Abstract
Background
Raloxifene and tamoxifen are FDA approved for breast cancer risk reduction; in 2013, the US Preventive Services Task Force (USPSTF) recommended these drugs for breast cancer risk reduction in high-risk women. Information on use of raloxifene and tamoxifen for breast cancer risk reduction in the general population is believed low; however, there is little literature on this.
Objective
To assess the use of breast cancer risk reduction medications by breast cancer risk level in an older cohort of women.
Methods
Women enrolled in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial were assessed for use of raloxifene, tamoxifen and other medications. The data sources for use of the drugs were a mailed medication use questionnaire (MUQ) in 2013 and linked Medicare Part D claims files from 2010–2014. Estimated breast cancer risk over 5 years (BrCa5) was assessed using the modified Gail model and self-reported breast cancer risk factors; comorbidities were assessed through a questionnaire.
Results
A total of 22235 women completed the MUQ; of these, 13640 (61%) had linked Part D data. In 2013, 45% were aged 65–74 and 55% aged 75–84. From the MUQ, raloxifene use (past month) was 1.8%, 2.5% and 4.0% for women with BrCa5 of <1.66%, 1.66–3.0% and ≥3%, respectively (p-value trend < 0.0001). From Part D, for any use during the period, among women with coverage, raloxifene rates were 3.3%, 4.0% and 6.6% for the three BrCa5 categories (p-value trend < 0.0001); use was 7.4% and 3.3% in women with and without osteoporosis, respectively. Raloxifene use significantly decreased over 2010 to 2014, and specifically from 2012 to 2014, both for all women and for women with BrCa5 ≥3%. Tamoxifen use from Part D was 0.36%, 0.45% and 0.85% for the three BrCa5 categories (p-value trend=0.009).
Conclusion
Raloxifene use was low overall but increased modestly with breast cancer risk. Use decreased from 2010 to 2014. Tamoxifen use was very low.
Keywords: breast cancer, chemoprevention, raloxifene, risk, tamoxifen
Introduction
Chemoprevention is a potential strategy for reducing morbidity and mortality from breast cancer. Tamoxifen and raloxifene have both been demonstrated to reduce breast cancer incidence and are both currently FDA approved for that purpose 1–3. Raloxifene, which was previously approved (in 1997) for osteoporosis treatment and prevention, was approved by FDA in 2007 for breast cancer risk reduction in postmenopausal women who either had osteoporosis or were at high risk for invasive breast cancer 4. High risk was defined as either at least one breast biopsy showing lobular carcinoma in situ (LCIS) or atypical hyperplasia, a 5-year predicted breast cancer risk of at least 1.66% based on the Gail Model, or a first degree relative with breast cancer. Adverse effects of raloxifene were noted, including risk of venous thromboembolism. Tamoxifen was approved by FDA in 1998 for breast cancer risk reduction high-risk women; it had previously been approved for treatment of breast cancer 5.
In September 2013, the U.S. Preventive Services Task Force (USPSTF) gave a B recommendation for use of breast cancer risk-reducing medications, including tamoxifen and raloxifene, in women at increased risk for breast cancer 6. Specifically, for women aged 50 years or older, the USPSTF concluded that “many women with an estimated 5-year breast cancer risk of 3% or greater are likely to have more benefit than harm from using tamoxifen or raloxifene”.
Notwithstanding the FDA approvals and USPSTF recommendation, population usage rates of tamoxifen and raloxifene for breast cancer risk reduction are believed to be low; however, the literature on such usage rates is sparse 7–8. A few published reports show limited overall use among post-menopausal women 8–9. To our knowledge there are no published data on population usage rates of raloxifene by breast cancer risk level.
In this manuscript, we assess the use of raloxifene and tamoxifen as a function of breast cancer risk level, osteoporosis status, and time period (before and after the USPSTF recommendation) in a large cohort of older women, specifically, women enrolled in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. PLCO was a randomized trial examining the effect of screening for four cancers undertaken at ten centers across the U.S. Data on prescription medication use were available from two main sources - self-reported use from a questionnaire administered in 2013 and prescription data from linked Medicare Part D for 2010–2014. In addition, information on the factors used to predict breast cancer risk, as well as osteoporosis status, were available from questionnaire data. Therefore, the PLCO cohort allows for the assessment of the use of breast cancer risk reducing medications in relation to breast cancer risk over the time period of the USPSTF recommendation. For context, we also assessed use of statins in this population, as another example of a class of drugs approved for disease risk reduction.
Methods
PLCO Design
The design of PLCO has been described previously 10. Briefly, the trial was designed to determine the effect of specific screening tests on cause-specific mortality. Enrollment at 10 U.S. screening centers was carried out from 1993–2001. Women were considered eligible if they were between ages 55 and 74 and had no previous diagnosis of lung, colorectal or ovarian cancer. Two initial PLCO exclusion criteria – previous oophorectomy and current tamoxifen use - were dropped in 1996 and 1999, respectively. Women were screened for lung cancer with chest radiographs annually for 4 years, for ovarian cancer with CA-125 annually for 6 years and with trans-vaginal ultrasound annually for 4 years, and were screened for colorectal cancer with sigmoidoscopy at baseline and year 3 or 5. Women with previous bilateral oophorectomy were included in the trial and screened for lung and colorectal cancer, but not ovarian cancer. All subjects provided informed consent and each screening center’s IRB approved the trial.
