Abstract
Objective
To perform a population-based analysis to first examine the changes in surgeon and hospital procedural volume for hysterectomy over time and then, to explore the association between very low surgeon procedural volume and outcomes.
Methods
All women who underwent hysterectomy in New York State from 2000 to 2014were examined. Surgeons were classified based on the average annual procedural volume as very low-volume surgeons if they performed 1 procedure per year. We used multivariable models to examine the association between very low-volume surgeon status and morbidity, mortality, transfusion, length of stay, and cost.
Results
Among 434,125 women who underwent hysterectomy, very low-volume surgeons accounted for 3197 (41.0%) of the surgeons performing the procedures and operated on 4488 (1.0%) of the patients. The overall complication rates were 32.0% for patients treated by very low-volume surgeons vs. 9.9% for those treated by other surgeons (P<0.001) (aRR=1.97; 95% CI, 1.86–2.09). Specifically, the rates of intraoperative (11.3% vs. 3.1%), surgical site (15.1% vs. 4.1%) and medical complications (19.5% vs. 4.8%), and transfusion (38.5% vs. 11.8%) were higher for very low-volume compared to higher volume surgeons (P<0.001 for all). Patients treated by very low-volume surgeons were also more likely to have a prolonged LOS (62.0% vs. 22.0%) and excessive hospital charges (59.8% vs. 24.6%) compared to higher-volume surgeons (P<0.001 for both). Mortality rate was 2.5% for very low-volume surgeons compared to 0.2% for higher volume surgeons (P<0.001) (aRR=2.89; 95% CI, 2.32–3.61).
Conclusion
A substantial number of surgeons performing hysterectomy are very low-volume surgeons. Performance of hysterectomy by very low-volume surgeons is associated with increased morbidity, mortality, and resource utilization.
Introduction
The relationship between surgical volume and outcomes has long been recognized; patients operated on by high-volume surgeons and at high-volume centers have superior outcomes.1–8 These findings are most marked for operations associated with substantial morbidity and have led to efforts to concentrate some procedures to high-volume surgeons and centers.9,10 Evidence suggests that efforts to regionalize care have been successful for some procedures. More importantly, it has been demonstrated that regionalization of care has led to decreased morbidity and mortality for some operations.11–13 To date, these efforts have primarily focused on high-risk oncologic and cardiovascular surgeries.9,12–15
For gynecologic surgery, a number of trends over the last decade have likely influenced surgical patterns of care. First, the number of hysterectomies performed annually has decreased substantially.16 Second, there has been an impetus to refer many gynecologic procedures including cancer surgeries, pelvic reconstructive operations, and advanced minimally invasive procedures to sub-specialist surgeons.17–19 These trends have likely altered practice patterns for many gynecologic surgeons. Particularly among practitioners for whom gynecology is not the exclusive focus of practice, these trends have the potential to reduce procedural volumes for a significant number of gynecologic surgeons.
Despite these changing trends in gynecologic surgery, relatively little is known about the impact of these changes on surgical volume and outcomes for hysterectomy. We performed a population-based analysis to first examine the changes in surgeon and hospital procedural volume for hysterectomy over time and then, to explore the associations between very low surgeon procedural volume and outcomes.
Materials and Methods
We used the New York Statewide Planning and Research Cooperative System (SPARCS)20 database for our analysis. SPARCS was established in 1979 as a state wide comprehensive data reporting system, collecting information on hospital discharges, inpatient surgeries, ambulatory surgeries, and emergency department admissions. SPARCS allows the identification of physicians across hospitals so that an accurate volume assessment of surgeons can be obtained. Therefore, a specific surgeons’ procedural volume and associated perioperative complications can be evaluated. The SPARCS database has been validated and previously used in a variety of outcomes studies.2,21 The study used de-identified data and was deemed exempt by the Columbia University Institutional Review Board.
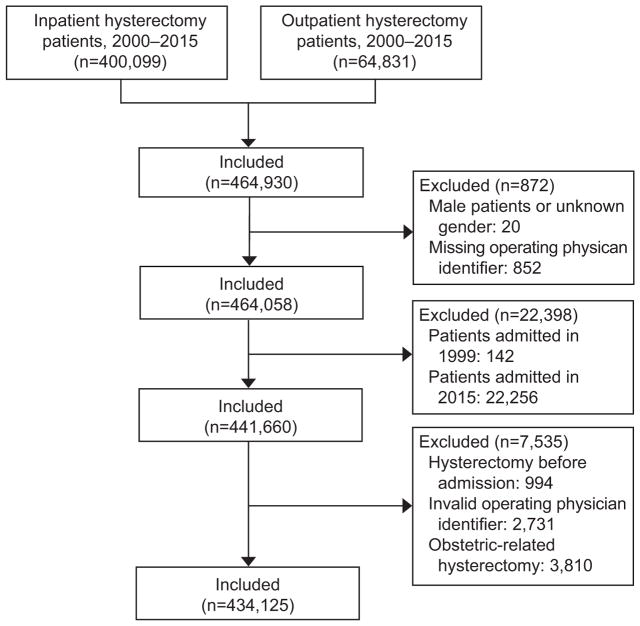
Women who underwent hysterectomy from 2000–2014 were identified for analysis. Procedures were selected based on ICD-9 and CPT coding with the route of hysterectomy stratified as abdominal, laparoscopic, robotic-assisted, or vaginal (Appendix 1, available online at http://links.lww.com/xxx). The primary operating physician of each patient was captured. Those patients missing a physician identification number (n=852) were excluded from the analysis. Additionally, patients who underwent obstetric hysterectomy were excluded from the analysis (n=3,810) (Figure 1).
