Abstract
Background
Anti-androgenic phthalates are reproductive toxicants that may have additive effects on male development. Diet is the primary exposure source for most phthalates, which contaminate the food supply through food contact materials and industrialized production.
Objective
To compare dietary sources of cumulative phthalates exposure between “food at home” (e.g. food consumed from a grocery store) and “food away from home” (e.g. food consumed from fast food/restaurants and cafeterias) in the U.S. general population.
Methods
We estimated cumulative phthalates exposure by calculating daily intake from metabolite concentrations in urinary spot samples for 10,253 participants (≥ 6 years old) using National Health and Nutrition Examination Survey (NHANES, 2005–2014) data. We constructed a biologically relevant metric of phthalates daily intake (Σandrogen-disruptor, μg/kg/day) by converting phthalates into anti-androgen equivalent terms prior to their summation. Particular foods and the percent of total energy intake (TEI) consumed from multiple dining out sources were ascertained from 24-hour recall surveys. Associations with Σandrogen-disruptor levels were estimated for children, adolescents, and adults using multivariable linear regression.
Results
We observed a consistent positive association between dining out and Σandrogen-disruptor levels across the study population (p-trend < 0.0001). Among adolescents, high consumers of foods outside the home had 55% (95% CI: 35%, 78%) higher Σandrogen-disruptor levels compared to those who only consumed food at home. The contribution of specific dining out sources to Σandrogen-disruptor levels varied by age group. For example, cafeteria food was associated with 15% (95% CI: 4.0%, 28%) and 64% (95% CI: 40%, 92%) higher Σandrogen-disruptor levels in children and adults, respectively. Particular foods, especially sandwiches (i.e. cheeseburgers), were associated with increased Σandrogen-disruptor levels only if they were purchased away from home (p < 0.01).
Conclusion
Dining out may be an important source of biologically relevant cumulative phthalates exposure among the U.S. population. Future studies should evaluate modifiable production practices that remove phthalates from the food supply in addition to the efficacy of interventions that promote eating fresh foods prepared at home.
Keywords: chemical mixtures, endocrine disruption, fast food, cumulative assessment, food contact materials, consumer product chemicals
1. Introduction
Endocrine disrupting chemicals (EDCs) are associated with hormone-mediated health outcomes, such as reproductive issues, metabolic disease, and neurodevelopmental problems.1,2 In the United States alone, researchers recently estimated the disease cost of EDCs at $340 billion, for which phthalates were the second-leading driver.3 Anti-androgenic phthalates may have additive adverse effects on fetal sex differentiation that are associated with testosterone inhibition during critical stages of pregnancy (i.e. reproductive organ development).4 Cumulative phthalate assessments are therefore more biologically relevant for human reproductive health than chemical-by-chemical approaches.5,6 Although pregnancy is one critical stage of toxicity, phthalates may also contribute to health impacts across the life course, including reduced semen quality, obesity, diabetes, and cancer.7–10 Low testosterone may influence adverse adult metabolic outcomes, suggesting a possible mode of action for androgen-disrupting phthalates along this disease pathway.11 Thus, efforts to identify opportunities for phthalates exposure reduction may have significant implications for preventing metabolic and other hormone-mediated illnesses and decreasing the economic burden of EDCs.
Phthalates have many uses in commerce, including food contact materials (e.g. plastic and recycled cardboard food packaging), personal care products, medical tubing, and/or any material containing polyvinyl chloride (PVC).12–14 Consequently, human exposure is ubiquitous, with multiple phthalates simultaneously detected in the vast majority of the U.S. population.12,15 Diet is the dominant exposure pathway for most anti-androgenic phthalates, especially for high molecular weight compounds such as di(2-ethylhexyl) phthalate (DEHP) and di-isononyl phthalate (DiNP).16–19 These phthalates are predominantly found in fatty foods such as meat and dairy,14,20 although they have also been linked to grains and spices,18,21 and likely enter the food supply through packaging, processing, and handling.22–27 Thus, it is plausible that a significant source of phthalates exposure may come from foods prepared outside the home which undergo substantial industrialized production practices, such as in the fast food and restaurant industries, school cafeterias, street food vendors, and sports and entertainment facilities.
Consumption of food prepared away from home, rather than food purchased in a store and prepared at home, has grown steadily over the last few decades in the United States. Between 1970 and 2014, household food expenditures devoted to dining out increased from 25.9% to 43. 7%, respectively, and over half of total U.S. food dollars are currently spent on foods purchased outside the home.28,29 Like adults, children 2-17 years old are dining out more, with 35% of their total calories sourced from food prepared away from home in 2003–6 compared to 20% in 1977–8.30 Among children, younger kids are more likely to eat meals offered by their school cafeterias, while adolescents are more likely to eat from competing vendors, such as fast food chains.30 Although research has primarily focused on nutrition and diet quality with regard to dining out,29–32 chemical exposures introduced through increased food packaging, handling, and processing are also important public health considerations.
Several studies have investigated the connection between food prepared outside the home and phthalate exposures. Zota et al. (2016) recently reported a consistent, positive association between fast food consumption and measured urinary metabolites of DEHP and DiNP in the U.S. general population,33 and fast food intake has been associated with urinary DiNP and butyl benzyl phthalate (BBzP) metabolites in a cohort of young children.34 In several smaller studies outside the United States, phthalates have been detected in takeout/delivery food containers as well as pre-cooked and immediately packaged cafeteria-style meals.23,27,35,36 However, a broader analysis across age groups in the U.S. population which evaluates multiple sources of food prepared away from and at home is warranted to assess the extent to which these and other dietary intake sources are associated with biologically relevant phthalate exposures.
Accordingly, this study compares cumulative phthalates exposure between consumers of “food away from home” (such as fast food and food from full-service restaurants and/or cafeterias) with consumers of “food at home” (such as food purchased from a grocery store) among children, adolescents, and adults in the United States.
2. Materials and Methods
2.1 Study population
The U.S. Centers for Disease Control and Prevention (CDC) administers the National Health and Nutrition Examination Survey (NHANES) as a nationally representative interview and physical examination of the civilian, non-institutionalized general population. We combined five cycles of laboratory, questionnaire, and dietary NHANES data between 2005 and 2014 for this study (http://www.cdc.gov/nchs/nhanes.htm). Our original study population included all participants ≥ 6 years old for which phthalate data, urinary creatinine measurements, kilocalorie and dietary intake source information were available (N = 12,134). Participants with missing information on household income and educational attainment (n = 920) were excluded from the study population because we wanted to evaluate these demographic characteristics in relation to phthalates exposure. We also excluded participants who did not self-identify as Hispanic, Mexican American, non-Hispanic white, or non-Hispanic black (n = 961) due to racial/ethnic ambiguity of other or multi-racial classifications. The final sample size included 10,253 study participants.
2.2 Cumulative phthalates exposure assessment
The NHANES survey provides phthalate metabolite measurements for one-third of study participants in each survey cycle. Spot urine samples are collected as part of the medical examination and analyzed at the CDC in Atlanta, GA., with analytical methods detailed elsewhere.37,38 In summary, phthalate metabolites are quantified using high performance liquid chromatography coupled with tandem mass spectrometry. Laboratory files for 2005-12 survey cycles were downloaded from the NHANES website in March 2015 and included impurity corrections for certain previously used analytical standards.39 NHANES 2013-14 data were downloaded in January 2017. The maximum limit of detection (LODmax) was used to standardize variable detection limits across survey cycles and concentrations below LODmax were substituted with LODmax divided by √2 (Supplementary Table S1).12 We used data for nine urinary metabolites to estimate exposure for six parent phthalates: di-n-butyl phthalate (DnBP), di-isobutyl phthalate (DiBP), BBzP, DEHP, DiNP, and diethyl phthalate (DEP). Because DiNP’s primary metabolite, mono-isononyl phthalate (MiNP), was below the detection limit for most NHANES samples, the secondary metabolite, mono(carboxy-isooctyl) phthalate (MCOP), was the only metabolite used to assess DiNP exposure.
We constructed a biologically relevant metric of cumulative phthalates exposure by summing phthalates based on their relative anti-androgenic potencies, according to our previously published method.40 In summary, phthalates were converted into anti-androgen equivalent terms by applying unitless relative potency factors (RPF) to daily intake estimates of individual parent compounds prior to their summation. A potency-weighted sum of estimated daily intake (Σandrogen-disruptor, μg/kg/day) for di-n-butyl phthalate (DnBP), di-isobutyl phthalate (DiBP), BBzP, DEHP, DiNP, and diethyl phthalate (DEP) was then calculated as follows (where i = individual phthalate): Σandrogen-disruptor (μg/kg/day) = Σ(Daily Intakei × RPFi) = (DnBP × 1.0) + (DiBP × 0.24) + (BBzP × 0.26) + (DEHP × 0.61) + (DiNP × 0.26) + (DEP × 0.024) (Eq. 1).40 RPFs were constructed previously by scaling each phthalate to a reference anti-androgen (DnBP).40 The RPF calculation involved comparing benchmark doses (BMDs) associated with fetal testosterone inhibition that were published in a 2008 National Academy of Sciences (NAS) report (although DiNP’s RPF relied on more recent toxicology data and DEP was assumed to be less potent by one order of magnitude).5,40 While the NAS BMDs were based on a singular endpoint, their underlying dose response data have since been shown to predict phthalate mixture effects on a broader range of postnatal male endpoints,4,6,41 suggesting these RPFs may be appropriate for other androgen-mediated outcomes. However, their applicability to neurodevelopment and metabolic disease have yet to be determined.
We back-calculated oral daily intake of parent phthalates in μg/kg/day using biomarkers (metabolites) in urine because our potency comparisons were based on atomic mass doses of parent phthalate compounds administered to rats. Consistent with prior studies and biomonitoring guidance from the NAS, we used the following pharmacokinetic equation to estimate daily intake, or “exposure dose”, from urinary metabolite concentrations: Daily Intakei = [(MEi × CE)/(FUE,i × 1000)] × [(MWp)/(MWm)]; i = individual phthalate (Eq. 2).42–44 The equation converts creatinine-adjusted urinary metabolite concentrations (ME) (μg/g) into molar equivalents from which molar daily intakes are estimated first by multiplying with body weight-adjusted creatinine excretion rate (CE) (mg/kg/day) and subsequently dividing by the metabolite’s molecular weight (MWm) (g/mol). The fractional molar ratio of excreted metabolite to ingested parent phthalate (FUE), determined in human kinetic and metabolism studies by measuring urinary metabolite concentrations in relation to known intake of labeled parent compound over a 24-hour period,16,45,46 is then used to calculate molar daily intake of the parent phthalate. The parent phthalate’s molecular weight (MWp) (g/mol) facilitates final conversion to μg/kg/day, units that are comparable to toxicology doses and human population reference values. Phthalate-specific RPF, BMD, FUE, and MW values are listed in Supplementary Table S1.
