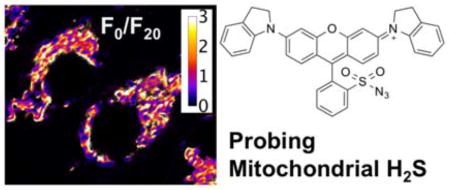
Abstract
Hydrogen sulfide (H2S) is an important gasotransmitter. Although a large number of fluorescent probes for cellular H2S have been reported, only a few can detect H2S in mitochondria, a cellular organelle connecting H2S with mitochondrial function and metabolic pathways. We hereby describe a novel near-infrared fluorescent probe, nimazide, by introducing sulfonyl azide to the core structure of a QSY-21 dark quencher. Nimazide responded quickly to H2S, resulting in robust fluorescence turn-off changes. This conversion displayed high specificity and fast kinetics. More impressively, we observed robust fluorescence decrease in live cells loaded with mitochondrial nimazide in response to extracellular addition of nanomolar H2S, and successfully imaged biologically-generated mitochondrial H2S in live mammalian cells. Nimazide is one of the most sensitive fluorescent probes for mitochondrial H2S.
Keywords: near-infrared fluorescent sensor, mitochondrial targeting, nanomolar hydrogen sulfide, fluorescence imaging, membrane activation
Graphical Abstract
Hydrogen sulfide (H2S), mainly converted from cysteine or homocysteine in biological systems, is a gasotransmitter playing pivotal roles in diverse biological processes, such as protein post-translational modification, smooth muscle relaxation, neuronal transmission, and regulation of ion channels and the mitochondrial respiratory chain.1 Dysregulation of H2S has been linked to a variety of diseases, including Alzheimer’s disease, Huntington’s disease, diabetes, cardiovascular diseases, Down syndrome, and lung diseases.2,3
Fluorescent probes for H2S are important research tools for understanding the dynamics, metabolism, and signaling of H2S in biological systems.4–8 On the basis of the unique chemoreactivity of H2S (i.e. reduction, metal binding, and nucleophilicity), a large number of fluorescent probes for H2S have been developed in recent years.9 Some of these probes have been utilized to image H2S, either exogenously supplemented or in situ synthesized by enzymes in live cells and animals. Although several fluorescent H2S probes with limits of detection (LODs) in the low nanomolar range have recently been reported, our detailed analysis concludes that these studies utilized in vitro assays to determine the LODs, and that 20–250 μM H2S (in the format of pH-buffered NaHS or Na2S) was often supplemented to imaging media to induce live-cell fluorescence changes.10–12 There is still a pressing need for fluorescent probes that can effectively detect low concentrations of H2S in cells.
The signaling of H2S is associated with specific organelles, so there is great interest in developing fluorescent H2S probes that can be targeted to subcellular domains. In particular, mitochondrial H2S has been reported to modulate mitochondrial biogenesis and cellular bioenergetics,13,14 as well as cancer cell proliferation and angiogenesis.15,16 To date, there are only a few mitochondria-targeted fluorescent H2S probes.12,17–20 Again, despite that some have been claimed for nanomolar LODs, these studies determined these numbers using in vitro assays.11,12 It remains a challenge to detect low amounts of H2S endogenously formed in mitochondria of live mammalian cells.11,12
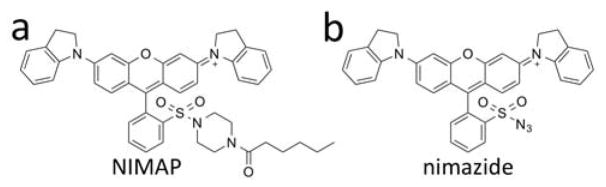
To reduce phototoxicity and increase tissue penetration, fluorescent probes with near-infrared absorption and emission are preferred.21 Our group previously reported a near-infrared fluorescent probe, NIMAP (Fig. 1a), derived from the dark quencher QSY-21, for monitoring mitochondrial membrane potentials in live cells.22 The fluorescence of NIMAP is activatable by the lipid membrane, resulting in low fluorescence background and high fluorescence contrast. Because of a number of favorable features of NIMAP, including mitochondrial localization, high photostability, and near-infrared fluorescence, we therefore developed a novel fluorescent H2S probe based on the QSY-21 scaffold. We named this new molecule “nimazide” for its near-infrared fluorescence, mitochondrial localization, and inclusion of an azide functional group. As one of the most sensitive fluorescent probe for mitochondrial H2S, nimazide responded to extracellularly supplemented, nanomolar H2S and was highly responsive to mitochondrial H2S biosynthesized in situ.