Figure 1.
Flowchart of cohort selection.
We calculated the average annual procedural volume of each operating physician in the cohort. For each physician, we determined the average annualized volume as the sum of all hysterectomies performed by a given physician divided by the number of years in which the physician performed at least 1 operation. As each physician has a unique identification number, the estimation of volume includes all procedures performed at any hospital in the state of New York.
Physicians were then classified based on annualized volume as very low volume surgeons if their annualized procedural volume was 1, or as higher-volume surgeons if their annualized volume was >1 as has been previously reported.21 In a similar fashion, each hospital’s annualized hysterectomy volume was calculated. Hospitals were stratified into tertiles with an approximately equal number of facilities: low volume (≤40 hysterectomies per year), intermediate (40–116 hysterectomies/year) and high volume (>116 hysterectomies per year).
Demographic data analyzed included age (<40, 40–49, 50–59, 60–69, ≥70 years), race and ethnicity (white, black, Hispanic, other, unknown), and insurance status (private insurance, Medicaid, Medicare, uninsured, none, unknown). Comorbidity was estimated using the Elixhauser Comorbidity Index and categorized as 0, 1, or ≥2.22 Each operation was classified as elective or emergent/urgent. Each hospital’s location was categorized as in New York City versus the remainder of New York State, as previously described.21
Concomitant procedures performed at the time of hysterectomy included anterior colporrhaphy, posterior colporrhaphy, incontinence repair, oophorectomy, colpopexy, exenteration, omentectomy, cytoreduction, lymph node dissection (LND), small bowel resection, colon resection, rectosigmoid resection, liver resection, bladder resection, diaphragm resection and splenectomy. We also analyzed the indications for the procedure and recorded the following diagnoses based on ICD-9 coding: leiomyoma, endometriosis, abnormal menstruation and bleeding, benign neoplasms and cysts, pelvic organ prolapse, endometrial hyperplasia with and without atypia, uterine cancer, cervical cancer, and ovarian/fallopian/peritoneal cancers.23
Outcomes were categorized based on prior studies relevant to hysterectomy complications24–26 and classified into: intraoperative complications (bladder injury, ureteral injury, intestinal injury, vascular injury, other operative injury), surgical site complications (hemorrhage, wound complication, abscess, gastrointestinal complication), and medical complications (vascular thrombosis, urinary complications, pulmonary complications, cardiovascular complications, neurologic complications, shock, infection). A composite of any complication (the occurrence of any intraoperative, surgical site, or medical complication) was also examined. In-hospital mortality was defined as death during the hospitalization in which the hysterectomy was performed. We calculated the length of stay for each procedural hospitalization and analyzed the rates of blood transfusion. An excessive length of stay (LOS) was defined as LOS >75th percentile. We also analyzed hospital charges reported for the procedural hospitalization and defined excessive charges as charges of >75th percentile.
The percentage of very low volume surgeons and patients was reported by year and compared using Cochran-Armitage trend tests. Patient demographics, hospital characteristics, concomitant procedures, and indications for surgery were reported as frequencies stratified by very low-volume surgeons and other surgeons and compared using χ2 tests.
We fit mixed-effects log-Poisson models to examine the predictors of treatment by a very low volume surgeon. The model included route of hysterectomy, elective surgery, age, year, race, insurance status, comorbidity, hospital location and volume, concomitant procedures and indications for surgery. Surgeon and hospital identifiers were included as nested random intercepts to account for clustering. Results are reported as rate ratios (RR) with 95% confidence intervals.
Outcome measures are reported as frequencies. Outcomes among very low volume surgeons were compared to other surgeons using χ2 tests. To further examine the effect of treatment by a very low-volume surgeon on each outcome, we fit mixed-effects log-Poisson models adjusted for the clinical and demographic characteristics described above. Surgeon and hospital identifiers were included as nested random intercepts to account for clustering. We also stratified the cohort by route of hysterectomy, and fit similar models to examine the association between surgeon volume and outcomes.
As sensitivity analyses, we performed a matched propensity score (PS) analysis. The propensity score was estimated as the probability that a patient had a hysterectomy performed by a very low volume surgeon. A multivariable logistic regression model was constructed and assessed based on the goodness of fit. The final model included all clinical and demographic characteristics of the study. Each patient’s propensity score was calculated from the model, and then a 1-to-1 match was performed. These analyses were performed for the entire cohort and for each type of hysterectomy individually. All analyses were performed with SAS version 9.4 (SAS Institute Inc, Cary, North Carolina). All statistical tests were two-sided. A P-value of <0.05 was considered statistically significant.
Results
We identified a total of 434,125 patients (Figure 1). Very low-volume surgeons accounted for 3,197 (41.0%) of the surgeons performing hysterectomy. The percentage of surgeons classified as very low-volume surgeons was 14.8% (95% CI, 13.5%–16.1%) in 2000, gradually declined to 10.4% (95% CI, 9.3%–11.6%) by 2007, and then rose to 13.7% (95% CI, 12.2%–15.1%) by 2014 (P<0.001) (Figure 2). A total of 4,488 (1.0%) patients were treated by very low-volume surgeons while 429,637 (99.0%) had higher volume surgeons (Table 1). The percentage of patients operated on by very low-volume surgeons ranged from 0.8–1.4% during the study period (Figure 2).
Figure 2.
