures 1D and 1B, respectively), can compete with H2O2 at the active site of the enzyme until H2O2 concertation stands out above the threshold.
Figure 5. LGM2605 Interferes with the H2O2 Active Site of Myeloperoxidase.
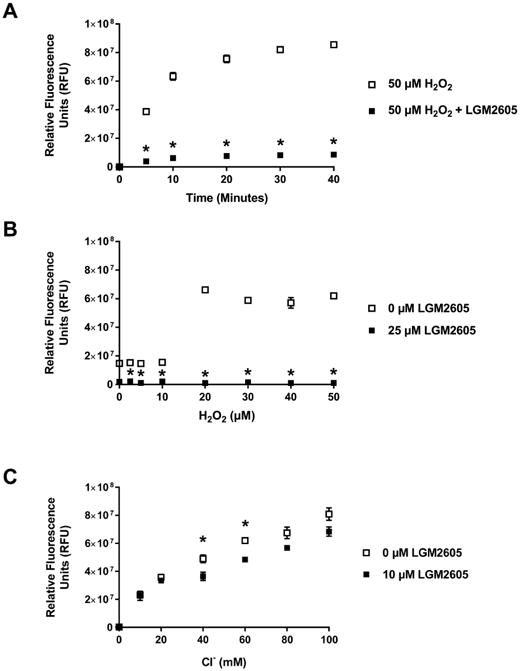
Figure 5A and Figure 5B show the effect of LGM2605 on peroxidase cycle of human MPO (neutrophils) with time (up to 40 minutes) and at H2O2 concentration ranging from 2.5-50 μM (at 15 minutes), respectively. The assay was performed in the absence of chloride as in Figure 4. Figure 5C shows the effect of LGM2605 on chlorination cycle of MPO at Cl− concentrations ranging from 10 -100 mM (at 10 minutes). All samples were run in triplicates. All data points have been adjusted by subtracting the average value for 0 mM chloride, 0 μM LGM2605 at time 0. The data are presented as mean value ± SEM. * (p<0.05) indicates a statistically significant difference as compared to control.
The significant (p<0.05) decrease in MPO activity at various H2O2 concentrations indicates that LGM2605 interferes with H2O2 binding site of MPO. The rate of reaction at increasing concentrations of H2O2 is presented in Supplemental Figure 3. The results show a 98.46% decrease in Vmax in the presence of 25 μM LGM2605 (Vmax = 6.41 ± 1.28 × 106 RFUs without LGM2605 compared to 9.90 ± 2.12 × 104 RFUs with LGM2605). In addition, in the presence LGM2605 near flattening of the activation slope shows absence of H2O2 dependent activation of MPO indicating an infinite increase in Km, reflecting a complete loss of affinity of MPO active site for H2O2. These results indicate LGM2605 interferes with H2O2 binding to its active site. Figure 5C shows that LGM2605 did not affect the Cl− concentration (10-100 mM)-dependent increase in MPO activity indicating that potentially LGM2605 does not interfere with Cl− access to the active site. Taken together, these results demonstrate that LGM2605 interferes with the peroxidase reaction perhaps by interfering with H2O2 access, but not Cl− access to the active site.
3.7. LGM2605 Decreases [Fe (IV)=O] Oxidation State of Iron in the Heme Pocket of MPO (EPR Analysis)
High resolution low temperature EPR is a novel and highly sensitive methodology to directly detect substrate/inhibitor bindings to the paramagnetic iron in the heme pocket of MPO [35, 36]. To gain insight on the LGM2605 protection mechanism, we further investigated the effects of LGM2605 on the heme center covalently linked to MPO by EPR spectroscopy. First, the EPR spectrum of the native state of MPO in a pH 7.4 buffer was measured at 4K (black trace, Figure 6A). The spectrum showed a typical high-spin rhombic Fe3+ spectrum with gz = ∼6.84, gy = ∼5.05, and gx = ∼2.00 and a small amount of non-heme Fe3+ with g = ∼4.3. However, in the presence of either H2O2 or LGM2605, the new signals at g = 3.63 (and 3.97) appeared together with the broad increase in the region between g = 4.5 and 6, which overlapped the native EPR spectrum without causing any shift or change in intensity of the native MPO signals (red and green traces, Figure 6A). The new g = 3.63 signal likely arises from the low-spin ferric species in MPO, partially converted by the interaction with H2O2 or LGM2605 molecules. Interestingly, however, when LGM2605 was added prior to the exposure to H2O2, the EPR spectrum became almost identical to the native MPO (blue trace, Figure 6A), albeit there are some remaining signals by the effect of either H2O2 or LGM2605. These data strongly suggest that both LGM2605 and H2O2 could enter inside the heme pocket, interact with the heme iron with a similar affinity, compete each other, and cancel out their effects on the heme center in MPO.
Figure 6. X-band EPR Spectra of MPO Purified from Human Leukocytes in the Broad Magnetic Field (800-4000 G) at 4.2 K (A) and in the g = ∼2.0 Region (3250-3450 G) at 30 K (B).

Black trace: Native untreated MPO. Red trace: MPO at 5 sec after the addition of H2O2 (60 μM). Blue trace: MPO incubated with LGM2605 (500 μM) for 5 min prior to the H2O2 addition. Green trace: MPO incubated with LGM2605. MPO concentrations are 1 mg/ml in all conditions. EPR conditions: microwave frequency, 9.45 GHz; modulation frequency, 100 kHz; microwave power was 10 mW for (A) and 0.05 mW for (B); modulation amplitude was 10 G for (A) and 8 G for (B); time constant, 82 ms.