Fig. 1.
Chemical structures of NIMAP (a) and nimazide (b), which are fluorescent probes for mitochondrial membrane potentials (MMP) and mitochondrial hydrogen sulfide, respectively.
EXPERIMENTAL SECTION
Materials and general methods
All chemicals and reagents were purchased from Fisher Scientific or Sigma-Aldrich. The scheme and procedure for organic synthesis are outlined in the Supporting Information. The structure and purity of nimazide were analyzed with ESI-MS, 1H-NMR and 13C-NMR. Except for endogenous H2S, we used freshly prepared, pH-buffered NaHS solutions to deliver H2S.
Spectroscopic characterization
A monochromator-based Synergy Mx Microplate Reader (BioTek, Winooski, VT) was used to record all spectra. To determine the fluorescence spectra of nimazide and the product upon H2S reduction, we prepared solutions of nimazide (10 μM) in 50 mM phosphate buffer (pH 7.4) with or without H2S (100 μM), which were further diluted with nine volumes of glycerol. To determine the pH sensitivity of nimazide, we dissolved nimazide (10 μM) in 50 mM 2-(N-morpholino)ethanesulfonic acid (MES, pH 5.5 or 6.5), or 50 mM (3-(N-morpholino)propanesulfonic acid (MOPS, pH 7.5 or 8.5), or 50 mM 3-(cyclohexylamino)-1-propanesulfonic acid (CAPS, pH 9.5 or 10.5), further diluted the solutions with nine volumes of glycerol, and recorded fluorescence emission at 710 nm with excitation at 650 nm. To characterize the chemoselectivity of nimazide, we prepared liposomes as previously described.22 Briefly, we vacuumed cholesterol (2.5 mg) and L-α-Lecithin egg yolk (10 mg) in chloroform: methanol (v/v 3: 2) on a rotary evaporator for 3 h to form a thin, oily film on the glass wall of the flask. Next, we added double distilled water (ddH2O, 1 mL) and continued the rotation of the rotary evaporator for another 15 min. Theoil/water mixture formed in the flask was next sonicated 5 min on a probe sonicator to yield a slightly hazy and transparent solution. Formed liposomes were diluted with equal volume phosphate buffered saline (PBS, pH 7.4), followed by addition of nimazide to a final concentration of 10 μM. Individual thiols and oxidants23 were next added and the resultant mixtures were incubated at room temperature for 30 min. Fluorescence emission at 710 nm was recorded with the excitation wavelength at 650 nm. To monitor the reaction kinetics between nimazide and H2S, we mixed nimazide (10 μM) with H2S (100 μM) in phosphate-buffered saline (PBS, pH 7.4) and measured the absorbance decrease at 650 nm at room temperature over time.
Mammalian cell culture, dye loading, and examination of cytotoxicity
Human embryonic kidney (HEK) 293T cells were maintained in T25 flasks containing 5 mL Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and incubated at 37 °C with 5% CO2 in humidified air. Cells at 80% confluence were subcultured into 35 mm culture dishes at a ratio of 1:10 for dye loading on the following day. To reduce the basal H2S level, we used DMEM without cysteine (Thermo Scientific Cat# 21013024 supplemented with glutamine and methionine) to culture cells the day before imaging. For typical experiments, cells were incubated with 3 μM nimazide in 1 mL of complete culture media at 37 °C for 60 min. To remove free dye molecules, cells were further incubated with 1 mL of fresh, complete culture media for 15 min and this washing procedure was repeated twice at 37 °C. To test the cytotoxicity of nimazide, we incubated HEK 293T cells with nimazide at various concentrations (500 nM to 5 μM) for 60 min and removed free dye molecules as aforementioned. Cells were then collected and mixed with 0.4% trypan blue. The percentages of viable cells were determined using a hemacytometer.