Distribution of very low volume by year. A. Percentage of very low-volume surgeons that performed any hysterectomy by year (P value from Cochran-Armitage trend test, P<.001). B. Percentage of any hysterectomy cases performed by very low-volume surgeons by year (P<.001). C. Percentage of very low-volume surgeons that performed abdominal, robotically assisted, laparoscopic, or vaginal hysterectomy by year (abdominal P<.001, robotically assisted P=.01, laparoscopic P<.001, vaginal P<.001). D. Percentage of abdominal, robotically assisted, laparoscopic, and vaginal hysterectomy cases performed by very low-volume surgeons by year (abdominal P=.001, robotically assisted P=.07, laparoscopic P<.001, vaginal P<.001).
Table 1.
Demographics of the patients who had hysterectomy by surgeon volume.
| Higher Volume Surgeons | Very Low Volume Surgeons | p-value | |||
|---|---|---|---|---|---|
|
| |||||
| N | (%) | N | (%) | ||
| Number of surgeons | 4,600 | (59.0%) | 3,197 | (41.0%) | |
| Number of patients | 429,637 | (99.0) | 4,488 | (1.0) | |
| Hysterectomy | <0.001 | ||||
| Abdominal | 256,202 | (59.6) | 3,582 | (79.8) | |
| Robotic | 17,696 | (4.1) | 89 | (2.0) | |
| Laparoscopic | 93,988 | (21.9) | 484 | (10.8) | |
| Vaginal | 61,751 | (14.4) | 333 | (7.4) | |
| Elective surgery | <0.001 | ||||
| Elective | 339,590 | (79.0) | 2,908 | (64.8) | |
| Emergent/urgent | 36,664 | (8.5) | 1,399 | (31.2) | |
| Other/unknown | 53,383 | (12.4) | 181 | (4.0) | |
| Age (years) | <0.001 | ||||
| <40 | 62,242 | (14.5) | 484 | (10.8) | |
| 40–49 | 186,456 | (43.4) | 1,531 | (34.1) | |
| 50–59 | 93,834 | (21.8) | 1,026 | (22.9) | |
| 60–69 | 49,733 | (11.6) | 665 | (14.8) | |
| ≥70 | 37,372 | (8.7) | 782 | (17.4) | |
| Year of admission | <0.001 | ||||
| 2000 | 29,711 | (6.9) | 422 | (9.4) | |
| 2001 | 30,017 | (7.0) | 375 | (8.4) | |
| 2002 | 31,189 | (7.3) | 368 | (8.2) | |
| 2003 | 29,727 | (6.9) | 358 | (8.0) | |
| 2004 | 30,173 | (7.0) | 330 | (7.4) | |
| 2005 | 29,685 | (6.9) | 299 | (6.7) | |
| 2006 | 28,783 | (6.7) | 296 | (6.6) | |
| 2007 | 27,923 | (6.5) | 263 | (5.9) | |
| 2008 | 27,797 | (6.5) | 288 | (6.4) | |
| 2009 | 28,140 | (6.5) | 256 | (5.7) | |
| 2010 | 28,608 | (6.7) | 227 | (5.1) | |
| 2011 | 27,667 | (6.4) | 227 | (5.1) | |
| 2012 | 27,076 | (6.3) | 235 | (5.2) | |
| 2013 | 27,158 | (6.3) | 256 | (5.7) | |
| 2014 | 25,983 | (6.0) | 288 | (6.4) | |
| Race/ethnicity | <0.001 | ||||
| White | 266,010 | (61.9) | 2,497 | (55.6) | |
| Black | 69,315 | (16.1) | 913 | (20.3) | |
| Hispanic | 39,788 | (9.3) | 454 | (10.1) | |
| Other | 38,437 | (8.9) | 434 | (9.7) | |
| Unknown | 16,087 | (3.7) | 190 | (4.2) | |
| Insurance status | <0.001 | ||||
| None | 10,390 | (2.4) | 115 | (2.6) | |
| Private | 321,633 | (74.9) | 2,875 | (64.1) | |
| Medicare | 54,674 | (12.7) | 979 | (21.8) | |
| Medicaid | 33,654 | (7.8) | 453 | (10.1) | |
| Other | 848 | (0.2) | 16 | (0.4) | |
| Unknown | 8,438 | (2.0) | 50 | (1.1) | |
| Comorbidity | <0.001 | ||||
| 0 | 190,897 | (44.4) | 1,340 | (29.9) | |
| 1 | 124,184 | (28.9) | 1,189 | (26.5) | |
| ≥2 | 114,556 | (26.7) | 1,959 | (43.6) | |
| New York city hospital | 154,826 | (36.0) | 2,146 | (47.8) | <0.001 |
| Hospital volume | <0.001 | ||||
| Low | 13,043 | (3.0) | 263 | (5.9) | |
| Medium | 81,670 | (19.0) | 854 | (19.0) | |
| High | 334,924 | (78.0) | 3,371 | (75.1) | |
| Other procedures | |||||
| Exenteration | 109 | (0.03) | 19 | (0.4) | <0.001 |
| Omentectomy | 27,519 | (6.4) | 555 | (12.4) | <0.001 |
| Debulk | 1,272 | (0.3) | 11 | (0.2) | 0.53 |
| Lymphadenectomy | 43,574 | (10.1) | 559 | (12.5) | <0.001 |
| Small bowel resection | 1,859 | (0.4) | 176 | (3.9) | <0.001 |
| Colon resection | 2,835 | (0.7) | 348 | (7.8) | <0.001 |
| Rectosigmoid resection | 3,657 | (0.9) | 528 | (11.8) | <0.001 |
| Liver resection | 323 | (0.1) | 26 | (0.6) | <0.001 |
| Bladder resection | 554 | (0.1) | 173 | (3.9) | <0.001 |
| Diaphragm resection | 943 | (0.2) | 14 | (0.3) | 0.19 |
| Splenectomy | 550 | (0.1) | 21 | (0.5) | <0.001 |
| Anterior repair | 36,820 | (8.6) | 201 | (4.5) | <0.001 |
| Posterior repair | 30,752 | (7.2) | 162 | (3.6) | <0.001 |
| Incontinence repair | 29,149 | (6.8) | 191 | (4.3) | <0.001 |