Next, we decided to look at the free radical region (g = ∼2) of the EPR spectra to investigate the inhibitory effect of LGM2605 on the formation of Compound I [36]. Since the inhibitory effect of LGM2605 was observed in presence as well as in the absence of Cl− demonstrating that both chlorination and peroxidase cycles of MPO are inhibited by LGM2605, we hypothesized that LGM2605 interacts with MPO, and suppresses the formation of Compound I. Compound I is the first reaction intermediate of MPO and H2O2, and mediates both one- and two-electron oxidation reactions, thus, it could be involved in both chlorination and peroxidase reactions of MPO. In the native MPO enzyme, only a small background free radical signal was detected (black trace), while this background signal became smaller in the presence of LGM2605 (green trace, Figure 6B). In contrast, after the exposure to H2O2, the free radical signal became about 5.46 times larger (red trace, Figure 6B). But, this signal decreased by 75% in the sample that received prior incubation with LGM2605 (blue trace, Figure 6B). This result clearly indicates that in the presence of LGM2605 the amount of Compound I dramatically decreases. One reason for this decrease can be the prevention of H2O2 access into the heme pocket of the native MPO. Another reason for this decrease can be free radical scavenging by LGM2605 (Compound I is a radical). It is important to note that no signals of the reduced (ferrous) MPO Fe (II) (Scheme 1) were detected in the EPR spectra.
3.8. LGM2605 Inhibits MPO by Binding to the Active Site of the Enzyme
Given the biophysical EPR binding data that shows that LGM2605 binds in the active site in such a way that it affects paramagnetic Fe(IV)=O signals, we next asked if computational docking of LGM2605 could help us begin to characterize possible binding poses for LGM2605. The chemical structures of SDG (LGM2605) and quercetin used for computational docking are shown in Supplemental Figures 1A and 1B. Docking LGM2605 to the recently published structure of MPO (PDB code 5MFA) yielded a top pose shown in Figure 7 (Panels A and B). To compare to previously-published docking, Quercetin was also docked to MPO using the same parameters and the top pose is shown in Figure 7 (Panels C and D). The top pose for quercetin yielded a similar pose to that found in previous studies [30] and a score of -8.72 kcal/mole. Docking of LGM2605 resulted in a similar position close to the heme group in the active site and a comparable score of -8.2 kcal/mole. Five of the 10 LGM2605 poses were part of this same active site cluster, and the top pose shown includes a hydrogen bond contact between MPO active site residue R239 and a hydroxyl group in LGM2605. Additional hydrogen bonds observed in the top pose include a bond to E116, and another hydrogen bond between an LGM2605 hydroxyl group to a propionate in the heme group. While this pose may not directly displace H2O2 binding, it's blocking of the substrate channel might slow H2O2 access to the heme. In future work, more comprehensive pose prediction with clustering of large numbers of poses will lay the foundation for the structure activity relationship experiments, which will focus on optimization of LGM2605 derivatives for superior inhibition of MPO.
Figure 7. Computational Docking of SDG to MPO's Active Heme Site.

The top two panels (Panels A and B) portray the top pose of SDG docked to MPO (PDB code 5MFA), with Panel A showing the entire MPO protein and the search space box in green. Protein alpha helices are shown in orange, coil in gray, and beta strands in light blue. The heme group is portrayed as large spheres, and the SDG molecule as ball-and-stick representation with carbons colored purple, and oxygens as red, and the large green sphere represents the calcium ion near the active site. Active site residues H95 and R239 that are important in heme binding are shown as stick representation and labeled in green. Potential hydrogen bonds to R239 and E116 are shown as yellow springs (a third to a heme oxygen is hard to see in this view). The bottom two panels (Panels C and D) show the docking of quercetin (carbons show in magenta), and other coloring and labeling as above.
For SDG:
H-bonds (donor, acceptor, hydrogen, D..A dist, D-H..A dist):
#2 ARG239.A NH1 #3.1 UNK 1 O9 #2 ARG 239.A HH11 3.346 2.651
#3.1 UNK 1 O12 #2 HEM 812.A O1A #3.1 UNK 1 H38 3.087 2.366
#3.1 UNK 1 O6 #2 GLU 116.A OE2 #3.1 UNK 1 H26 3.087 2.309
3 hydrogen bonds found
For Quercetin:
H-bonds (donor, acceptor, hydrogen, D..A dist, D-H..A dist):
#2 ARG 405.A NH1 #3.1 UNK 1.A O7 #2 ARG 405.A HH11 2.851 2.154
1 hydrogen bond found
4. Discussion
The results of the present study provide evidence that LGM2605 inhibits MPO activity based on the following: i) LGM2605, a known lignan antioxidant and free radical scavenger, inhibited the activity of human leukocyte (neutrophils) myeloperoxidase; ii) LGM2605 inhibited the activity of myeloperoxidase from mouse macrophages; iii) LGM2605 also inhibited myeloperoxidase from murine RAW 264.7 macrophages and iv) LGM2605 inhibited MPO in murine elicited neutrophils. Regarding mechanism, LGM2605 inhibited the H2O2-dependent MPO activity by interfering with H2O2 access to the active site of MPO without affecting Cl− access; LGM2605 can also scavenge radical Compound I. Thus, LGM2605 inhibits the peroxidase and chlorination cycles by suppressing the formation of Compound I as demonstrated by EPR. Computational docking simulation confirmed an energetically favorable binding of LGM2605 to MPO's active site, providing further evidence that this agent could bind proximal to the active site in a position that would interfere with MPO enzymatic function. These results demonstrate the MPO inhibitory characteristic of LGM2605 in three different biological systems and identify its potential mechanism and site of action.
In our previous studies, using a mouse model of thoracic radiation damage, we have established the protection in lung tissue by whole grain dietary flaxseed [37, 38], a grain rich in lignan polyphenols, as well as of flaxseed lignan formulations enriched in SDG [39, 40]. These studies emphasized the protective and mitigating properties of the lignan SDG against radiation-induced lung tissue damage in vivo. SDG, purified from flaxseed or synthetic, is a potent antioxidant in vitro as well as in vivo [33, 34, 41]. In order to explore the therapeutic potential of SDG we have synthesized SDG by a novel chemical reaction and determined its antioxidant properties by assessing their reducing power, metal chelating potential, and free radical scavenging activity for •OH, peroxyl and DPPH radicals [16]. We also demonstrated that synthetic LGM2605 prevents radiation-induced DNA damage by assessing the potential for preventing γ-radiation-induced damage to plasmid DNA (pBR322) and calf thymus DNA [17].