Fluorescence imaging
Fluorescence imaging was performed on a Leica TCS SPE inverted confocal fluorescence microscope with the spectral imaging capability. A 40× water objective or a 63× oil objective was used for imaging studies. Unless otherwise noted, nimazide was imaged using a 635 nm laser line (18 mW at full power) at 15% laser power for excitation. The detector gain was set to 900 mW. The emission was set from 650 nm to 800 nm. To image Rhodamine 123, the excitation was set at 488 nm (10 mW at full power) with 20% laser power and the detector gain was set to 900 mV. The emission was set from 500 nm to 570 nm. Time-lapse images were acquired every minute. After the initial five frames, H2S was added and additional frames were recorded. To demonstrate the use of nimazide to detect endogenous H2S, DL-cysteine (100 μM) was added to the cell culture medium to stimulate H2S production in HEK 293 at 37 °C for 30 min. DL-propargylglycine (DL-PPG, 50 mg/L) was applied as a control to inhibit cellular H2S production. Cell images were processed with ImageJ. We followed a previously reported procedure to derive ratios of two images.24,25 Briefly, images were cropped, subtracted for background, and applied for registration. We next converted background pixels of the denominator image to ‘not a number’ (NaN) by manually adjusting threshold parameters. This procedure did not change intensity values of bright pixels for mitochondria. We next divided the numerator image by the denominator image to gain ratios for every pixel. The resultant ratio image was pseudocolored by selecting a preset ‘fire’ lookup table.
Determination of the dose response of nimazide-loaded mammalian cells
H2S at concentrations from 10 nM to 10 μM was used to treat nimazide-loaded HEK 293T cells. After incubation at 37 °C for 20 min, cells were collected and resuspended in PBS. Fluorescence intensities for each group were determined from 5×106 cells. Average values from three repeating experiments were plotted and linearly fitted against the logarithms of H2S concentrations.
RESULTS AND DISCUSSION
Designing and preparation of nimazide
Azide has been widely utilized to sense H2S via H2S-induced reduction of azide to amine.9 In particular, Wang et al. previously demonstrated that when the azide functional group was linked to an electron-withdrawing group such as the sulfonyl group, its reactivity toward H2S could be greatly enhanced.26 Therefore, we designed our new probe by directly installing an azide functional group to QSY-21 through a sulfonyl linkage (Fig. 1b). We expected that H2S could reduce nimazide into a sulfonamide, which would further cyclize to form a spirolactam (Supporting Information Fig. S1a), thereby leading to the decrease of near-infrared fluorescence. We developed a synthetic scheme to prepare nimazide in 5 steps from inexpensive, commercialized starting materials (Scheme S1).
In vitro characterization of nimazide
Similar to NIMAP, nimazide itself is non-fluorescent in aqueous solutions. When its molecular rotation is restricted, nimazide turns fluorescent. We determined the fluorescence spectra of nimazide in glycerol and the excitation and emission maxima were 671 and 733 nm, respectively (Fig. 2a). The fluorescence of nimazide was insensitive to pH from 5.5 to 10.5 (Fig. 2b). Upon addition of H2S, the fluorescence of nimazide decreased dramatically. Furthermore, we used electrospray ionization mass spectrometry (ESI-MS) to characterize the reduction product and confirmed the formation of sulfonamide or spirolactam (Fig. S1b).
Fig. 2.
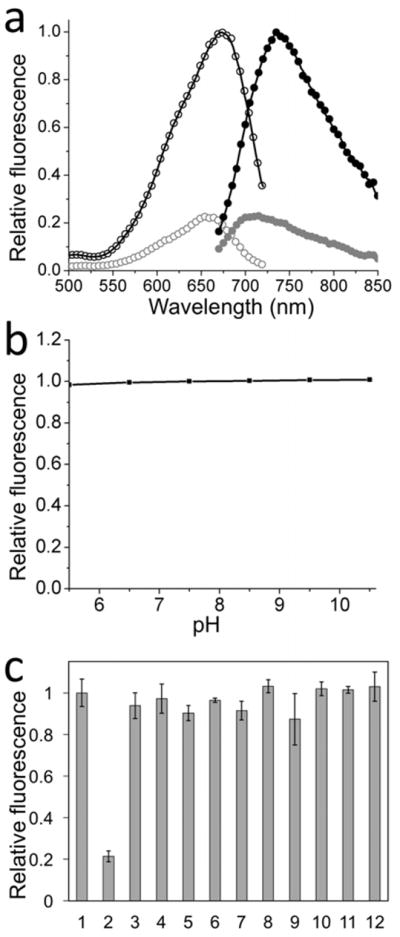
(a) Fluorescence excitation (open circle) and emission (closed circle) spectra of nimazide in glycerol before (black) and after (gray) reaction with H2S. (b) Fluorescence of nimazide under different pH conditions. (c) Chemoselectivity of nimazide in liposomes against a panel of redox-active chemicals: (1) PBS, (2) 40 μM H2S, (3) 1 mM H2O2, (4) 100 μM NaOCl, (5) 100 μM HOOtBu, (6) •OH (1 mM Fe2+ and 100 μM H2O2), (7) •OtBu (1 mM Fe2+ and 100 μM HOOtBu), (8) 5 mM L-cysteine, (9) 20 μM ONOO−, (10) 5 mM reduced glutathione, (11) 1 mM ascorbate, (12) 100 μM O2•−. Data are presented as means and standard deviations (n = 3).