| Oophorectomy | 256,682 | (59.7) | 3,214 | (71.6) | <0.001 |
| Colpopexy | 29,765 | (6.9) | 163 | (3.6) | <0.001 |
| Indications for surgery | |||||
| Leiomyoma | 249,778 | (58.1) | 2,220 | (49.5) | <0.001 |
| Endometriosis | 115,310 | (26.8) | 790 | (17.6) | <0.001 |
| Abnormal menstruation and bleeding | 158,590 | (36.9) | 994 | (22.1) | <0.001 |
| Benign neoplasms and cysts | 118,933 | (27.7) | 1,076 | (24.0) | <0.001 |
| Pelvic organ prolapse | 68,312 | (15.9) | 404 | (9.0) | <0.001 |
| Endometrial hyperplasia with atypia | 5,697 | (1.3) | 25 | (0.6) | <0.001 |
| Endometrial hyperplasia without atypia | 15,887 | (3.7) | 102 | (2.3) | <0.001 |
| Uterine cancer | 45,062 | (10.5) | 356 | (7.9) | <0.001 |
| Cervical cancer | 6,517 | (1.5) | 53 | (1.2) | 0.07 |
| Ovarian, fallopian tube, peritoneal cancer | 17,402 | (4.1) | 353 | (7.9) | <0.001 |
VLV: very low volume.
Surgeon volume and hospital volume were calculated for any hysterectomy. Hospital volume tertiles were calculated at hospital-level.
Among very low-volume surgeons, the most common route of hysterectomy was abdominal, which accounted for 79.8% of cases, followed by laparoscopic hysterectomy in 10.8%. For higher-volume surgeons, abdominal hysterectomy was performed in 59.6%, laparoscopic in 21.9%, and vaginal in 14.4% of women (P<0.0001). In a multivariable model, patients who underwent abdominal hysterectomy were more likely to have a very low-volume surgeon (Table 2). Older patients, those with greater comorbidity, and women who underwent emergent or urgent surgery (aRR=3.39; 95% CI, 3.16–3.64) were more likely to have had a very low-volume surgeon. Patients insured through Medicare (aRR=1.14; 95% CI, 1.03–1.26) and operated in a low-volume hospital were also more likely to have had a very low-volume surgeon.
Table 2.
Predictors of having a surgeon with very low volume.
| aRR | |
|---|---|
| Hysterectomy | |
| Abdominal | Referent |
| Robotic | 0.44 (0.35–0.55)* |
| Laparoscopic | 0.59 (0.53–0.66)* |
| Vaginal | 0.53 (0.45–0.62)* |
| Elective surgery | |
| Elective | Referent |
| Emergent/urgent | 3.39 (3.16–3.64)* |
| Other/unknown | 0.65 (0.53–0.78)* |
| Age | |
| <40 | Referent |
| 40–49 | 1.03 (0.93–1.15) |
| 50–59 | 1.27 (1.13–1.43)* |
| 60–69 | 1.53 (1.34–1.76)* |
| ≥70 | 1.94 (1.67–2.25)* |
| Year of admission | |
| 2000 | 1.05 (0.90–1.23) |
| 2001 | 0.94 (0.80–1.11) |
| 2002 | 0.90 (0.77–1.06) |
| 2003 | 0.94 (0.80–1.11) |
| 2004 | 0.87 (0.74–1.03) |
| 2005 | 0.79 (0.67–0.94)* |
| 2006 | 0.82 (0.69–0.97)* |
| 2007 | 0.77 (0.65–0.91)* |
| 2008 | 0.86 (0.73–1.01) |
| 2009 | 0.76 (0.64–0.90)* |
| 2010 | 0.69 (0.58–0.82)* |
| 2011 | 0.72 (0.60–0.85)* |
| 2012 | 0.80 (0.67–0.95)* |
| 2013 | 0.87 (0.74–1.03) |
| 2014 | Referent |
| Race/ethnicity | |
| White | Referent |
| Black | 0.96 (0.87–1.05) |
| Hispanic | 0.92 (0.82–1.04) |
| Other | 0.87 (0.77–0.97)* |
| Unknown | 1.06 (0.89–1.25) |
| Insurance status | |
| Private | Referent |
| Medicare | 1.14 (1.03–1.26)* |
| Medicaid | 1.05 (0.95–1.18) |
| Other | 2.02 (1.21–3.36)* |
| None | 0.98 (0.80–1.19) |
| Unknown | 2.24 (1.62–3.10)* |
| Comorbidity | |
| 0 | Referent |
| 1 | 1.26 (1.16–1.36)* |
| ≥2 | 1.83 (1.69–1.98)* |
| New York city hospital | 1.33 (1.12–1.57)* |
| Hospital volume | |
| Low | Referent |
| Medium | 0.68 (0.54–0.85)* |
| High | 0.60 (0.48–0.75)* |
| Other procedures | |
| Omentectomy | 0.91 (0.80–1.03) |
| Lymphadenectomy | 1.13 (1.01–1.27)* |
| Anterior repair | 0.72 (0.57–0.91)* |
| Posterior repair | 0.87 (0.69–1.10) |
| Incontinence repair | 1.00 (0.84–1.18) |
| Oophorectomy | 1.22 (1.13–1.33)* |
| Colpopexy | 0.65 (0.54–0.79)* |
| Indications for surgery | |
| Leiomyoma | 0.62 (0.58–0.67)* |
| Endometriosis | 0.75 (0.69–0.81)* |
| Abnormal menstruation and bleeding | 0.56 (0.52–0.61)* |
| Benign neoplasms and cysts | 0.68 (0.63–0.73)* |
| Pelvic organ prolapse | 0.67 (0.56–0.80)* |
| Endometrial hyperplasia with atypia | 0.43 (0.29–0.65)* |
| Endometrial hyperplasia without atypia | 0.55 (0.45–0.68)* |
| Uterine cancer | 0.25 (0.22–0.28)* |
| Cervical cancer | 0.34 (0.25–0.45)* |
| Ovarian, fallopian tube, peritoneal cancer | 0.46 (0.40–0.53)* |
aRR: adjusted risk ratio. VLV: very low volume.
