Abstract
Cerebellar abiotrophy (CA) is a neurodegenerative disorder affecting the cerebellum and occurs in multiple species. Although CA is well researched in humans and mice, domestic species such as the dog, cat, sheep, cow, and horse receive little recognition. This may be due to few studies addressing the mechanism of CA in these species. However, valuable information can still be extracted from these cases. A review of the clinicohistologic phenotype of CA in these species and determining the various etiologies of CA may aid in determining conserved and required pathways necessary for proper cerebellar development and function. This review outlines research approaches of studies of CA in domestic species, compared to the approaches used in mice, with the objective of comparing CA in domestic species while identifying areas for further research efforts.
Keywords: Cerebellar abiotrophy, Domestic species, Histology
Introduction
The gross structure and circuitry of the cerebellum is relatively conserved across species, with the cerebellar volume consistently occupying approximately 10% of the entire brain volume [1]. While some reports contradict that cerebellar size evolves in concert with the cerebral cortex size, there is agreement that the surface area and absolute mass are proportional to the cerebral cortex [2]. Across 19 species of varying sizes (spanning between rodents and primates, including humans), there was an estimated 3.6 neurons in the cerebellum to every neuron in the cerebral cortex [3]. However, in humans, it has been found that the cerebellum contains a remarkable 80% of the CNS neurons [4], whereas in mice, this proportion is 60%. Does this reflect the functional importance of the cerebellum in these species? This issue remains unclear. However, there are examples of cerebellar adaptation in species more reliant on sensory information for their movement decisions, such as cetacea and microchiroptera, which rely on echolocation [2]. Additionally, information processing capacity in the cerebellum has been directly correlated with the number of granular neuron-Purkinje neuron synapses [5]. It has been shown that there is a marked increase in granule-Purkinje synapses from the rodent to the human, which exceeds the proportional change expected based upon the mass and volume changes seen in the cerebral cortex [5]. Thus, despite the similar gross and circuitry structures across species, synapse-level differences must be taken into consideration when doing a cross-species studies.
When the cerebellum is damaged, motor movement is impaired, often in the form of ataxia and uncoordinated movement. Examples of these cerebella-based motor deficits can be found in well-known diseases such as Parkinson’s [6] and fragile X syndrome [7, 8]. Here, we will focus on heritable cerebellar abiotrophies seen across domestic species, which is often overlooked, but constitutes a large portion of neurodegeneration seen in domestic animals, as reviewed last in 1990 by de Lahunta [9]. As referenced in the name, cerebellar abiotrophy is due to a specific atrophy within the cerebellum resulting from an inborn metabolic defect during development [10]. Histologically, cerebellar abiotrophies often result in loss of the Purkinje neurons of the cerebellum within a few days to a few months after birth. There is usually no gross reduction in cerebellar size, as seen in cerebellar hypoplasias, and the loss of Purkinje neurons is often considered diffuse throughout the cerebellum. Despite CA often being inherited in an autosomal recessive fashion, definitive diagnosis of CA remains to be based upon post-mortem histological findings. Neurologically, there are often head tremors associated with voluntary movement, a lack of menace response (often due to the lack of blink response), bilaterally symmetric hypermetric gait (exaggerated movement of all four limbs) and abnormal postural behavior (often delayed then exaggerated when performing a neurological exam). CA demonstrates substantial variation on all scales of its phenotype across and within species. This is most likely due to the nature of CA as a broad spectrum of disorders affecting the cerebellum and its description of the end result of the disorder, rather than the mechanism of neurodegeneration. However, discovering causative mutations or mechanisms of CA in various species helps to explain this variability and may be relevant to the rare and early onset cases of CA seen in humans that are yet to have mutations associated with them [11].
CA was first documented in cattle in 1951 [12] and has since been researched in other species including dogs, cats, sheep, and horses. Among these species, 18 breeds of dogs and eight breeds of cattle have been reported to be affected by CA. In other species, including the horse, only a single breed is affected by CA (Table 1). Several iterations of CA have been well characterized in the mouse. There are currently eight spontaneously inherited CAs found in mice. This review seeks to outline the status of CA characterization across domestic species relative to characterization standards set forth by CA research done in mice.
Table 1.
