Abstract
Background
Per- and polyfluoroalkyl substances (PFASs) are suspected developmental toxicants, but epidemiological evidence on neurodevelopmental effects of PFAS exposure is inconsistent. We examined associations of prenatal and childhood PFAS exposure with performance on assessments of cognition in children.
Methods
We included mother-child pairs from Project Viva, a longitudinal Boston-area birth cohort enrolled during 1999–2002. We quantified concentrations of eight PFASs, including perfluorooctane sulfonate (PFOS), perfluorooctanoate (PFOA), and perfluorohexane sulfonate (PFHxS), in plasma collected from women during pregnancy (median 9.7 weeks gestation) and from children at a visit in mid-childhood (median age 7.7 years). In early childhood (median age 3.2 years) we administered standardized assessments of visual motor skills and vocabulary comprehension, and in mid-childhood we assessed visual motor skills, visual memory, and verbal and non-verbal intelligence. Using multivariable regression, we estimated associations of prenatal and childhood PFAS plasma concentrations with children’s cognitive assessment scores, adjusted for relevant covariates including breastfeeding, maternal intelligence, parental education, and household income. Samples sizes ranged from 631–971, depending on analysis.
Results
Prenatal PFAS concentrations were associated with both better and worse cognitive performance; children with top quartile prenatal concentrations of some PFASs had better visual motor abilities in early childhood and non-verbal IQ and visual memory in mid-childhood, while children with upper quartile prenatal PFOA and PFOS had lower mid-childhood visual-motor scores. In cross-sectional analyses of mid-childhood PFAS concentrations and cognitive assessments, visual-motor scores on the Wide Range Assessment of Visual Motor Abilities (WRAVMA) (standardized mean=100, standard deviation=15) were lower among children with higher PFHxS (fourth quartile (Q4) vs. Q1: −5.0, 95% confidence interval (CI): −9.1, −0.8). Upper quartiles of childhood PFOA and PFOS were also associated with somewhat lower childhood WRAVMA scores, but childhood PFASs were not associated with verbal or non-verbal IQ or visual memory.
Conclusions
We present evidence suggesting associations of prenatal and childhood PFAS exposure with lower childhood visual motor abilities. Other results were inconsistent, with higher prenatal PFASs associated in some cases with better cognitive outcomes.
Keywords: Neurodevelopment, cognition, per- and polyfluoroalkyl substances, PFAS, persistent organic pollutants
1. INTRODUCTION
Per- and polyfluoroalkyl substances (PFASs) are synthetic chemicals used in consumer and industrial products including oil-resistant coatings for food packaging and stain-resistant fabric treatments.1,2 Several PFASs, including perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA), have been identified as persistent and bioaccumulative chemicals that may have neurotoxic properties.2 PFASs are frequently detected in human biosamples, including those of pregnant women1–5 and children,6–8 and cross the placenta.9–11
Rodent studies suggest that PFASs may disrupt neurodevelopment,12–14 but epidemiological evidence is inconsistent and relatively limited. In prospective cohort studies, prenatal PFAS concentrations were associated with worse motor skills at age two years,15 behavior problems at age 5–9 years,16 and poorer executive function at age 5–8 years.17 Other cohort studies of prenatal or perinatal PFAS exposures and neurobehavioral outcomes in children have reported null18–24 or protective60,64,65,66 associations. Cross-sectional studies in U.S. children and adolescents have linked serum concentrations of PFASs with attention deficit hyperactivity disorder (ADHD) diagnosis6,25 and greater impulsivity.26
Effects of PFASs on neurodevelopment may vary depending on of timing of exposure, but few prior studies have examined PFAS exposure at multiple time points in relation to childhood cognition or behavior.24,27 A cohort study from the Faroe Islands (n=539) reported associations of PFASs measured at age 5 with behavior problems assessed at age 7, but no associations with prenatal PFASs,24 while in a small U.S. cohort (n=167), higher prenatal and childhood PFASs were associated with better child reading skills.27 In a prospective cohort of U.S. mothers and children, we examined associations of prenatal (maternal plasma from pregnancy) and childhood plasma PFAS concentrations with children’s performance on assessments of visual motor skills and vocabulary comprehension in early childhood and visual motor skills, visual memory, and verbal and non-verbal intelligence in mid-childhood. We hypothesized that higher PFAS exposure would be associated with poorer cognitive outcomes, and sought to identify potentially sensitive exposure periods for effects of PFAS exposure on cognitive development.