Urinary creatinine is often used as a surrogate for urine dilution, although its use is controversial because creatinine excretion rates vary across racial/ethnic groups, sex, age, and body mass index (BMI).47 We used average creatinine excretion rates of 23 mg/kg/day and 18 mg/kg/day for men and women, respectively. For boys and girls < 20 years old, we calculated average creatinine excretion rates of 21 mg/kg/day and 19 mg/kg/day, respectively, from the literature.48,49 Alternatively, urine flow rate can be used as a more direct volume-based measure of urine dilution if the data are available, although urine flow rate has also been shown to vary across demographic groups.50 To use the volume-based approach, one would simply replace (MEi × CE) with (UMEi × UE) in the numerator of the daily intake equation, where UME is the unadjusted measured urinary metabolite concentration (ug/L) and UE is the daily urine flow rate normalized by body weight (mL/kg/day). Sample calculations for both approaches are provided in supplementary materials of our original method publication.40
2.3 Dietary intake exposure assessment
As part of NHANES, the CDC also collects 24-hour dietary recall data from study participants that includes extensive information about what foods were eaten, time of eating occasion, and food source (where obtained or purchased).51 Energy and nutrient intake are later quantified for each food recorded during participant surveys,51–53 and cup-equivalent data are also made available by the U.S. Department of Agriculture (USDA).54 We used dietary data from the day prior to urine sample collection because phthalates have short metabolic half-lives (12-24 hours), and it is reasonable to assume that urinary metabolite concentrations measured within ~ 24 hours of parent compound exposure would appropriately reflect dietary intake the previous day.46,55 Participants 12 years and older completed the survey independently unless they chose otherwise, while proxy-assisted interviews were automatically provided for children 6-11 years old.
Based on the methods in Zota et al. (2016), we calculated total energy intake (TEI) in kilocalories (kcals) the prior day for each participant by summing NHANES-provided kcals for all foods recorded during each participant’s dietary interview.33 We determined total fat in grams by summing NHANES-provided grams of fat for all recorded food items, since dining out is positively associated with total caloric and fat intake among the U.S. general population,30,31 and high-fat foods have been linked to phthalates exposure.14,20 Total fat in kcals was calculated using nine fat grams per calorie as the conversion factor, and the percent of TEI from fat was then determined by dividing fat kcals by TEI.56,57 Calculating “nutrient density” (i.e. % kcals from fat) is an energy adjustment method commonly used by nutritional epidemiologists to reduce the influence of exposure misclassification from dietary recall surveys and isolate diet composition from body size, metabolism, and physical activity.56,58,59
We categorized all foods reported by each participant as either food away from home (prepared at a restaurant, cafeteria, etc.) or food at home (purchased or obtained at a grocery store and potentially prepared at home), ascertained from the NHANES survey question regarding where the food item (or the majority of its ingredients) was obtained or purchased. Seventeen mutually exclusive responses were provided by NHANES. We defined food away from home as follows: 1) Fast food, defined by NHANES as food purchased/obtained from restaurants without table service, pizza restaurants regardless of waiter/waitress service, and all carryout and delivery food; 2) Full-service restaurants, or restaurants with table service, including bars, taverns, and lounges; 3) Cafeterias, including K-12 school cafeterias and other types; and 4) All other marginal away-from-home sources that each contributed < 2% to TEI (and together contributed < 5% to TEI), which included child/family care centers, soup kitchen/shelter/food pantry, Meals on Wheels, community food programs, vending machines, sport/recreation/entertainment facilities, street vendor/vending trucks, residential dining facilities, and fundraiser sales. We defined food at home as items purchased at a store, anything home grown or caught, and “from someone else/gift”, which the USDA has characterized as dinner cooked by a friend.30 We summed the total NHANES-provided kcals consumed for each participant from sources of either food away from home or food at home. The contribution of each source to total dietary intake, or the “energy density” of food away from home and food at home were determined by dividing total kcals (and total fat kcals) from each source by TEI.
2.4 Statistical analysis
All statistical analyses were performed in SAS version 9.4 (SAS Institute Inc., Cary, NC) with new sample weights calculated according to analytical guidelines for combining multiple cycles of data.37 We used survey-weighted SAS procedures (i.e. PROC SURVEYMEANS, PROC SURVEYFREQ, and PROC SURVEYREG) to adjust models for population weights as well as the stratified multi-stage sample design. Statistical significance was defined at p < 0.05 for two-sided tests. Degrees of freedom for variance estimation were determined by subtracting the number of strata by the number of unique clusters. Phthalate exposure estimates were log-transformed prior to statistical testing to normalize their skewed distributions.
We used linear regression to examine cumulative phthalates daily intake (Σandrogen-disruptor), our primary outcome of interest, across the following study population characteristics: Age (children 6-11, adolescents 12-19, adults 20-59, and older adults ≥ 60 years old); sex (male or female); race/ethnicity [non-Hispanic white, non-Hispanic black, or Hispanic]; body mass index (BMI) [underweight (< 18.5 kg/m2), normal weight (18.5–25 kg/m2), overweight (25–30 kg/m2), or obese (≥ 30 kg/m2)]; poverty-to-income ratio, or PIR [ratio of household income to poverty threshold adjusted to family size and inflation: < 1 (beneath poverty threshold), 1–2.99, or ≥ 3]; educational attainment (less than high school, high school graduate, or any post high school education) in adults only; NHANES survey cycle (2005–6, 2007–8, 2009–10, 2011–12, or 2013–14); time of sampling session (morning, afternoon, or evening); and dietary intake of food prepared away from home [any (participants who reported dining out the prior day) or none (participants who reported consuming only food at home the prior day)]. Univariate statistics for Σandrogen-disruptor levels were calculated from these models. We also added NHANES survey cycle and a statistical interaction term to the dining out model to observe whether the association with Σandrogen-disruptor levels varied over time.
We then compared dietary intake of food away from home (our primary exposure of interest) across several population characteristics. Specifically, we used the Rao Scott chi-square test of independence, first across age (children, adolescents, adults, and older adults), then by sex, and finally, across NHANES survey cycle. The remainder of statistical analyses focus on age-specific subgroups, since differences in Σandrogen-disruptor levels and dietary behavior across age were more pronounced than between men and women or across time.
For our primary multivariable analysis, we followed the approach of Zota et al. (2016)33 and modeled dining out as both the dietary intake of food away from home (% TEI), or the percent of TEI consumed outside the home, and away from home-derived fat intake (% TEI), or the percent of TEI consumed as fat kcals from dining out sources. Because many participants did not consume any calories outside the home (resulting in highly left-truncated distributions for these variables), we categorized these dietary intake source variables into three groups: none (participants who consumed 100% of their calories, or fat calories, from food at home the prior day), low (participants who consumed < weighted median of those who had some dietary intake of food away from home > 0), and high (participants who consumed ≥ the weighted median of those who had a dietary intake of food away from home > 0). Covariates included all population characteristics evaluated in unadjusted regression analyses (with age modeled continuously) except for BMI, which could potentially be on the causal pathway between our exposure and outcome of interest. We did not adjust for creatinine as an independent covariate since it was included in the calculation of phthalates daily intake (Eq. 2).
We additionally assessed the influence of total dietary intake on phthalates exposure by replacing the away-from-home dietary intake variables in our main analysis with TEI and total fat intake (% TEI), which were each categorized into low, middle, and high tertiles from age-specific weighted distributions. These “baseline” dietary intake variables were not added as independent covariates in core regression models because our primary dietary exposures of interest were normalized by TEI, which reduces bias from dietary recall surveys.56,58,59
Third, we constructed separate multivariable models for specific sources of food away from home, including fast food, full-service restaurants, and cafeterias (for children, adolescents, and adults). Specific away-from-home food sources were modeled as categorical independent variables because the high proportion of away-from-home “non-consumers” results in truncated distributions that pose statistical challenges in survey-weighted data analysis. Instead, we compared each “consumer” group to the same referent population in separate models to reduce the impact of potential confounding and correlation among dietary variables. Specifically, we compared consumers of “any” fast food (followed by any restaurant food and any cafeteria food) to a standard “none” referent category consisting of participants who did not consume any food away from home the prior day (e.g. all of their calories came from foods purchased at a store), with 31% of children and 43% of adolescents and adults meeting this criteria.
We also examined the influence of food type on phthalates exposure by performing multivariable regression to estimate associations between particular foods (fruits/vegetables, sandwiches, fried potatoes, and pizza, each modeled separately as binary or categorical independent variables) and Σandrogen-disruptor levels. First, we summed USDA-converted cup-equivalents for fruit (including 100% fruit juice) and vegetables (including legumes but excluding fried potatoes) from each participant’s 24-hour food record.54 We grouped participants into yes/no categories based on whether or not their daily dietary recommendations were met for either fruit or vegetable intake.60 Next we constructed a common referent group restricted to non-consumers of sandwiches, fried potatoes, and pizza, and we compared consumers of each food to the common referent group in separate at-home and away-from home models (since a small number of participants consumed foods from both sources). For example, away-from-home sandwich consumers (who may have also consumed at-home sandwiches but not fried potatoes or pizza) were compared to participants who did not eat any sandwiches, fried potatoes, or pizza (whether purchased at or away from home) the prior day. We selected energy-dense foods as potential sources of phthalates exposure regardless of source, restricting to sandwiches containing animal protein (meat/poultry/fish, dairy, and/or egg). Similar to Sebastian et al. (2015),66 we counted sandwiches recorded as single items (e.g. one food record described as “cheeseburger”) and those recorded as combinations of individual ingredients (e.g. multiple food records for cheese, beef patty, bun, and tomato consumed in the same meal). We added total fat and energy intake to these models to evaluate them as potential confounders or mediators.
From log-level regression models, the percent difference in cumulative phthalates exposure 95% confidence interval (95% CI) were estimated as (e(β) −1) * 100 and (e(β ± critical value × SE) −1) * 100, respectively, where β is the beta coefficient and SE is the standard error. We tested for linear trends in our association of interest by modeling categorical dietary intake variables as ordinal terms in multivariable models. Finally, adjusted associations with dining out were evaluated for each phthalate separately. We also calculated the percent geometric mean (GM) contribution to Σandrogen-disruptor from individual phthalate daily intake estimates (μg/kg/day).
2.5 Sensitivity analysis
We performed a sensitivity analysis to evaluate alternate approaches to urine dilution correction and daily intake estimation, since these factors may introduce uncertainty and variability into regression models.47,50,62 We specifically assessed whether alternate measures of urine dilution (i.e. urine flow rate rather than creatinine) and/or the application of potency-weights directly to metabolites impacted our results. Adjusted associations were compared between Σandrogen-disruptor (μg/kg/day) and four other potency-weighted cumulative metrics (with more details provided in the method publication)40: 1) Phthalate daily intake metric calculated with individual urine flow rate rather than average creatinine excretion rate (Σurine-flow, μg/kg/day); 2) Metabolite-based analyte excretion rate (mass/time) calculated using urine flow rate (Σexrate-rpf, μg/day); 3) Metabolite concentration-based metric without urine dilution correction (Σmetab-rpf, μg/L); and 4) Metabolite concentration-based metric correcting for creatinine as an independent variable in regression models (Σmetab-rpf + creat, μg/L).40 We also re-calculated these cumulative metrics using molar mass to compare adjusted associations with atomic mass-based metrics. These models were restricted to 2009-14 data because NHANES did not report urine flow rate data prior to 2009. We selected the largest age-specific subgroup evaluated in core regression models for this analysis (adults 20-59 years old, N = 2695).