To investigate whether the response of nimazide to H2S is affected by other common cellular thiols or reactive oxygen and nitrogen species (ROS/RNS), we incubated nimazide with a series of redox-active chemicals in a phosphate-buffered solution supplemented with 90% (v/v) glycerol. H2S dramatically reduced the fluorescence of nimazide, whereas other tested molecules at physiologically relevant or higher concentrations caused no or small changes (Fig. 2c). The result suggests that nimazide may be utilized to selectively detected H2S in biological systems.
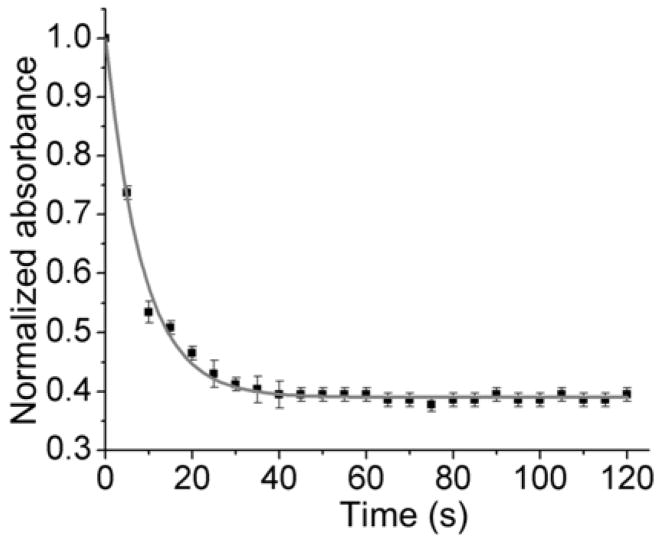
We next examined the reaction kinetics between nimazide and H2S. We mixed nimazide (10 μM) with H2S (100 μM) in phosphate-buffered saline (PBS, pH 7.4) and monitored the absorbance decrease at 650 nm at room temperature over time (Fig. 3). Most of the change happened in the first 20 s. Since H2S was in excess, we fitted the data for pseudo-first-order kinetics and obtained a pseudo first-order rate constant of 0.119 s−1. Correspondingly, the second-order rate constant was estimated to be 1.19×103 M−1·S−1. This rate of reaction is three to four orders of magnitude faster than that between aryl azide and H2S,27 suggesting that nimazide can respond to H2S much faster than common fluorescent H2S probes, including our previously reported hsGFP.28,29
Fig. 3.
The reaction kinetics of nimazide (10 μM) with H2S (100 μM) in PBS (pH 7.4), monitored by the absorbance decrease at 650 nm. Data are presented as means and standard deviations (n = 3).