We have shown that the maximum protection of genomic DNA by LGM2605 against radiation is achieved at approximately one tenth the concentration of the EC50 values for their free radical scavenging and antioxidant effects, typically in the range of 130-200 μM [16, 34]. We have shown that the protection of genomic DNA by LGM2605 against radiation [17] is due in part to scavenging of damaging ACS, including ClO− [21]. The results of the present study showing that LGM2605 inhibits human MPO, indicating that LGM2605 is protective by inhibiting MPO as well as by scavenging HOCl in biological systems.
LGM2605, by inhibiting MPO, will decrease generation of HOCl that causes chlorination and oxidation of nucleobases and proteins. In addition, we have shown that LGM2605 scavenges HOCl as well as •OH free radicals that produce ACS by reacting with chloride ions. Thus, LGM2605 can decrease HOCl generation by MPO-mediated enzymatic pathway as well as •OH-mediated non-enzymatic pathway. In addition, LGM2605, by associating with DNA base pairs, can protect DNA like several flavonoids such as luteolin, kempferol and quercetin [42-44]. Therefore, LGM2605 as an inhibitor of MPO, scavenger of HOCl as well as being an antioxidant and free radical scavenger, can function as a radioprotector and potentially a radiation mitigator.
MPO is expressed in hematopoietic cells and located in azurophil (primary) granules [45, 46]. In neutrophils, MPO makes from 2-5% of total cellular protein or 2-4 μg per million cells [47, 48]. In phagosomes, MPO is present as a peroxidase along with NADPH oxidase as a source for H2O2. Activated neutrophils produce H2O2 and superoxide anion O2•-. In addition, cystic fibrosis transmembrane conductance regulator transports Cl− from the cytoplasm (high in Cl− concentration) to the phagosomal lumen [49, 50]. The MPO-H2O2-Cl− system generates the potent antimicrobial oxidant hypochlorous acid/ hypochlorite (HOCl/ OCl−, pKa 7.53). However, increased and persistent activation of MPO-H2O2-Cl− system may lead to tissue damage by modifying lipids, nucleobases of DNA and proteins by chlorination and oxidative reactions.
Hypochlorite produced by myeloperoxidase from activated neutrophils using hydrogen peroxide generated by NADPH oxidase and chloride ions as substrates [51, 52] can cause genotoxicity by modifying nucleobases. Chlorinated nucleosides have been identified and linked to inflammation and cancer [53]. HOCl and its conjugate base ClO− oxidize amino acids, peptides, proteins and lipids [54-57]; and chlorinate nuclear bases in cellular DNA and RNA [54, 58, 59]. Therefore, it is important to develop therapeutic intervention strategies for: 1) inhibiting MPO at different levels, and 2) scavenging HOCl to prevent initiation and propagation of inflammatory diseases. As an inhibitor of MPO, LGM2605 could be a potential drug for a number of inflammatory and immunological diseases.
Developing MPO inhibitors is an attractive strategy to block tissue damage associated with inflammation. However, the main issue with the development of a therapeutic MPO inhibitor is that highly potent and irreversible inhibitors could harm the physiological role of MPO in innate host defenses. In addition, a natural, non-toxic substance would be desired that can be obtained in large quantities with high purity without batch to batch variations. Some flavonoids, based on epidemiological studies, have been suspected to prevent or ameliorate several diseases including lowering the risk for frequency of cardiovascular diseases and cancer [60-62]. Quercetin was found to have a strong inhibitory effect on MPO catalyzed oxidation [63, 64]. It is also the strongest inhibitor of MPO catalyzed dityrosine formation, followed by >luteolin >fisetin >taxifolin >kaemferol [30]. However, the strongest inhibitor quercetin and several radioprotective agents have been linked to adverse side effects, high toxicities and unwanted morbidities [65-69].
There could be several potential mechanisms by which LGM2605 may inhibit MPO: 1) by preventing the oxidation of the native MPO to oxidized MPO intermediate, Compound I; 2) by converting the oxidized MPO intermediate, Compound I, to native state by two-electron as well as by one-electron reduction; 3) by interacting with oxidized MPO, LGM2605 can interrupt the MPO cycles of chlorination and peroxidation reactions. There could be several reasons for the varying inhibition of MPO by LGM2605 (50-85%) in the different enzyme and cell systems we investigated: 1) The interaction of LGM2605 on sites other than the H2O2 active site may affect its interaction with the active site. 2) The redox state of the active site as well as that of LGM2605. 3) Based on our preliminary work, MPO activity is potentially regulated by additional mechanisms such as phosphorylation/dephosphorylation.
In Scheme 1 we propose a potential mechanism of MPO inhibition by LGM2605 by a combination of both preventing H2O2 access to the active center of the native MPO Fe (III) and chemically reducing the oxidized MPO forms. Specifically, we propose that LGM2605 (*AH2 in Scheme 1) inhibits MPO activity by: 1) blocking the H2O2 biding site of the native MPO; 2) reducing (a two-electron reduction) Compound I, the oxidized MPO intermediate (Fe(IV)=O), to native MPO (Scheme 1, reaction 4 with *AH2); 3) reducing (single-electron reduction [70-72]) Compound I to Compound II (Scheme 1, reaction 5 with *AH2) and subsequently to the native MPO enzyme Fe(III) (Scheme 1, reaction 6 with *AH2). A possible reduction of the native ferric MPO Fe (III) to the reduced (ferrous) MPO Fe (II) (Scheme 1, reaction 7) was not validated by the conducted experiments; no signal of the reduced MPO was detected by EPR spectroscopy. Thus, we conclude that the MPO reactions are seized at the Compound I intermediate stage and both chlorination and peroxidase cycles are suppressed.
