Abstract
Rates of short-interval pregnancies that result in unintended pregnancies remain high in the United States and contribute to adverse reproductive health outcomes. Long-acting reversible contraception methods have annual failure rates of <1%, compared with 9% for oral contraceptive pills, and are an effective strategy to reduce unintended pregnancies. To increase access to long-acting reversible contraception in the immediate postpartum period, several State Medicaid programs, which include those in Iowa and Louisiana, recently established reimbursement policies to remove the barriers to reimbursement of immediate postpartum long-acting reversible contraception insertion. We used a mixed-methods approach to analyze 2013–2015 linked Medicaid and vital records data from both Iowa and Louisiana and to describe trends in immediate postpartum long-acting reversible contraception provision 1 year before and after the Medicaid reimbursement policy change. We also used data from key informant interviews with state program staff to understand how provider champions affected policy uptake. We found that the monthly average for the number of insertions in Iowa increased from 4.6 per month before the policy to 6.6 per month after the policy; in Louisiana, the average number of insertions increased from 2.6 per month before the policy to 45.2 per month. In both states, the majority of insertions occurred at 1 academic/teaching hospital. In Louisiana, the additional increase may be due to the engagement of a provider champion who worked at both the state and facility level. Recruiting, training, engaging, and supporting provider champions, as facilitators, with influence at state and facility levels, is an important component of a multipart strategy for increasing successful implementation of state-level Medicaid payment reform policies that allow reimbursement for immediate postpartum long-acting reversible contraception insertions.
Keywords: champion, insertions, inter-pregnancy intervals, long acting reversible contraception, Medicaid, policy, postpartum, pregnancy spacing, providers, provider champions, state, reimbursement policy, uptake
Based on the 2006–2010 National Survey of Family Growth, 38% of pregnancies, after a previous birth, were mistimed or unwanted.1 These unintended pregnancies are significantly more likely to have short interpregnancy intervals (<18 months apart) compared with pregnancies reported as intended (unadjusted odds ratios, 4.3 and 1.8, respectively).1 Short interpregnancy intervals also increase risks for poor maternal and infant outcomes,2–4 which makes the reduction in short-interval unintended pregnancies a public health priority.
Long-acting reversible contraceptive methods (LARC [ie, contraceptive implants and intrauterine devices (IUD)]) are effective contraceptive strategies to promote optimal interpregnancy intervals, reduce unintended pregnancies, and improve maternal and child outcomes.5–7 These methods have failure rates of <1% for 3–10 years, depending on the method.8,9 Although only an estimated 11% of parous US women, 15–44 years old, use LARC,10 use among postpartum women is higher (7–31%).11 Therefore, providing LARC immediately after birth can further improve effectiveness. However, reasons for low immediate postpartum LARC uptake are complex. For instance, a survey of 1221 obstetricians/gynecologists found that, although almost all offered IUDs, fewer offered implants, with 32% of respondents citing lack of insertion training as a barrier to offering the implant.12 Additionally, a major system-level barrier to immediate post-partum LARC insertion is its high cost, which historically has not been reimbursed outside of the bundled obstetrics delivery rate.13,14 To address this, several state Medicaid programs recently established policies and issued guidance to support inpatient reimbursement of LARC insertion and device placement immediately after delivery.13,15,16 However, experiences of early adopting states revealed that reimbursement policy change alone was insufficient to overcome access barriers.17 Rather, additional provider and systems barriers must be addressed, as components of a multipart strategy, that leads to immediate postpartum LARC uptake in birthing facilities across states.
This call to action summarizes the experiences in 2 states regarding immediate postpartum LARC insertion policy implementation, with a focus on the provider champion’s role in policy implementation. We describe trends in immediate postpartum LARC adoption in Iowa and Louisiana 1 year before and after state Medicaid reimbursement policy changes that occurred in February and June of 2014, respectively16,18,19 (Table 1; for other states with LARC reimbursement policy changes, please see reference 16 and http://www.astho.org/Maternal-and-Child-Health/Increasing-Access-to-Contraception/Resources-Map/). Although these results cannot be generalized beyond the participating states, this information may benefit other states as they consider adopting reimbursement policies to increase immediate postpartum LARC uptake at birthing facilities.