Characterization of nimazide in live cells
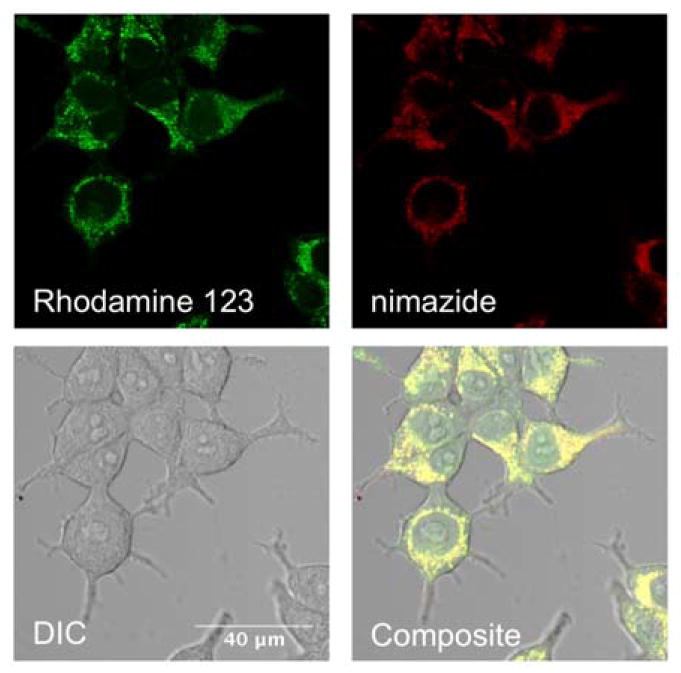
We next stained human embryonic kidney (HEK) 293T cells with nimazide. Nimazide was localized to mitochondria, as confirmed by its colocalization with Rhodamine 123, a widely-used mitochondrial stain (Fig. 4).30 Similar localization patterns were also observed in human cervical cancer HeLa cells. We further investigated the cytotoxicity of nimazide using a trypan blue assay, which specifically stains dead cells. We observed no to minimal cytotoxicity in our cell-staining concentration range (Fig. S2).
Fig. 4.
Colocalization of Rhodamine 123 (green channel) and nimazide (NIR channel) in the mitochondria of HEK 293T cells (Pearson coefficient r = 0.91; scale bar: 40 μm). This experiment was repeated twice.
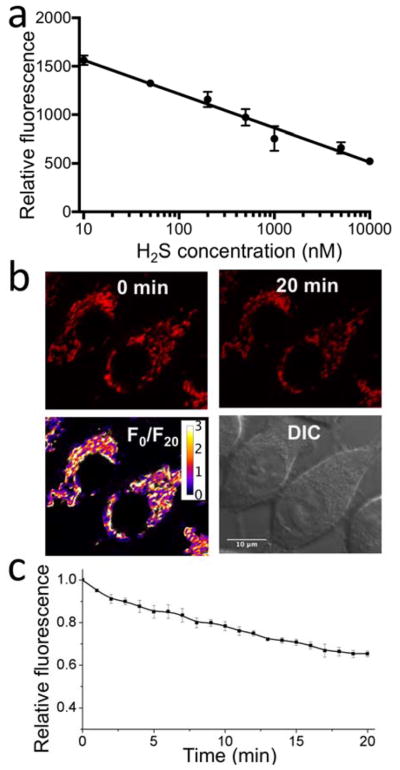
We treated nimazide-loaded HEK 293T cells with various concentrations of H2S and observed robust fluorescence changes upon addition of nanomolar H2S (Fig. 5a). Moreover, there is an empirical, linear relationship between fluorescence intensities and the logarithms of extracellular H2S concentrations (from 10 nM to 10 μM). The data suggest that, at lower concentrations, a higher percentage of H2S reached mitochondria. The transport of extracellular H2S to mitochondria is of course very complicated. Presumably, at low concentrations, H2S may preferably enter the membrane phase because of its lipophilicity. At high concentration, the membrane phase may be near-saturated by H2S. A preferable accumulation of nimazide in mitochondria may also occur, as the maximal fluorescence change requires H2S more than the stoichiometry deduced from the dye-loading concentration. Furthermore, mitochondrial nimazide responded to extracellular addition of nanomolar H2S, suggesting that nimazide is one of the most sensitive fluorescent probes for live-cell mitochondrial H2S. We also challenged cells with 500 nM H2S and followed time-lapse changes (Fig. 5bc). As expected, robust fluorescence decrease was observed. In the absence of H2S, we observed little fluorescence decrease during our imaging of nimazide-loaded HEK 293T cells for 1 h (Fig. S3).
Fig. 5.