General statistics regarding CA research across domestic species relative to the human and mouse
| Animal | No. of papers (case studies) | Onset post parturition | Number of breeds | Mode of inheritance determined? | Mass of cerebellum (g)a | Mass of brain (g)a | Number of predicted Purkinje neurons (millions) |
|---|---|---|---|---|---|---|---|
| Cat | 7 (7) | 10 months old to 9 years old | 3 | No | 3.8 | 17.3 | 1.735 |
| Dog | 24 (12) | 6 months old to 5 years old | 18 | Yes | 12.0 | 54.9 | 3.858 |
| Horse | 10 (4) | Days to 6 years old | 1 | Yes | 85.1 | 598.63 | 15.05 |
| Cow | 6 (4) | Days to 2 years old | 3 | No | 48.06 (2.25) | 480.54 (3.93) | NA |
| Sheep | 5 (2) | 4 weeks old to 5 months old | 3 | No | 9.05 | 130 | 3.171 |
| Goat | 2 (2) | 6 to 9 months old | 2 | No | 15.9 | 55 | 4.691 |
| Mouse | > 1000 | Birth to 3 weeks old | 8 | Yes | 0.08 | 0.3 | 0.119 |
| Human | > 1000 | – | – | Yes | 154.02 | 1508.91 | 15.85 |
NA not available
References for these weights: (77–81)
CA Research in Mice
Attributing to their well-characterized genetics, ease of breeding, and ability to generate colonies demonstrating a CA genotype and phenotype, a gold standard of approaches on characterizing CA in mice has been established. As outlined by Lalonde and Strazielle [13], approaches to describe spontaneously induced CA in eight mouse lines include identification of the mutation associated with the CA, neurologic consequences and age of onset of CA, neuropathology, areas of secondary neurodegeneration, and the neurochemistry underlying the CA pathology. For each of these eight mouse lines, a genotype associating with CA has been established. With the exceptions of the lurcher and weaver mice, the other six CAs are inherited in an autosomal recessive fashion. The lurcher and weaver mice have semi-dominant mutations, where the homozygotes die at birth because of a severe phenotype [14, 15]. Onset of the common ataxic phenotype and wide-based gait is often detected within two to 3 weeks postpartum, exceptions being the staggerer mouse, which shows signs at 1 week [16] and the weaver from day of birth [17]. Histologically, six of the eight mouse lines show primary degeneration of the Purkinje neurons, while reeler and weaver mice show primary granule neurodegeneration [18, 19]. The weaver mouse also exhibits selective degeneration in the cerebellar midline versus the lateral hemispheres [20]. There is further selectivity of degeneration seen in the molecular landscape, where the nervous and Purkinje cell degeneration mice show degeneration in the Purkinje neurons positive for Zebrin II [21], whereas the leaner mouse shows degeneration of Purkinje neurons that are negative for Zebrin II [22]. Secondary loss of neurons is often limited to other populations of neurons within the cerebellum, such as granular neurons, or neurons in the inferior olivary nucleus. However, in the Purkinje neuron degeneration and nervous mice, there is concurrent neurodegeneration in mitral and retinophotoreceptor cells [23] or the dorsal cochlear and retinal photoreceptors [24], respectively. With the exception of the nervous mouse, the other seven mouse lines have candidate genes confirmed to be associated with the mutation and CA phenotype. How these genes belong to pathways prompting the CA phenotype is elucidated by focusing on regional cerebellar metabolism as well as concentrations of receptors and neurotransmitters.
There has been a standard procedure accompanying the description of CA in mice, in terms of phenotyping via behavioral tests, histopathological analysis, and observation of the genetic and protein components associated with the CA. Examples of behavioral tests include the square beam, round beam, rotorod, and coat hanger, which have been applied to all the CA-affected mice and test the ability of the mice to remain on the structure. Variations of the T-maze and water mazes have also been used on CA-affected mice to test for working memory. The detection of the differing spectra of severity in the neurologic phenotype between these mice can be achieved by applying and then comparing the results of these tests across the mice. Focusing on neuronal subpopulations specifically affected by CA, regional neuronal metabolism and activity are often assessed using cytochrome oxidase (CO) staining and determination of neurotransmitter concentration in the cerebellar cortex of the CA-affected mice. Increased CO staining indicates neuron-specific activity and, in cases such as the lurcher mouse, CO activity was found to be higher in the remaining Purkinje neurons [25]. This was most likely due to chronic depolarized membrane potential, leading to Purkinje neuron autophagy [15]. Upon observing the neurotransmitter levels across the cerebellar cortex of CA-affected mice, serotonin is increased in all mice, except the dystonia musculorum [13]. Could this reflect the developmental role of serotonin known in the cerebellum [26] and reveal the temporal component of CA? These examples certainly illustrate the expansive characterizations of CA in the mouse, and by considering the behavioral outcome, localization and molecular pathways encompassed by the CA phenotype, more intricate and detailed comparisons can be made.
Localization of Cerebellar Abiotrophy in Domestic Animals
The cerebellar cortex is often considered as a series of repeating computational units, differing in their input and the targets of their output. When cerebellar degeneration is reported in domestic animals, it is often done with a lack of discernible local patterns of degeneration. In all the domestic species mentioned in this review, diffuse degeneration throughout the cerebellar cortex has been reported, with specific incidences of confined neurodegeneration. In horses and dogs, a reduction in cerebellar size relative to cerebral size has been determined using magnetic resonance imaging (MRI) in a small number of cases affected with CA [27, 28]. In Scottish Terriers, more severe neurodegeneration was identified in the dorsal versus ventral regions of the cerebellar cortex [29]. Specific neurodegeneration in the anterior vermis was identified in specific cases of CA in Wiltshire sheep [30]. Examples of true random or diffuse loss of Purkinje neurons are quite rare and are potentially a misobservation due to inaccurate analysis of the entire cerebellar cortex, as reviewed by Sarna and Hawkes [31]. However, comparisons of neurodegeneration through the entire cerebellar cortex are rarely performed in domestic species. Future comparative studies would benefit from evaluating the entire cerebellum.
Regarding the specific cell types affected by CA across domestic species, neurodegeneration of Purkinje neurons is most frequently reported. This observation is often likely a “default” due to the ease of evaluating Purkinje neuron loss and insult versus granular neuron loss and phenotype. More studies specifying which population of neurons are undergoing apoptosis need to be conducted, such as in the horse, where the TUNEL assay confirmed apoptosis primarily in the Purkinje neurons [32]. Often, granular neuron loss is reported as secondary to Purkinje neuron loss in CA. However, in two breeds of dog, the Brittany spaniel and rough-coated collie, granular neuron loss has been documented as the primary neuronal population affected, suggesting a more complex pathogenesis [33, 34]. The various cases of apoptosis in the granular versus the Purkinje neurons in CA highlight the variation underlying CA, and the need to characterize CAwith regards to CA progression and cell type.
Clinical and Histological Findings of CA in Domestic Species
CA is often defined by the characteristic neurologic and histological phenotype. Across the species in this review, the neurologic phenotype involves head tremors, lack of coordination, and lack of menace response. A wide-based stance is also commonly reported in Arabian horses [35], Holsteiner cows [36] and border collies [37]. In the horse, cow, goat, and many breeds of dogs, CA is often not accompanied with neuroanatomic lesions outside of the cerebellum. There are examples, however, of species showing other signs of neurodegeneration. For example, in a domestic shorthair cat affected with CA, retinal degeneration was also identified [38]. In another case in, a Staffordshire terrier, intermittent seizure-like and opisthotonus episodes were noted [39]. There are also differing descriptions of the movement appearing with CA, such as hopping of the pelvic limbs seen in the Brittany spaniel dog [34], Wiltshire and Charollais sheep [30, 40], the truncal sway seen in the Staffordshire terrier [39], or the limb stiffness reported in Poll Hereford bulls [41]. Beyond the neurologic phenotype, CA is most often confirmed via histology.