2. METHODS
2.1 Study population
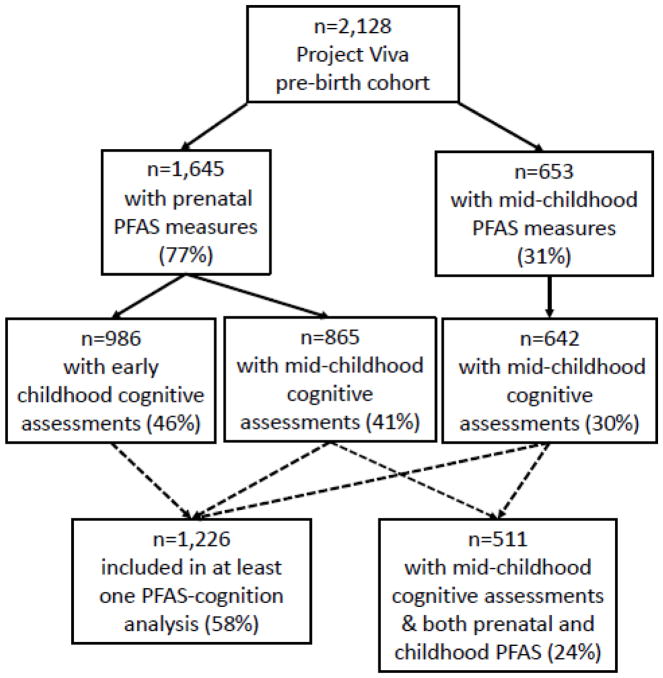
Participants were drawn from Project Viva, a prospective pre-birth cohort enrolled 1999–2002 at mothers’ first prenatal visit (median 9.9 weeks of gestation) at eight locations of Atrius Harvard Vanguard Medical Associates, a multispecialty group practice in urban and suburban Eastern Massachusetts.28 Eligibility criteria included gestational age <22 weeks at enrollment, ability to answer questions in English, and no plans to move away from the study area before delivery. A total of 2,128 mothers with live, singleton births was initially enrolled, and a subset (n=1,668; 78%) agreed to contribute blood samples at an in-person visit in pregnancy (median 9.7 weeks gestation, range 4.8–21.4). Mother-child pairs were followed prospectively; mothers provided demographic and health-related information through annual questionnaires and children attended periodic in-person visits assessing health and development, including a visit in in early childhood (2.8–6.3 years) attended by 1,294 pairs (61%) and a visit in mid-childhood (6.6–10.9 years), attended by 1,116 pairs (52%). A subset of children (n=702; 63% of those attending visit, 34% of those enrolled at birth) agreed to contribute blood samples at the mid-childhood visit in 2007–10. Additional description of the Project Viva cohort and participation flow is available in a published cohort profile.28
The Institutional Review Boards of participating institutions approved all study protocols. Mothers provided written informed consent, and children provided verbal assent at the mid-childhood visit. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects research. All study forms are available at https://www.hms.harvard.edu/viva/.
2.2 Cognitive assessments
A pediatric neuropsychologist trained Project Viva staff to administer standardized assessments of cognitive development to child participants during in-person study visits in early childhood (median age 3.2 years) and mid-childhood (median age 7.7 years) (Table 1).
Table 1.
Cognitive assessments administered at Project Viva in-person study visits in early childhood (median age 3.2 years; range 2.8–6.3) and mid-childhood (median age 7.7 years; range 6.6–10.9).