(a) Fluorescence response of nimazide-loaded HEK 293T cells to H2S (20 min incubation at RT). Data are presented as means and standard deviations (n = 3). (b) Live-cell imaging of nimazide-stained HEK 293T cells upon addition of 500 nM H2S. In the top row are fluorescence images at 0 and 20 min. In the bottom row are the pseudocolored ratio of fluorescence images at 0 and 20 min (F0/F20) and the DIC image of corresponding HEK 293T cells. (c) Time-lapse responses of nimazide-stained HEK 293T cells to 500 nM H2S. Data are presented as means and standard deviations of five cells from two independent replicates.
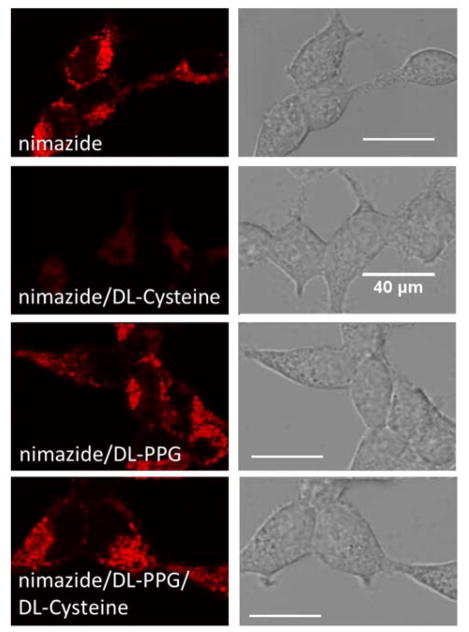
The superior sensitivity of nimazide prompted us to use it to detect biologically generated H2S in mitochondria. Cysteine is a major precursor for H2S, so we utilized DL-cysteine (100 μM) to stimulate nimazide-loaded HEK 293T cells. Not surprisingly, we observed drastic fluorescence decrease after a 30-min incubation (Fig. 6). For comparison, we treated cells with DL-PPG (50 mg/L, 440 μM), an inhibitor for cystathionine γ lyase, a H2S production enzyme.31 DL-PPG alone did not affect the fluorescence of nimazide-stained cells, but it rescued the fluorescence of nimazide-stained cells in the presence of DL-cysteine. These results strongly suggest that nimazide can respond to mitochondrial H2S biologically converted from DL-cysteine.
Fig. 6.
Fluorescence Imaging of mitochondrial H2S in HEK 293T (from top to bottom: nimazide-loaded cells without additional stimulation, nimazide-loaded cells stimulated with 100 μM DL-cysteine for 30 min, nimazide-loaded cells treated with 50 mg/L DL-PPG for 20 min, and nimazide-loaded cells sequentially treated with 50 mg/L DL-PPG for 20 min and 100 μM DL-Cysteine for 30 min). This experiment was repeated three times.
CONCLUSIONS
In conclusion, a novel near-infrared, fluorescence turn-off probe was developed for sensitive detection of mitochondrial H2S. This probe, nimazide, was found to be selective toward H2S among various cellular thiols and oxidants. Moreover, the reaction between nimazide and H2S displayed a fast kinetics, making nimazide a promising sensor for real-time imaging of H2S dynamics. Furthermore, it is particularly exciting that nimazide can respond to extracellular addition of nanomolar H2S. Because of its near-infrared absorption and emission, nimazide may also be utilized for deep tissue imaging. Taken together, nimazide is a most promising fluorescent probe for monitoring mitochondrial H2S.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (R21EB021651) and the National Science Foundation (CHE-1750660) for financial support.
ABBREVIATIONS
- LOD
limit of detection
- ESI-MS
electrospray ionization mass spectrometry
- NMR
nuclear magnetic resonance
- MES
2-(N-morpholino)ethanesulfonic acid
- MOPS
(3-(N-morpholino)propanesulfonic acid
- CAPS
3-(cyclohexylamino)-1-propanesulfonic acid
- PBS
phosphate buffered saline
- DMEM
Dulbecco’s Modified Eagle’s Medium
- HEK
human embryonic kidney
- DL-PPG
DL-propargylglycine
- ROS/RNS
reactive oxygen and nitrogen species
Footnotes
Notes
The authors declare no competing financial interest.
Supporting Information Available: The following files are available free of charge.
Supporting Information.pdf Supplementary figures, the procedure to synthesis nimazide, and NMR and MS characterization of nimazide.