High resolution low temperature EPR spectroscopy has been a major tool to characterize paramagnetic metal centers including hemes and has provided valuable information on reactive radical intermediates to understand the underlying reaction mechanism of redox enzymes. Therefore, we applied this novel technique to further investigate the inhibitory activity of LGM2605 toward MPO. The EPR study successfully led to unexpected new findings about LGM2605: LGM2605 reversibly interacts with the heme center in MPO and affects its spin state of Fe3+ to a certain extent. As shown in Figure 6A, we clearly detected the appearance of the new EPR signal at g = 3.63 from MPO in the presence of LGM2605. This signal is undoubtedly assigned to the low spin state of the heme. The similar low spin signals have been attributed to the b cytochromes of mitochondrial complex III [73] and cytochrome b6 in cytochrome b6f-complex from chloroplasts [74-77]. In cytochrome b6f-complex, both high spin (g = 6) and low spin (g = 3.6 and 3.85) signals have been detected and assigned to cytochrome b6, the intensities of their signals were varied depending on the reduction states and the freshness of the enzyme [74, 76, 77].
In fact, the appearance of the high spin signal at g = 6 is correlated with the loss of the enzyme activity and the high spin state indicates the loss of the sixth coordinate to the heme [74, 76, 77]. The EPR data suggest that likely the opposite phenomenon is occurring in the presence of LGM2605, allowing the speculation that LGM2605 facilitates the conversion from five to six coordinate. We observed similar changes in the EPR spectrum from MPO reacted with H2O2, but none in the presence of both LGM2605 and H2O2. This suggests that LGM2605 likely competes with H2O2 and prevents H2O2 from entering into the heme pocket leading to the suppression of the Compound I formation.
We originally thought that the scavenging action of LGM2605 on MPO-catalyzed generation of HOCl is similar to a general antioxidant effect. Since it is known that Compound I (generated by the reaction of Ferric MPO with hydrogen peroxide) oxidizes halides to produce hypohalous acid, we planned to investigate whether the amount of Compound I would be affected in the presence of LGM2605 in the reaction mixture. Therefore, we chose the EPR method, because only Compound I gives an EPR-detectable a porphyrin π-cation radical (g = ∼2.00). Thus, we can avoid any involvement of the EPR-silent Compound II and directly monitor changes in Compound I. During the course of our EPR experiments, to our surprise, we noticed that LGM2605 directly interacts with heme iron of MPO, when analyzing EPR signals.
Given the biophysical EPR binding data that show that LGM2605 binds near the active site, it follows that computational docking of SDG (LGM2605) would yield possible binding poses for SDG in the same location. Docking SDG was also compared to previously-published docking of quercetin using the same parameters. The top pose for quercetin yielded a similar pose to that found in previous studies [30], and the docking of SDG resulted in a similar position (Figure 7- upper panel, close to the heme group in the active site) with a comparable score to that of quercetin. Three possible hydrogen bonds between SDG atoms and MPO side chains (R239, and E116) or heme atoms demonstrate that this top pose holds the potential for drug specificity. In future work, more comprehensive pose prediction with clustering of large numbers of poses will lay the foundation for the structure activity relationship experiments which will permit lead optimization of SDG (LGM2605) derivatives for superior inhibition of MPO. While SDG is highly soluble (xLogP3-AA of -0.7), it is a very flexible molecule with 15 rotatable bonds. Lead optimization that targets specific active site side chains could improve the mechanism of this class of MPO inhibitors. However, inhibition of MPO activity by SDG may also depend on the phosphorylation of amino acid residues in the MPO protein under in vivo physiological as well as pathological conditions.
Regarding amino acids which are important for the binding of aromatic substrates/inhibitors in the active site of MPO, we compared our findings with those by Gau et al. where they show interaction of flavonoids with amino acids of the substrate channel near the active center of MPO [78]. Specifically, drug HX1 in the Gau et al. study is shown to be in a pose much more intimately binding to the heme than our current top pose of LGM2605. This crystal structure with the HX1 drug actually shows that the drug might well displace H2O2 binding. Quercetin and this LGM2605 pose are not as close. This is not to say that there are not better poses to be discovered. However, a deeper docking investigation of LGM2605 and its derivatives is the subject of future studies, and beyond the scope of this manuscript.
The inhibitory effect of LGM2605 on MPO activity was evaluated in various MPO enzyme systems and MPO-containing cell types. We observed differences in the max inhibition (ranging from 50%-80%) of MPO activity depending on the MPO enzyme system and MPO-containing cell type evaluated. For example, the IC50 value for LGM2605 on MPO activity was 63.47 μM when tested on human MPO, 88.97 μM when tested on MPO from elicited murine macrophages, and 106.34 μM when tested on MPO from RAW 264.7 murine macrophages (Supplemental Table 1). The variation in the IC50 values may represent the various cell types (murine macrophages and neutrophils, and human enzyme commercial preparation), enzyme preparations, and the level of enzyme purification, in addition to the various conditions under which the inhibitory effect of LGM2605 on MPO activity was measured.
In addition to blocking the H2O2 binding site of the native MPO, LGM2605 can scavenge Compound I by a radical mechanism. The LGM2605 molecule contains 4 labile benzyl hydrogen atoms (Supplemental Figure 1A) that can be captured by the radical Compound I. In our previous publications we demonstrated that LGM2605 scavenge free radicals [16] and chlorine atoms [21]. A possible mechanism of the Compound I scavenging by LGM2605 is hydrogen atom capture.
In summary, we have demonstrated that LGM2605 inhibits the activity of MPO from human leukocytes (neutrophils), the elicited mouse macrophages and the murine RAW 264.7 macrophages. Using high resolution low temperature EPR spectroscopy we successfully demonstrated that LGM2605 disrupts both the peroxidase and chlorination cycles of MPO by drastically suppressing the formation of the highly reactive intermediate Compound I, which is critical to both the cycles. Our findings strongly support that LGM2605 would be an ideal candidate for the development as a potential compound to inhibit and control MPO activities to prevent a number of inflammatory diseases and lung tissue damage associated with radiation therapy in cancer patients or in normal population following accidental radiation exposure.