| Assessment | Subtest | Primary cognitive domains assessed | Administered at early childhood study visit | Administered at mid- childhood study visit |
|---|---|---|---|---|
| Peabody Picture Vocabulary Test (PPVT-III) | – | Word knowledge | × | |
| Wide Range Assessment of Visual Motor Abilities (WRAVMA) | Drawing | Visual-motor | × | × |
| Pegboard | Fine motor | × | ||
| Matching | Visual-spatial perception | × | ||
| Kaufman Brief Intelligence Test (KBIT-2) | Verbal IQ | Word knowledge, crystallized intelligence | × | |
| Non-verbal IQ | Visual-spatial perception, fluid intelligence | × | ||
| Visual Memory Index of the Wide Range Assessment of Memory and Learning (WRAML2) | Design Memory | Visual memory, visual-spatial perception | × | |
| Picture Memory | Visual memory | × |
In early childhood, staff administered the Peabody Picture Vocabulary Test, 3rd edition (PPVT-III),29 which assesses vocabulary comprehension, and the Wide Range Assessment of Visual Motor Abilities (WRAVMA),30 which assesses visual-motor (drawing subtest), fine motor (pegboard subtest) and visuospatial (matching subtest) skills. The PPVT-III is valid for use in children and adults from 2.5 to 90+ years and was standardized based on a sample representative of the general U.S. population; PPVT-III scores in children correlate well (r ≥ 0.90) with verbal and full-scale intelligence quotient (IQ) scores on the full length Wechsler Intelligence Scale for Children-III (WISC-III).29 The WRAVMA is designed for children aged 3 to 17 and was normed using a representative sample of U.S. children; WRAVMA composite scores are moderately correlated (r=0.62) with WISC-III full-scale IQ and moderately to highly correlated with other standardized tests of visual-motor ability.30
In mid-childhood, staff assessed verbal and non-verbal intelligence using the Kaufman Brief Intelligence Test, Second Edition (KBIT-2),31 visual-motor skills using the WRAVMA drawing subtest, and visual memory (design memory and picture memory) using the Visual Memory Index of the Wide Range Assessment of Memory and Learning, Second Edition (WRAML2).32 The KBIT-2 is a valid and reliable measure for children and adults age 4 to 90, standardized using a representative U.S. sample; KBIT-2 scores are moderately to highly correlated with relevant subscores on the WISC-III (r=0.76 for KBIT-2 IQ composite and WISC-III full-scale IQ).31 The WRAML2 is standardized for ages 5 to 90 based on a representative U.S sample; among children aged 6–16, the WRAML2 Visual Memory Index showed moderate correlation with relevant indices from the Children’s Memory Scale (r=0.48 for General Memory, 0.52 for Attention/Concentration).32
Assessors rated their confidence in the accuracy of each assessment on a scale of 1–5 (based on testing conditions, child’s mood, etc.); assessments with low confidence (≤2) were excluded from primary analyses. Project Viva staff double-scored all cognitive assessments using published scoring guidelines; in cases where two scorers disagreed, group adjudication was used. WRAVMA visual-motor scoring also employed supplementary guidelines developed by a pediatric neuropsychologist to ensure consistency among scorers.33 Scaled scores were standardized to mean=100, standard deviation (SD)=15 for the PPVT-III, KBIT-2 and WRAVMA, and mean=10, SD=3 for WRAML2 design memory and picture memory subscores, using published reference data.29–32 Staff administering and scoring assessments had no knowledge of participants’ PFAS plasma concentrations, nor did participants themselves.
2.3 PFAS measurements
We quantified PFAS concentrations in maternal plasma samples from pregnancy (4.8–21.4 weeks gestation) and child plasma samples from mid-childhood (6.6–10.9 years). Following collection, plasma specimens were stored in PFAS-free cryovials in liquid nitrogen freezers. Samples were subsequently thawed, aliquoted, shipped to the Division of Laboratory Sciences at CDC and analyzed for concentrations of eight PFASs: PFOS, PFOA, perfluorohexane sulfonate (PFHxS), perfluorononanoate (PFNA), 2-(N-ethyl-perfluorooctane sulfonamido) acetate (EtFOSAA; also known as Et-PFOSA-AcOH), 2-(N-methyl-perfluorooctane sulfonamido) acetate (MeFOSAA; also known as Me-PFOSA-AcOH), perfluorodecanoate (PFDeA), and perfluorooctane sulfonamide (FOSA; also known as PFOSA) using on-line solid-phase extraction coupled with isotope dilution high-performance liquid chromatography-tandem mass spectrometry. Analytical methods for the maternal and child samples were the same as those used to analyze PFAS concentrations in the 2011–201234 and 2013–201435 National Health and Nutrition Examination Survey (NHANES) cycles, respectively. To ensure accuracy and reliability, the laboratory analyzed low and high-concentration quality control materials, analytical standards, and reagent and serum blanks along with the study samples; the laboratory successfully participated in external Quality Assessment schemes.36 PFOS and PFOA measures represented total PFOS and PFOA (sum of linear and branched isomers). Prenatal samples were shipped and analyzed in 2014 and child plasma samples were shipped and analyzed in 2015; 1,645 prenatal plasma samples (of 1,668; 99%) and 653 child plasma samples (of 702; 93%) had sufficient volume for PFAS quantification. Limits of detection (LOD) were 0.1 ng/mL, except for prenatal PFOS measures, for which the LOD was 0.2 ng/mL; values below LOD were estimated as LOD/√2.37