As mentioned in the localization of CA section above, the loss of Purkinje neurons is the most frequent reported finding in affected individuals. With this loss, all species mentioned in this review also demonstrate a concordant disorganization of the three layers of the cerebellar cortex, along with gliosis (Fig. 1a) and resulting empty baskets in the Purkinje neuron layer (Fig. 1b). Of the Purkinje neurons remaining, common features include cytoplasmic vacuolization (Fig. 1c), swollen axons (Fig. 1b) and chromatolysis. When a genetic mutation associating with CA is absent, as is the case with most domestic species, these histological hallmarks are required for a definitive diagnosis of CA.
Fig. 1.
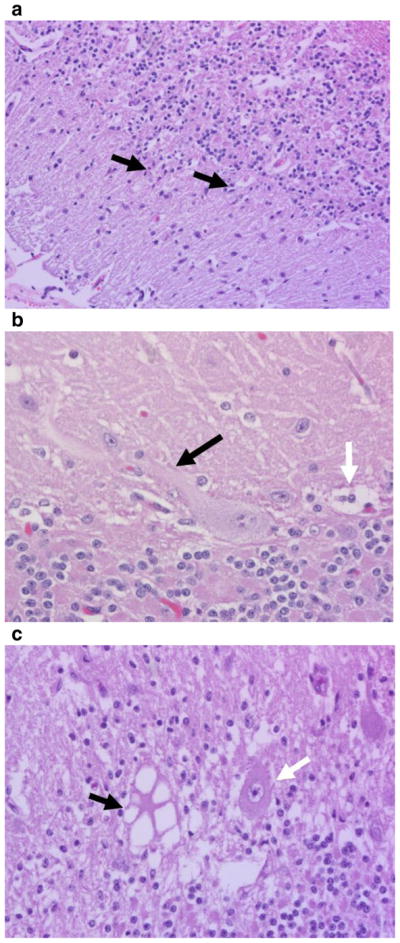
H&E histology from CA-affected horses showing a the disorganization of the three layers of the cerebellar cortex as well as gliosis (black arrows), b swollen axon (black arrow) and the empty baskets (white arrow), and c cytoplasmic vacuolization (black arrow) versus a normal Purkinje neuron (white arrow)
Onset of Neurologic Phenotype in Domestic Animals
In addition to the spectra of clinical and histologic findings with CA, there is variability in its onset and progression. In cats and dogs, CA has been commonly reported as late onset, with rapid progression followed by a stabilization period [27, 38, 39, 42–45]. There are also examples of early onset disease in 6-week-old kittens [46], 9-week-old Labrador retrievers [47], and 4-month-old border collie puppies [37], exhibiting similar rapid CA progression patterns. The larger domestic species, such as the cow, goat, sheep, and horse, have an earlier onset of CA with a slower progression. This ranges from the day of birth to almost 2 years in the cow [36, 48], 6 to 9 months in the goat [49], 4 weeks to 5 months in sheep [30, 40, 50], and 6 to 9 months in the horse [32, 51–53]. In order to properly resolve CA onset in these species, time series experiments capturing the onset of CA and the mechanisms underlying the onset are necessary.
Proposed Mechanisms Underlying the CA Phenotypes in Domestic Animals
As most of the information about CA in domestic animals originates from case reports, little information has been discovered in terms of underlying causal factors. It has been generally recognized that CA is often inherited in an autosomal recessive manner. This has been proposed in mixed bred Japanese cats [54], Charolais calves [55], several Hereford cross breeds [41], mixed breed goats [49], Kerry blue terrier dogs [56], Stafford terriers [57], Rhodesian ridgeback dogs [58], and the Coton de Tulear dogs [59]. CA associated genomic regions have been identified in Scottish terriers [29] and Australian Kelpies [60] using pedigree analyses, genome wide association studies (GWAS), and homozygosity mapping. Furthermore, a single nucleotide polymorphism (SNP), inherited in an autosomal recessive manner, has been associated with CA in the Finnish hound [61], Hungarian Vizsla dog [62], and the Arabian horse [63]. In terms of elucidating the molecular manifestations of these variants in CA-affected individuals, this has only been attempted in the Arabian horse, Finnish hound and Hungarian Vizsla dog. Using whole-genome sequencing, a mutation affecting a splice donor site in sorting nexin 14 gene (SNX14) has been identified in the Hungarian Vizsla, with PCR and western blotting used to confirm alternate splicing of SNX14 products at the gene and protein level [62]. In the Finnish hound, GWAS studies and subsequent sequencing uncovered a homozygous missense mutation in the conserved sel-1 suppressor of lin-12 like gene (SEL1L) [61]. Further profiling of this mutation revealed that it may also be linked to endoplasmic reticulum (ER) protein degradation [61]. In the Arabian horse, GWAS, homozygosity analysis and targeted Sanger sequencing identified a SNP in the protein coding target of Egr1 gene (TOE1), which could also affect the promoter, and therefore expression, of MUTYH [63]. The exact molecular ramifications of this SNP have not been elucidated. However, apoptosis of Purkinje neurons has been established [32], with a marked decrease in transcription, specifically of calcium homeostasis pathways in Purkinje neurons [64]. Recently, we have shown that MUTYH has differential promoter behavior, splice variant expression, and cerebellar localization in CA-affected horses [65]. Due to the opportunistic nature of sample retrieval in most CA studies in domestic animals, functional characterization of the molecular pathways affected in CA is understated. This can potentially be mitigated with more targeted biochemistry approaches to candidate gene questions and by obtaining larger treatment groups to establish more concrete conclusions.