Annualized surgeon and hospital volume were calculated for any hysterectomy. Mixed-effects log-Poisson models included route of hysterectomy, elective surgery, age, year of admission, race, insurance status, comorbidity, NYC hospital, hospital-level tertiles of hospital volume, concomitant procedures (omentectomy, lymphadenectomy, anterior, posterior and incontinence repair, oophorectomy and colpopexy), indications (leiomyoma, endometriosis, abnormal menstruation and bleeding, benign neoplasms and cysts, pelvic organ prolapse, endometrial hyperplasia with or without atypia, uterine, cervical, and ovarian/fallopian tube/peritoneal cancer). Hospital identifiers were included as random intercept to account for hospital level of clustering.
P-value<0.05
The overall complication rates were 32.0% for patients treated by very low-volume surgeons vs. 9.9% for those treated by other physicians (P<0.001) (aRR=1.97; 95% CI, 1.86–2.09) (Table 3). Specifically, each individual intraoperative complication was increased for very low-volume surgeons (Appendix 2, available online at http://links.lww.com/xxx). The rates of intraoperative complications (11.3% vs. 3.1%), surgical site complications (15.1% vs. 4.1%), medical complications (19.5% vs. 4.8%), and transfusion (38.5% vs. 11.8%) were all higher for very low-volume compared to higher volume surgeons (P<0.001 for all). Patients treated by very low-volume surgeons were also more likely to have a prolonged LOS (62.0% vs. 22.0%) and excessive hospital charges (59.8% vs. 24.6%) compared to higher-volume surgeons (P<0.001 for both). Lastly, the in-hospital mortality rate was 2.5% for very low-volume surgeons compared to 0.2% for higher-volume surgeons (P<0.001) (aRR=2.89; 95% CI, 2.32–3.61).
Table 3.
Rates of outcomes and adjusted risk ratio of surgeon volume in patients with any hysterectomy.
| Other | VLV | p-value | aRR, VLV vs. other | |||
|---|---|---|---|---|---|---|
| Outcomes | N | % | N | % | ||
| Any morbidity | 42,423 | 9.9 | 1,437 | 32.0 | <0.001 | 1.97 (1.86–2.09)* |
| Intraoperative complication | 13,528 | 3.2 | 509 | 11.3 | <0.001 | 2.41 (2.19–2.65)* |
| Surgical site complication | 17,668 | 4.1 | 677 | 15.1 | <0.001 | 1.96 (1.80–2.13)* |
| Medical complication | 20,833 | 4.9 | 877 | 19.5 | <0.001 | 2.18 (2.02–2.35)* |
| Mortality | 666 | 0.2 | 113 | 2.5 | <0.001 | 2.89 (2.32–3.61)* |
| Transfusion | 50,740 | 11.8 | 1,728 | 38.5 | <0.001 | 1.78 (1.68–1.88)* |
| LOS >75% | 94,537 | 22.0 | 2,781 | 62.0 | <0.001 | 1.55 (1.48–1.62)* |
| Total charges>75% | 105,845 | 24.6 | 2,686 | 59.9 | <0.001 | 1.89 (1.80–1.99)* |
VLV: very low volume. aRR: adjusted risk ratio. LOS: length of stay.
Annualized surgeon and hospital volume were calculated for any hysterectomy. Mixed-effects log-Poisson models included surgeon volume, elective surgery, age, year of admission, race, insurance status, comorbidity, NYC hospital, hospital-level tertiles of hospital volume, concomitant procedures (omentectomy, lymphadenectomy, anterior, posterior and incontinence repair, oophorectomy and colpopexy), indications (leiomyoma, endometriosis, abnormal menstruation and bleeding, benign neoplasms and cysts, pelvic organ prolapse, endometrial hyperplasia with or without atypia, uterine, cervical, and ovarian/fallopian tube/peritoneal cancer) and route of hysterectomy. Surgeon and hospital identifiers were included as nested random intercepts to account for surgeon and hospital level of clustering.
p-value<0.05
When stratified by route of hysterectomy, similar trends were noted with higher complication rates for very low-volume surgeons (Figure 2, Table 4). Among women who underwent abdominal hysterectomy, the overall morbidity rate was 35.2% for patients treated by very low-volume surgeons compared to 12.8% for higher volume surgeons (P<0.001) (aRR=1.89; 95% CI, 1.79–2.01). The corresponding morbidity rates for very low-volume vs. higher-volume surgeons were 19.9% vs. 6.8% for robotic-assisted hysterectomy (P<0.001), 10.2% vs. 4.7% for laparoscopic hysterectomy (P<0.001) and 8.4% vs. 6.1% (P<0.001) for vaginal hysterectomy. These trends were similar for the individual complication classes, transfusion, prolonged LOS and excessive hospital charges. We performed a series of sensitivity analyses after propensity score matching of patients treated by a very low-volume vs. higher-volume surgeon and the outcomes for the entire cohort and for each individual type of hysterectomy were largely unchanged (Appendixes 3–5, available online at http://links.lww.com/xxx).