According to the original trial design, women were followed at each screening center for up to 13 years for all-cancer incidence and mortality. In 2011, PLCO transitioned to centralized follow-up, with subjects having to reconsent to either continue active follow-up, switch to passive follow-up, or refuse further follow-up. Only the active follow-up group could be contacted with further questionnaires or consent requests. Active and passive participants continued to be followed for mortality and cancer incidence, through linkage with the National Death Index and state cancer registries.
Baseline and Supplemental Questionnaires
A baseline questionnaire (BQ) was administered at randomization and a supplemental questionnaire (SQX) was administered in 2006–2007, corresponding to study years 6–13. Together, these questionnaires inquired about the breast cancer risk factors utilized in predictive models, as well as comorbidities, including osteoporosis, high cholesterol, diabetes, and history of heart attack or stroke.
Breast Cancer Risk Model
The breast cancer risk model utilized here is the Gail Model, updated in 2012 and available on the NCI website, and which is mentioned in the USPSTF statement as a model that can be used to assess breast cancer risk level 11. The model includes the following factors: current age, age at first live birth, age at menarche, family history of cancer in a first degree relative, number of prior breast biopsies and race/ethnicity. To apply the model, race/ethnicity and ages at first live birth and menarche were derived from the corresponding BQ variables. Number of breast biopsies was derived from the SQX responses. Family history was included in both the BQ and SQX; the SQX value was used if non-missing, otherwise, the BQ variable was used. Current age was age at the time medication use was being assessed. The Gail Model also included BRCA status, which was not available for PLCO and was presumed negative since the prevalence of BRCA mutations is under 0.5% in the general population. The 5-year risk of breast cancer, denoted BrCa5, was computed and used in the analysis.
Medication Information
Information on prescription medication use came from two sources. First, a medication use questionnaire (MUQ) was administered in 2013. The MUQ asked participants to list all prescription drugs taken during the past 30 days. For each listed drug, respondents also filled in number of years taken and number of days taken per month. The second source came from linked Medicare Part D (prescription drug) data. Participants receiving the MUQ were asked to consent to have their study data linked to Medicare claims data. For those consenting, enrollment and claims data were linked for the years 2010–2014. Medicare Part D data includes information on months of coverage, prescriptions filled (not actual use), and days supply for each prescription.
In addition, the SQX, in a section on women’s health issues, included the question “Did you ever take any of the following medications to strengthen your bones or for any other reason” and listed eight specific medications, including raloxifene and tamoxifen, as well as various bisphosphonates. For each medication, there was a question on whether the woman ever took the drug and, if ever, whether they were currently taking it. The SQX data were used to assess duration of use among women with medication as reported from Part D.
Analysis Cohort
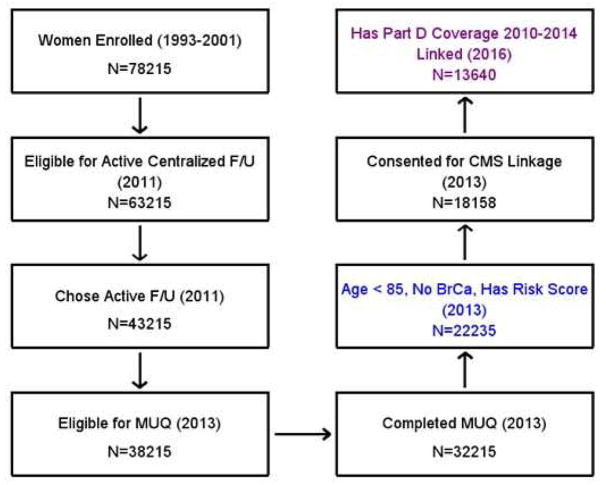
Figure 1 shows a flowchart of who was included in the cohort for this analysis. The analysis cohort consisted of all women who completed the MUQ and who were under age 85 and with no history of breast cancer at that time; they also had to have a computable breast cancer risk score. To have been eligible for the MUQ, women must have previously been eligible for and chosen to transfer to active follow-up status (Figure 1). Of the analysis cohort, a subset had useable Part D data; specifically, women who consented to Medicare linkage and had Part D coverage for ≥12 months during 2010–2014.
Figure 1. Study flowchart.
Flowchart shows which PLCO women were eligible for the current analysis. Year(s) in parenthesis is time of the event. To be eligible for transfer to active centralized follow-up in 2011 (2nd box), subjects had to be alive, not lost to follow-up, and not at the University of Alabama at Birmingham (UAB); UAB subjects transferred to centralized follow-up later and were not eligible for the MUQ. Having part D coverage was defined as at least 12 months of coverage during the period. Colored boxed represent women included in the overall analysis (blue box) and the sub-analysis of Medicare claims data (purple box).
To assess the representativeness of the analysis cohort within PLCO, we compared their baseline demographics to those of the cohort in PLCO who potentially could have been included in the analysis cohort but were not. Specifically, this was all women eligible for active transfer status in 2011, and who were alive, under age 85 and with no history of breast cancer in 2013. Thus, this included women who were eligible for but did not choose active follow-up status and women who were eligible for but did not participate in the MUQ.