Potential Sources of Variation Underlying the CA Phenotype
CA is a general description of a phenotype, rather than a specific disease or disease mechanism, leading to large variability in mechanisms underlying CA. In domestic animals, a majority of this variability cannot be explained due to lack of determination of definite molecular mechanisms explaining the disease phenotype. Aside from establishing CA as postnatal, there is also no detection of the exact moment of disease onset. How could there be so many different avenues leading to a similar result?
One reason could be due to the developmental characteristics of the cerebellum. Although it is one of the first discernible structures of the brain, its mature organization is often not achieved until several months after birth [66]. This long developmental period provides several opportunities for insult, ranging from affecting migration of Purkinje neurons via the Reelin signaling pathway [67] or granular neuron migration via the Dcc/netrin pathway [68] to later pathways in development affecting Purkinje neuron synapse formation via Wnt3 signaling [69].
Another source of variability can emerge from the subpopulations of neurons affected by CA. Despite having apparent homogeneity of cytoarchitecture throughout the cerebellar cortex, there has been recent research illuminating the several distinct subpopulations of Purkinje and granular neurons [31, 70]. These subpopulations have macro-level differences, different physiologies relative to location and distinct population-specific molecular markers. These three factors must be considered, as this removes the equality of losing one Purkinje neuron over another. An example of macro-level differences has been found in rat cerebella, where the packing densities of the Purkinje neurons are higher in the anterior versus the posterior lobe of the cerebellum [71]. There has also been divergence of physical characteristics of Purkinje neurons based on location. Purkinje neurons located in phylogenetically older areas of the brain, such as the vermis, have larger cell diameters as well as organelles [72]. Regarding the synapse forming areas of the Purkinje neuron, there are differences in axon diameter in different sections of the white matter [73] and different dendrite morphologies relative to the base and apex of the cerebellar cortex folium [74]. These kinds of physical differences have foreseeable effects on the physiology of these neurons. With regard to Purkinje neuron excitability in the vermis, depolarization-induced slow current is higher in the posterior versus the anterior lobes [75], while Purkinje neurons in the most posterior flocculonodular lobe seem to show lower levels of excitability and more variable firing patterns due to various passive and active membrane properties [76]. Molecular markers present in these sub-populations demonstrate divergence relative to both region and physiology. Here we will focus on the most popular zebrin II marker; however the list of molecular markers in the cerebellar cortex is far more extensive [70]. Most obvious about zebrin II expression in Purkinje neurons is the rostrocaudal oriented banding pattern of zebrin II+ and zebrin II− seen throughout the cerebellar cortex. This banding pattern also has distinct physiological differences, such as reduced LTD capabilities of parallel fiber-Purkinje synapses in the zebrin II+ bands [77]. This may be associated with differential input from mossy and climbing fibers, which seems to align with zebrin II [78]. Collectively, the variability encompassed in the macro, physiological, and molecular differences of Purkinje neurons, combined with changes accompanying cerebellar development, need to be addressed when resolving mechanisms underlying the CA phenotype.
Conclusion
Studies of CA across domestic species demonstrate that several altered pathways can lead to a similar phenotype: cerebellar ataxia. Although descriptions of CA across domestic species are mainly limited to case reports and thus lack mechanistic explanation, they are useful at identifying which species to target for further analysis. Currently, CA comparability across species is often restricted to the neurologic phenotype, but with further characterization of CA on a molecular level across species, better understanding of which pathways is essential to the cerebellum could be elucidated. Ideally, if CA research across species adhered to certain standards of classification, including neurologic, histological and molecular phenotyping, as seen in mice, they could be much more effective at drawing comparisons across species. One of the major limitations preventing this standardized procedure is sample availability. If there were more coordinated efforts to efficiently acquire samples from individuals affected with CA, perhaps studies beyond individual-based case reports could be done. Efficient sample procurement should include complete ante-mortem phenotyping with video-documented behavioral tests, collection of genomic DNA, evaluation of a representative sampling of histologic samples from the entire cerebellum, and flash-frozen cerebellar tissue for gene and protein expression analysis. If this type of coordinated work could be performed, trends concerning CA could be identified and perhaps these CA-affected domestic animals could be more applicable for translational studies. For example, the extensive studies performed on equine CA have shown relevant similarities to a human pontocerebellar hypoplasia in the involvement of the gene Target of Egr1 (TOE1) [79]. CA across domestic species has been beneficial in identifying that CA exists in various species and that there are several circumstances leading to cerebellar degeneration. With a detailed characterization of CA in domestic species, translational implications would be strengthened and our understanding of cerebellar development will be expanded.