In additional sensitivity analyses, we performed multivariable regression to evaluate the continuous association between total energy intake (TEI) and cumulative phthalates exposure, stratified between consumers of food away from home and non-consumers (participants who consumed all of their calories from food at home). Finally, we added BMI to core multivariable regression models to evaluate its potential influence on the association between dietary intake of food away from home (% TEI) and cumulative phthalates daily intake (Σandrogen-disruptor).
3. Results
The majority of study participants were adults with at least some post-high school education, non-Hispanic white, above normal weight, and in the middle income category (Table 1). More participants reported dining out than eating only at home the prior day (61 vs 39%). Unadjusted cumulative phthalates daily intake (Σandrogen-disruptor) was 35% (95% CI: 29%, 41%) higher among consumers of food away from home compared to those who ate only at home (p < 0.0001). Higher Σandrogen-disruptor levels were observed in earlier NHANES cycles, with a 50% total decrease in exposure between 2005 and 2014 (p < 0.0001). Cumulative phthalates were also elevated in males; Hispanic and white compared to black participants; underweight compared to normal weight participants; those in the highest (≥ 3) compared the middle (1 – 3) PIR; adults with the least (< high school) compared to the most (> high school) education; and in evening rather than morning sample collection times. Unadjusted Σandrogen-disruptor levels were fairly similar for adolescents, adults, and older adults (GM range: 3.9 to 4.3 μg/kg/day), while the GM for children (GM = 6.6 μg/kg/day; 95% CI: 6.2, 6.9) was 50–70% higher (p < 0.0001) (Table 1 and Figure 1).
Table 1.
Unadjusted association between cumulative phthalates daily intake (Σandrogen-disruptor) and study population characteristicsa in NHANES 2005–14 (N = 10,253)
|
Σandrogen-disruptor (μg/kg/day)
|
|||
|---|---|---|---|
| Population Characteristics | n (%) | Percent diff (95% CI) | GM (95% CI) |
| Age (years) | |||
| ≥ 60 | 2244 (22) | Referent | 3.9 (3.7, 4.2) |
| 20-59 | 4601 (45) | 9.2 (2.4, 17)* | 4.3 (4.1, 4.5) |
| 12-19 | 1887 (18) | 9.2 (1.3, 18)* | 4.3 (4.0, 4.6) |
| 6-11 | 1521 (15) | 70 (56, 81)* | 6.6 (6.2, 6.9) |
| Sex | |||
| Male | 5089 (50) | Referent | 4.5 (4.3, 4.6) |
| Female | 5164 (50) | −4.4 (−8.2, −0.4)* | 4.3 (4.1, 4.5) |
| Race/ethnicity | |||
| Black | 2633 (26) | Referent | 3.9 (3.7, 4.1) |
| White | 4592 (45) | 15 (7.1, 26)* | 4.4 (4.2, 4.8) |
| Mexican American/Hispanic | 3028 (30) | 16 (7.1, 26)* | 4.5 (4.2, 4.8) |
| Body mass index (BMI)b | |||
| Normal (18.5-25 kg/m2) | 3211 (32) | Referent | 4.3 (4.1, 4.5) |
| Underweight (< 18.5 kg/m2) | 1230 (12) | 39 (29, 49)* | 6.0 (5.6, 6.3) |
| Overweight (25-30 kg/m2) | 2751 (27) | −0.8 (−6.2, 4.8) | 4.3 (4.0, 4.5) |
| Obese (≥ 30 kg/m2) | 2985 (29) | −2.6 (−8.1, 3.2) | 4.3 (4.1, 4.5) |
| Poverty:income ratio (PIR) | |||
| ≥ 3 (Highest income) | 3389 (33) | Referent | 4.5 (4.3, 4.8) |
| 1-3 (Moderate income) | 4260 (42) | −7.4 (−13, −1.8)* | 4.2 (4.0, 4.4) |
| < Poverty line | 2604 (25) | −5.2 (−12, 1.8) | 4.3 (4.1, 4.5) |
| Educationc | |||
| > High School | 3420 (50) | Referent | 4.3 (4.1, 4.5) |
| = High School | 1652 (24) | −4.0 (−10, 2.3) | 4.1 (3.9, 4.4) |
| < High School | 1773 (26) | −9.3 (−15, −2.8)* | 3.9 (3.7, 4.1) |
| Survey cycle | |||
| 2013-14 | 1873 (18) | Referent | 3.5 (3.3, 3.7) |
| 2011-12 | 1735 (17) | 16 (3.4, 30)* | 4.1 (3.7, 4.5) |
| 2009-10 | 2241 (22) | 21 (10, 33)* | 4.3 (4.0, 4.6) |
| 2007-8 | 2193 (21) | 39 (25, 55)* | 4.9 (4.5, 5.3) |
| 2005-6 | 2211 (22) | 49 (34, 66)* | 5.2 (4.8, 5.7) |
| Sampling session | |||
| Morning | 4833 (47) | Referent | 4.2 (4.0, 4.4) |
| Afternoon | 3629 (35) | −2.0 (−7.0, 3.2) | 4.1 (4.0, 4.4) |
| Evening | 1791 (18) | 22 (15, 30)* | 5.2 (4.9, 5.4) |
| Self-reported dietary intake of food away from home (prior day) | |||
| None | 4024 (39) | Referent | 3.6 (3.5, 3.8) |
| Any | 6229 (61) | 35 (29, 41)* | 4.9 (4.7, 5.1) |
GM = Geometric mean.
From linear regression models where null hypothesis assumes no difference from referent category.
Sample size restricted to 10,177 due to missing BMI data.
Educational attainment restricted to adults only (N = 6845).
p<0.05
Figure 1.
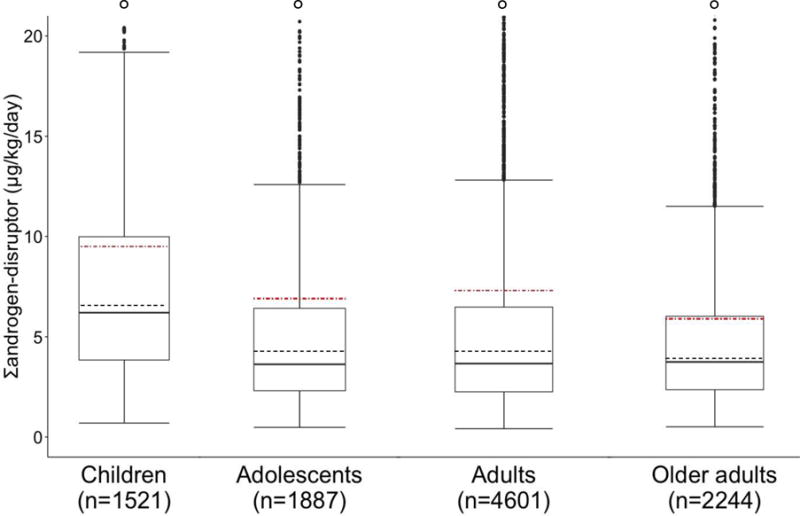
Univariate distribution of cumulative phthalates daily intake (Σandrogen-disruptor, μg/kg/day) among age-specific subgroups in NHANES 2005-14 (N = 10,253). Boxes represent interquartile range (IQR: 25th to 75th percentiles). Dark lines represent medians. Dashed lines represent geometric means. Red dot-dashed lines represent arithmetic means. Whiskers extend to min and max (Max = Most extreme values within 1.5 • IQR of the median). Outliers were defined as values below the min or max and are represented by dark points, and hollow points denote outliers off the y-axis scale. A total of 127 (8.3%), 205 (11%), 469 (10%), and 200 (8.9%) outliers were observed among children, adolescents, adults, and older adults, respectively. P < 0.0001 from statistical test of difference between age-specific subgroups.
Dietary consumption patterns varied significantly by age and sex (p < 0.0001), with 52% of older adults (compared to ~ 30-35% of younger participants) eating only food at home the prior day (Table 2). A higher percentage of children ate cafeteria food compared to adolescents, but a higher proportion of adolescents than children consumed calories from fast food and full-service restaurants. Among all age groups, adults (20-39 years) obtained the highest percentage of their total calories from fast food and full-service restaurants. Men were more likely to consume food outside the home than women, but the differences were less pronounced than those observed across age (Table 2). Dining out differences remained fairly consistent over time, ranging from 58% in 2009–10 to just under 65% in other years (data not shown). Differences in Σandrogen-disruptor levels between consumers of food away from home ranged from 29% (95% CI: 16%, 44%) in 2005–6 to 44% (95% CI: 32%, 58%) in 2011–12; however, we did not observe evidence of effect modification by survey cycle year (pinteraction = 0.48) for the association between eating out and Σandrogen-disruptor levels.
Table 2.
Survey-weighted percent of participants who reported consumption of food away from home the prior day across age and by sex among the U.S. general populationa in NHANES 2005–14 (N = 10,253)
| Age (years) | Sex (Adults ≥ 20 years) | |||||||
|---|---|---|---|---|---|---|---|---|
| 6-11 n=1521 |
12-19 n = 1887 |
20-59 n = 4601 |
≥ 60 n = 2244 |
Men n = 3337 |
Women n = 3508 |
|||
| Dietary Intakebb Food away from home |
% (SE) | % (SE) | % (SE) | % (SE) | p-value | % (SE) | % (SE) | p-value |
|
NONE Away Only food at home |
31 (1.7) | 34 (1.3) | 35 (1.2) | 52 (1.5) | <0.0001 | 35 (1.1) | 43 (1.0) | <0.0001 |
|
TOTAL Away Any food away from home |
69 (1.7) | 67 (1.3) | 65 (0.9) | 48 (1.5) | <0.0001 | 65 (1.1) | 57 (1.0) | <0.0001 |
| Fast food Any |
36 (1.7) | 42 (1.2) | 39 (0.9) | 21 (1.5) | <0.0001 | 38 (1.1) | 33 (0.8) | <0.0001 |
| Full-service restaurant Any |
14 (1.2) | 18 (1.5) | 28 (0.8) | 22 (1.1) | <0.0001 | 27 (0.9) | 22 (0.8) | <0.0001 |
| Cafeteria Any |
28 (1.8) | 16 (1.1) | 4.0 (0.6) | 3.0 (0.5) | <0.0001 | 8.0 (0.5) | 7.0 (0.5) | 0.420 |
| Other (marginal) Any |
17 (1.2) | 12 (1.0) | 17 (0.8) | 12 (1.0) | <0.0001 | 17 (0.7) | 13 (0.7) | <0.0001 |
Percentages are weighted due to the NHANES multi-level sampling strategy. Differences evaluated using Rao Scott chi-square test for independence, where null hypothesis equates to no significant difference between age subgroups or between men and women.
NONE Away indicates participants who did not consume any food away from home the prior day (i.e. 100% of their calories came from food at home, such as food purchased from the grocery store). TOTAL Away includes all participants who consumed any food away from home (including food consumed from full-service/fast food restaurants, cafeterias, and/or “other” marginal sources that together contributed less than 5% to total energy intake, or TEI).