Quantitative Methods
From the MUQ, the medication usage rate was defined as the proportion of women reporting use during the prior month; from Part D, it was defined as the proportion filling any prescription between 2010–2014. We examined usage rates of raloxifene and tamoxifen by BrCa5 level, and also by osteoporosis status for raloxifene. For BrCa5, we utilized the categories of <1.66%, 1.66–3.0% and ≥3.0% based on the FDA and USPSTF recommended cutoffs. To examine the intensity of medication use from Part D, we added up the total days supply of raloxifene (or tamoxifen) over all filled prescriptions during the coverage period and divided this by the total number days of Part D coverage to produce a daily usage rate.
To analyze time trends in Part D data over 2010–2014, we developed a longitudinal GEE model (Proc Genmod, SAS version 9.4) with one observation per person per calendar year of Part D coverage and use (prescription claim) of raloxifene (or tamoxifen) per year as the outcome variable. The model accounts for within-person correlation based on the repeated statement and an autoregressive correlation matrix. The model controlled for age, breast cancer risk score and osteoporosis status. We also examined specifically the change in use between 2012 and 2014, i.e., between the year before and after the USPSTF recommendation was issued.
For context, we also assessed use of other drugs, specifically oral bisphosphonates and statins. Bisphosphonates, like raloxifene, are used to treat osteoporosis, and statins are an example of another drug class approved for disease risk reduction, in this case cardiovascular disease.
Finally, to assess the concordance between the MUQ and Part D data, we compared MUQ responses to Part D claims over a similar time period. Because the majority of raloxifene and tamoxifen prescriptions were for 90 days, we used the 90-day period prior to the MUQ survey date for Part D claims. The kappa statistic was used to measure concordance.
Results
Analysis Cohort
Of 78215 women enrolled in PLCO, 22235 were included in the analysis cohort (Figure 1). All of the 22235 were age 65 or over by 2013, and thus Medicare eligible for ≥12 months during 2010–2014, the period with Part D data. Of the analysis cohort, 18158 (81.7%) consented for linkage with CMS. Of those consenting, 103 (0.6%) could not be matched to a Medicare record, 4094 (22.5%) had a Medicare record but no Part D coverage, and 321 (1.8%) had less than 12 months of Part D coverage, leaving 13640 (75.1%) included in the Part D analysis based on ≥12 months of Part D coverage. Among these 13640 women, the mean length of coverage was 4.4 years, with 71% having coverage for the entire 5-year period.
Table 1 gives the baseline characteristics of the analysis cohort. Most (92.1%) were non-Hispanic white, 20% were age 65+ at enrollment, and 38.2% were college educated. At the time of the MUQ, 45% were 75–84 and the rest (55%) were age 65–74. As compared to the potentially eligible (but excluded) cohort, the analysis cohort was more non-Hispanic white (92.1% vs. 83.6%) and more likely to be a college graduate (38.2% vs. 23.8%). There was no difference in family history of breast cancer (13.6% vs 13.2%). Within the analysis cohort, those with Part D data were similar to the overall cohort (Table 1).
Table 1.
Demographics and medical history of analysis cohort and potentially eligible cohort
| Analysis Cohort | Potentially Eligible Cohort 1 | ||
|---|---|---|---|
| All | Has Medicare Part D data | ||
| At Baseline | N=22235 | N=13640 | N=25878 |
| Non-Hispanic White | 92.1% | 92.5% | 83.6% ** |
| College Educated | 38.2% | 38.5% | 23.8% * |
| Married | 73.9% | 72.7% | 71.7% ** |
| Family History of Breast Cancer | 13.6% | 13.5% | 13.2% |
| Mammography in Prior 3 Years | 93.4% | 93.6% | 89.6% ** |
| History of hysterectomy | 34.0% | 34.3% | 38.3% ** |
| Age ** | |||
| 55–64 | 83.5% | 83.9% | 79.4 |
| 65–74 | 16.5% | 16.1% | 20.6 |
| At time of SQX (2006–7) | |||
| Family History of Breast Cancer | 17.6% | 17.5% | 17.7%2 |
| Mammography in Prior 3 Years | 96.5% | 96.7% | 95.2%2 ** |
| History of hysterectomy | 39.0% | 39.3% | 43.7% 2 ** |
| At time of MUQ (2013) | |||
| Age | |||
| 65–74 | 44.9% | 45.3% | N/A |
| 75–84 | 55.1% | 54.7% | N/A |
Significant Difference Across Cohorts, p < 0.05 (*), p < 0.01 (**).
Potentially eligible cohort are women alive, not at the UAB center, under age 85 and without breast cancer at the time of Medication Use Questionnaire (MUQ) but who did not complete the MUQ. See text and Figure 1 for more details.
Among the 59% of cohort who filled out the SQX
Among all women, 36.5% had BrCa5 < 1.66%, 45.2% had BrCa5 within 1.66–3.0%, and 18.2% had BrCa5 ≥3.0% (Table 2).
Table 2A.