Table 4.
Rates of outcomes and adjusted risk ratio of surgeon volume in patients with abdominal, robotically-assisted, laparoscopic, or vaginal hysterectomy.
| Abdominal | Robotic | Laparoscopic | Vaginal | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Other (%) |
VLV (%) |
aRR, VLV vs. other |
Other (%) |
VLV (%) |
aRR, VLV vs. other |
Other (%) |
VLV (%) |
aRR, VLV vs. other |
Other (%) |
VLV (%) |
aRR, VLV vs. other |
|
| Outcomes | ||||||||||||
| Any morbidity | 12.8% | 35.2% | 1.89 (1.79–2.01)* | 6.8% | 19.9% | 2.49 (1.90–3.27)* | 4.7% | 10.2% | 1.70 (1.47–1.98)* | 6.1% | 8.4% | 1.24 (1.06–1.46)* |
| Intraoperative complication | 3.7% | 12.6% | 2.34 (2.13–2.58)* | 3.2% | 9.9% | 2.87 (1.97–4.19)* | 2.3% | 4.0% | 1.79 (1.42–2.26)* | 2.0% | 2.8% | 1.21 (0.92–1.59) |
| Surgical site complication | 5.9% | 17.1% | 1.91 (1.76–2.08)* | 1.7% | 6.8% | 2.90 (1.82–4.63)* | 1.3% | 3.5% | 1.76 (1.37–2.27)* | 1.6% | 2.1% | 1.16 (0.85–1.60) |
| Medical complication | 6.5% | 21.0% | 2.03 (1.88–2.19)* | 2.9% | 9.0% | 2.46 (1.64–3.69)* | 1.7% | 5.1% | 1.98 (1.60–2.44)* | 3.2% | 4.9% | 1.42 (1.15–1.76)* |
| Transfusion | 16.3% | 43.2% | 1.76 (1.66–1.86)* | 4.4% | 15.5% | 2.44 (1.78–3.36)* | 4.4% | 12.4% | 1.52 (1.31–1.75)* | 6.0% | 11.7% | 1.41 (1.21–1.63)* |
| LOS >75% | 17.5% | 58.3% | 2.02 (1.92–2.13)* | 23.3% | 46.9% | 1.61 (1.34–1.93)* | 8.6% | 31.5% | 1.84 (1.68–2.03)* | 23.3% | 36.8% | 1.28 (1.18–1.40)* |
| Total charges>75% | 24.4% | 61.2% | 1.93 (1.83–2.03)* | 24.6% | 48.1% | 1.69 (1.41–2.03)* | 24.7% | 39.2% | 1.35 (1.24–1.47)* | 24.4% | 41.6% | 1.31 (1.21–1.42)* |
VLV: very low volume. aRR: adjusted risk ratio.
P-values of univariate analysis <0.001, except intraoperative complication (p=0.01), and surgical site complication (p=0.04) in vaginal hysterectomy patients.
Surgeon and hospital volume were calculated separately for each route of hysterectomy. For each outcome, separate mixed-effects log-Poisson models included surgeon volume, elective surgery, age, year of admission, race, insurance status, comorbidity, NYC hospital, hospital-level tertiles of hospital volume, concomitant procedures (omentectomy, LND, anterior, posterior and incontinence repair, oophorectomy and colpopexy), indications (leiomyoma, endometriosis, abnormal menstruation and bleeding, benign neoplasms and cysts, pelvic organ prolapse, endometrial hyperplasia with or without atypia, uterine, cervical, and ovarian/fallopian tube/peritoneal cancer). Surgeon and hospital identifiers were included as nested random intercepts to account for surgeon and hospital level of clustering. In the group of robotically-assisted hysterectomy, the model for surgical site complication did not adjust for race and insurance status, and the models for medical complication and transfusion did not adjust for insurance status because of convergence issues.
P<0.05
Discussion
Among women who underwent hysterectomy, complication rates, hospital charges, length of stay and perioperative mortality are significantly greater when the operation is performed by a very low volume surgeon. While very low-volume surgeons performed a small number of hysterectomies annually, these providers comprise over 40% of the physicians performing hysterectomy in New York State.
Prior studies examining surgical volume and outcomes for hysterectomy have demonstrated that, while higher procedural volume is associated with superior outcomes, the association between volume and outcomes is more modest than for other higher-risk procedures.1–5,8,18,27–29 In one analysis, the morbidity rate was 17% among surgeons who performed <10 hysterectomies per year vs. 12% in those performing >10 procedures, the corresponding mortality rates were 0.2% vs. 0.06%, respectively.2 For patients undergoing laparoscopic hysterectomy, the complication rate of patients treated by low-volume surgeons was 6% compared to 4% for high-volume surgeons.28 Similarly, among women undergoing vaginal hysterectomy, higher surgeon volume is associated with a small, but statistically significant, reduction in morbidity.30 In contrast, we noted a marked association between performance of a very low number of hysterectomies annually and an increased risk of adverse events. Compared to patients operated on by higher-volume surgeons, the risk of a perioperative complication was doubled in women treated by a very low-volume surgeon. In addition to increased risk of adverse outcomes, very low-volume surgeons were much less likely to offer minimally invasive surgery.