TABLE 1.
Summary of the 2014 revised Medicaid policies from Iowa and Louisiana to provide immediate postpartum long-acting reversible contraception reimbursement17–19
| Iowa’s policy (March 2014) | Louisiana’s policy (June 2014) |
|---|---|
| 1. Medicaid will cover the cost of inserting intrauterine devices and other long-acting reversible contraception devices before the beneficiary leaves the hospital after delivery. | 1. Medicaid will cover the cost of inserting intrauterine devices and other long-acting reversible contraception devices before the beneficiary leaves the hospital after delivery. |
| 2. Payment for these services is allowed for both practitioners and hospitals. | 2. Payment for these services is allowed for both practitioners and hospitals. |
| 3. Practitioners who provide this service in the hospital setting will need to bill with the appropriate place of service code. | 3. Practitioners who provide this service in the hospital setting will need to bill as an add-on service in addition to their professional services rate. |
| 4. For hospitals that provide this service, this payment will be separate from the diagnosis-related group payment for the inpatient admission that is associated with the delivery. | 4. Hospitals that provide this service will need to bill as an add-on service in addition to their daily per diem rate for the inpatient hospital stay or diagnosis-related group rate. |
Methods for monitoring immediate postpartum LARC adoption
Quantitative approach
Women with Medicaid-paid claims for deliveries in Iowa and Louisiana during the 12 months before and 12 months after reimbursement policy implementation (March 2014 in Iowa and July 2014 in Louisiana) were included. In 2014, 38.4% (n=15,212) of Iowa’s 39,013 resident births and 66.7% (n=42,682) of Louisiana’s 64,038 resident births were reimbursed by Medicaid.
Medicaid databases were queried for paid claims with International Classification of Diseases, 9th Revision, Clinical Modification Diagnosis and Procedure codes or Current Procedural Terminology codes for a LARC insertion, prescription claims for National Drug Codes for copper or hormonal IUD and hormonal implants (collectively, LARC devices), or Healthcare Common Procedure Coding System codes for LARC devices (Table 2). Claims were restricted to those with dates of service within 3 days of an inpatient live birth delivery, which is consistent with the National Quality Forum recommended measure #2902.20 The immediate post-partum LARC claims were linked to birth certificate records for validation, which resulted in mother/infant dyad records matched from the mother’s and infant’s Medicaid records to the birth certificate (University of Louisiana Monroe, Office of Outcomes Research and Evaluation, High Risk Pregnancy Registry Matching Process White Paper, unpublished data, 2014).21,22 Linked data were used to de-duplicate claims and validate delivery for Medicaid patients. We categorized facilities by geographic location (urban vs rural) and facility type (community vs academic/teaching hospital). We defined academic/teaching hospital as a flagship facility or major affiliate of a medical school (ie, academic health or medical center).22 We identified 76 birthing hospitals in Iowa (2014), 1 of which was a teaching hospital, and 56 birthing hospitals in Louisiana (2014) and 8 were teaching hospitals.
TABLE 2.
Codes used to identify immediate postpartum long-acting reversible contraception in the inpatient Medicaid claim files within 3 days of an inpatient claim for a delivery
| Code type | Codes |
|---|---|
| Clinical Modification Diagnosis and Procedure Codes | 69.7, V25.11, V25.5 |
| Current Procedural Terminology Codes | 11981, 58300 |
| National Drug Codes | J7300, J7301, J7302, J7306, J7307, Q0090, S4981, S4989 |
| Healthcare Common Procedure Coding System Codes | 50419042101, 50419042301, 00052027201, 00052027401, 00052433001, 51285020401, 51285020402, 50419042201 |
Statistical methods
To examine immediate postpartum LARC uptake during the 12 months before and after the reimbursement policy release date, we calculated the mean of the monthly insertion counts and compared the monthly within-state means before and after policy implementation using paired, 1-sample t-tests (α=.05). Graphic representations of the monthly insertion counts were scaled based on the maximum count for each state. This was a retrospective de-identified analysis approved by Iowa’s Research and Ethics Review Committee and considered exempt from Louisiana’s Department of Health’s Institutional Review Board. The Centers for Disease Control and Prevention determined that the staff members at the Centers for Disease Control and Prevention were not engaged in human subject research.