Supplementary Material
Summary.
This study characterizes the ability of synthetic SDG (LGM2605) to inhibit myeloperoxidase (MPO), the key enzyme in inflammatory cells that generates hypochlorite (HOCl), a potent cytotoxic oxidant that contributes to tissue damage during inflammation. Using electron paramagnetic resonance (EPR), we demonstrate that LGM2605 suppressed the formation of Compound I, an oxoiron (IV) intermediate [Fe(IV) = O] containing a porphyrin π-radical, of the catalytic cycle of MPO. Computational results suggest that SDG (LGM2605) can act as an inhibitor by binding at the active site of MPO. This study identifies a novel mechanism of LGM2605 action as an inhibitor of MPO and highlights LGM2605 as a promising potential attenuator of oxidant-dependent inflammatory tissue damage.
Highlights.
Synthetic SDG (LGM2605) inhibits myeloperoxidase (MPO) activity in murine inflammatory cells and human MPO.
We demonstrate that LGM2605 directly interferes with Compound I an oxoiron (IV) intermediate [Fe(IV) = O] containing a porphyrin π-radical, on the catalytic cycle of MPO
SDG inhibits MPO by interacting at the active site of MPO decreasing damaging HOCl formation.
This study identified MPO inhibition as a novel mechanism of LGM2605 action associated with the known anti-inflammatory properties of this agent.
LGM2605 is a promising potential attenuator of oxidant-dependent inflammatory tissue damage.
Acknowledgments
Funding: This work was funded in part by: NIH-R01 CA133470 (MCS), NIH-1R21AT008291-01A1 (MCS), NIH-R03 CA180548 (MCS), 1P42ES023720-01 (MCS) and by pilot project support from 1P30 ES013508-02 awarded to MCS (its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH). MA and the Fox Chase Cancer Center Molecular Modelling is supported by P30 CA006927. The authors would like to thank Dr. Edward J. Delikatny for critical review of the manuscript.
Abbreviations
- •OH
hydroxyl radical
- ACS
active chlorine species
- APF
3′-(p-aminophenyl) fluorescein
- ClŌ
hypochlorite anion
- ELISA
enzyme-linked immunosorbent assay
- EPR
electron paramagnetic resonance
- H2O2
hydrogen peroxide
- HOCl
hypochlorous acid
- MPO
myeloperoxidase
- LGM2605
synthetic SDG
- LPS
lipopolysaccharide
- PBS
phosphate-buffered saline
- PMA
phorbol 12-myristate 13-acetate
- RFU
relative fluorescence units
- ROS
reactive oxygen species
- SDG
secoisolariciresinol diglucoside
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lazarevic-Pasti T, Leskovac A, Vasic V. Myeloperoxidase Inhibitors as Potential Drugs. Curr Drug Metab. 2015;16:168–190. doi: 10.2174/138920021603150812120640. [DOI] [PubMed] [Google Scholar]
- 2.Nussbaum C, Klinke A, Adam M, Baldus S, Sperandio M. Myeloperoxidase: a leukocyte-derived protagonist of inflammation and cardiovascular disease. Antioxid Redox Signal. 2013;18:692–713. doi: 10.1089/ars.2012.4783. [DOI] [PubMed] [Google Scholar]
- 3.Davies MJ. Myeloperoxidase-derived oxidation: mechanisms of biological damage and its prevention. J Clin Biochem Nutr. 2011;48:8–19. doi: 10.3164/jcbn.11-006FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pattison DI, Davies MJ, Hawkins CL. Reactions and reactivity of myeloperoxidase-derived oxidants: differential biological effects of hypochlorous and hypothiocyanous acids. Free Radic Res. 2012;46:975–995. doi: 10.3109/10715762.2012.667566. [DOI] [PubMed] [Google Scholar]
- 5.Malle E, Furtmuller PG, Sattler W, Obinger C. Myeloperoxidase: a target for new drug development? Br J Pharmacol. 2007;152:838–854. doi: 10.1038/sj.bjp.0707358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeitner T, Lawrence D. Pulmonary autoimmunity and inflammation. In: Cohen MD, Zelikoff JT, Schlesinger RB, editors. Pulmonary Immunotoxicology. Kluwer Academic Publishers; New York: 2000. pp. 153–179. [Google Scholar]
- 7.Nauseef WM. Myeloperoxidase in human neutrophil host defence. Cell Microbiol. 2014;16:1146–1155. doi: 10.1111/cmi.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 9.Bernofsky C. Nucleotide chloramines and neutrophil-mediated cytotoxicity. FASEB J. 1991;5:295–300. doi: 10.1096/fasebj.5.3.1848195. [DOI] [PubMed] [Google Scholar]
- 10.Bernofsky C, Bandara BMR, Hinojosa O, Strauss SL. Hypochlorite-modified adenine nucleotides: structure, spin-trapping, and formation by activated guinea pig polymorphonuclear leukocytes. Free Radical Res Commun. 1990;9:303–315. doi: 10.3109/10715769009145689. [DOI] [PubMed] [Google Scholar]
- 11.Henderson JP, Byun J, Heinecke JW. Chlorination of nucleobases, RNA and DNA by myeloperoxidase: a pathway for cytotoxicity and mutagenesis by activated phagocytes. Redox Rep. 1999;4:319–320. doi: 10.1179/135100099101535025. [DOI] [PubMed] [Google Scholar]
- 12.Arnhold J, Flemmig J. Human myeloperoxidase in innate and acquired immunity. Arch Biochem Biophys. 2010;500:92–106. doi: 10.1016/j.abb.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Kettle AJ, Gedye CA, Winterbourn CC. Mechanism of inactivation of myeloperoxidase by 4-aminobenzoic acid hydrazide. Biochem J. 1997;321(Pt 2):503–508. doi: 10.1042/bj3210503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furtmuller PG, Zederbauer M, Jantschko W, Helm J, Bogner M, Jakopitsch C, Obinger C. Active site structure and catalytic mechanisms of human peroxidases. Arch Biochem Biophys. 2006;445:199–213. doi: 10.1016/j.abb.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Zamocky M, Jakopitsch C, Furtmuller PG, Dunand C, Obinger C. The peroxidase-cyclooxygenase superfamily: Reconstructed evolution of critical enzymes of the innate immune system. Proteins. 2008;72:589–605. doi: 10.1002/prot.21950. [DOI] [PubMed] [Google Scholar]