2.4 Covariates
Study staff collected data on participant demographics and health-related behaviors via study questionnaires and interviews and assessed mothers’ cognition using the PPVT-III (at the early childhood visit) and the KBIT-2 (mid-childhood visit). At the mid-childhood visit, mothers completed the Home Observation for Measurement of the Environment - Short Form (HOME-SF), which measures support for cognitive development in the child’s home. HOME-SF scores range from 0 to 22, with higher scores representing better support.38 Plasma creatinine and albumin were measured in the same maternal pregnancy plasma samples analyzed for PFASs. We used plasma creatinine measurements to estimate maternal glomerular filtration rate (GFR) at the time of pregnancy blood draw according to the Cockcroft-Gault equation.39
2.5 Statistical analyses
Missing covariates were imputed using a chained equation multiple imputation model (PROC MI in SAS) including all exposure and outcome variables, all study covariates, and auxiliary variables potentially predictive of exposures, outcomes, covariates or missingness, including maternal and child dietary factors and child anthropometric and clinical measurements. We generated 50 imputed data sets including all Project Viva participants (n=2,128)40 and pooled beta estimates from imputed data sets according to Rubin’s rules.41 The use of multiply imputed data allows for unbiased effect estimates if data are missing at random, meaning that missingness depends only on factors included in imputation model, a less restrictive assumption than is necessary for complete case analyses.40 Primary models included participants with imputed covariate data, but excluded participants lacking PFAS measures or cognitive outcome scores. In sensitivity analyses, we also ran complete case analyses and models including imputed exposures and outcomes for all participants with at least one measure of PFASs (prenatal and/or childhood) and at least one cognitive assessment (early childhood and/or mid-childhood) (n=1,226).
Correlations among PFASs plasma concentrations, outcomes, and covariates were determined using Spearman rank correlation coefficients. We examined associations of maternal plasma PFASs with early and mid-childhood cognitive assessments, as well as cross-sectional associations of child plasma PFAS with mid-childhood cognitive assessments. To assess the shape of exposure-outcome associations, we fit generalized additive models with cubic regression splines (three degrees of freedom) for continuous exposures.
All models were adjusted for child sex and age at cognitive testing, along with covariates hypothesized to be potential confounders of the studied associations based on prior knowledge, according to Directed Acyclic Graph (DAG) theory (Figures S1 and S2).42,43 Covariates included for all models were: year of blood collection (for prenatal or mid-childhood blood samples, depending on model), maternal race/ethnicity (black, white, Hispanic, Asian, other), age (<25, 25–34, ≥35 years), maternal and paternal education (<college/college/graduate degree), and maternal intelligence scores (PPVT-III for early childhood cognition models, KBIT-2 for mid-childhood cognition models). Models for early childhood cognition also included annual household income at enrollment (<$40K, $40-≤70K, >$70K), while models for mid-childhood cognition included mid-childhood annual household income (<$40K, $40-≤70K, $70-≤150K, >$150K) and HOME-SF score.
As changes in plasma volume expansion and renal function in pregnancy may affect measured plasma concentrations of PFASs and may also relate to pregnancy health,44 models for maternal plasma PFASs were additionally adjusted for estimated GFR and the gestational week in which plasma was collected, along with pre-pregnancy body mass index (kg/m2). Prenatal PFAS models were also adjusted for maternal smoking status in pregnancy, a predictor of maternal PFAS plasma concentrations in this population.45 Finally, because PFASs can partition into breastmilk, prior breastfeeding may influence maternal PFAS levels and breastfeeding may represent a source of PFAS exposure for children.46,47 Information on prior breastfeeding was not available in Project Viva, so we created a proxy indicator of prior breastfeeding derived from parity and report of breastfeeding (≥1 month) of the studied child. Prenatal PFAS models were adjusted for this indicator of prior breastfeeding incorporating maternal parity (nulliparous, parous with no breastfeeding, parous with breastfeeding), while childhood PFAS models were adjusted for breastfeeding duration (in months) of the studied child and maternal parity (0, ≥1).