In our main analysis, we found a positive association between dining out and cumulative phthalates daily intake (Σandrogen-disruptor), with evidence of a linear trend across exposure categories, among all age-specific subgroups (p for trend < 0.0001) (Figure 2 and Table 3). While associations were significant in all age groups, the magnitude of association was largest for adolescents, with high consumers of food away from home having 55% (95% CI: 35%, 78%) higher Σandrogen-disruptor levels than adolescents who ate only food at home the prior day. The weakest associations were observed among children, with high consumers of food away from home having 30% (95% CI: 16%, 45%) higher Σandrogen-disruptor levels than children who consumed all their calories from food at home. Similar positive associations were observed across age-specific subgroups between food away from home-derived fat intake and Σandrogen-disruptor levels, including evidence of a linear trend between exposure categories (p for trend < 0.0001). The measures of total dietary intake, TEI and total fat intake (% TEI), were positively associated with Σandrogen-disruptor levels among adults, with 14% (95% CI: 6.6%, 22%) higher levels in high compared to low fat consumers (p for trend = 0.0002) and 9.5% (95% CI: 1.0%, 19%) increased Σandrogen-disruptor levels in high relative to low TEI consumers (p for trend = 0.03); however, these baseline associations were substantially smaller than those observed for dining out (Table 3).
Figure 2.
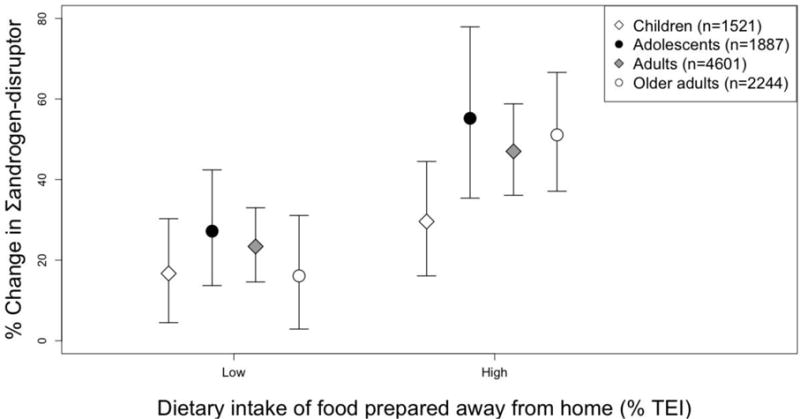
Adjusted percent difference and 95% CI of cumulative phthalates daily intake (Σandrogen-disruptor, μg/kg/day) among age-specific subgroups in NHANES 2005-14 (N = 10,253). Covariates: Sex, age, race/ethnicity, poverty-to-income (PIR), education (adults only), NHANES survey cycle, and time of sampling session. P for trend was < 0.0001 for all age subgroups. Low and high intake divided at weighted median of participants who consumed any food away from home the prior day. Referent groups include participants who did not consume any food away from home the prior day (i.e. 100% of their calories came from food at home, such as from a grocery store). TEI = Total energy intake (kcals).
Table 3.
Adjusted association between cumulative phthalates daily intake (Σandrogen-disruptor, μg/kg/day) and dietary intake (total and from food away from home)a in NHANES 2005–14 (N = 10,253)
|
Children 6-11 yrs (n = 1521) |
Adolescents 12-19 yrs (n = 1887) |
Adults 20-59 yrs (n = 4601) |
Older Adults ≥ 60yrs (n = 2244) |
|||||
|---|---|---|---|---|---|---|---|---|
| Dietary Intake | n | Percent difference (95% CI) |
n | Percent difference (95% CI) |
n | Percent difference (95% CI) |
n | Percent difference (95% CI) |
| Away from home (% TEI)b | ||||||||
| None | 471 | Referent | 630 | Referent | 1714 | Referent | 1209 | Referent |
| Low | 504 | 17 (4.5, 30)* | 650 | 27 (14, 42)* | 1462 | 23 (15, 33)* | 526 | 16 (2.9, 31)* |
| High | 546 | 30 (16, 45)* | 607 | 55 (35, 78)* | 1425 | 47 (36, 59)* | 509 | 51 (37, 67)* |
| p for trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| Away from home fat (% TEI)b | ||||||||
| None | 477 | Referent | 638 | Referent | 1756 | Referent | 1221 | Referent |
| Low | 506 | 18 (4.9, 32)* | 647 | 27 (14, 42)* | 1438 | 23 (14, 32)* | 525 | 18 (5.1, 31)* |
| High | 538 | 28 (14, 44)* | 602 | 54 (34, 78)* | 1407 | 46 (34, 58)* | 498 | 51 (36, 68)* |
| p for trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| Total fat (% TEI)c | ||||||||
| Low | 497 | Referent | 634 | Referent | 1634 | Referent | 795 | Referent |
| Mid | 498 | −0.16 (−11, 12) | 603 | 3.6 (−8.1, 17) | 1465 | 3.1 (−4.0, 11) | 759 | −9.3 (−18, 0.8) |
| High | 526 | 0.93 (−9.0, 12) | 650 | 9.8 (−2.9, 24) | 1502 | 14 (6.6, 22)* | 690 | 10 (−1.4, 23) |
| p for trend | 0.86 | 0.13 | 0.0002 | 0.09 | ||||
| Total energy intake (TEI) (kcals)c | ||||||||
| Low | 528 | Referent | 626 | Referent | 1570 | Referent | 848 | Referent |
| Mid | 494 | 4.5 (−6.7, 17) | 615 | −7.5 (−17.0, 3.0) | 1526 | 1.9 (−5.8, 10) | 672 | −8.1 (−18, 2.3) |
| High | 499 | 2.6 (−8.2, 15) | 646 | 12 (−4.6, 30) | 1505 | 9.5 (1.0, 19)* | 724 | −1.1 (−13, 12) |
| p for trend | 0.64 | 0.18 | 0.03 | 0.90 | ||||
Covariates: Sex, age, race/ethnicity, poverty-to-income ratio (PIR), education (adults only), NHANES survey cycle, and time of sampling session.
Referent groups include participants who did not consume any food away from home the prior day (i.e. 100% of their calories came from food at home). Low/High divided at weighted median of dining out consumers (% TEI), which was 40, 49, 44, and 38, respectively, for children, adolescents, adults, and older adults (range 0–100%). Weighted median (range) for away from home-derived fat intake (% TEI): 14 (0–42), 18 (0–62), 17 (0–56), and 15 (0–62) for children, adolescents, adults, and older adults, respectively.
Low/Mid/High calculated from weighted distributions of age-specific subgroups. Tertile divisions (range) for children, adolescents, adults, and older adults, respectively: 30, 36 (3.8–62), 30, 37 (1.8–69), 30, 37 (1.5–75), and 31, 38 (4.1–65) for total fat intake (% TEI); 1613, 2112 (171–6992), 1650, 2379 (193–9363), 1746, 2570 (89–13,133), and 1496, 2004 (188–6305) for TEI (kcals).
p<0.05
Each specific source of food away from home was significantly associated with cumulative phthalates daily intake (Σandrogen-disruptor) across our study population (Table 4). However, contributions from specific dining out sources to Σandrogen-disruptor levels varied by age group. For example, when compared to a common referent group (restricted to children who consumed only food at home the prior day), children who ate cafeteria food had 15% (95% CI: 4.0%, 28%) higher Σandrogen-disruptor levels while children who ate restaurant food had 46% (95% CI: 22%, 73%) higher levels. We observed stronger associations between cafeteria food intake and Σandrogen-disruptor levels among adolescents and adults (compared to their respective referent groups), with adult cafeteria consumers having 64% (95% CI: 40%, 92%) higher Σandrogen-disruptor levels in comparison to adult non-consumers. (Table 4).
Table 4.
Adjusted association between cumulative phthalates daily intake (Σandrogen-disruptor, μg/kg/day) and dietary intake from specific sources of food away from homea in NHANES 2005–14 (N = 10,253)
| Children 6–11 yrs (n = 1521) |
Adolescents 12–19 yrs (n = 1887) |
Adults ≥ 20 yrs (n = 6845) |
||||
|---|---|---|---|---|---|---|
| Children 6–11 yrs (n = 1521) |
||||||
| Dietary Intake (% TEI) |
n | Percent diff (95% CI) |
n | Percent diff (95% CI) |
n | Percent diff (95% CI) |
| Fast food restaurant | ||||||
| Noneb | 471 | Referent | 630 | Referent | 2923 | Referent |
| Any | 545 | 29 (16, 43)* | 798 | 47 (32, 64)* | 2313 | 39 (31, 48)* |
| Full-service restaurant | ||||||
| Noneb | 471 | Referent | 630 | Referent | 2923 | Referent |
| Any | 165 | 46 (22, 73)* | 251 | 52 (24, 86)* | 1503 | 41 (31, 51)* |
| Cafeteria | ||||||
| Noneb | 471 | Referent | 630 | Referent | 2923 | Referent |
| Any | 488 | 15 (4.0, 28)* | 381 | 45 (24, 68)* | 223 | 64 (40, 92)* |
Covariates: Sex, age, race/ethnicity, poverty-to-income ratio (PIR), education (adults only), NHANES survey cycle, and time of sampling session.
Common referent groups include participants who did not consume any calories from food away from home the prior day (i.e. 100% of their calories came from food at home, such food purchased from a grocery store).
p<0.05
Across age-specific subgroups, consuming sandwiches, fried potatoes, and pizza at home was not associated with cumulative phthalates daily intake (Σandrogen-disruptor), with the exception of fried potatoes, which were associated with reduced Σandrogen-disruptor levels among adolescents only (Table 5). On the other hand, consuming sandwiches outside the home was consistently associated with increased Σandrogen-disruptor levels across the study population. For example, away-from-home sandwich consumption was associated with > 30% higher Σandrogen-disruptor levels in all age groups (p < 0.005). Results were less consistent for other foods purchased away from home, although pizza and fried potatoes were positively associated with Σandrogen-disruptor levels in children and adults, respectively (p < 0.01). Meeting daily guidelines for fruit or vegetable intake was associated with reduced Σandrogen-disruptor levels in adolescents (p = 0.03), but the difference was no longer significant when models adjusted for TEI, indicating that total caloric intake may attenuate the association between fruit or vegetable intake and phthalates exposure (Supplementary Table S2).
Table 5.