Breast cancer risk and raloxifene use as assessed from the Medication Use Questionnaire (MUQ), 2013.
| All Subjects | Osteoporosis | No Osteoporosis | ||||
|---|---|---|---|---|---|---|
| Breast Cancer Risk Level 1 – BrCa5 | N in Cell (%) | Raloxifene Use – N (%) | N in Cell (%) | Raloxifene Use – N (%) | N in Cell (%) | Raloxifene Use – N (%) |
| All levels | 22235 (100) | 562 (2.5) | 5000 (100) | 198 (4.0) | 17235 (100) | 364 (2.1) |
| < 1.66% | 8121 (36.5) | 147 (1.8) | 1833 (36.7) | 56 (3.1) | 6288 (36.5) | 91 (1.5) |
| 1.66–3.0% | 10059 (45.2) | 254 (2.5) | 2228 (44.6) | 93 (4.2) | 7831 (45.4) | 161 (2.1) |
| ≥ 3.0% | 4055 (18.2) | 161 (4.0) | 939 (18.8) | 49 (5.2) | 3116 (18.1) | 112 (3.6) |
| P-value (trend) | <0.0001 | 0.005 | <0.0001 | |||
| < 3.0% | 18180 (81.8) | 401 (2.2) | 4061 (81.2) | 149 (3.7) | 14119 (81.9) | 1.8 |
| P-value (≥ 3% vs < 3%) | <0.0001 | 0.03 | <0.0001 | |||
| ≥ 1.66% | 14114 (63.5) | 415 (2.9) | 3167 (63.3) | 142 (4.5) | 10947 (63.5) | 2.5 |
| P-value (< 1.66% vs ≥ 1.66%) | <0.0001 | 0.01 | <0.0001 | |||
Five-year predicted risk of breast cancer
Raloxifene Use
Table 2A shows the results of the MUQ medication analysis. Usage rates were 2.5%, 4.0% and 2.1% for all women, women with osteoporosis and women without osteoporosis, respectively. Among all women, and among women with and without osteoporosis, raloxifene use significantly increased with BrCa5 level; usage rates for all women were 1.8%, 2.5% and 4.0% for BrCa5 < 1.66%, 1.66–3.0%, and ≥ 3.0%, respectively (p-value trend < 0.0001). Women without osteoporosis and BrCa5 ≥3.0% had a usage rate of 3.6%. Overall rates of raloxifene use from the MUQ were similar among women included in the part D analysis, 2.6%, as for women not included, 2.4%. Among reported raloxifene users, 97.3% reported usage of 30 days in the past month. For duration of use, 75% of users had used for at least 6 years; this proportion did not statistically differ by osteoporosis status or BrCa5 level.
Overall, older women (75–84 vs 65–74) had significantly higher rates of osteoporosis but lower overall rates of raloxifene use, though not statistically significantly so. Controlling for osteoporosis status, raloxifene use was border significantly lower among older women (OR=0.84; 95% CI: 0.7–1.0).
Patterns of raloxifene use by breast cancer risk and osteoporosis status from Part D data were similar to those observed with MUQ data, with rates higher among women with elevated BrCa5 and with osteoporosis (Table 2B). Among all women, raloxifene use was 3.3%, 4.0% and 6.6% for BrCa5 levels of <1.66%, 1.66–3.0% and ≥ 3.0%, respectively (p-value trend < 0.0001). Among women without osteoporosis, those with BrCa5 ≥ 3.0% had a raloxifene usage rate of 5.2%, compared to 2.9% in women with BrCa5 < 3.0% (p < 0.0001). Among all raloxifene users without osteoporosis, 28% (98/353) had BrCa5 ≥ 3.0%. Among ever-users of raloxifene from Part D data (N=577), 45.1% had a daily use rate above 75%, 20.5% had a rate under 25%, and the rest (34.5%) had a rate in between 25 and 75%. The proportion with daily use rates above 75% increased with increasing BrCa5 level; proportions were 37.6%, 45.8%, and 51.5% for BrCa5 < 1.66%, 1.66–3.0% and ≥3.0%, respectively (p-value trend=0.01). Daily use rates did not vary significantly by osteoporosis status.
Table 2B.
Breast cancer risk and raloxifene use as assessed from Medicare Part D 2010–2014.