Qualitative data sources
In 2014, the Association of State and Territorial Health Officials (ASTHO) with support of the Centers for Disease Control and Prevention, the Centers for Medicare and Medicaid Services, and the Office of Population Affairs convened a Learning Community to provide technical assistance to states that had newly implemented Medicaid immediate postpartum LARC insertion policies. The Learning Community identified promising practices to support states in their immediate postpartum LARC implementation process. ASTHO conducted key informant interviews with all participating state teams, including Iowa and Louisiana. State teams consisted of State Health Officials, Medicaid Medical Directors, Maternal and Child Health Directors, Family Planning Directors, American College of Obstetricians and Gynecologists state chapter members, and other state program staff. ASTHO asked a series of questions about the barriers and facilitators to immediate postpartum LARC implementation policies. ASTHO transcribed interviews and abstracted information on barriers and facilitators, validated the extractions, and coded the information. States identified provider champions as key facilitators to increase LARC uptake. States shared how provider champions were identified either at the state (Medicaid level) and facility level or both and how they were integrated and used in a multipart strategy to improve immediate postpartum LARC insertion implementation.23–25
Uptake of immediate postpartum LARCs after policy change
Iowa
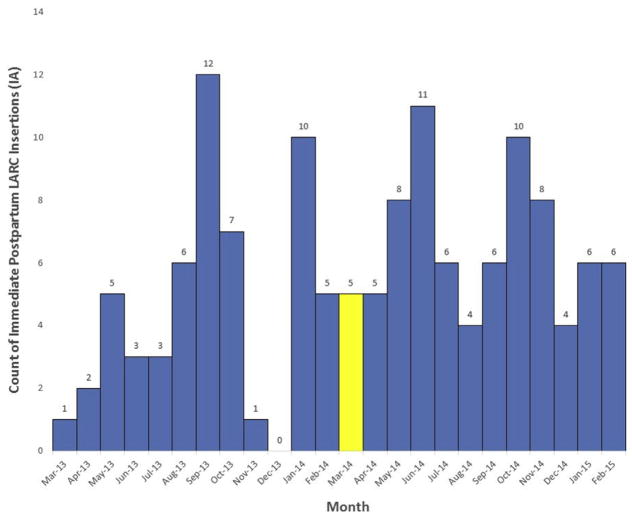
Of the >15,000 Medicaid-paid births in the 12 months after the reimbursement policy implementation, 0.5% had a LARC claim immediately after delivery. On average, clinicians inserted 4.6 immediate postpartum LARCs per month within the 12 months before policy change. In the 12 months after policy change, clinicians inserted on average, 6.6 immediate postpartum LARCs per month (P=.12; Figure 1). Before and after the policy change, approximately 80% of immediate postpartum LARC insertions took place at 1 academic hospital that I located in an urban area of Iowa. At the time of this analysis, the identified provider champion had worked to assure that hospital and pharmacy policies were in place to facilitate LARC uptake. However, based on Medicaid paid claims, it appeared that the provider champion’s facility had not yet implemented immediate postpartum LARC insertions. Iowa’s provider champion was an obstetrics/gynecology provider who championed facility and practice level efforts to increase immediate postpartum LARC adoption at her practicing facility.23,26 Iowa, however, was not successful in recruiting or retaining champions at the state level or within other facilities.
FIGURE 1. Monthly count of immediate postpartum long-acting reversible contraception claims in Iowa 2013–2015.
Monthly count of immediate postpartum long-acting reversible contraception insertion claims 12 months before and 12 months after implementation of long-acting reversible contraception reimbursment policy in Iowa. The yellow hashed bar highlights the month in which the long-acting reversible contraception reimbursement policy was implemented.