References
- 1.Sultan F, Glickstein M. The cerebellum: comparative and animal studies. Cerebellum. 2007;6(3):168–76. doi: 10.1080/14734220701332486. https://doi.org/10.1080/14734220701332486. [DOI] [PubMed] [Google Scholar]
- 2.Clark DA, Mitra PP, Wang SS. Scalable architecture in mammalian brains. Nature. 2001;411(6834):189–93. doi: 10.1038/35075564. https://doi.org/10.1038/35075564. [DOI] [PubMed] [Google Scholar]
- 3.Herculano-Houzel S. Coordinated scaling of cortical and cerebellar numbers of neurons. Front Neuroanat. 2010;4:12. doi: 10.3389/fnana.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarko DK, Catania KC, Leitch DB, Kaas JH, Herculano-Houzel S. Cellular scaling rules of insectivore brains. Front Neuroanat. 2009;3:8. doi: 10.3389/neuro.05.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Gammon SJ, Dieterle M, Huang RH, Likins L, Ricklefs RE. Dramatic increases in number of cerebellar granule-cell-Purkinje-cell synapses across several mammals. Mamm Biol—Z Säugetierkd. 2014;79(3):163–9. https://doi.org/10.1016/j.mambio.2013.12.003. [Google Scholar]
- 6.Wu T, Hallett M. The cerebellum in Parkinson’s disease. Brain. 2013;136(Pt 3):696–709. doi: 10.1093/brain/aws360. https://doi.org/10.1093/brain/aws360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellegood J, Pacey LK, Hampson DR, Lerch JP, Henkelman RM. Anatomical phenotyping in a mouse model of fragile X syndrome with magnetic resonance imaging. NeuroImage. 2010;53(3):1023–9. doi: 10.1016/j.neuroimage.2010.03.038. https://doi.org/10.1016/j.neuroimage.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Roy S, Zhao Y, Allensworth M, Farook MF, LeDoux MS, Reiter LT, et al. Comprehensive motor testing in Fmr1-KO mice exposes temporal defects in oromotor coordination. Behav Neurosci. 2011;125(6):962–9. doi: 10.1037/a0025920. https://doi.org/10.1037/a0025920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lahunta A. Abiotrophy in domestic animals: a review. Can J Vet Res. 1990;54(1):65–76. [PMC free article] [PubMed] [Google Scholar]
- 10.Siso S, Hanzlicek D, Fluehmann G, Kathmann I, Tomek A, Papa V, et al. Neurodegenerative diseases in domestic animals: a comparative review. Vet J. 2006;171(1):20–38. doi: 10.1016/j.tvjl.2004.08.015. https://doi.org/10.1016/j.tvjl.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Vermeer S, Meijer RP, Pijl BJ, Timmermans J, Cruysberg JR, Bos MM, et al. ARSACS in the Dutch population: a frequent cause of early-onset cerebellar ataxia. Neurogenetics. 2008;9(3):207–14. doi: 10.1007/s10048-008-0131-7. https://doi.org/10.1007/s10048-008-0131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jennings AR, Sumner GR. Cortical cerebellar disease in an Ayrshire calf. Vet Rec. 1951;63(4):60–1. doi: 10.1136/vr.63.4.60. [DOI] [PubMed] [Google Scholar]
- 13.Lalonde R, Strazielle C. Spontaneous and induced mouse mutations with cerebellar dysfunctions: behavior and neurochemistry. Brain Res. 2007;1140:51–74. doi: 10.1016/j.brainres.2006.01.031. https://doi.org/10.1016/j.brainres.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Goldowitz D. The weaver granuloprival phenotype is due to intrinsic action of the mutant locus in granule cells: evidence from homozygous weaver chimeras. Neuron. 1989;2(6):1565–75. doi: 10.1016/0896-6273(89)90045-7. https://doi.org/10.1016/0896-6273(89)90045-7. [DOI] [PubMed] [Google Scholar]
- 15.Zuo J, De Jager PL, Takahashi KA, Jiang W, Linden DJ, Heintz N. Neurodegeneration in Lurcher mice caused by mutation in delta2 glutamate receptor gene. Nature. 1997;388(6644):769–73. doi: 10.1038/42009. https://doi.org/10.1038/42009. [DOI] [PubMed] [Google Scholar]
- 16.Herrup K, Thoenen H. Properties of the nerve growth factor receptor of a clonal line of rat pheochromocytoma (PC12) cells. Exp Cell Res. 1979;121(1):71–8. doi: 10.1016/0014-4827(79)90445-2. https://doi.org/10.1016/0014-4827(79)90445-2. [DOI] [PubMed] [Google Scholar]
- 17.Smeyne RJ, Goldowitz D. Development and death of external granular layer cells in the weaver mouse cerebellum: a quantitative study. J Neurosci. 1989;9(5):1608–20. doi: 10.1523/JNEUROSCI.09-05-01608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirano A, Dembitzer HM. Cerebellar alterations in the weaver mouse. J Cell Biol. 1973;56(2):478–86. doi: 10.1083/jcb.56.2.478. https://doi.org/10.1083/jcb.56.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiffmann SN, Bernier B, Goffinet AM. Reelin mRNA expression during mouse brain development. Eur J Neurosci. 1997;9(5):1055–71. doi: 10.1111/j.1460-9568.1997.tb01456.x. https://doi.org/10.1111/j.1460-9568.1997.tb01456.x. [DOI] [PubMed] [Google Scholar]
- 20.Herrup K, Trenkner E. Regional differences in cytoarchitecture of the weaver cerebellum suggest a new model for weaver gene action. Neuroscience. 1987;23(3):871–85. doi: 10.1016/0306-4522(87)90164-3. [DOI] [PubMed] [Google Scholar]
- 21.Wassef M, Sotelo C, Cholley B, Brehier A, Thomasset M. Cerebellar mutations affecting the postnatal survival of Purkinje cells in the mouse disclose a longitudinal pattern of differentially sensitive cells. Dev Biol. 1987;124(2):379–89. doi: 10.1016/0012-1606(87)90490-8. https://doi.org/10.1016/0012-1606(87)90490-8. [DOI] [PubMed] [Google Scholar]