| All Subjects | Osteoporosis | No Osteoporosis | ||||
|---|---|---|---|---|---|---|
| Breast Cancer Risk Level 1 – BrCa5 | N in Cell (%) | Raloxifene Use – N (%) | N in Cell (%) | Raloxifene Use – N (%) | N in Cell (%) | Raloxifene Use - N (%) |
| All levels | 13640 (100) | 577 (4.2) | 3045 (100) | 224 (7.4) | 10595 (100) | 353 (3.3) |
| < 1.66% | 4999 (36.7) | 165 (3.3) | 1106 (36.3) | 66 (6.0) | 3893 (36.7) | 99 (2.5) |
| 1.66–3.0% | 6162 (45.2) | 249 (4.0) | 1342 (44.1) | 93 (6.9) | 4820 (45.5) | 156 (3.2) |
| ≥ 3.0% | 2479 (18.2) | 163 (6.6) | 597 (19.6) | 65 (10.9) | 1882 (17.8) | 98 (5.2) |
| P-value (trend) | <0.0001 | 0.0006 | <0.0001 | |||
| < 3.0% | 11161 (81.8) | 414 (3.7) | 2044 (80.4) | 159 (6.5) | 8713 (82.2) | 255 (2.9) |
| P-value (< 3.0 vs ≥ 3.0%) | <0.0001 | 0.0003 | 0.0003 | <0.0001 | ||
| ≥ 1.66% | 8641 (63.4) | 412 (4.8) | 1939 (63.7) | 158 (8.1) | 6702 (63.3) | 254 (3.8) |
| P-value (<1.66% vs ≥ 1.66%) | <0.0001 | 0.03 | 0.0006 | |||
Five-year predicted risk of breast cancer
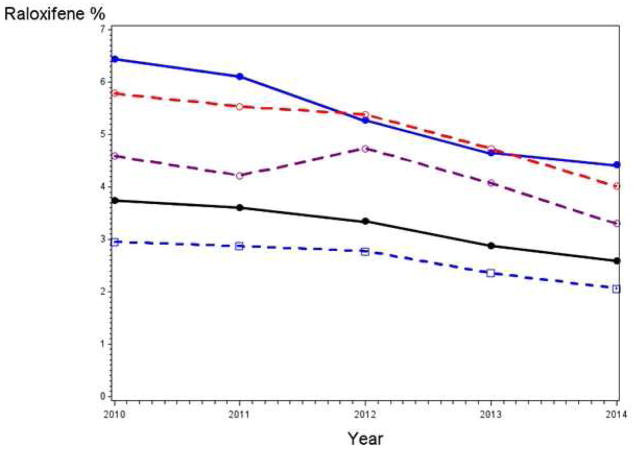
Figure 2 shows raloxifene use rates by calendar year from Part D data. Rates monotonically decreased from 2010 to 2014 - overall, among those with and without osteoporosis, and among those with BrCa5 ≥3.0%. Among all women, use was 3.7%, 3.3% and 2.6% in 2010, 2012 and 2014, respectively. The longitudinal model demonstrated a statistically significant decrease in use over time overall, among women with and without osteoporosis, and among women with BrCa5 ≥3.0% (Table 3). Overall, the OR for raloxifene use in the current versus prior year was 0.90 (95% CI: 0.87–0.92). The model comparing use in 2014 versus 2012 also showed significant decreases among all groups; the overall OR was 0.77 (95% CI: 0.71–0.83) and the OR for women with BrCa5 ≥3.0% and no osteoporosis was 0.73 (95% CI: 0.60–0.90).
Figure 2. Raloxifene rates over time.
Raloxifene use (prescriptions filled) by calendar year from Part D claims data. The usage rate in a given year was calculated as the proportion of women with full coverage in that year who had at least one prescription filled in that year. Black line is all women, blue solid and dotted line are women with and without osteoporosis, respectively, red line is all women with 5-year breast cancer risk of at least 3% and purple line is women with at least 3% breast cancer risk and no osteoporosis.
Table 3.
Model for Raloxifene and Tamoxifen Use Over Time
| Group | OR (95% CI) for current year versus prior year: 2010–20141 | OR (95% CI) for 2014 compared to 2012 |
|---|---|---|
| Raloxifene | ||
| All women 2,3 | 0.90 (0.87–0.92) p <0.0001 | 0.77 (0.71–0.83) p<0.0001 |
| With osteoporosis 3 | 0.88 (0.85–0.92) p <0.0001 | 0.82 (0.72–0.94) p=0.003 |
| No osteoporosis 3 | 0.90 (0.88–0.93) p <0.0001 | 0.75 (0.67–0.83) p<0.0001 |
| ≥3% Breast cancer risk 2 | 0.88 (0.85–0.92) p <0.0001 | 0.77 (0.65–0.91) p=0.002 |
| ≥3% Breast cancer risk and no osteoporosis | 0.90 (0.86–0.95) p < 0.0001 | 0.73 (0.60–0.90) p=0.003 |
| Tamoxifen | ||
| All women3 | 1.18 (1.06–1.32) p=0.002 | 1.12 (0.83–1.51) p=0.44 |
| ≥3% Breast cancer risk | 1.13 (0.89–1.27) p=0.31 | 1.17 (0.62–2.21) p=0.62 |
Calendar year was modeled as a linear variable over the time period.
Model controlled for osteoporosis status.
Model controlled for breast cancer risk.
Note: All models controlled for age.
Using the SQX medication data from 2006–7, the decrease in use from 2012 to 2014 was assessed in relation to past use at a time approximately five years prior. Among women with raloxifene use in 2012 and with part D coverage through 2014 (N=393), 31% had discontinued use in 2014. The discontinuation rate (from 2012 to 2014) was similar for women reporting raloxifene use in 2006–7, 29% (75/258), as for women not reporting raloxifene use in 2006–7, 34% (46/135).
Assessing the concordance of the MUQ and Part D sources of raloxifene use for the same time period, the kappa statistic was 0.88 (95% CI:0.85–0.91).