We assessed collinearity among covariates for each model by calculating variance inflation factors (VIF); VIFs were <2 for all variables, suggesting that covariate collinearity did not substantially reduce precision of effect estimates. As covariate-adjusted spline models suggested non-linearity in a number of the studied PFAS-cognitive assessment associations, we categorized PFAS concentrations into quartiles for primary analyses. Quartiles were determined separately for prenatal PFASs and childhood PFASs based on concentrations among all children with the relevant PFAS measurement and at least one measured cognitive outcome (n=1095 for prenatal PFASs and n=653 for childhood PFASs).
As primary analyses, we ran separate multivariable linear regression models assessing associations of prenatal plasma concentrations of each PFAS with each early childhood and mid-childhood cognitive assessment score, and cross-sectional associations of child plasma concentrations of each PFAS with each mid-childhood cognitive assessment score. As secondary analyses, to examine whether cross-sectional associations might be confounded by prenatal PFAS exposure, we ran cross-sectional models for each mid-childhood PFAS with additional adjustment for maternal pregnancy plasma concentrations of the same PFAS, modeling maternal PFAS concentrations as cubic regression splines (3 degrees of freedom) to allow for non-linear dose-response relationships; these analyses included only the subset of the study population with both prenatal and childhood PFAS measures (n=511). To assess whether sex might be an effect measure modifier, we re-ran models stratified by child sex, and conducted log-likelihood ratio tests to assess whether model fit improved with inclusion of multiplicative interaction terms for PFAS quartile × sex.
As sensitivity analyses, we re-ran prenatal PFAS models with additional adjustment for plasma albumin, as albumin is a primary binding site for PFASs and plasma albumin may also reflect hemodynamic variability in pregnancy. To evaluate the influence of adjusting for breastfeeding, we ran models excluding prior breastfeeding and breastfeeding duration covariates. To evaluate potential bias related to inter-rater variability, we ran models adjusted for assessor and excluding assessments from assessors who conducted <20 assessments. Finally, we ran models excluding assessments with assessor confidence scores of 3/5 (primary models excluded assessments with confidence scores ≤2).
We prepared data sets and completed multiple imputation in SAS Version 9.3 (SAS Institute Inc., Cary, NC); all other analyses were conducted in R Version 3.1.3 (R Foundation for Statistical Computing, Vienna, Austria). We based our conclusions about potential relationships between exposures and outcomes on the magnitude and precision of estimates and patterns observed across results.
3. RESULTS
3.1 Participant characteristics and PFAS concentrations
Our study included participants with at least one measure of PFAS plasma concentrations (prenatal and/or mid-childhood) and at least one completed cognitive assessment (in early childhood and/or mid-childhood). Sample sizes ranged from 631 to 971 (representing 30 to 46% of the 2,128 children enrolled at birth); Figure 1 outlines inclusion criteria and sample sizes for each analysis. Table 2 outlines characteristics of study participants, stratified by analysis. Mothers were predominantly white (62–73%, depending on analysis), tended to have high educational attainment (65–70% with college or graduate degrees), and the majority had household income >$70,000/year (60–69%). Distributions of covariates were similar when imputed covariate data were excluded (data not shown); proportions of imputed data were low (≤10%) (Table 2).
Figure 1.
Flow diagram for inclusion in study population (with % of original cohort included at each stage)
Table 2.