IA, Iowa; LARC, long-acting reversible contraception.
Louisiana
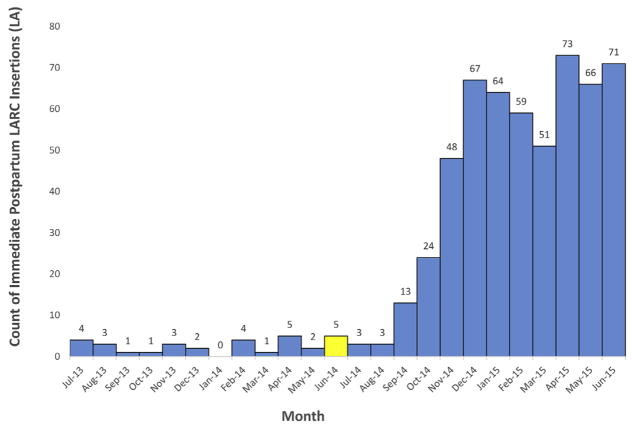
Approximately 1.3% of Medicaid-paid births had an associated LARC claims 12 months after reimbursement policy change. Compared with Iowa, average monthly counts for immediate postpartum LARC insertions increased more dramatically after policy implementation (Figure 2). Average per-month LARC insertions increased from 2.6–45.2 per month (P=.0002) from 1 year before to 1 year after policy change, a 1638% relative increase. Similar to Iowa, the majority of insertions occurred at 1 academic hospital in an urban area of Louisiana. In Louisiana, the obstetrics/gynecology provider champion emerged at the state level and identified immediate postpartum LARC as a priority through statewide quality improvement initiatives. The champion (R.E.G.) was the Medicaid Medical Director at the time of the initiative and worked closely with Louisiana’s Medicaid program to encourage reimbursement policy changes. Also, as a provider at the academic hospital, she worked with the facility to reassure its leadership that the devices would be reimbursable before ordering and stocking the devices. She supported additional reimbursement for in-hospital postpartum LARC device insertion, waiver expansion, an increase in Medicaid LARC reimbursement, and the removal of previous authorization for LARCs. She also conducted educational sessions for residents and other attendings on the importance and ease of immediate postpartum LARC placement and achieved buy-in for immediate postpartum LARC placement by other faculty.
FIGURE 2. Monthly count of immediate postpartum long-acting reversible contraception claims in Louisiana 2013–2015.
Monthly count of immediate postpartum long-acting reversible contraception insertion claims 12 months before and 12 months after implementation of long-acting reversible contraception reimbursment policy in Louisiana. The yellow hashed bar highlights the month in which the long-acting reversible contraception reimbursement policy was implemented.
LARC, long-acting reversible contraception; LA, Louisiana.
Early results of reimbursement policy
Based on the experiences of 2 states, establishment of an immediate post-partum LARC Medicaid reimbursement policy change alone did not substantially improve its uptake. By 12 months after the reimbursement policy change, only a fraction of the Medicaid-paid deliveries, from both states, had an associated claim for immediate postpartum LARC. Most insertions occurred at an urban academic hospital within each state. This may be partly due to the nature of academic hospitals as training facilities. For example, Whiteman et al27 found that women who delivered at teaching hospitals were 3 times as likely to undergo postpartum IUD insertion compared with those who delivered at nonteaching hospitals (adjusted odds ratio, 3.02; 95% confidence interval, 1.43–6.37). Lastly, uptake, although low overall, may have occurred more rapidly in Louisiana compared with Iowa because of the provider champion’s engagement at both the facility and state levels.