- 16.Mishra OP, Simmons N, Tyagi S, Pietrofesa R, Shuvaev VV, Valiulin RA, Heretsch P, Nicolaou KC, Christofidou-Solomidou M. Synthesis and antioxidant evaluation of (S,S)- and (R,R)-secoisolariciresinol diglucosides (SDGs) Bioorg Med Chem Lett. 2013;23:5325–5328. doi: 10.1016/j.bmcl.2013.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishra OP, Pietrofesa R, Christofidou-Solomidou M. Novel synthetic (S,S) and (R,R)-secoisolariciresinol diglucosides (SDGs) protect naked plasmid and genomic DNA from gamma radiation damage. Radiat Res. 2014;182:102–110. doi: 10.1667/RR13635.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexandrova M, Rozhko T, Vydryakova G, Kudryasheva N. Effect of americium-241 on luminous bacteria. Role of peroxides. J Environ Radioact. 2011;102:407–411. doi: 10.1016/j.jenvrad.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Kudryasheva NS, Rozhko TV. Effect of low-dose ionizing radiation on luminous marine bacteria: radiation hormesis and toxicity. J Environ Radioact. 2015;142:68–77. doi: 10.1016/j.jenvrad.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Petrova AS, Lukonina AA, Badun GA, Kudryasheva NS. Fluorescent coelenteramide-containing protein as a color bioindicator for low-dose radiation effects. Anal Bioanal Chem. 2017;409:4377–4381. doi: 10.1007/s00216-017-0404-9. [DOI] [PubMed] [Google Scholar]
- 21.Mishra OP, Popov AV, Pietrofesa RA, Christofidou-Solomidou M. Gamma-irradiation produces active chlorine species (ACS) in physiological solutions: Secoisolariciresinol diglucoside (SDG) scavenges ACS - A novel mechanism of DNA radioprotection. Biochim Biophys Acta, Gen Subj. 2016;1860:1884–1897. doi: 10.1016/j.bbagen.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallund J, Tetens I, Bugel S, Tholstrup T, Ferrari M, Teerlink T, Kjaer A, Wiinberg N. Daily consumption for six weeks of a lignan complex isolated from flaxseed does not affect endothelial function in healthy postmenopausal women. J Nutr. 2006;136:2314–2318. doi: 10.1093/jn/136.9.2314. [DOI] [PubMed] [Google Scholar]
- 23.Hallund J, Ravn-Haren G, Bugel S, Tholstrup T, Tetens I. A lignan complex isolated from flaxseed does not affect plasma lipid concentrations or antioxidant capacity in healthy postmenopausal women. J Nutr. 2006;136:112–116. doi: 10.1093/jn/136.1.112. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Wang X, Liu Y, Tian H, Flickinger B, Empie MW, Sun SZ. Dietary flaxseed lignan extract lowers plasma cholesterol and glucose concentrations in hypercholesterolaemic subjects. Br J Nutr. 2008;99:1301–1309. doi: 10.1017/S0007114507871649. [DOI] [PubMed] [Google Scholar]
- 25.Fukumitsu S, Aida K, Shimizu H, Toyoda K. Flaxseed lignan lowers blood cholesterol and decreases liver disease risk factors in moderately hypercholesterolemic men. Nutr Res. 2010;30:441–446. doi: 10.1016/j.nutres.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Alcorn J, Whiting S, Viveky N, Di Y, Mansell K, Fowler S, Thorpe L, Almousa A, Cheng PC, Jones J, Billinsky J, Hadjistavropoulos T. Protocol for a 24-Week Randomized Controlled Study of Once-Daily Oral Dose of Flax Lignan to Healthy Older Adults. JMIR Res Protoc. 2017;6:e14. doi: 10.2196/resprot.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aasa R, Vanngard T, Dunford HB. EPR studies on compound I of horseradish peroxidase. Biochim Biophys Acta, Enzymol. 1975;391:259–264. doi: 10.1016/0005-2744(75)90249-1. [DOI] [PubMed] [Google Scholar]
- 28.Svistunenko DA. Reaction of heme containing proteins and enzymes with hydroperoxides: The radical view. Biochim Biophys Acta, Bioenerg. 2005;1707:127–155. doi: 10.1016/j.bbabio.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Ohnishi ST, Shinzawa-Itoh K, Ohta K, Yoshikawa S, Ohnishi T. New insights into the superoxide generation sites in bovine heart NADH-ubiquinone oxidoreductase (Complex I): The significance of protein-associated ubiquinone and the dynamic shifting of generation sites between semiflavin and semiquinone radicals. Biochim Biophys Acta, Bioenerg. 2010;1797:1901–1909. doi: 10.1016/j.bbabio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Shiba Y, Kinoshita T, Chuman H, Taketani Y, Takeda E, Kato Y, Naito M, Kawabata K, Ishisaka A, Terao J, Kawai Y. Flavonoids as substrates and inhibitors of myeloperoxidase: molecular actions of aglycone and metabolites. Chem Res Toxicol. 2008;21:1600–1609. doi: 10.1021/tx8000835. [DOI] [PubMed] [Google Scholar]
- 31.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 33.Moree SS, Rajesha J. Investigation of in vitro and in vivo antioxidant potential of secoisolariciresinol diglucoside. Mol Cell Biochem. 2013;373:179–187. doi: 10.1007/s11010-012-1487-4. [DOI] [PubMed] [Google Scholar]
- 34.Moree SS, Khanum SA, Rajesha J. Secoisolariciresinol diglucoside - a phytoestrogen nutraceutical of flaxseed: synthesis and evaluation of antioxidant potency. Free Radicals Antioxid. 2011;1:31–38. [Google Scholar]