Tamoxifen Use
Table 4 shows rates of tamoxifen use by BrCa5 category. From the MUQ, rates for the BrCa5 categories of <1.66%, 1.66–3.0%, and >3.0% were 0.17%, 0.24% and 0.39%, respectively (p-value trend=0.03), whereas for Part D, rates were 0.36%, 0.45% and 0.85%, respectively (p-value trend=0.009). Among ever-users (N=67), 17.9% had a daily use rate above 75%, 38.8% had a rate under 25% and 43.3% had a rate between 25 and 75%. Concordance between the MUQ and Part D data sources on tamoxifen usage was good (kappa=0.83). In all women, tamoxifen use significantly increased over 2010–2014, with OR=1.18 (95% CI: 1.06–1.32) for the current compared to prior year (Table 3).
Table 4.
Breast cancer risk and tamoxifen use as assessed from the Medication Use Questionnaire (MUQ) (2013) and from Medicare Part D (2010–2014).
| MUQ | Medicare Part D | |||
|---|---|---|---|---|
| Breast Cancer Risk Level 1 – BrCa5 | N in cell (%) | Tamoxifen Use – N (%) | N in cell (%) | Tamoxifen Use – N (%) |
| All levels | 22235 (100) | 54 (0.24) | 13640 (100) | 67 (0.49) |
| < 1.66% | 8121 (36.5) | 14 (0.17) | 4999 (36.7) | 18 (0.36) |
| 1.66–3.0% | 10059 (45.2) | 24 (0.24) | 6162 (45.2) | 28 (0.45) |
| ≥ 3.0% | 4055 (18.2) | 16 (0.39) | 2479 (18.2) | 21 (0.85) |
| P-value (trend) | 0.03 | 0.009 | ||
Five-year predicted risk of breast cancer
Use of Other Drugs
Rates of oral bisphosphonate use, from Part D data, were 22.4% overall and 41.9% in women with osteoporosis. Rates decreased markedly over time, from 41% in 2010 to 14% in 2014 in women with osteoporosis.
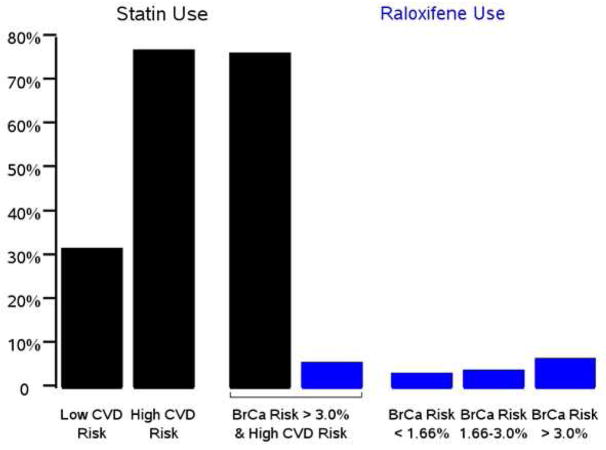
From Part D, use of statins was 58.0%. Among women at high cardiovascular risk, defined as those reporting a history of diabetes, high cholesterol, heart attack or stroke, and who comprised 58% of the cohort, use was 76.9%. Figure 3 displays use rates for statins and raloxifene according to cardiovascular and breast cancer risk. Among women with high cardiovascular risk and BrCa5 ≥ 3%, 76.3% used statins and 5.8% used raloxifene. Statin use was relatively steady over time.
Figure 3. Usage rates of statins and raloxifene by risk level.
Rates of use of raloxifene (blue bars) and statins (black bars) from Part D data by cardiovascular disease (CVD) risk level and five-year breast cancer risk category. High CVD risk is defined as history of diabetes, high cholesterol, heart attack or stroke; low risk is all women not at high risk.
Discussion
This analysis of an older cohort showed that use of raloxifene overall was low, 2.5% in a short-term period (one month) and 4.2% over a longer (5-year) period. Among both women with and without osteoporosis, raloxifene use increased significantly with breast cancer risk level. Based on longer term (Part D) data, among women without osteoporosis, who were more likely using raloxifene for breast cancer prevention, use increased 2-fold for the highest (≥3%) compared to lowest (<1.66%) breast cancer risk group, although even in this highest risk group, usage (from Part D data) was only 5.2%. Tamoxifen use was substantially lower here than raloxifene use. This was presumably due to the better side-effect profile of raloxifene.
Interestingly, there was a statistically significant decrease in raloxifene use from 2010 to 2014 and specifically from the year before (2012) to after (2014) the USPSTF B recommendation concerning breast cancer risk reducing medications. Further, it decreased in not only in women overall but also in those women recommended by the USPSTF for raloxifene use, namely, those with a ≥3% 5-year risk of breast cancer, who had a 23% reduction in use from 2012 to 2014. This decrease from 2012 to 2014 was not due to women discontinuing raloxifene due to duration of use because discontinuation rates were similar, if slightly lower, among women who had been using raloxifene five years prior as for women who had not been using raloxifene five years prior. Note that the USPSTF recommendation statement shows the standard dose of raloxifene for breast cancer risk reduction as being 60 mg for five years, based on the duration of use in the major clinical trials of raloxifene. Data from the MUQ, though, shows that most women (75%) currently using raloxifene had been using for over five years.