A number of factors likely contributed to the increased rate of adverse outcomes noted for very low-volume surgeons. Intuitively, one would predict that decreased technical proficiency contributes to the increased morbidity and the prolonged length of stay we noted for very low-volume surgeons. Additionally, very low-volume surgeons were more likely to operate on women undergoing urgent procedures, older women and those with more comorbidities. While we adjusted for these factors in our multivariable models, these patients were at higher risk for complications and other adverse outcomes. Very low-volume surgeons were more likely to perform their operations in low-volume hospitals which may impact postoperative care, especially among women with complications.13,31 Lastly, a portion of very low-volume surgeons are likely new graduates who recently completed training. Clearly balancing surgical “learning curves” and patient outcomes is a difficult balance.
Very low-volume surgeons accounted for over 40% of the physicians performing hysterectomy in New York state from 2000 to 2014. During the years of study, the percentage of surgeons classified as very low-volume providers was relatively constant and ranged from 10% to 15%. These findings are somewhat surprising given efforts to promote the referral of many women who require hysterectomy to sub-specialist gynecologic surgeons and may reflect insurance-mediated limitations on where patients can receive care or performance of emergent procedures at low volume centers. As trends towards referring women who require hysterectomy to higher-volume providers increase in combination with the declining rate of hysterectomy, the number of very low-volume surgeons may increase over time. The strong association between performance of a very low number of hysterectomies and adverse outcomes suggests that very low procedure volume may be a possible metric for credentialing or targeted quality improvement initiatives.
Our findings should be interpreted in light of a number of important limitations. First while we adjusted for numerous clinical factors, a number of unmeasured confounders such as prior surgical history and intraoperative technical factors undoubtedly influenced the risk of complications. Second, the very low-volume physicians likely include a heterogenous group of providers. Some physicians in this group may be non-gynecologic surgeons performing emergent cases and others gynecologic surgeons who just perform a very low number of hysterectomies. Third, while the benefit of the SPARCS dataset is the ability to capture physician volume across hospitals within New York State, data on procedures performed in other states were unavailable. However, we believe that any underestimation of volume due to procedures performed in other states is likely to be very small. Inherent to any study of administrative data is possible errors in coding or classification. Any error in classification of the procedures or outcomes is likely to be small. Lastly, our analysis focused only on patients in one state. While New York is geographically diverse, our findings may not be applicable to other regions of the U.S. where higher-volume surgeons may not be readily available.
These findings have important policy implications for the practice of gynecologic surgery. Efforts to improve outcomes for low volume surgeons typically rely on either regionalization of care or targeted quality improvement initiatives. Referral to higher-volume surgeons is clearly an attractive option for patients who would receive care by a very low-volume provider. Practice patterns in gynecology are already likely shifting, with increased referral of a larger number of women to gynecologic sub-specialists or to practitioners who focus exclusively on gynecology. However, regionalization of care is sometimes not feasible due to geographic limitations and patients often have a strong preference to receive care locally.32 Some prior studies have suggested that outcomes of low volume physicians and hospitals can be improved with strict adherence to quality of care guidelines.19,33 As such, strict adherence to evidence-based guidelines for care may be particularly important for very low-volume surgeons and an actionable approach to improve outcomes.
In conclusion, we noted that a relatively large number of gynecologic surgeons perform a very low number of hysterectomies annually. Treatment by very low-volume surgeons is associated with increased morbidity, mortality, and increased resource utilization. Targeted efforts to improve outcomes among very low-volume surgeons or to reduce the number of very low volume surgeons performing hysterectomy may help to reduce the morbidity associated with hysterectomy.
Supplementary Material
Acknowledgments
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01 CA166084) are recipients of grants from the National Cancer Institute. Dr. Hershman is the recipient of a grant from the Breast Cancer Research Foundation/Conquer Cancer Foundation.
Footnotes
Financial Disclosure: Dr. Wright has served as a consultant for Tesaro and Clovis Oncology. Dr. Neugut has served as a consultant to Pfizer, Teva, Otsuka, and United Biosource Corporation. He is on the medical advisory board of EHE, Intl. The other authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal’s requirements for authorship.