References
- 1.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 2.Martelli A, Testai L, Breschi MC, Blandizzi C, Virdis A, Taddei S, Calderone V. Hydrogen sulphide: novel opportunity for drug discovery. Med Res Rev. 2012;32(6):1093–1130. doi: 10.1002/med.20234. [DOI] [PubMed] [Google Scholar]
- 3.Renga B. Hydrogen sulfide generation in mammals: the molecular biology of cystathionine-beta- synthase (CBS) and cystathionine-gamma-lyase (CSE) Inflamm Allergy Drug Targets. 2011;10(2):85–91. doi: 10.2174/187152811794776286. [DOI] [PubMed] [Google Scholar]
- 4.Lin VS, Chang CJ. Fluorescent probes for sensing and imaging biological hydrogen sulfide. Curr Opin Chem Biol. 2012;16(5–6):595–601. doi: 10.1016/j.cbpa.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao M, Yu F, Lv C, Choo J, Chen L. Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy. Chem Soc Rev. 2017;46(8):2237–2271. doi: 10.1039/c6cs00908e. [DOI] [PubMed] [Google Scholar]
- 6.Yu F, Han X, Chen L. Fluorescent probes for hydrogen sulfide detection and bioimaging. Chem Commun (Camb) 2014;50(82):12234–12249. doi: 10.1039/c4cc03312d. [DOI] [PubMed] [Google Scholar]
- 7.Gao M, Yu F, Chen H, Chen L. Near-infrared fluorescent probe for imaging mitochondrial hydrogen polysulfides in living cells and in vivo. Anal Chem. 2015;87(7):3631–3638. doi: 10.1021/ac5044237. [DOI] [PubMed] [Google Scholar]
- 8.Wang R, Yu F, Chen L, Chen H, Wang L, Zhang W. A highly selective turn-on near-infrared fluorescent probe for hydrogen sulfide detection and imaging in living cells. Chem Commun (Camb) 2012;48(96):11757–11759. doi: 10.1039/c2cc36088h. [DOI] [PubMed] [Google Scholar]
- 9.Lin VS, Chen W, Xian M, Chang CJ. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem Soc Rev. 2015;44(14):4596–4618. doi: 10.1039/c4cs00298a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Zheng XE, Zou F, Shang Y, Meng W, Lai E, Xu Z, Liu Y, Zhao J. A highly selective and sensitive near-infrared fluorescent probe for imaging of hydrogen sulphide in living cells and mice. Scientific reports. 2016;6:18868. doi: 10.1038/srep18868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Sun J, Zhang W, Ma X, Lv J, Tang B. A near-infrared ratiometric fluorescent probe for rapid and highly sensitive imaging of endogenous hydrogen sulfide in living cells. Chemical Science. 2013;4(6):2551–2556. [Google Scholar]
- 12.Velusamy N, Binoy A, Bobba KN, Nedungadi D, Mishra N, Bhuniya S. A bioorthogonal fluorescent probe for mitochondrial hydrogen sulfide: new strategy for cancer cell labeling. Chem Commun. 2017;53(62):8802–8805. doi: 10.1039/c7cc05339h. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu Y, Polavarapu R, Eskla KL, Nicholson CK, Koczor CA, Wang R, Lewis W, Shiva S, Lefer DJ, Calvert JW. Hydrogen sulfide regulates cardiac mitochondrial biogenesis via the activation of AMPK. J Mol Cell Cardiol. 2018;116:29–40. doi: 10.1016/j.yjmcc.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modis K, Bos EM, Calzia E, van Goor H, Coletta C, Papapetropoulos A, Hellmich MR, Radermacher P, Bouillaud F, Szabo C. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part II. Pathophysiological and therapeutic aspects. Br J Pharmacol. 2014;171(8):2123–2146. doi: 10.1111/bph.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellmich MR, Szabo C. Hydrogen Sulfide and Cancer. Handb Exp Pharmacol. 2015;230:233–241. doi: 10.1007/978-3-319-18144-8_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szabo C, Papapetropoulos A. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br J Pharmacol. 2011;164(3):853–865. doi: 10.1111/j.1476-5381.2010.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pak YL, Li J, Ko KC, Kim G, Lee JY, Yoon J. Mitochondria-Targeted Reaction-Based Fluorescent Probe for Hydrogen Sulfide. Anal Chem. 2016;88(10):5476–5481. doi: 10.1021/acs.analchem.6b00956. [DOI] [PubMed] [Google Scholar]