Adjusted association between cumulative phthalates daily intake (Σandrogen-disruptor, μg/kg/day) and dietary intake of particular foods at home and away from homea in NHANES 2005–14 (N = 10,253)
| Children 6-11 yrs (N = 1521) |
Adolescents 12-19 yrs (N = 1887) |
Adults ≥ 20 yrs (N = 6845) |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| Dietary Intake | n | Percent difference (95% CI) |
n | Percent difference (95% CI) |
n | Percent difference (95% CI) |
| ≥ Fruit/veggie guidelineb | ||||||
| Yes | 498 | Referent | 415 | Referent | 1890 | Referent |
| No | 751 | 3.0 (−8.0, 15) | 1158 | −13 (−23, −1.9)* | 3668 | 4.1 (−1.7, 10) |
|
| ||||||
| Sandwichc | ||||||
| None | 474 | Referent | 619 | Referent | 3043 | Referent |
| At home | 253 | 7.4 (−4.0, 20) | 323 | −5.0 (−19, 11) | 1485 | 1.8 (−4.9, 9.0) |
|
| ||||||
| None | 474 | Referent | 619 | Referent | 3043 | Referent |
| Away from home | 145 | 35 (10, 64)* | 218 | 37 (12, 68)* | 729 | 31 (17, 47)* |
|
| ||||||
| Fried potatoesc | ||||||
| None | 474 | Referent | 619 | Referent | 3043 | Referent |
| At home | 35 | 11 (−12, 39) | 39 | −21 (−37, −0.3)* | 154 | −0.8 (−22, 25) |
|
| ||||||
| None | 474 | Referent | 619 | Referent | 3043 | Referent |
| Away from home | 61 | 8.2 (−18, 42) | 68 | 7.6 (−23, 51) | 192 | 26 (6.9, 49)* |
|
| ||||||
| Pizzac | ||||||
| None | 474 | Referent | 619 | Referent | 3043 | Referent |
| At home | 50 | 6.3 (−17, 37) | 54 | −2.0 (−19, 19) | 131 | 12 (−6.1, 34) |
|
| ||||||
| None | 474 | Referent | 619 | Referent | 3043 | Referent |
| Away from home | 177 | 22 (5.2, 40)* | 167 | 3.2 (−14, 24) | 247 | 9.1 (−5.3, 26) |
Covariates: Sex, age, race/ethnicity, poverty-to-income ratio (PIR), education (adults only), NHANES survey cycle, and time of sampling session.
Fruit/veggies were evaluated separately from other foods. Referent group includes participants who met either their daily fruit or vegetable guideline (in cup-equivalents, or c-eq); “No” group includes participants who did not meet either requirement.65 Sample sizes restricted by data availability.
Six models were performed for each age-specific subgroup to separately compare At home and Away from home consumers to a common referent group of non-consumers of any of these foods. For example, consumers of sandwiches away-from-home were compared to participants who did not eat any at-home or away-from-home sandwiches, fried potatoes, or pizza the prior day. The At home and Away from home categories included a small number of overlapping participants who consumed particular foods from both sources the prior day (e.g. n = 58, 7, and 9 children consumed at-home and away-from-home sandwiches, fried potatoes, and pizza, respectively).
p<0.05
When evaluated separately, daily intake of DEHP and DiNP were both positively associated with dining and we observed a linear trend between exposure categories (p < 0.0001), with high consumers of food away from home having 74% increased DiNP exposure compared to participants who consumed all of their calories from food at home, such as food purchased from the grocery store (Table 6). These high molecular weight phthalates were the top contributors to Σandrogen-disruptor (45% and 30%, respectively), while DnBP ranked third (16%). However, DnBP was negatively associated with dining out (p = 0.056), and all other phthalates were not individually associated with dietary intake of food away from home. Thus, dining out associations were largely driven by DEHP and DiNP (Table 6).
Table 6.
Individual phthalate contributions to cumulative daily intake (Σandrogen-disruptor, μg/kg/day) and their adjusted associations with dietary intake of food away from homea in NHANES 2005–14 (N = 10,253)
| Dietary Intakeb (% TEI) |
n | DEHP | DiNP | DnBP | DEP | BBzP | DiBP |
|---|---|---|---|---|---|---|---|
| None (0) | 4024 | Referent | Referent | Referent | Referent | Referent | Referent |
| Low (0.04 – 43.3) | 3178 | 15 (9.1, 21)* | 37 (27, 47)* | −1.3 (−5.9, 3.5) | −2.4 (−10, 6.1) | −2.3 (−8.3, 4.1) | −0.8 (−5.4, 3.9) |
| High (43.4 – 100) | 3051 | 31 (22, 40)* | 74 (60, 901)* | −5.8 (−11, 0.1) | −6.9 (−14, 1.0) | −3.0 (−8.7, 3.0) | −2.7 (−7.8, 2.8) |
| p for trend | <0.0001 | <0.0001 | 0.056 | 0.088 | 0.314 | 0.333 | |
|
| |||||||
| Contribution (%) | 45 | 30 | 16 | 4 | 2 | 2 | |
Covariates: Sex, age, race/ethnicity, poverty-to-income ratio (PIR), education (adults only), NHANES survey cycle, and time of sampling session.
Low and high dietary intake divided at the weighted median of participants who consumed food away from home the prior day. None includes participants who did not consume any food away from home (i.e. 100% of their calories were consumed from food at home, such as food purchased from the grocery store.
p<0.05
Consumption of food away from home was positively associated with cumulative phthalates exposure across all supplementary metrics that evaluated alternate approaches to urine dilution correction and daily intake estimation (Supplementary Table S3). However, the magnitude of associations varied across models. Adjusted associations for daily intake metrics, including the main outcome variable (Σandrogen-disruptor) and the metric that uses urine flow rate rather than creatinine to estimate phthalate daily intakes (Σurine-flow), were more similar to each other and larger than those observed for metabolite-based metrics. Of the three metabolite-based metrics, the metric that applied RPF weights directly to non-creatinine-corrected urinary metabolite concentrations (Σmetab-rpf) generally produced the strongest associations, followed by the metric combining RPF-weighted metabolite excretion rates (Σexrate-rpf). The metric that applied RPF weights directly to urinary metabolite concentrations and adjusted for creatinine as an independent covariate in the multivariable model (Σmetab-rpf + creat) generally produced the weakest associations. Associations were slightly attenuated when RPFs were applied directly to molar mass-based metrics (i.e. molar urinary concentrations, molar excretion rates, and molar intake rates) (Supplementary Table S3).
Results from the sensitivity analysis evaluating the continuous association between TEI and cumulative phthalates daily intake (Σandrogen-disruptor), stratified by consumption of food at home and food away from home, were reasonably consistent with our main analysis among children, adolescents, and adults 20-59 years old (Supplementary Table S4). Results were less consistent for older adults. Among older adults, an increase of 100 kcals was associated with 1.6% (95% CI: −2.6%, −0.58%) lower Σandrogen-disruptor levels among consumers (p = 0.002) and 0.83% (95% CI: 0.13%, 1.5%) higher Σandrogen-disruptor levels among non-consumers (p = 0.02) (Supplementary Table S4).
Finally, including BMI in core regression models did not substantially influence associations between dietary intake of food away from home and Σandrogen-disruptor levels (data not shown).
4. Discussion
In this cross-sectional study of NHANES participants sampled between 2005 and 2014, dining out was positively associated with potency-adjusted exposure to multiple phthalates across age groups in the U.S. general population. Among adolescents, consuming food away from home the prior day was associated with as much as 55% higher cumulative phthalates exposure compared to eating food at home only (such as food purchased from the grocery store).
To our knowledge this is the first study to compare biologically relevant cumulative phthalates exposure between individuals eating foods from multiple sources away from home to those eating predominantly store-bought foods. While prior work has shown that phthalate exposures are associated with fast food intake among the U.S. general population,33 this study demonstrates that other sources of food away from home, such as full-service restaurants and cafeterias, are also important sources of phthalates exposure. It also shows, for the first time, that the contribution of specific food sources varies by age group. For example, cafeteria food intake was associated with a smaller percent difference in phthalates exposure among children than teenagers and adults, even though the proportion of children who consumed cafeteria food was seven times higher than adults and double that of adolescents. This suggests that food choices in the cafeteria setting may vary with age. Indeed, adolescents have greater autonomy than younger children in their cafeteria food choices, and high-school meals are generally richer in fat and lower in nutritional quality than those prepared for younger kids.30
Children in our study had substantially higher cumulative phthalates exposure than other age groups (70% higher than older adults), despite the fact that adolescents and adults consumed more overall calories from restaurants and/or fast food establishments the prior day. This finding is consistent with previous research on phthalates in children, which has suggested that several age-dependent biological and behavioral differences may explain the disparity.63,64 For example, children consume a higher proportion of food to body size, and phthalate metabolism varies with age.63–65 Also, younger kids may ingest more phthalates from consumer and personal care products that settle in house dust by playing on the floor and engaging in hand-to-mouth activity.66–68 Children also eat more snack foods than older groups69 and may be consuming additional phthalates from processed or packaged foods eaten at home. For example, a recent analysis by the Coalition for Safer Food Processing and Packaging detected higher phthalate levels in processed macaroni and cheese powder than in other U.S. cheese products.70
Older adults consumed fewer calories than younger groups in this study (with the highest TEI reported at ~ 6300 kcals compared to > 9000 kcals in adolescents and younger adults), yet unlike other groups their TEI was negatively associated with phthalates exposure among dining out consumers in the stratified sensitivity analysis. Possible reasons for this result could be that differential food preferences and/or use of meal services influence the types of food consumed by older adults (e.g. ordering a salad instead of a sandwich at a restaurant, eating nutrition-oriented meals provided by Meals on Wheels, etc.),71,72 and older adults who dine out appear to consume more calories overall compared to those who do not dine out (~130 more kcals on average in this study). Eating more calories away from home would reduce the estimate of association among dining out consumers if TEI is negatively associated with phthalates, as we observed in our main analysis. Moreover, a linear model with TEI and phthalates exposure may not be appropriate for this age group; we did not find a linear trend across TEI groups in our main analysis (with TEI modeled categorically), and we observed minimal linearity when we modeled TEI continuously in the sensitivity analysis. Finally, adults are more likely to use medications or require outpatient services and hospitalizations as they age,73 which may be important non-dietary sources of phthalate exposures that we were unable to account for in this study.74,75 Future investigation of phthalate exposures among older adults should assess the role of unique dietary patterns in conjunction with non-dietary exposure sources in this subgroup.
Interestingly, we found that particular foods, especially sandwiches (i.e. cheeseburgers), were associated with increased cumulative phthalates exposure only if consumed from fast food/restaurant, cafeteria, or other dining out establishments. While previous studies have identified important at-home sources of dietary phthalates exposure, including store-bought meats, dairy products, olive oil, cooking spices, and bread,21,25,76–78 our findings suggest that consuming foods from the grocery store may reduce exposures relative to dining out. However, few studies have evaluated phthalates in restaurant and cafeteria food. Research on pre-cooked and immediately packaged school and hospital meals in Europe and Japan23,26,79 may be relevant for U.S. schools, which are increasingly outsourcing their food preparations off-site.80,81 The influence of cooking methods (i.e. phthalate levels after boiling compared to frying foods)82 may also be pertinent for restaurants or cafeterias that cook foods on-site. However, many data gaps regarding specific contamination pathways in U.S. food industries have yet to be addressed.
Food contamination sources are difficult to distinguish between industries because phthalates may enter the food supply from many different food contact materials both upstream (e.g. processing equipment such as conveyer belts and industrial tubing) and downstream (e.g. food preparation products such as plastic wrap and food handling gloves).14,22,26,83 Focusing on key differences between industries may help guide research directions going forward. For example, compared to large-scale supermarkets, which may have company-owned processing plants and distribution centers, restaurants and cafeterias typically rely on local distributors that potentially receive products from a variety of sources.81 Less in-house control may be one factor that contributes to increased phthalates contamination along dining out supply channels, though more research is required to make this determination. High fat meals and large portion sizes typically offered by the U.S. food service industry may further compound dietary exposures along this pathway,29,30 which should also be evaluated in future work.