- 22.Herrup K, Wilczynski SL. Cerebellar cell degeneration in the leaner mutant mouse. Neuroscience. 1982;7(9):2185–96. doi: 10.1016/0306-4522(82)90129-4. https://doi.org/10.1016/0306-4522(82)90129-4. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Gonzalez A, La Spada AR, Treadaway J, Higdon JC, Harris BS, Sidman RL, et al. Purkinje cell degeneration (pcd) phenotypes caused by mutations in the axotomy-induced gene, Nna1. Science. 2002;295(5561):1904–6. doi: 10.1126/science.1068912. https://doi.org/10.1126/science.1068912. [DOI] [PubMed] [Google Scholar]
- 24.LaVail MM, White MP, Gorrin GM, Yasumura D, Porrello KV, Mullen RJ. Retinal degeneration in the nervous mutant mouse. I. Light microscopic cytopathology and changes in the interphotoreceptor matrix. J Comp Neurol. 1993;333(2):168–81. doi: 10.1002/cne.903330204. https://doi.org/10.1002/cne.903330204. [DOI] [PubMed] [Google Scholar]
- 25.Vogel MW, Fan H, Sydnor J, Guidetti P. Cytochrome oxidase activity is increased in +/Lc Purkinje cells destined to die. Neuroreport. 2001;12(14):3039–43. doi: 10.1097/00001756-200110080-00012. https://doi.org/10.1097/00001756-200110080-00012. [DOI] [PubMed] [Google Scholar]
- 26.Oostland M, Buijink MR, Teunisse GM, von Oerthel L, Smidt MP, van Hooft JA. Distinct temporal expression of 5-HT(1A) and 5-HT(2A) receptors on cerebellar granule cells in mice. Cerebellum. 2014;13(4):491–500. doi: 10.1007/s12311-014-0565-4. https://doi.org/10.1007/s12311-014-0565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertalan A, Glass EN, Kent M, De LaHunta A, Bradley C. Late-onset cerebellar abiotrophy in a Labrador retriever. Aust Vet J. 2014;92(9):339–42. doi: 10.1111/avj.12211. https://doi.org/10.1111/avj.12211. [DOI] [PubMed] [Google Scholar]
- 28.Cavalleri JM, Metzger J, Hellige M, Lampe V, Stuckenschneider K, Tipold A, et al. Morphometric magnetic resonance imaging and genetic testing in cerebellar abiotrophy in Arabian horses. BMC Vet Res. 2013;9(1):105. doi: 10.1186/1746-6148-9-105. https://doi.org/10.1186/1746-6148-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urkasemsin G, Linder KE, Bell JS, de Lahunta A, Olby NJ. Hereditary cerebellar degeneration in Scottish terriers. J Vet Intern Med. 2010;24(3):565–70. doi: 10.1111/j.1939-1676.2010.0499.x. https://doi.org/10.1111/j.1939-1676.2010.0499.x. [DOI] [PubMed] [Google Scholar]
- 30.Johnstone AC, Johnson CB, Malcolm KE, Jolly RD. Cerebellar cortical abiotrophy in Wiltshire sheep. N Z Vet J. 2005;53(4):242–5. doi: 10.1080/00480169.2005.36552. https://doi.org/10.1080/00480169.2005.36552. [DOI] [PubMed] [Google Scholar]
- 31.Sarna JR, Hawkes R. Patterned Purkinje cell death in the cerebellum. Prog Neurobiol. 2003;70(6):473–507. doi: 10.1016/s0301-0082(03)00114-x. https://doi.org/10.1016/S0301-0082(03)00114-X. [DOI] [PubMed] [Google Scholar]
- 32.Blanco A, Moyano R, Vivo J, Flores-Acuna R, Molina A, Blanco C, et al. Purkinje cell apoptosis in Arabian horses with cerebellar abiotrophy. J Vet Med A Physiol Pathol Clin Med. 2006;53(6):286–7. doi: 10.1111/j.1439-0442.2006.00836.x. https://doi.org/10.1111/j.1439-0442.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 33.Hartley WJ, Barker JSF, Wanner RA, Farrow BRH. Inherited cerebellar degeneration in rough coated collie. Aust Vet Pract. 1978;8(2):79–85. [Google Scholar]
- 34.Tatalick LM, Marks SL, Baszler TV. Cerebellar abiotrophy characterized by granular cell loss in a Brittany. Vet Pathol. 1993;30(4):385–8. doi: 10.1177/030098589303000411. https://doi.org/10.1177/030098589303000411. [DOI] [PubMed] [Google Scholar]
- 35.DeBowes RM, Leipold HW, Turner-Beatty M. Cerebellar abiotrophy. Vet Clin North Am Equine Pract. 1987;3(2):345–52. doi: 10.1016/s0749-0739(17)30677-6. https://doi.org/10.1016/S0749-0739(17)30677-6. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen F, George C, Douart A, Cherel Y, Lars F, Wyers M. Late onset of cerebellar abiotrophy in a Holstein heifer. Eur J Vet Pathol. 2001;7(1):27–9. [Google Scholar]
- 37.Sandy JR, Slocombe RE, Mitten RW, Jedwab D. Cerebellar abiotrophy in a family of border collie dogs. Vet Pathol. 2002;39(6):736–8. doi: 10.1354/vp.39-6-736. https://doi.org/10.1354/vp.39-6-736. [DOI] [PubMed] [Google Scholar]
- 38.Barone G, Foureman P, deLahunta A. Adult-onset cerebellar cortical abiotrophy and retinal degeneration in a domestic shorthair cat. J Am Anim Hosp Assoc. 2002;38(1):51–4. doi: 10.5326/0380051. https://doi.org/10.5326/0380051. [DOI] [PubMed] [Google Scholar]
- 39.Speciale J, de Lahunta A. Cerebellar degeneration in a mature Staffordshire terrier. J Am Anim Hosp Assoc. 2003;39(5):459–62. doi: 10.5326/0390459. https://doi.org/10.5326/0390459. [DOI] [PubMed] [Google Scholar]
- 40.Milne EM, Schock A. Cerebellar abiotrophy in a pedigree Charollais sheep flock. Vet Rec. 1998;143(8):224–5. doi: 10.1136/vr.143.8.224. https://doi.org/10.1136/vr.143.8.224. [DOI] [PubMed] [Google Scholar]