- 35.Chavali B, Masquelin T, Nilges MJ, Timm DE, Stout SL, Matter WF, Jin N, Jadhav PK, Deng GG. ESR and X-ray Structure Investigations on the Binding and Mechanism of Inhibition of the Native State of Myeloperoxidase with Low Molecular Weight Fragments. Appl Magn Reson. 2015;46:853–873. doi: 10.1007/s00723-015-0698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dolphin D, Forman A, Borg DC, Fajer J, Felton RH. Compounds I of catalase and horse radish peroxidase: pi-cation radicals. Proc Natl Acad Sci U S A. 1971;68:614–618. doi: 10.1073/pnas.68.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JC, Krochak R, Blouin A, Kanterakis S, Chatterjee S, Arguiri E, Vachani A, Solomides CC, Cengel KA, Christofidou-Solomidou M. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol Ther. 2009;8:47–53. doi: 10.4161/cbt.8.1.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christofidou-Solomidou M, Tyagi S, Tan KS, Hagan S, Pietrofesa R, Dukes F, Arguiri E, Heitjan DF, Solomides CC, Cengel KA. Dietary flaxseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice. BMC Cancer. 2011;11:269. doi: 10.1186/1471-2407-11-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christofidou-Solomidou M, Tyagi S, Pietrofesa R, Dukes F, Arguiri E, Turowski J, Grieshaber PA, Solomides CC, Cengel KA. Radioprotective role in lung of the flaxseed lignan complex enriched in the phenolic secoisolariciresinol diglucoside (SDG) Radiat Res. 2012;178:568–580. doi: 10.1667/RR2980.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pietrofesa R, Turowski J, Tyagi S, Dukes F, Arguiri E, Busch TM, Gallagher-Colombo SM, Solomides CC, Cengel KA, Christofidou-Solomidou M. Radiation mitigating properties of the lignan component in flaxseed. BMC Cancer. 2013;13:179. doi: 10.1186/1471-2407-13-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu C, Yuan YV, Kitts DD. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem Toxicol. 2007;45:2219–2227. doi: 10.1016/j.fct.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 42.Rusak G, Piantanida I, Masic L, Kapuralin K, Durgo K, Kopjar N. Spectrophotometric analysis of flavonoid-DNA interactions and DNA damaging/protecting and cytotoxic potential of flavonoids in human peripheral blood lymphocytes. Chem-Biol Interact. 2010;188:181–189. doi: 10.1016/j.cbi.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Zhang S, Ling B, Qu F, Sun X. Investigation on the interaction between luteolin and calf thymus DNA by spectroscopic techniques. Spectrochim Acta, Part A. 2012;97:521–525. doi: 10.1016/j.saa.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 44.Marinic M, Piantanida I, Rusak G, Zinic M. Interactions of quercetin and its lanthane complex with double stranded DNA/RNA and single stranded RNA: Spectrophotometric sensing of poly G. J Inorg Biochem. 2006;100:288–298. doi: 10.1016/j.jinorgbio.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Yamada M, Kurahashi K. Regulation of myeloperoxidase gene expression during differentiation of human myeloid leukemia HL-60 cells. J Biol Chem. 1984;259:3021–3025. [PubMed] [Google Scholar]
- 46.Koeffler HP, Ranyard J, Pertcheck M. Myeloperoxidase: its structure and expression during myeloid differentiation. Blood. 1985;65:484–491. [PubMed] [Google Scholar]
- 47.Schultz J, Kaminker K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys. 1962;96:465–467. doi: 10.1016/0003-9861(62)90321-1. [DOI] [PubMed] [Google Scholar]
- 48.Bos A, Wever R, Roos D. Characterization and quantification of the peroxidase in human monocytes. Biochim Biophys Acta, Enzymol. 1978;525:37–44. doi: 10.1016/0005-2744(78)90197-3. [DOI] [PubMed] [Google Scholar]
- 49.Aiken ML, Painter RG, Zhou Y, Wang G. Chloride transport in functionally active phagosomes isolated from Human neutrophils. Free Radic Biol Med. 2012;53:2308–2317. doi: 10.1016/j.freeradbiomed.2012.10.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y, Song K, Painter RG, Aiken M, Reiser J, Stanton BA, Nauseef WM, Wang G. Cystic fibrosis transmembrane conductance regulator recruitment to phagosomes in neutrophils. J Innate Immun. 2013;5:219–230. doi: 10.1159/000346568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Moscow) 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 52.Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hawkins CL, Pattison David I, Whiteman Matthew, Davies Michael J. Chlorination and Nitration of DNA and Nucleic Acid Components. In: Cooke MDEMS, editor. Oxidative damage to nucleic acids. Springer; New York: 2007. pp. 14–39. [Google Scholar]
- 54.Hawkins CL, Davies MJ. Hypochlorite-Induced Damage to DNA, RNA, and Polynucleotides: Formation of Chloramines and Nitrogen-Centered Radicals. Chem Res Toxicol. 2002;15:83–92. doi: 10.1021/tx015548d. [DOI] [PubMed] [Google Scholar]
- 55.Hawkins CL, Pattison DI, Davies MJ. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids. 2003;25:259–274. doi: 10.1007/s00726-003-0016-x. [DOI] [PubMed] [Google Scholar]