It is important to note that this decreasing trend over time was for a cohort of older women, all well past menopause at the start of the observation period (2010). Therefore, these data are not informative about whether women newly reaching post-menopausal status had decreasing rates in initiating raloxifene use over this time period.
There is relatively little literature on tamoxifen and especially raloxifene use for breast cancer prevention in the general population outside of a chemoprevention trial or a study examining decision aids and/or attitudes about breast cancer chemoprevention 8. A study using the 2010 NHIS survey found 0.9% of women 50–79 taking raloxifene, with about one-fourth taking the drug for breast cancer prevention 9. Use of tamoxifen for breast cancer prevention among women aged 35–79 in the NHIS was 0.03%. This rate of raloxifene use is lower than that reported here of 2.5% for women 65–84.
Studies of decision aids or attitudes about raloxifene and tamoxifen generally show low levels of interest in taking either drug. In one study of a decision aid, 5.7% of intervention and 3.2% of control arm subjects indicated an intention to take a breast cancer prevention drug, but only 0.5% of intervention arm and no control subjects actually took either drug 12. A majority of women, in each arm, did not perceive significant benefits from these drugs. In another study, factors influencing the decision to take raloxifene were the time having to take it (women preferred one year versus five years), the risk of side effects, and not having a test available to determine if the drugs were working 13. Another study showed that among women discussing breast cancer risk-reducing medications with their health care provider, the provider’s recommendation was the most important determinant of intention to use tamoxifen or raloxifene 14. It has also been suggested that, in the setting of a preventive intervention, formal quantitative comparison of risk reduction versus adverse effects may be less important than subjects’ feelings, and that, while “relief” is an important motivator for some preventive behaviors (e.g., mammography), taking tamoxifen or raloxifene is unlikely to afford a sense of relief 15,16.
Surveys have generally found low rates of prescribing breast cancer chemopreventive agents among physicians 17,18. Barriers to physicians, and especially those in primary care, in recommending breast cancer chemoprevention include difficulty in identifying patients who might benefit from it, lack of time to discuss it with patients, lack of knowledge in risk reducing medication options, unfamiliarity with the Gail model and breast cancer risk prediction, insufficient training in risk counselling, and concerns about side effects 18–19. Because of findings like these in both patients and physicians, investigators are exploring dual interventions providing personalized information to both the patient and health-care provider to increase informed decision making and chemoprevention uptake 20. Other suggestions for increasing the use of breast cancer chemopreventive agents include incorporating training on this topic into residency and fellowship programs and having the breast cancer advocacy community take a more active role in promoting chemoprevention 21.
In contrast to the relatively low rates of raloxifene use for breast cancer risk reduction, use of statins for cardiovascular disease prevention was high, 58% overall. Strikingly, for women at both high cardiovascular risk and high breast cancer risk (BrCa5 ≥ 3.0%), the use rate for statins was 13-fold higher than for raloxifene (76.3% versus 5.8%). There is little or no literature on comparative attitudes of patients or physicians on use of statins versus breast cancer risk reducing medications; however, factors that may be affecting the difference in usage rates include physician attitudes and knowledge, perceived risks of breast cancer versus cardiovascular disease, and real and believed side-effect profiles of the medications. In addition, statins have a well-validated intermediate endpoint, cholesterol level, that can be evaluated as a marker of effectiveness, whereas there is no such intermediate endpoint for raloxifene. Breast density has been shown to be a potential surrogate for tamoxifen efficacy; however, this same relationship has not been shown for raloxifene, potentially because most of the studies were retrospective and enrolled mainly women with low mammographic density 22,23. Interestingly, the new statin guidelines indicate use for those with 10-year cardiovascular disease risk ≥ 7.5% 24. For a five-year period, that would translate into a risk of roughly 3.75%, which is similar to the 3% five-year breast cancer risk threshold for use recommended by the USPSTF.
A limitation of this study is that cohort analyzed here, all enrolled in PLCO, were volunteers for a cancer screening trial and were more highly educated and health conscious than the general population in their age group; additionally, their rate of mammography use was also higher than average 25,26. Therefore, the rates observed here of raloxifene and tamoxifen use may overestimate rates in the overall population. Another limitation is that the indication for raloxifene use, whether for osteoporosis or breast cancer prevention, or both, was not known. An indication for breast cancer risk reduction can be inferred, however, in a subset of users based on the gradient of increasing usage rates with increasing breast cancer risk. There were some missing data in terms of calendar years with no part D coverage, but the rate was a relatively low 12% (based on a mean of 4.4 of 5 years (88%) with Part D data), so this should have had only minimal impact on the results. A strength of the analysis, in addition to large numbers, is that two sources of drug usage information were used, with excellent agreement.
Conclusion
Use of raloxifene in this Medicare-aged cohort was low overall, but increased modestly with breast cancer risk. Raloxifene use in this cohort significantly declined in the period from before to after the USPSTF B recommendation for breast cancer chemoprevention. Use of tamoxifen was very low.
Acknowledgments
This work was funded in part by NIH contract number HHSN261201600007I.