- 41.Whittington RJ, Morton AG, Kennedy DJ. Cerebellar abiotrophy in crossbred cattle. Aust Vet J. 1989;66(1):12–5. doi: 10.1111/j.1751-0813.1989.tb09705.x. https://doi.org/10.1111/j.1751-0813.1989.tb09705.x. [DOI] [PubMed] [Google Scholar]
- 42.Biolatti C, Gianella P, Capucchio MT, Borrelli A, D’Angelo A. Late onset and rapid progression of cerebellar abiotrophy in a domestic shorthair cat. J Small Anim Pract. 2010;51(2):123–6. doi: 10.1111/j.1748-5827.2009.00852.x. https://doi.org/10.1111/j.1748-5827.2009.00852.x. [DOI] [PubMed] [Google Scholar]
- 43.de Lahunta A, Fenner WR, Indrieri RJ, Mellick PW, Gardner S, Bell JS. Hereditary cerebellar cortical abiotrophy in the Gordon setter. J Am Vet Med Assoc. 1980;177(6):538–41. [PubMed] [Google Scholar]
- 44.Negrin A, Bernardini M, Baumgartner W, Castagnaro M. Late onset cerebellar degeneration in a middle-aged cat. J Feline Med Surg. 2006;8(6):424–9. doi: 10.1016/j.jfms.2006.04.007. https://doi.org/10.1016/j.jfms.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shamir M, Perl S, Sharon L. Late onset of cerebellar abiotrophy in a Siamese cat. J Small Anim Pract. 1999;40(7):343–5. doi: 10.1111/j.1748-5827.1999.tb03095.x. https://doi.org/10.1111/j.1748-5827.1999.tb03095.x. [DOI] [PubMed] [Google Scholar]
- 46.Taniyama H, Takayanagi S, Izumisawa Y, Kotani T, Kaji Y, Okada H, et al. Cerebellar cortical atrophy in a kitten. Vet Pathol. 1994;31(6):710–3. doi: 10.1177/030098589403100614. https://doi.org/10.1177/030098589403100614. [DOI] [PubMed] [Google Scholar]
- 47.Bildfell RJ, Mitchell SK, de Lahunta A. Cerebellar cortical degeneration in a Labrador retriever. Can Vet J. 1995;36(9):570–2. [PMC free article] [PubMed] [Google Scholar]
- 48.Schild AL, Riet-Correa F, Portiansky EL, Mendez MC, Graca DL. Congenital cerebellar cortical degeneration in Holstein cattle in Southern Brazil. Vet Res Commun. 2001;25(3):189–95. doi: 10.1023/a:1006477508776. https://doi.org/10.1023/A:1006477508776. [DOI] [PubMed] [Google Scholar]
- 49.Koehler JW, Newcomer BW, Holland M, Caldwell JMA. Novel inherited cerebellar abiotrophy in a cohort of related goats. J Comp Pathol. 2015;153(2–3):135–9. doi: 10.1016/j.jcpa.2015.06.001. https://doi.org/10.1016/j.jcpa.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Harper PA, Plant JW, Walker KH, Timmins KG. Progressive ataxia associated with degenerative thoracic myelopathy in Merino sheep. Aust Vet J. 1991;68(11):357–8. doi: 10.1111/j.1751-0813.1991.tb00735.x. https://doi.org/10.1111/j.1751-0813.1991.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 51.Baird JD, Mackenzie CD. Cerebellar hypoplasia and degeneration in part-Arab horses. Aust Vet J. 1974;50(1):25–8. doi: 10.1111/j.1751-0813.1974.tb09367.x. https://doi.org/10.1111/j.1751-0813.1974.tb09367.x. [DOI] [PubMed] [Google Scholar]
- 52.Foley A, Grady J, Almes K, Patton K, Davis E. Cerebellar abiotrophy in a 6-year-old Arabian mare. Equine Vet Educ. 2011;23(3):130–4. https://doi.org/10.1111/j.2042-3292.2010.00166.x. [Google Scholar]
- 53.Sadaba SA, Madariaga GJ, Botto CM, Carino MH, Zappa ME, Garcia PP, et al. First report of cerebellar abiotrophy in an Arabian foal from Argentina. Open Vet J. 2016;6(3):259–62. doi: 10.4314/ovj.v6i3.17. https://doi.org/10.4314/ovj.v6i3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inada S, Mochizuki M, Izumo S, Kuriyama M, Sakamoto H, Kawasaki Y, et al. Study of hereditary cerebellar degeneration in cats. Am J Vet Res. 1996;57(3):296–301. [PubMed] [Google Scholar]
- 55.Kemp J, McOrist S, Jeffrey M. Cerebellar abiotrophy in Holstein Friesian calves. Vet Rec. 1995;136(8):198. doi: 10.1136/vr.136.8.198. https://doi.org/10.1136/vr.136.8.198. [DOI] [PubMed] [Google Scholar]
- 56.Deforest ME, Eger CE, Basrur PK. Hereditary cerebellar neuronal abiotrophy in a Kerry Blue Terrier dog. Can Vet J. 1978;19(7):198–202. [PMC free article] [PubMed] [Google Scholar]
- 57.Olby N, Blot S, Thibaud JL, Phillips J, O’Brien DP, Burr J, et al. Cerebellar cortical degeneration in adult American Staffordshire Terriers. J Vet Intern Med. 2004;18(2):201–8. doi: 10.1892/0891-6640(2004)18<201:ccdiaa>2.0.co;2. https://doi.org/10.1111/j.1939-1676.2004.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 58.Chieffo C, Stalis IH, Van Winkle TJ, Haskins ME, Patterson DF. Cerebellar Purkinje’s cell degeneration and coat color dilution in a family of Rhodesian Ridgeback dogs. J Vet Intern Med. 1994;8(2):112–6. doi: 10.1111/j.1939-1676.1994.tb03207.x. https://doi.org/10.1111/j.1939-1676.1994.tb03207.x. [DOI] [PubMed] [Google Scholar]
- 59.Coates JR, O’Brien DP, Kline KL, Storts RW, Johnson GC, Shelton GD, et al. Neonatal cerebellar ataxia in Coton de Tulear dogs. J Vet Intern Med. 2002;16(6):680–9. doi: 10.1892/0891-6640(2002)016<0680:ncaicd>2.3.co;2. https://doi.org/10.1111/j.1939-1676.2002.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 60.Shearman JR, Cook RW, McCowan C, Fletcher JL, Taylor RM, Wilton AN. Mapping cerebellar abiotrophy in Australian Kelpies. Anim Genet. 2011;42(6):675–8. doi: 10.1111/j.1365-2052.2011.02199.x. https://doi.org/10.1111/j.1365-2052.2011.02199.x. [DOI] [PubMed] [Google Scholar]