Despite our findings, highly processed store-bought foods may also contribute to phthalates exposure. For example, higher phthalate levels in frozen compared to home-made French fries were recently attributed to increased transport activity and contact with factory equipment.82 Prepared foods are also rising in popularity among U.S. supermarkets,81,84,85 and NHANES recently acknowledged that foods purchased from the store are now considered a suboptimal proxy for home preparation due to the proliferation of prepared or ready-to-eat foods among U.S. grocery stores (https://wwwn.cdc.gov/nchs/nhanes/2011-2012/DR1IFF_G.htm). However, we evaluated NHANES survey data from earlier years in which store-bought foods were classified as “prepared away from home” rather than “away from home” and did not observe changes in the association between dining out and cumulative phthalates exposure across time. Moreover, our findings suggest there may be true differences in phthalate exposures between food purchased away from home and food purchased from the store (regardless of where prepared). Future efforts to characterize food production practices that increase dietary phthalates exposure should target prepared, processed, and/or packaged foods across multiple food industries, with particular emphasis placed on fast food/restaurants and cafeterias.
Together, DEHP and DiNP comprised 75% of our cumulative phthalates exposure metric. Although DEHP contributed more to the metric than DiNP (45% compared to 30%, respectively), DiNP was more strongly associated with dining out (74% compared to 30%, respectively). This is consistent with previous research suggesting that fast food consumption may be a unique source of DiNP exposure.34 Although fewer studies have assessed DiNP sources, especially in the United States, evidence suggests that DiNP is replacing DEHP in the global plasticizers market and in food contact materials specifically.18,33,86 Additionally, U.S. biomonitoring trends have reported DiNP increases (coupled with steady DEHP declines) in recent years.12,40 In this study, we observed decreased cumulative phthalates exposure over time, likely due to DiNP’s lower relative potency (though it is a recognized anti-androgen of concern),16,86 while the association between dining out and cumulative phthalates exposure remained relatively constant. Thus, if dining out trends remain constant or increase over time, food service products may continue to be important sources of anti-androgenic exposure going forward. Moreover, while DnBP, DiBP, BBzP, and DEP were not individually associated with dining out in our study, diet is suspected to be the dominant exposure pathway for most phthalates.16,19 Thus, future studies should assess food contamination pathways more broadly for all phthalates, with a focus on emerging anti-androgens such as DiNP. Forthcoming work should also examine sources of phthalates in combination, which may inform strategies to reduce biologically relevant cumulative exposures.5,6
Although our cumulative metric is biologically meaningful, several limitations should be addressed in future research. First, the method assumes that relative potencies are accurately predicted from toxicology studies and are appropriate for multiple endpoints. While fetal testosterone inhibition predicts additive effects on other male developmental endpoints in laboratory studies, reproductive organ malformations,4,6 more work is needed to examine the method’s applicability to other hormone-mediated outcomes. For example, phthalates may influence risk of obesity and diabetes by disrupting androgen (or even thyroid hormone) activity in adult men and women,87–89 and their affinity for binding the peroxisome proliferator-activated receptor (PPAR-γ) has gained more recent attention, particularly since PPAR-γ effects may contribute to fetal programming of metabolic disease.90–93 However, current data gaps preclude our ability to compare anti-androgenic and PPAR-γ potencies, since metabolic toxicology studies vary in terms of whether experiments were performed with phthalate metabolites or parent compounds, mouse or human cells, and so forth.94–98 Mixture studies on PPAR-γ could resolve these issues and inform whether the metric is appropriate for metabolic disease. Relative potency data for phthalates and thyroid function during pregnancy may also help discern the method’s applicability to neurological outcomes in children, since thyroid hormones are critical for fetal brain development.99 Moreover, while our results were robust across alternate cumulative exposure metrics, future efforts to identify optimal approaches for urine dilution correction and daily intake estimation that reduce potential bias in regression models47,50,62 would provide useful guidance for exposure scientists, risk assessors, and epidemiologists who seek to evaluate phthalate exposures in combination.
Other study limitations include the cross-sectional design of NHANES, which makes causality difficult to determine and can lead to misclassification of phthalate exposures (i.e. through use of spot samples that may not capture within-person variation over time).100,101 However, we assessed dietary intake specifically for the day prior to urine sample collection, providing temporal support for a causal association between dining out and phthalates exposure. The NHANES sampling strategy also minimizes the effect of this misclassification at the population-level by collecting urine from study participants at varying points throughout the day (morning, afternoon, and evening). The 24-hour dietary recall survey is also prone to misclassification from inaccurate self-reporting of foods and/or portion sizes,56,59,102 but this form of bias would likely under-estimate associations in this study. For example, the weaker associations we observed in children might be partially attributable to proxy-assisted interviews in which kids with high phthalates intake may not be honest about what foods they consumed away from home when interviewed in front of their parents. Converting self-reported foods into kcals may introduce additional uncertainty due to assumptions about accurate recording and interpretation of the amount, size, and mass-to-energy conversion of reported foods.102 While our nutrient density approach to energy adjustment (converting to kcals and dividing by TEI) has been shown to reduce this bias,56–59 a longitudinal dietary intervention or elimination study may be more appropriate for future identification of dietary sources. Finally, although we examined foods that are likely to contain phthalates regardless of their source (i.e. sandwiches containing meat and cheese), our results may be influenced by residual confounding, since meal type is often correlated with source. For example, ~60% of cheeseburgers and 50% of poultry sandwiches consumed by the U.S. population are from fast food restaurants whereas ~75% of cold cut sandwiches are from the store.103 Future research should assess whether different sandwich types, which may be more or less likely to be prepared away from rather than at home (with store-bought ingredients), may confound associations between dining out and cumulative phthalates exposure.
Despite these limitations, our study provides important information about dietary sources of cumulative phthalates exposure across age groups in the U.S. general population. We found that relative to food consumed from a grocery store (and potentially prepared at home), food consumed from full-service restaurants, fast food establishments, and cafeterias (prepared away from home) was associated with increased potency-adjusted exposure to multiple anti-androgenic phthalates. Efforts should be made to identify modifiable production practices that mitigate food product contamination and ultimately remove phthalates from the food supply. The effectiveness of eating and preparing food more frequently at home as a way to reduce biologically relevant phthalate exposures should also be examined in future studies, with continued emphasis placed on less processed and packaged store-bought foods.
Supplementary Material
Highlights.
Dining out (restaurants and cafeterias) may increase cumulative phthalates exposure
Sandwiches purchased away from home are associated with higher phthalate levels
Two thirds of the U.S. population eat at least some food outside the home daily
In general, children have higher phthalate levels than adolescents or adults
Acknowledgments
We would like to thank Dr. Kimberly Robien at George Washington University Milken Institute School of Public Health for providing expertise in nutritional epidemiology.
Funding
This work was supported by the U.S. Environmental Protection Agency Science to Achieve Results (STAR) [grant numbers FP-91750801-1 and RD-83543301], the National Science Foundation Systems Approach to Green Energy [grant number 1144885], Passport Foundation, and the National Institute of Environmental Health Sciences [grant numbers R00ES019881 and P01ES022841].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
None
References
- 1.Gore AC, Chappell VA, Fenton SE, et al. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015;36(6):E1–E150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization/UNEP. State of the Science of Endocrine Disrupting Chemicals 2012. World Health Organization (WHO) and United Nations Environment Programme (UNEP); 2013. p. 296. http://www.who.int/ceh/publications/endocrine/en/. Accessed May 23, 2017. [Google Scholar]
- 3.Attina TM, Hauser R, Sathyanarayana S, et al. Exposure to endocrine-disrupting chemicals in the USA: a population-based disease burden and cost analysis. Lancet Diabetes Endocrinol. 2016;4(12):996–1003. doi: 10.1016/S2213-8587(16)30275-3. [DOI] [PubMed] [Google Scholar]
- 4.Howdeshell KL, Rider CV, Wilson VS, Furr JR, Lambright CR, Gray LE. Dose Addition Models Based on Biologically Relevant Reductions in Fetal Testosterone Accurately Predict Postnatal Reproductive Tract Alterations by a Phthalate Mixture in Rats. Toxicol Sci. 2015;148(2):488–502. doi: 10.1093/toxsci/kfv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Research Council. Phthalates and Cumulative Risk Assessment: The Task Ahead. Washington, D.C.: The National Academies Press; 2008. https://doi.org/10.17226/12528. Accessed October 15, 2011. [PubMed] [Google Scholar]
- 6.Howdeshell KL, Hotchkiss AK, Gray LE., Jr Cumulative effects of antiandrogenic chemical mixtures and their relevance to human health risk assessment. Int J Hyg Environ Health. 2017;220(2, Part A):179–188. doi: 10.1016/j.ijheh.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giulivo M, Lopez de Alda M, Capri E, Barceló D. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ Res. 2016;151:251–264. doi: 10.1016/j.envres.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered Semen Quality in Relation to Urinary Concentrations of Phthalate Monoester and Oxidative Metabolites. Epidemiol Novemb 2006. 2006;17(6):682–691. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- 9.James-Todd T, Stahlhut R, Meeker JD, et al. Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ Health Perspect. 2012;120(9):1307–1313. doi: 10.1289/ehp.1104717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of Urinary Phthalate Metabolites Are Associated with Increased Waist Circumference and Insulin Resistance in Adult U.S. Males. Environ Health Perspect. 2007;115(6):876–882. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trasande L, Zoeller RT, Hass U, et al. Estimating Burden and Disease Costs of Exposure to Endocrine-Disrupting Chemicals in the European Union. J Clin Endocrinol Metab. 2015;100(4):1245–1255. doi: 10.1210/jc.2014-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: Findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Perspect. 2014;122(3):235–241. doi: 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang K, Noonan GO, Begley TH. Determination of 2,6-diisopropylnaphthalene (DIPN) and n-dibutylphthalate (DBP) in food and paper packaging materials from US marketplaces. Food Addit Contam Part A. 2008;25(11):1416–1423. doi: 10.1080/02652030802163380. [DOI] [PubMed] [Google Scholar]
- 14.Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health. 2014;13(1):43. doi: 10.1186/1476-069X-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodruff TJ, Zota AR, Schwartz JM. Environmental Chemicals in Pregnant Women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119(6) doi: 10.1289/ehp.1002727. http://ehp03.niehs.nih.gov/article/fetchArticle.action?articleURI=info%3Adoi%2F10.1289%2Fehp.1002727. Accessed July 24, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CHAP. Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives. Bethesda, MD: U.S. Consumer Product Safety Commission, Directorate for Health Sciences; 2014. http://www.cpsc.gov/en/Regulations-Laws–Standards/Statutes/The-Consumer-Product-Safety-Improvement-Act/Phthalates/Chronic-Hazard-Advisory-Panel-CHAP-on-Phthalates/ [Google Scholar]
- 17.Koch HM, Lorber M, Christensen KL, Palmke C, Koslitz S, Bruning T. Identifying sources of phthalate exposure with human biomonitoring: Results of a 48h fasting study with urine collection and personal activity patterns. Int J Hyg Env Health. 2013 Jan; doi: 10.1016/j.ijheh.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Sakhi AK, Lillegaard ITL, Voorspoels S, et al. Concentrations of phthalates and bisphenol A in Norwegian foods and beverages and estimated dietary exposure in adults. Environ Int. 2014;73:259–269. doi: 10.1016/j.envint.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K. What Are the Sources of Exposure to Eight Frequently Used Phthalic Acid Esters in Europeans? Risk Anal. 2006;26(3):803–824. doi: 10.1111/j.1539-6924.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 20.Trasande L, Sathyanarayana S, Messito M, Gross R, Attina T, Mendelsohln A. Phthalates and the diets of US children and adolescents. Env Res. 2013;126:84–90. doi: 10.1016/j.envres.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Sathyanarayana S, Alcedo G, Saelens B, et al. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. J Expo Sci Env Epidemiol. 2013;23(4):378–384. doi: 10.1038/jes.2013.9. [DOI] [PubMed] [Google Scholar]
- 22.Cao X. Phthalate esters in foods: sources, occurrence, and analytical methods. Compr Rev Food Sci Food Saf. 2010;9:21–43. doi: 10.1111/j.1541-4337.2009.00093.x. [DOI] [PubMed] [Google Scholar]
- 23.Cirillo T, Fasano E, Castaldi E, Montuori P, Amodio Cocchieri R. Children’s Exposure to Di(2-ethylhexyl)phthalate and Dibutylphthalate Plasticizers from School Meals. J Agric Food Chem. 2011;59(19):10532–10538. doi: 10.1021/jf2020446. [DOI] [PubMed] [Google Scholar]
- 24.Petersen JH, Jensen LK. Phthalates in soft PVC products used in food production equipment and in other food contact materials on the Danish and the Nordic Market 2013-2014. Int J Food Contam. 2016;3(1):3. doi: 10.1186/s40550-016-0026-6. [DOI] [Google Scholar]
- 25.Rudel RA, Gray JM, Engel CL, et al. Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention. Environ Health Perspect. 2011;119(7):914–920. doi: 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsumura Y, Ishimitsu S, Saito I, Sakai H, Tsuchida Y, Tonogai Y. Estimated daily intake of plasticizers in 1-week duplicate diet samples following regulation of DEHP-containing PVC gloves in Japan. Food Addit Contam. 2003;20(4):317–324. doi: 10.1080/0265203031000122021. [DOI] [PubMed] [Google Scholar]
- 27.Tsumura Y, Ishimitsu S, Saito I, Sakai H, Kobayashi Y, Tonogai Y. Eleven phthalate esters and di(2-ethylhexyl) adipate in oneweek duplicate diet samples obtained from hospitals and their estimated daily intake. Food Addit Contam. 2001;18(5):449–460. doi: 10.1080/02652030117484. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Department of Agriculture, Economic Research Service. Food Expenditures. https://www.ers.usda.gov/data-products/food-expenditures/food-expenditures/#FoodExpenditures. Published 2016. Accessed April 29, 2017.