- 18.Wu Z, Liang D, Tang X. Visualizing Hydrogen Sulfide in Mitochondria and Lysosome of Living Cells and in Tumors of Living Mice with Positively Charged Fluorescent Chemosensors. Anal Chem. 2016;88(18):9213–9218. doi: 10.1021/acs.analchem.6b02459. [DOI] [PubMed] [Google Scholar]
- 19.Liu X-L, Du X-J, Dai C-G, Song Q-H. Ratiometric Two-Photon Fluorescent Probes for Mitochondrial Hydrogen Sulfide in Living Cells. The Journal of Organic Chemistry. 2014;79(20):9481–9489. doi: 10.1021/jo5014838. [DOI] [PubMed] [Google Scholar]
- 20.Bae SK, Heo CH, Choi DJ, Sen D, Joe EH, Cho BR, Kim HM. A ratiometric two-photon fluorescent probe reveals reduction in mitochondrial H2S production in Parkinson’s disease gene knockout astrocytes. J Am Chem Soc. 2013;135(26):9915–9923. doi: 10.1021/ja404004v. [DOI] [PubMed] [Google Scholar]
- 21.Yeh HW, Karmach O, Ji A, Carter D, Martins-Green MM, Ai HW. Red-shifted luciferase-luciferin pairs for enhanced bioluminescence imaging. Nat Methods. 2017;14(10):971–974. doi: 10.1038/nmeth.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren W, Ji A, Karmach O, Carter DG, Martins-Green MM, Ai HW. A membrane-activatable near-infrared fluorescent probe with ultra-photostability for mitochondrial membrane potentials. Analyst. 2016;141:3679–3685. doi: 10.1039/c5an01860a. [DOI] [PubMed] [Google Scholar]
- 23.Chen ZJ, Ren W, Wright QE, Ai HW. Genetically encoded fluorescent probe for the selective detection of peroxynitrite. J Am Chem Soc. 2013;135(40):14940–14943. doi: 10.1021/ja408011q. [DOI] [PubMed] [Google Scholar]
- 24.Kardash E, Bandemer J, Raz E. Imaging protein activity in live embryos using fluorescence resonance energy transfer biosensors. Nat Protoc. 2011;6(12):1835–1846. doi: 10.1038/nprot.2011.395. [DOI] [PubMed] [Google Scholar]
- 25.Ren W, Ai HW. Photocontrol of the Src Kinase in Mammalian Cells with a Photocaged Intein. Methods Mol Biol. 2017;1495:217–226. doi: 10.1007/978-1-4939-6451-2_14. [DOI] [PubMed] [Google Scholar]
- 26.Peng HJ, Cheng YF, Dai CF, King AL, Predmore BL, Lefer DJ, Wang BH. A Fluorescent Probe for Fast and Quantitative Detection of Hydrogen Sulfide in Blood. Angew Chem Int Edit. 2011;50(41):9672–9675. doi: 10.1002/anie.201104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henthorn HA, Pluth MD. Mechanistic Insights into the H(2)S-Mediated Reduction of Aryl Azides Commonly Used in H(2)S Detection. J Am Chem Soc. 2015;137(48):15330–15336. doi: 10.1021/jacs.5b10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen ZJ, Ai HW. A Highly Responsive and Selective Fluorescent Probe for Imaging Physiological Hydrogen Sulfide. Biochemistry. 2014;53(37):5966–5974. doi: 10.1021/bi500830d. [DOI] [PubMed] [Google Scholar]
- 29.Chen S, Chen ZJ, Ren W, Ai HW. Reaction-based genetically encoded fluorescent hydrogen sulfide sensors. J Am Chem Soc. 2012;134(23):9589–9592. doi: 10.1021/ja303261d. [DOI] [PubMed] [Google Scholar]
- 30.Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. BioTechniques. 2011;50(2):98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, Papapetropoulos A. Selectivity of commonly used pharmacological inhibitors for cystathionine beta synthase (CBS) and cystathionine gamma lyase (CSE) Br J Pharmacol. 2013;169(4):922–932. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.