- 61.Kyostila K, Cizinauskas S, Seppala EH, Suhonen E, Jeserevics J, Sukura A, et al. A SEL1L mutation links a canine progressive early-onset cerebellar ataxia to the endoplasmic reticulum-associated protein degradation (ERAD) machinery. PLoS Genet. 2012;8(6):e1002759. doi: 10.1371/journal.pgen.1002759. https://doi.org/10.1371/journal.pgen.1002759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fenn J, Boursnell M, Hitti RJ, Jenkins CA, Terry RL, Priestnall SL, et al. Genome sequencing reveals a splice donor site mutation in the SNX14 gene associated with a novel cerebellar cortical degeneration in the Hungarian Vizsla dog breed. BMC Genet. 2016;17(1):123. doi: 10.1186/s12863-016-0433-y. https://doi.org/10.1186/s12863-016-0433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brault LS, Cooper CA, Famula TR, Murray JD, Penedo MC. Mapping of equine cerebellar abiotrophy to ECA2 and identification of a potential causative mutation affecting expression of MUTYH. Genomics. 2011;97(2):121–9. doi: 10.1016/j.ygeno.2010.11.006. https://doi.org/10.1016/j.ygeno.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 64.Scott EY, Penedo MC, Murray JD, Finno CJ. Defining trends in global gene expression in Arabian horses with cerebellar abiotrophy. Cerebellum. 2017;16(2):462–72. doi: 10.1007/s12311-016-0823-8. https://doi.org/10.1007/s12311-016-0823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scott EY, Woolard KD, Finno CJ, Penedo MCT, Murray JD. Variation in MUTYH expression in Arabian horses with cerebellar abiotrophy. Brain Res. 2018;1678:330–6. doi: 10.1016/j.brainres.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nat Rev Neurosci. 2001;2(7):484–91. doi: 10.1038/35081558. https://doi.org/10.1038/35081558. [DOI] [PubMed] [Google Scholar]
- 67.Miyata T, Nakajima K, Mikoshiba K, Ogawa M. Regulation of Purkinje cell alignment by reelin as revealed with CR-50 antibody. J Neurosci. 1997;17(10):3599–609. doi: 10.1523/JNEUROSCI.17-10-03599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wingate ME. SLD is not stuttering. J Speech Lang Hear Res. 2001;44(2):381–3. doi: 10.1044/1092-4388(2001/031). https://doi.org/10.1044/1092-4388(2001/031) [DOI] [PubMed] [Google Scholar]
- 69.Salinas PC, Fletcher C, Copeland NG, Jenkins NA, Nusse R. Maintenance of Wnt-3 expression in Purkinje cells of the mouse cerebellum depends on interactions with granule cells. Development. 1994;120(5):1277–86. doi: 10.1242/dev.120.5.1277. [DOI] [PubMed] [Google Scholar]
- 70.Cerminara NL, Lang EJ, Sillitoe RV, Apps R. Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat Rev Neurosci. 2015;16(2):79–93. doi: 10.1038/nrn3886. https://doi.org/10.1038/nrn3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Armstrong DM, Schild RF. A quantitative study of the Purkinje cells in the cerebellum of the albino rat. J Comp Neurol. 1970;139(4):449–56. doi: 10.1002/cne.901390405. https://doi.org/10.1002/cne.901390405. [DOI] [PubMed] [Google Scholar]
- 72.Muller U, Heinsen H. Regional differences in the ultrastructure of purkinje cells of the rat. Cell Tissue Res. 1984;235(1):91–8. doi: 10.1007/BF00213728. [DOI] [PubMed] [Google Scholar]
- 73.Voogd J. Cerebellar zones: a personal history. Cerebellum. 2011;10(3):334–50. doi: 10.1007/s12311-010-0221-6. https://doi.org/10.1007/s12311-010-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nedelescu H, Abdelhack M. Comparative morphology of dendritic arbors in populations of Purkinje cells in mouse sulcus and apex. Neural Plast. 2013;2013:948587. doi: 10.1155/2013/948587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim YS, Shin JH, Hall FS, Linden DJ. Dopamine signaling is required for depolarization-induced slow current in cerebellar Purkinje cells. J Neurosci. 2009;29(26):8530–8. doi: 10.1523/JNEUROSCI.0468-09.2009. https://doi.org/10.1523/JNEUROSCI.0468-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim CH, Oh SH, Lee JH, Chang SO, Kim J, Kim SJ. Lobule-specific membrane excitability of cerebellar Purkinje cells. J Physiol. 2012;590(2):273–88. doi: 10.1113/jphysiol.2011.221846. https://doi.org/10.1113/jphysiol.2011.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wadiche JI, Jahr CE. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat Neurosci. 2005;8(10):1329–34. doi: 10.1038/nn1539. https://doi.org/10.1038/nn1539. [DOI] [PubMed] [Google Scholar]
- 78.Pijpers A, Apps R, Pardoe J, Voogd J, Ruigrok TJ. Precise spatial relationships between mossy fibers and climbing fibers in rat cerebellar cortical zones. J Neurosci. 2006;26(46):12067–80. doi: 10.1523/JNEUROSCI.2905-06.2006. https://doi.org/10.1523/JNEUROSCI.2905-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lardelli RM, Schaffer AE, Eggens VR, Zaki MS, Grainger S, Sathe S, et al. Biallelic mutations in the 3′ exonuclease TOE1 cause pontocerebellar hypoplasia and uncover a role in snRNA processing. Nat Genet. 2017;49(3):457–64. doi: 10.1038/ng.3762. https://doi.org/10.1038/ng.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]