- 29.Todd JE, Mancino L, Lin BH. The Impact of Food Away From Home on Adult Diet Quality. U.S. Department of Agriculture, Economic Research Service; 2010. https://www.ers.usda.gov/webdocs/publications/46352/8170_err90_1_.pdf?v=41056. [Google Scholar]
- 30.Mancino L, Todd JE, Guthrie J, Biing-Hwan L. How Food Away From Home Affects Children’s Diet Quality. U.S. Department of Agriculture, Economic Research Service; 2010. p. 32. https://www.ers.usda.gov/publications/err104. Accessed April 29, 2017. [Google Scholar]
- 31.Lin B-H, Guthrie J. Nutritional Quality of Food Prepared at Home and Away From Home, 1977-2008. US Department of Agriculture, Economic Research Service; 2012. p. 24. https://www.ers.usda.gov/publications/pub-details/?pubid=43699. Accessed April 29, 2017. [Google Scholar]
- 32.Drewnowski A, Rehm CD. Energy intakes of US children and adults by food purchase location and by specific food source. Nutr J. 2013;12(1) doi: 10.1186/1475-2891-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zota AR, Phillips CA, Mitro SD. Population in NHANES. 10. Vol. 124. Environ Health Perspect; 2016. Recent Fast Food Consumption and Bisphenol A and Phthalates Exposures among the U.S; pp. 2003–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watkins DJ, Eliot M, Sathyanarayana S, et al. Variability and Predictors of Urinary Concentrations of Phthalate Metabolites during Early Childhood. Environ Sci Technol. 2014;48(15):8881–8890. doi: 10.1021/es501744v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bononi M, Tateo F. Identification of diisobutyl phthalate (DIBP) suspected as possible contaminant in recycled cellulose for take-away pizza boxes. Packag Technol Sci. 2009;22(1):53–58. doi: 10.1002/pts.805. [DOI] [Google Scholar]
- 36.Lopez-Espinosa M-J, Granada A, Araque P, et al. Oestrogenicity of paper and cardboard extracts used as food containers. Food Addit Contam. 2007;24(1):95–102. doi: 10.1080/02652030600936375. [DOI] [PubMed] [Google Scholar]
- 37.Johnson C, Paulose-Ram R, Ogden C, et al. National Health and Nutrition Examination Survey: Analytic Guidelines, 1999–2010. National Center for Health Statistics, Department of Health and Human Services; 2013. http://www.cdc.gov/nchs/data/series/sr_01/sr01_056.pdf. [PubMed] [Google Scholar]
- 38.Silva MJ, Samandar E, Preau JL, Jr, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;860(1):106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Langlois É, LeBlanc A, Simard Y, Thellen C. Accuracy Investigation of Phthalate Metabolite Standards. J Anal Toxicol. 2012;36(4):270–279. doi: 10.1093/jat/bks016. [DOI] [PubMed] [Google Scholar]
- 40.Varshavsky JR, Zota AR, Woodruff TJ. A novel method for calculating potency-weighted cumulative phthalates exposure with implications for identifying racial/ethnic disparities among U.S. reproductive-aged women in NHANES 2001–2012. Environ Sci Technol. 2016;50(19):10616–10624. doi: 10.1021/acs.est.6b00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rider CV, Furr JR, Wilson VS, Gray LE. Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. Int J Androl. 2010;33(2):445–462. doi: 10.1111/j.1365-2605.2009.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.David RM. Exposure to Phthalate Esters. Environ Health Perspect. 2000;108(10):A440–A443. doi: 10.1289/ehp.108-a440a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marsee K, Woodruff TJ, Axelrad DA, Calafat AM, Swan SH. Estimated Daily Phthalate Exposures in a Population of Mothers of Male Infants Exhibiting Reduced Anogenital Distance. Environ Health Perspect. 2006;114(6):805–809. doi: 10.1289/ehp.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Academy of Sciences’ National Research Council. Human Biomonitoring for Environmental Chemicals. Washington, D.C.: The National Academies Press; 2006. http://www.nap.edu/openbook.php?record_id=11700. [Google Scholar]
- 45.Anderson WA, Castle L, Scotter MJ, Massey RC, Springall C. A biomarker approach to measuring human dietary exposure to certain phthalate diesters. Food Addit Contam. 2001;18:1068–1074. doi: 10.1080/02652030110050113. [DOI] [PubMed] [Google Scholar]
- 46.Anderson WA, L C, Hird S, Jeffery J, Scotter MJ. A twenty-volunteer study using deuterium labelling to determine the kinetics and fractional excretion of primary and secondary urinary metabolites of di-2-ethylhexyl phthalate and di-iso-nonyl phthalate. Food Chem Toxicol. 2011;49:2022–2029. doi: 10.1016/j.fct.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary Creatinine Concentrations in the US Population: Implications for Urinary Biologic Monitoring Measurements. 2. Vol. 113. Environ Health Perspect; 2005. pp. 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohn MC, Parham F, Masten SA, Portier CJ, Shelby MD, Brock JW, Needham LL. Human exposure estimates for phthalates. 10. Vol. 108. Environ Health Perspect; 2000. pp. A440–A442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Remer T, Neubert A, Maser-Gluth C. Anthropometry-based reference values for 24-h urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am J Clin Nutr. 2002;75(3):561–569. doi: 10.1093/ajcn/75.3.561. [DOI] [PubMed] [Google Scholar]
- 50.Hays SM, Aylward LL, Blount BC. Variation in Urinary Flow Rates According to Demographic Characteristics and Body Mass Index in NHANES: Potential Confounding of Associations between Health Outcomes and Urinary Biomarker Concentrations. Environ Health Perspect. 2015;123(4):293–300. doi: 10.1289/ehp.1408944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.CDC (Centers for Disease Control and Prevention) MEC In-Person Dietary Interviewers Procedures Manual. 2002 https://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/DIETARY_MEC.pdf.
- 52.CDC (Centers for Disease Control and Prevention) NHANES 2013-2014: Dietary Interview - Individual Foods, First Day Data Documentation, Codebook, and Frequencies. https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/DR1IFF_H.htm. Published 2013. Accessed April 29, 2017.
- 53.U.S. Department of Agriculture, Agricultural Research Service. What We Eat In America/NHANES Overview. https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweianhanes-overview/. Accessed April 29, 2017.
- 54.U.S. Department of Agriculture, Agricultural Research Service. MyPyramid Equivalents Database for USDA Survey Food Codes Version 1.0. https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/mypyramid-equivalents-product-downloads/. Accessed July 11, 2017.
- 55.Johns LE, Cooper GS, Galizia A, Meeker JD. Exposure Assessment Issues in Epidemiology Studies of Phthalates. Environ Int. 2015;85:27–39. doi: 10.1016/j.envint.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Cancer Institute. Learn More about Energy Adjustment | Dietary Assessment Primer. https://dietassessmentprimer.cancer.gov/learn/adjustment.html. Accessed October 27, 2017.
- 57.National Research Council. Diet and Health: Implications for Reducing Chronic Disease Risk. Washington, D.C.: The National Academies Press; 1989. [DOI] [PubMed] [Google Scholar]
- 58.Rhee JJ, Cho E, Willett WC. Energy-adjustment of nutrient intakes is preferable to adjustment using body weight and physical activity in epidemiologic analyses. Public Health Nutr. 2014;17(5):1054–1060. doi: 10.1017/S1368980013001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kipnis V, Subar AF, Midthune D, et al. Structure of Dietary Measurement Error: Results of the OPEN Biomarker Study. Am J Epidemiol. 2003;158(1):14–21. doi: 10.1093/aje/kwg091. [DOI] [PubMed] [Google Scholar]
- 60.U.S. Department of Agriculture, Health and Human Services. 2015-2020 Dietary Guidelines for Americans. (8th) 2015 https://health.gov/dietaryguidelines/2015/guidelines/. Accessed April 29, 2017.