The role of provider champions as facilitators
The Iowa and Louisiana state teams had identified provider champions as a component of a multipart strategy and an important facilitator to advance immediate postpartum LARC policy implementation. Further, the experiences from Louisiana suggest that provider champions operate at 2 different levels: state and facility level. State-level champions can exert influence through authority and status and build consensus to implement new practices across the state.28 Facility-level champions motivate key individuals by relying on personal relationships, established credibility within the facility, and displaying persistence in garnering organizational support. They can also help secure resources and overcome institutional obstacles.29,30 Champions at both levels may maximize the adoption of complex population-based interventions, such as the implementation of Medicaid reimbursement policies within and across clinical sites throughout the state.13,28,31–36
For immediate postpartum LARC, state-level champions could support uptake by educating Medicaid partners and other payers about LARC safety, cost, and clinical benefits to women who desire these methods in the immediate postpartum period. Additionally, champions could build state-level partnerships and coalitions to support implementation efforts, such as educational outreach and training to providers across the state.14 Facility-level champions can support efforts by role-modeling and providing peer-to-peer training within the facility and by helping coordinate across administration, pharmacy, and billing departments to ensure device availability and reimbursement.24
We acknowledge that provider champion engagement is 1 intervention that states can use to achieve optimal immediate postpartum LARC uptake and that a multipart intervention is needed. For instance, we found only 1 hospital in both Iowa and Louisiana that actively implemented immediate postpartum LARC, even with the presence of a state level champion. This may be due to the level of hospital administrative support to purchase and stock LARC devices in advance of reimbursement, availability of trained providers to place LARCs, and women’s preference for LARCs in the immediate postpartum period. For example, the Louisiana provider champion used established credibility to assure hospital administrators about the minimal revenue decreases from stocking LARC devices before reimbursement. Although these factors could be influenced by a state and/or facility-level provider champion, we acknowledge that such issues can also be out of the provider champion’s sphere of influence.
Some approaches to provider champion engagement beyond 1 facility may include hosting provider training sessions on billing and insertion techniques and sharing information about successful implementation strategies within and across states. However, additional research in other states that are implementing immediate postpartum LARC policies is warranted to better understand barriers and facilitators for increasing uptake.
How can a provider support LARC reimbursement changes and improve implementation?
Increasing access to immediate post-partum LARC requires systems change, which includes state and facility level provider champions. Provider champions are positioned to promote immediate postpartum LARC access expansion in their sphere of influence, whether at the state, local, facility, or at multiple levels. Champions are needed as facilitators to engage in multidisciplinary partnership between payer systems, local and state public health agencies, and the healthcare system as direct clinical providers.
THE PROBLEM
States with Medicaid payment reform policies, which allow reimbursement for immediate postpartum long-acting reversible contraception devices and insertion, have found that policy changes alone are not sufficient to ensure access to these highly effective contraceptive methods.
A SOLUTION
Recruiting, training, engaging, and supporting provider champions, whether at the state, local, facility, or at multiple levels, are important components of the multipart strategy to implement state-level Medicaid payment reform policies that allow reimbursement for immediate postpartum long-acting reversible contraception insertions.