Footnotes
The authors have no conflicts of interest to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fisher B, Constantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Ca Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 2.Vogel VG, Constantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes. JAMA. 2006;23:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 3.Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in post411 menopausal women treated with raloxifene: 4-year results from the MORE trial. Breast Cancer Res Treat. 2001;65:125–134. doi: 10.1023/a:1006478317173. [DOI] [PubMed] [Google Scholar]
- 4.Cancer.Gov. [Accessed Oct 2nd, 2017];FDA approval for raloxifene hydrochloride. https://www.cancer.gov/aboutcancer/treatment/drugs/fda-raloxifene-hydrochloride.
- 5.U.S. Department of Health and Human Services, HHS News. [Accessed Oct 2nd, 2017];Tamoxifen approved for reducing breast cancer incidence. http://www3.scienceblog.com/community/older/archives/M/2/fda1168.htm.
- 6.Moyer VA. Medications for risk reduction of primary breast cancer in women: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2013;159:698–708. doi: 10.7326/0003-4819-159-10-201311190-00717. [DOI] [PubMed] [Google Scholar]
- 7.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28:3090–3095. doi: 10.1200/JCO.2009.27.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27:575–590. doi: 10.1093/annonc/mdv590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waters EA, McNeel TS, McCaskill Stevens W, Freedman AN. Use of tamoxifen and raloxifene for breast cancer chemoprevention in 2010. Breast Cancer Res Treat. 2012;134:875–880. doi: 10.1007/s10549-012-2089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinsky PF, Yu K, Kramer BS, et al. Extended mortality results for ovarian cancer screening in the PLCO trial with median 15 years follow-up. Gyn Oncol. 2016;143:270–275. doi: 10.1016/j.ygyno.2016.08.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cancer Institute. [Accessed July 8th, 2017];Breast cancer risk assessment SAS Macro (Gail Model) https://dceg.cancer.gov/tools/risk-assessment/bcrasasmacro.
- 12.Fagerlin A, Dillard AJ, Smith DM, et al. Women’s interest in taking tamoxifen and raloxifene for breast cancer prevention: response to a tailored decision aid. Breast Cancer Res Treat. 2011;127:681–688. doi: 10.1007/s10549-011-1450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez KA, Fagerlin A, Witteman HO, Holmberg C, Hawley ST. What matters to women when making decisions about chemoprevention. Patient. 2016;9:149–159. doi: 10.1007/s40271-015-0134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmberg C, Bandos H, Fagerlin A, et al. NRG Oncology/National Surgical Adjuvant Breast and Bowel Project Decision-making Project-1 results: decision making in breast cancer risk reduction. Cancer Prev Res. 2017;10:625–634. doi: 10.1158/1940-6207.CAPR-17-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narod SA. Tamoxifen chemoprevention – end of the road? JAMA Oncology. 2015;8:1033–1034. doi: 10.1001/jamaoncol.2015.2247. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum L. Invisible risks, emotional choices. N Engl J Med. 2014;371:1549–1552. doi: 10.1056/NEJMms1409003. [DOI] [PubMed] [Google Scholar]
- 17.Sabatino SA, McCarthy EP, Phillips RS, Burns RB. Breast cancer risk assessment and management in primary care: provider attitudes, practices and barriers. Cancer Detect Prev. 2007;31:375–383. doi: 10.1016/j.cdp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Corbelli J, Borrero S, Bonnema R, et al. Use of the Gail Model and breast cancer preventive therapy among three primary care specialties. J Women’s Health. 2014;23:746–752. doi: 10.1089/jwh.2014.4742. [DOI] [PubMed] [Google Scholar]
- 19.Hum S, Wu M, Pruthi S, Heisey R. Physician and patient barriers to breast cancer preventive therapy. Curr Breast Cancer Rep. 2016;8:158–164. doi: 10.1007/s12609-016-0216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. National Library of Medicine. Study of Web-based decision aids for increasing breast cancer chemoprevention in the primary care setting. [Accessed Oct 10th, 2017];ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03069742.
- 21.Wickerham DL, Vogel VG. Breast cancer chemoprevention: the saga of underuse continues. J Natl Cancer Inst. 2015;107:399. doi: 10.1093/jnci/dju399. [DOI] [PubMed] [Google Scholar]
- 22.Cuzick J, Warwick J, Pinney E, Warren R, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96:621–628. doi: 10.1093/jnci/djh106. [DOI] [PubMed] [Google Scholar]
- 23.Pearman L, Kagan R, Arsenault J, Muram D. The effects of raloxifene on mammographic breast density: a review of clinical trials. Menopause. 2010;17:654–659. doi: 10.1097/gme.0b013e3181c29e56. [DOI] [PubMed] [Google Scholar]
- 24.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 25.Pinsky PF, Miller A, Kramer BS, et al. Evidence of a healthy volunteer effect in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Am J Epidemiol. 2007;165:874–881. doi: 10.1093/aje/kwk075. [DOI] [PubMed] [Google Scholar]
- 26.Royce TJ, Hendrix LH, Stokes WA, et al. Cancer screening rates in individuals with different life expectancies. JAMA Intern Med. 2014;174:1558–1565. doi: 10.1001/jamainternmed.2014.3895. [DOI] [PubMed] [Google Scholar]