- 61.Sebastian RS, Wilkinson Enns C, Goldman JD, Hoy MK, Moshfegh AJ. Sandwiches Are Major Contributors of Sodium in the Diets of American Adults: Results from What We Eat in America, National Health and Nutrition Examination Survey 2009–2010. J Acad Nutr Diet. 2015;115(2):272–277. doi: 10.1016/j.jand.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 62.Christensen K, Sobus J, Phillips M, Blessinger T, Lorber M, Tan Y-M. Changes in epidemiologic associations with different exposure metrics: A case study of phthalate exposure associations with body mass index and waist circumference. Environ Int. 2014;73:66–76. doi: 10.1016/j.envint.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 63.Koch HM, Drexler H, Angerer J. Internal exposure of nursery-school children and their parents and teachers to di(2-ethylhexyl)phthalate (DEHP) Int J Hyg Environ Health. 2004;207(1):15–22. doi: 10.1078/1438-4639-00270. [DOI] [PubMed] [Google Scholar]
- 64.Wittassek M, Koch H, Angerer J, Bruning T. Assessing exposure to phthalates - the human biomonitoring approach. Mol Nutr Food Res. 2011;55:7–31. doi: 10.1002/mnfr.201000121. [DOI] [PubMed] [Google Scholar]
- 65.Frederiksen H, Aksglaede L, Sorensen K, Skakkebaek NE, Juul A, Andersson A-M. Urinary excretion of phthalate metabolites in 129 healthy Danish children and adolescents: Estimation of daily phthalate intake. Environ Res. 2011;111(5):656–663. doi: 10.1016/j.envres.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Mitro SD, Dodson RE, Singla V, et al. Consumer Product Chemicals in Indoor Dust: A Quantitative Meta-analysis of U.S. Studies. Environ Sci Technol. 2016;50(19):10661–10672. doi: 10.1021/acs.est.6b02023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beko G, Weschler CJ, Langer S, Callesen M, Toftum J, Clausen G. Children’s phthalate intakes and resultant cumulative exposures estimated from urine compared with estimates from dust ingestion, inhalation and dermal absorption in their homes and daycare centers. PLoS One. 2013;8(4):e62442. doi: 10.1371/journal.pone.0062442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Becker K, Seiwert M, Angerer J, et al. DEHP metabolites in urine of children and DEHP in house dust. Int J Hyg Environ Health. 2004;207(5):409–417. doi: 10.1078/1438-4639-00309. [DOI] [PubMed] [Google Scholar]
- 69.Trends in Snacking Among U. S. Children. Health Aff Proj Hope. 2010;29(3):398–404. doi: 10.1377/hlthaff.2009.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coalition for Safer Food Processing and Packaging. Testing Finds Industrial Chemical Phthalates in Cheese. 2017 http://kleanupkraft.org/data-summary.pdf.
- 71.Magdalena Krondl PhD R, MS PC, Daisy Lau PhD R. Helping Older Adults Meet Nutritional Challenges. J Nutr Elder. 2008;27(3–4):205–220. doi: 10.1080/01639360802261755. [DOI] [PubMed] [Google Scholar]
- 72.Drewnowski A, Shultz JM. Impact of aging on eating behaviors, food choices, nutrition, and health status. J Nutr Health Aging. 2001;5(2):75–79. [PubMed] [Google Scholar]
- 73.Cameron KA, Song J, Manheim LM, Dunlop DD. Gender Disparities in Health and Healthcare Use Among Older Adults. J Womens Health. 2010;19(9):1643–1650. doi: 10.1089/jwh.2009.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kelley KE, Hernández-Díaz S, Chaplin EL, Hauser R, Mitchell AA. Identification of Phthalates in Medications and Dietary Supplement Formulations in the United States and Canada. Environ Health Perspect. 2012;120(3):379–384. doi: 10.1289/ehp.1103998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yan X, Calafat A, Lashley S, et al. Phthalates Biomarker Identification and Exposure Estimates in a Population of Pregnant Women. Hum Ecol Risk Assess HERA. 2009;15(3):565–578. doi: 10.1080/10807030902892554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Colacino J, Harris T, Schecter A. Dietary intake is associated with phthalate body burden in a nationally representative sample. Env Health Perspect. 2010;118:998–1003. doi: 10.1289/ehp.0901712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lorber M, Calafat AM. Dose reconstruction of di(2-ethylhexyl) phthalate using a simple pharmacokinetic model. Env Health Perspect. 2012;120(12):1705–1710. doi: 10.1289/ehp.1205182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schecter A, Lorber M, Guo Y, et al. Phthalate concentrations and dietary exposure from food purchased in New York State. Env Health Perspect. 2013;121(4):473–494. 494e1–4. doi: 10.1289/ehp.1206367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cirillo T, Fasano E, Esposito F, Montuori P, Amodio Cocchieri R. Di(2-ethylhexyl)phthalate (DEHP) and di-n-butylphthalate (DBP) exposure through diet in hospital patients. Food Chem Toxicol. 2013;51:434–438. doi: 10.1016/j.fct.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 80.Komisar L. School Lunches and the Food Industry. The New York Times. http://www.nytimes.com/2011/12/04/opinion/sunday/school-lunches-and-the-food-industry.html. Published December 3, 2011. Accessed July 31, 2017.
- 81.Davies T, Konisky DM. Environmental Implications of the Foodservice and Food Retail Industries. Washigton, D.C.: Resources for the Future; 2000. http://www.rff.org/files/sharepoint/WorkImages/Download/RFF-DP-00-11.pdf. [Google Scholar]
- 82.Fierens T, Vanermen G, Van Holderbeke M, Henauw S, Sioen I. Effect of cooking at home on the levels of eight phthalates in foods. Food Chem Toxicol. 2012;50:4428–4435. doi: 10.1016/j.fct.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 83.Fierens T, Servaes K, Van Holderbeke M, Geerts L, Hanauw S, Sioen I. Analysis of phthalates in food products and packaging materials sold on the Belgian market. Food Chem Toxicol. 2012;50:2575–2583. doi: 10.1016/j.fct.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 84.Millennials driving sales of grocery prepared foods. Restaur Hosp. 2016 Jul; http://www.restaurant-hospitality.com/consumer-trends/millennials-driving-sales-grocery-prepared-foods.
- 85.Sebastian RS, Enns CW, Steinfeldt LC, Goldman JD, Moshfegh AJ. Monitoring Sodium Intake of the US Population: Impact and Implications of a Change in What We Eat in America, National Health and Nutrition Examination Survey Dietary Data Processing. J Acad Nutr Diet. 2013;113(7):942–949. doi: 10.1016/j.jand.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 86.ECHA (European Chemicals Agency) Evaluation of New Scientific Evidence Concerning DINP and DIDP: In Relation to Entry 52 of Annex XVII to REACH Regulation (EC) No 1907/2006. 2012 https://echa.europa.eu/documents/10162/31b4067e-de40-4044-93e8-9c9ff1960715.
- 87.Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85(5):1319–1340. doi: 10.1016/j.fertnstert.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 88.Lovejoy JC, Sainsbury A, the Stock Conference 2008 Working Group Sex differences in obesity and the regulation of energy homeostasis. Obes Rev. 2009;10(2):154–167. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- 89.Knudsen N, Laurberg P, Rasmussen LB, et al. Small Differences in Thyroid Function May Be Important for Body Mass Index and the Occurrence of Obesity in the Population. J Clin Endocrinol Metab. 2005;90(7):4019–4024. doi: 10.1210/jc.2004-2225. [DOI] [PubMed] [Google Scholar]
- 90.Adibi JJ, Buckley JP, Lee MK, et al. Maternal urinary phthalates and sex-specific placental mRNA levels in an urban birth cohort. Environ Health. 2017;16 doi: 10.1186/s12940-017-0241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Desvergne B, Feige JN, Casals-Casas C. PPAR-mediated activity of phthalates: A link to the obesity epidemic? Mol Cell Endocrinol. 2009;304(1–2):43–48. doi: 10.1016/j.mce.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 92.Zhang W, Shen X-yue, Zhang W, Chen H, Xu W, Wei W. Di-(2-ethylhexyl) phthalate could disrupt the insulin signaling pathway in liver of SD rats and L02 cells via PPARγ. Toxicol Appl Pharmacol. 2017;316:17–26. doi: 10.1016/j.taap.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 93.Stojanoska MM, Milosevic N, Milic N, Abenavoli L. The influence of phthalates and bisphenol A on the obesity development and glucose metabolism disorders. Endocrine. 2016 Nov; doi: 10.1007/s12020-016-1158-4. [DOI] [PubMed] [Google Scholar]
- 94.Kaya T, Mohr SC, Waxman DJ, Vajda S. Computational Screening of Phthalate Monoesters for Binding to PPARγ. Chem Res Toxicol. 2006;19(8):999–1009. doi: 10.1021/tx050301s. [DOI] [PubMed] [Google Scholar]
- 95.Bility MT, Thompson JT, McKee RH, et al. Activation of Mouse and Human Peroxisome Proliferator-Activated Receptors (PPARs) by Phthalate Monoesters. Toxicol Sci. 2004;82(1):170–182. doi: 10.1093/toxsci/kfh253. [DOI] [PubMed] [Google Scholar]
- 96.Hurst CH, Waxman DJ. Activation of PPAR and PPAR by Environmental Phthalate Monoesters. Toxicol Sci. 2003;74(2):297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- 97.Maloney EK, Waxman DJ. trans-Activation of PPARα and PPARγ by Structurally Diverse Environmental Chemicals. Toxicol Appl Pharmacol. 1999;161(2):209–218. doi: 10.1006/taap.1999.8809. [DOI] [PubMed] [Google Scholar]
- 98.Lampen A, Zimnik S, Nau H. Teratogenic phthalate esters and metabolites activate the nuclear receptors PPARs and induce differentiation of F9 cells. Toxicol Appl Pharmacol. 2003;188(1):14–23. doi: 10.1016/S0041-008X(03)00014-0. [DOI] [PubMed] [Google Scholar]
- 99.Woodruff TJ, Zeise L, Axelrad DA, et al. Meeting report: moving upstream-evaluating adverse upstream end points for improved risk assessment and decision-making. Environ Health Perspect. 2008;116(11):1568–1575. doi: 10.1289/ehp.11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aylward LL, Hays SM, Zidek A. Variation in urinary spot sample, 24 h samples, and longer-term average urinary concentrations of short-lived environmental chemicals: implications for exposure assessment and reverse dosimetry. J Expo Sci Environ Epidemiol. 2017;27(6):582–590. doi: 10.1038/jes.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv Nutr Int Rev J. 2016;7(1):121–134. doi: 10.3945/an.115.009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thompson FE, Subar AF. Chapter 1 - Dietary Assessment Methodology. In: Coulston AM, Boushey CJ, Ferruzzi MG, Delahanty LM, editors. Nutrition in the Prevention and Treatment of Disease (Fourth Edition) Academic Press; 2017. pp. 5–48. [DOI] [Google Scholar]
- 103.Sebastian RS, Enns C, Goldman JD, Hoy MK, Moshfegh AJ. Sandwich Consumption by Adults in the US: What We Eat In America, NHANES 2009–2012. Beltsville Human Nutrition Research Center: U.S. Department of Agriculture, Food Surveys Research Group; 2015. https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/DBrief/14_sandwich_consumption_0912.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