Footnotes
The authors report no conflict of interest.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the Association of American Medical Colleges.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Gemmill A, Lindberg LD. Short interpregnancy intervals in the United States. Obstet Gynecol. 2013;122:64–71. doi: 10.1097/AOG.0b013e3182955e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKinney D, House M, Chen A, Muglia L, DeFranco E. The influence of interpregnancy interval on infant mortality. Am J Obstet Gynecol. 2017;216:316e1–9. doi: 10.1016/j.ajog.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809–23. doi: 10.1001/jama.295.15.1809. [DOI] [PubMed] [Google Scholar]
- 4.Conde-Agudelo A, Belizan JM. Maternal morbidity and mortality associated with interpregnancy interval: cross-sectional study. BMJ. 2000;321:1255–9. doi: 10.1136/bmj.321.7271.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celen S, Möröy P, Sucak A, Aktulay A, Danişman N. Clinical outcomes of early postplacental insertion of intrauterine contraceptive devices. Contraception. 2004;69:279–82. doi: 10.1016/j.contraception.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481e1–7. doi: 10.1016/j.ajog.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Washington CI, Jamshidi R, Thung SF, Nayeri UA, Caughey AB, Werner EF. Timing of postpartum intrauterine device placement: a cost-effectiveness analysis. Fertil Steril. 2015;103:131–7. doi: 10.1016/j.fertnstert.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Trussell J. Contraceptive failure in the United States. Contraception. 2011;82:397–404. doi: 10.1016/j.contraception.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Committee on Gynecologic Practice Long-Acting Reversible Contraception Working Group. Committee Opinion No 642: Increasing Access to Contraceptive Implants and Intra-uterine Devices to Reduce Unintended Pregnancy. Obstet Gynecol. 2015;126:e44–8. doi: 10.1097/AOG.0000000000001106. [DOI] [PubMed] [Google Scholar]
- 10.Branum A, Jones J. Trends in long acting reversible contraceptive use among women aged 15–44. NCHS Brief. 2015;188:1–8. [PubMed] [Google Scholar]
- 11.Boulet SL, D’Angelo DV, Morrow B, et al. Contraceptive use among nonpregnant and postpartum women at risk for unintended pregnancy, and female high school students, in the context of Zika preparedness: United States, 2011–2013 and 2015. MMWR Morb Mortal Wkly Rep. 2016;65:780–7. doi: 10.15585/mmwr.mm6530e2. [DOI] [PubMed] [Google Scholar]
- 12.Luchowski AT, Anderson BL, Power ML, Raglan GB, Espey E, Schulkin J. Obstetrician-gynecologists and contraception: long-acting reversible contraception practices and education. Contraception. 2014;89:578–83. doi: 10.1016/j.contraception.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Moniz MH, Dalton VK, Davis MM, et al. Characterization of Medicaid policy for immediate postpartum contraception. Contraception. 2015;92:523–31. doi: 10.1016/j.contraception.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Fox J, Barfield W. Decreasing unintended pregnancy: opportunities created by the Affordable Care Act. JAMA. 2016;316:815–6. doi: 10.1001/jama.2016.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Association of State and Territorial Health Officials. Long-Acting Reversible Contraception: Medicaid Policies, Codes, and Guidance. Association of State and Territorial Health Officials website; [Accessed July 5, 2016]. Available at: http://www.astho.org/Programs/Maternal-and-Child-Health/Long-Acting-Reversible-Contraception-LARC/Medicaid-Policies/ [Google Scholar]
- 16.Wachino V. State Medicaid Payment Approaches to Improve Access to Long-Acting Reversible Contraception. [Accessed March 21, 2018];CMCS Informational Bulletin. Available at: https://www.medicaid.gov/federal-policy-guidance/downloads/CIB040816.pdf.
- 17.Rankin KM, Kroelinger CD, DeSisto CL, et al. Application of implementation science methodology to immediate postpartum long-acting reversible contraception policy roll-out across states. Matern Child Health J. 2016;20(suppl1):173–9. doi: 10.1007/s10995-016-2002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iowa Department of Human Services. [Accessed July 16, 2016];Informational Letter NO. 1349. Available at: https://dhs.iowa.gov/sites/default/files/1349%20Long%20Acting%20Reversible%20Contraception.pdf.
- 19.Louisiana Department of Health and Hospitals. [Accessed July 16, 2016];Health Plan Advisory 14-9. Available at: http://dhh.louisiana.gov/assets/docs/BayouHealth/HealthPlanAdvisories/2014/HPA14-9.pdf.
- 20.Perinatal and Reproductive Health Project 2015–2016. [Accessed December 7, 2016];National Quality Forum web-site. Available at: http://www.qualityforum.org/ProjectMeasures.aspx?projectID=80680.