- 56.Cadet J, Wagner JR. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harbor Perspect Biol. 2013;5:A012559/012551–A012559/012518. doi: 10.1101/cshperspect.a012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeitner TM, Xu H, Gibson GE. Inhibition of the α-ketoglutarate dehydrogenase complex by the myeloperoxidase products, hypochlorous acid and mono-N-chloramine. J Neurochem. 2005;92:302–310. doi: 10.1111/j.1471-4159.2004.02868.x. [DOI] [PubMed] [Google Scholar]
- 58.Masuda M, Suzuki T, Friesen MD, Ravanat JL, Cadet J, Pignatelli B, Nishino H, Ohshima H. Chlorination of guanosine and other nucleosides by hypochlorous acid and myeloperoxidase of activated human neutrophils: catalysis by nicotine and trimethylamine. J Biol Chem. 2001;276:40486–40496. doi: 10.1074/jbc.M102700200. [DOI] [PubMed] [Google Scholar]
- 59.Badouard C, Masuda M, Nishino H, Cadet J, Favier A, Ravanat JL. Detection of chlorinated DNA and RNA nucleosides by HPLC coupled to tandem mass spectrometry as potential biomarkers of inflammation. J Chromatogr B: Anal Technol Biomed Life Sci. 2005;827:26–31. doi: 10.1016/j.jchromb.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 60.Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med. 1996;156:637–642. [PubMed] [Google Scholar]
- 61.Hertog MG, Hollman PC. Potential health effects of the dietary flavonol quercetin. Eur J Clin Nutr. 1996;50:63–71. [PubMed] [Google Scholar]
- 62.Le Marchand L, Murphy SP, Hankin JH, Wilkens LR, Kolonel LN. Intake of flavonoids and lung cancer. J Natl Cancer Inst. 2000;92:154–160. doi: 10.1093/jnci/92.2.154. [DOI] [PubMed] [Google Scholar]
- 63.Pincemail J, Deby C, Thirion A, de Bruyn-Dister M, Goutier R. Human myeloperoxidase activity is inhibited in vitro by quercetin. Comparison with three related compounds, Experientia. 1988;44:450–453. doi: 10.1007/BF01940544. [DOI] [PubMed] [Google Scholar]
- 64.Kato Y, Nagao A, Terao J, Osawa T. Inhibition of myeloperoxidase-catalyzed tyrosylation by phenolic antioxidants in vitro. Biosci Biotechnol Biochem. 2003;67:1136–1139. doi: 10.1271/bbb.67.1136. [DOI] [PubMed] [Google Scholar]
- 65.Zhang JL, Souders C Laurence, 2nd, Denslow ND, Martyniuk CJ. Quercetin, a natural product supplement, impairs mitochondrial bioenergetics and locomotor behavior in larval zebrafish (Danio rerio) Toxicol Appl Pharmacol. 2017;327:30–38. doi: 10.1016/j.taap.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 66.Chen Y, Okunieff P. Radiation and third-generation chemotherapy. Hematol Oncol Clin North Am. 2004;18:55–80. doi: 10.1016/s0889-8588(03)00145-x. [DOI] [PubMed] [Google Scholar]
- 67.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32:311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 68.Parveen N, Shadab GG. The dual clastogenic and anti-clastogenic properties of quercetin is dose dependent. Front Biosci (Schol Ed) 2017;9:139–153. doi: 10.2741/s478. [DOI] [PubMed] [Google Scholar]
- 69.N NL, Lopez-Gil S, Chavez-Tapia N, Uribe M, Barbero-Becerra VJ. Liver toxicity mechanisms of herbs commonly used in Latin America. Drug Metab Rev. 2017;49:338–356. doi: 10.1080/03602532.2017.1335750. [DOI] [PubMed] [Google Scholar]
- 70.Coelho JFJ, Carvalho EY, Marques DS, Popov AV, Percec V, Gil MH. Influence of the isomeric structures of butyl acrylate on its single-electron transfer-degenerative chain transfer living radical polymerization in water catalyzed by Na2S2O4, J. Polym. Sci. Part A: Polym Chem. 2008;46:6542–6551. [Google Scholar]
- 71.Coelho JFJ, Carvalho EY, Marques DS, Popov AV, Percec V, Goncalves PMFO, Gil MH. Synthesis of poly(ethyl acrylate) by single electron transfer-degenerative chain transfer living radical polymerization in water catalyzed by Na2S2O4. J Polym Sci, Part A: Polym Chem. 2007;46:421–432. [Google Scholar]
- 72.Percec V, Popov AV. Functionalization of the active chain ends of poly(vinyl chloride) obtained by single-electron-transfer/degenerative-chain-transfer mediated living radical polymerization: synthesis of telechelic α,ω -di(hydroxy)poly(vinyl chloride) J Polym Sci, Part A: Polym Chem. 2005;43:1255–1260. [Google Scholar]
- 73.Salerno JC. Cytochrome electron spin resonance line shapes, ligand fields, and components stoichiometry in ubiquinol-cytochrome c oxidoreductase. J Biol Chem. 1984;259:2331–2336. [PubMed] [Google Scholar]
- 74.Nitschke W, Hauska G. Structural Studies on Cytochrome b6 and the Rieske FeS-center. In: Biggins J, editor. Progress in Photosynthesis Research. Vol. 2. Nijhoff; Dordrecht: 1987. pp. 165–171. [Google Scholar]
- 75.Zhang H, Primak A, Cape J, Bowman MK, Kramer DM, Cramer WA. Characterization of the high-spin heme x in the cytochrome b6f complex of oxygenic photosynthesis. Biochemistry. 2004;43:16329–16336. doi: 10.1021/bi048363p. [DOI] [PubMed] [Google Scholar]
- 76.Nitschke W, Hauska G. On the nature of the g = 6 EPR signal in isolated cytochrome b6f complex from spinach chloroplasts. Biochim Biophys Acta, Bioenerg. 1987;892:314–319. [Google Scholar]
- 77.Nitschke W, Hauska G. An asymmetric low-spin EPR signal of cytochrome b6 in the cytochrome b6f-complex from spinach chloroplasts. FEBS Lett. 1987;213:453–455. [Google Scholar]
- 78.Gau J, Furtmuller PG, Obinger C, Prevost M, Van Antwerpen P, Arnhold J, Flemmig J. Flavonoids as promoters of the (pseudo-)halogenating activity of lactoperoxidase and myeloperoxidase. Free Radic Biol Med. 2016;97:307–319. doi: 10.1016/j.freeradbiomed.2016.06.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