References
- 1.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 2.Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909–15. doi: 10.1097/AOG.0b013e3181f395d9. [DOI] [PubMed] [Google Scholar]
- 3.Goodney PP, Lucas FL, Stukel TA, Birkmeyer JD. Surgeon specialty and operative mortality with lung resection. Ann Surg. 2005;241:179–84. doi: 10.1097/01.sla.0000149428.17238.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hannan EL, Radzyner M, Rubin D, Dougherty J, Brennan MF. The influence of hospital and surgeon volume on in-hospital mortality for colectomy, gastrectomy, and lung lobectomy in patients with cancer. Surgery. 2002;131:6–15. doi: 10.1067/msy.2002.120238. [DOI] [PubMed] [Google Scholar]
- 5.Hanstede MFWL, Stewart EA, Feldman S. The relation of annual surgeon case volume to clinical outcomes and resource utilization in abdominal hysterectomy. J Reprod Med. 2009;54:193–202. [PubMed] [Google Scholar]
- 6.Hendren S, Birkmeyer JD, Yin H, Banerjee M, Sonnenday C, Morris AM. Surgical complications are associated with omission of chemotherapy for stage III colorectal cancer. Dis Colon Rectum. 2010;53:1587–93. doi: 10.1007/DCR.0b013e3181f2f202. [DOI] [PubMed] [Google Scholar]
- 7.Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–41. doi: 10.1097/01.sla.0000179621.33268.83. discussion 41–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mercado C, Zingmond D, Karlan BY, et al. Quality of care in advanced ovarian cancer: the importance of provider specialty. Gynecol Oncol. 2010;117:18–22. doi: 10.1016/j.ygyno.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 9.Birkmeyer JD. High-risk surgery--follow the crowd. JAMA. 2000;283:1191–3. doi: 10.1001/jama.283.9.1191. [DOI] [PubMed] [Google Scholar]
- 10.Learn PA, Bach PB. A decade of mortality reductions in major oncologic surgery: the impact of centralization and quality improvement. Med Care. 2010;48:1041–9. doi: 10.1097/MLR.0b013e3181f37d5f. [DOI] [PubMed] [Google Scholar]
- 11.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–51. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 12.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 13.Buettner S, Gani F, Amini N, et al. The relative effect of hospital and surgeon volume on failure to rescue among patients undergoing liver resection for cancer. Surgery. 2016;159:1004–12. doi: 10.1016/j.surg.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 14.Macedo FIB, Jayanthi P, Mowzoon M, Yakoub D, Dudeja V, Merchant N. The Impact of Surgeon Volume on Outcomes After Pancreaticoduodenectomy: a Meta-analysis. J Gastrointest Surg. 2017;21:1723–31. doi: 10.1007/s11605-017-3498-7. [DOI] [PubMed] [Google Scholar]
- 15.Scali STGK, Huber TS, Beck AW. RS01 Hospital volume impact on patient safety indicators and failure to rescue following open abdominal aortic aneurysm repair. J Vasc Surg. 2017;65:45S–6S. doi: 10.1016/j.jvs.2019.06.194. [DOI] [PubMed] [Google Scholar]
- 16.Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122:233–41. doi: 10.1097/AOG.0b013e318299a6cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earle CC, Schrag D, Neville BA, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst. 2006;98:172–80. doi: 10.1093/jnci/djj019. [DOI] [PubMed] [Google Scholar]
- 18.Mowat A, Maher C, Ballard E. Surgical outcomes for low-volume vs high-volume surgeons in gynecology surgery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215:21–33. doi: 10.1016/j.ajog.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 19.Wright JD, Chen L, Gabor L, et al. Patterns of Specialty-Based Referral and Perioperative Outcomes for Women With Endometrial Cancer Undergoing Hysterectomy. Obstet Gynecol. 2017;130:81–90. doi: 10.1097/AOG.0000000000002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(SPARCS) SPARCS. Bureau of Health Informatics. Office of Quality and Patient Safety. New York State Departmetn of Health.
- 21.Mao J, Goodney P, Cronenwett J, Sedrakyan A. Association of Very Low-Volume Practice With Vascular Surgery Outcomes in New York. JAMA Surg. 2017;152:759–66. doi: 10.1001/jamasurg.2017.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–33. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 23.Jones NL, Chen L, Chatterjee S, et al. National Trends in Extended Procedures for Ovarian Cancer Debulking Surgery. Int J Gynecol Cancer. 2017 doi: 10.1097/IGC.0000000000001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guy MS, Sheeder J, Behbakht K, Wright JD, Guntupalli SR. Comparative outcomes in older and younger women undergoing laparotomy or robotic surgical staging for endometrial cancer. Am J Obstet Gynecol. 2016;214:350e1–e10. doi: 10.1016/j.ajog.2015.09.085. [DOI] [PubMed] [Google Scholar]
- 25.Wright JD, Devine P, Shah M, et al. Morbidity and mortality of peripartum hysterectomy. Obstet Gynecol. 2010;115:1187–93. doi: 10.1097/AOG.0b013e3181df94fb. [DOI] [PubMed] [Google Scholar]
- 26.Wright JD, Lewin SN, Barrena Medel NI, et al. Morbidity and mortality of surgery for endometrial cancer in the oldest old. Am J Obstet Gynecol. 2011;205:66e1–8. doi: 10.1016/j.ajog.2011.02.067. [DOI] [PubMed] [Google Scholar]
- 27.Wright JD, Hershman DL, Burke WM, et al. Influence of surgical volume on outcome for laparoscopic hysterectomy for endometrial cancer. Ann Surg Oncol. 2012;19:948–58. doi: 10.1245/s10434-011-2090-8. [DOI] [PubMed] [Google Scholar]
- 28.Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709–16. doi: 10.1097/AOG.0b013e318248f7a8. [DOI] [PubMed] [Google Scholar]
- 29.Bristow RE, Zahurak ML, Diaz-Montes TP, Giuntoli RL, Armstrong DK. Impact of surgeon and hospital ovarian cancer surgical case volume on in-hospital mortality and related short-term outcomes. Gynecol Oncol. 2009;115:334–8. doi: 10.1016/j.ygyno.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 30.Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341–7. doi: 10.1097/AOG.0b013e3181fca8c5. [DOI] [PubMed] [Google Scholar]
- 31.Wright JD, Ananth CV, Ojalvo L, et al. Failure to rescue after major gynecologic surgery. Am J Obstet Gynecol. 2013;209:420e1–8. doi: 10.1016/j.ajog.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finlayson SRG, Birkmeyer JD, Tosteson ANA, Nease RFJ. Patient Preferences for Location of Care: Implications for Regionalization. Medical Care. 1999;37:204–9. doi: 10.1097/00005650-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Auerbach AD, Hilton JF, Maselli J, Pekow PS, Rothberg MB, Lindenauer PK. Shop for quality or volume? Volume, quality, and outcomes of coronary artery bypass surgery. Ann Intern Med. 2009;150:696–704. doi: 10.7326/0003-4819-150-10-200905190-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.