- 21.Kane DJ, Sappenfield WM. Ascertainment of Medicaid payment for delivery on the Iowa birth certificate: is accuracy sufficient for timely policy and program relevant analysis? Matern Child Health J. 2014;18:970–7. doi: 10.1007/s10995-013-1325-7. [DOI] [PubMed] [Google Scholar]
- 22.Ayanian JZ, Weissman JS. Teaching hospitals and quality of care: a review of the literature. Milbank Q. 2002;80:569–93. doi: 10.1111/1468-0009.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Association of State and Territorial Health Officials. [Accessed July 25, 2016];Long-Acting Reversible Contraception (LARC) Learning Community Launch Report. 2014 Aug 19; Available at: http://www.astho.org/Programs/Prevention/Maternal-and-Child-Health/LARCLearning-Community-Launch-Report/
- 24.Rankin KM, DeSisto C UIC-SPH. ASTHO IPP LARC Learning Community: Findings from Key Informant Interviews with Cohort 1 States. [Accessed July 25, 2016];Prepared for ASTHO. 2016 Apr; Available at: http://www.astho.org/Programs/Maternal-and-Child-Health/Documents/Key-Informant-Interview-Cohort-1-Year-2/
- 25.Association of State and Territorial Health Officials. [Accessed April 19, 2016];Immediate Postpartum LARC Learning Community: Findings from Key Informant Interviews with Cohort 2 States. Available at: http://www.astho.org/Programs/Maternal-and-Child-Health/Documents/LARC-Key-Informant-Interviews-Cohort-2-Year-2/
- 26.US Department of Health and Human Services. About the Secretary’s Advisory Committee on Infant Mortality. U.S. Department of Health and Human Services website; [Accessed November 29, 2016]. Available at: http://www.hrsa.gov/advisorycommittees/mchbadvisory/InfantMortality/About/about.html. [Google Scholar]
- 27.Whiteman MK, Cox S, Tepper NK, et al. Postpartum intrauterine device insertion and postpartum tubal sterilization in the United States. Am J Obstet Gynecol. 2012;206:127e1–17. doi: 10.1016/j.ajog.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 28.National Collaborating Centre for Methods and Tools. Engaging public health champions to garner support for innovations. Hamilton, ON: McMaster University; 2011. [Accessed November 29, 2016]. Available at: http://www.nccmt.ca/resources/search/91. [Google Scholar]
- 29.Howell JM, Shea CM, Higgins CA. Champions of product innovations: defining, developing, and validating a measure of champion behavior. Journal of Business Venturing. 2005;20:641–61. [Google Scholar]
- 30.Weiner BJ, Haynes-Maslow L, Kahwati LC, Kinsinger LS, Campbell MK. Implementing the MOVE! weight-management program in the Veterans Health Administration, 2007–2010: a qualitative study. Prev Chronic Dis. 2012;9:E16. [PMC free article] [PubMed] [Google Scholar]
- 31.Zavalkoff S, Korah N, Quach C. Presence of a physician safety champion is associated with a reduction in urinary catheter utilization in the pediatric intensive care unit. PLoS One. 2015;10:e0144222. doi: 10.1371/journal.pone.0144222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locock L, Dopson S, Chambers D, Gabbay J. Understanding the role of opinion leaders in improving clinical effectiveness. Soc Sci Med. 2001;53:745–57. doi: 10.1016/s0277-9536(00)00387-7. [DOI] [PubMed] [Google Scholar]
- 33.Aagaard EM, Gonzales R, Camargo CA, Jr, et al. Physician champions are key to improving antibiotic prescribing quality. Jt Comm J Qual Patient Saf. 2010;36:109–16. doi: 10.1016/s1553-7250(10)36019-3. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez MI, Evans M, Espey E. Advocating for immediate postpartum LARC: increasing access, improving outcomes, and decreasing cost. Contraception. 2014;90:468–71. doi: 10.1016/j.contraception.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Soni A, Amin A, Patel DV, et al. The presence of physician champions improved Kangaroo mother care in rural western India. Acta Paediatr. 2016;105:e390–5. doi: 10.1111/apa.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofler LG, Cordes S, Cwiak CA, Goedken P, Jamieson DJ, Kottke M. Implementing immediate postpartum long-acting reversible contraception programs. Obstet Gynecol. 2017;129:3–9. doi: 10.1097/AOG.0000000000001798. [DOI] [PubMed] [Google Scholar]