Characteristics of study participants, stratified by analysis
| Characteristic | N (%) or mean ± standard deviation | ||
|---|---|---|---|
|
| |||
| Included in analyses of prenatal PFASs and early childhood cognition (n=986) | Included in analyses of prenatal PFASs and mid-childhood cognition (n=865) | Included in analyses of childhood PFASs and mid-childhood cognition (n=642) | |
| Early childhood cognitive assessments | |||
| Peabody Picture Vocabulary Test (PPVT-III)a (n=948) | 104.4 ± 14.1 | - | - |
| Wide Range Assessment of Visual Motor Abilities (WRAVMA) Composite Scorea (n=919) | 102.1 ± 11.5 | - | - |
| WRAVMA Visual-Motor (a (n=971) | 99.0 ± 11.3 | - | - |
| WRAVMA Fine Motora (n=968) | 98.0 ± 10.8 | - | - |
| WRAVMA Visual-Spatiala (n=937) | 108.1 ± 13.8 | - | - |
| Mid-childhood cognitive assessments | |||
| Kaufman Brief Intelligence Test (KBIT-2) Verbal IQa (n=851 with prenatal PFASs, 631 with child PFASs) | - | 112.1 ± 14.9 | 111.1 ± 15.4 |
| KBIT-2 Non-Verbal IQa (n=862 with prenatal PFASs, 640 with child PFASs) | - | 106.5 ± 16.9 | 105.8 ± 16.9 |
| WRAVMA Visual-Motor a (n=853 with prenatal PFASs, 635 with child PFASs) | - | 92.1 ± 16.7 | 93.1 ± 17.2 |
| Visual Memory Index of the Wide Range Assessment of Memory and Learning (WRAML2) Design Memory (WRAML2)b (n=853 with prenatal PFASs, 636 with child PFASs) | - | 8.0 ± 2.8 | 8.1 ± 2.7 |
| WRAML 2 Picture Memoryb (n=855 with prenatal PFASs, 635 with child PFASs) | - | 8.9 ± 3.0 | 9.0 ± 3.0 |
| Maternal characteristics | |||
| Year of enrollment | |||
| 1999 | 245 (25) | 212 (25) | - |
| 2000 | 362 (37) | 329 (38) | - |
| 2001 | 343 (35) | 289 (33) | - |
| 2002 | 36 (4) | 35 (4) | - |
| Age at enrollment (years) | 32.4 ± 5.1 | 32.1 ± 5.3 | 31.8 ± 5.6 |
| Parity at enrollment | |||
| Nulliparous | 468 (47) | 415 (48) | 271 (42) |
| Parous | 518 (53) | 450 (52) | 371 (58) |
| Proxy for breastfeeding of a prior childc | |||
| Nulliparous (assume no prior breastfeeding) | 468 (47) | 415 (48) | 271 (42) |
| Parous, did not breastfeed Project Viva child | 100 (10) | 76 (9) | 57 (9) |
| Parous, breastfed Project Viva child | 391 (40) | 343 (40) | 283 (44) |
| Missing data | 27 (3) | 31 (4) | 31 (5) |
| Education (at enrollment) | |||
| Less than college degree | 286 (29) | 275 (32) | 225 (35) |
| College degree | 378 (38) | 300 (35) | 230 (36) |
| Graduate degree | 320 (32) | 286 (33) | 183 (29) |
| Missing data | 2 (<1) | 4 (1) | 4 (1) |
| Race/ethnicity | |||
| White | 722 (73) | 594 (69) | 397 (62) |
| Black | 115 (12) | 129 (15) | 133 (21) |
| Asian | 40 (4) | 39 (5) | 29 (5) |
| Hispanic | 60 (6) | 59 (7) | 46 (7) |
| Other | 47 (5) | 40 (5) | 33 (5) |
| Missing data | 2 (<1) | 4 (<1) | 4 (1) |
| Smoking in pregnancy | |||
| Smoked in pregnancy | 110 (11) | 166 (19) | 73 (11) |
| Former smoker | 206 (21) | 88 (10) | 122 (19) |
| Never smoker | 666 (68) | 608 (71) | 446 (70) |
| Missing data | 4 (<1) | 3 (<1) | 1 (<1) |
| Pre-pregnancy body mass index (kg/m2) | 24.8 ± 5.3 | 24.8 ± 5.3 | 25.0 ± 5.3 |
| Missing data | 2 (<1) | 6 (1) | 4 (1) |
| Gestational duration at time of pregnancy blood draw (weeks) | 9.6 ± 2.2 | 10.1 ± 2.3 | - |
| Estimated glomerular filtration rate at time of pregnancy blood draw (mL/min per 1.73 m2) | 110.5 ± 49.5 | 109.4 ± 38.5 | - |
| Missing data | 7 (<1) | 13 (2) | - |
| Plasma albumin at time of pregnancy blood draw (g/dL) | 8.3 ± 2.8 | 8.2 ± 2.0 | - |
| Missing data | 46 (5) | 44 (5) | - |
| PPVT-III score at early childhood cognitive assessment | 106.1 ± 14.6 | - | - |
| Missing data | 23 (2) | - | - |
| IQ (KBIT-2 composite) at mid-childhood cognitive assessment | - | 106.9 ± 15.2 | 105.1 ± 15.6 |
| Missing data | - | 15 (2) | 13 (2) |
| Paternal characteristics | |||
| Education (at enrollment) | |||
| Less than college degree | 287 (29) | 251 (29) | 199 (31) |
| College degree | 332 (34) | 284 (33) | 199 (31) |
| Graduate degree | 281 (28) | 241 (28) | 168 (26) |
| Missing data | 86 (9) | 89 (10) | 76 (12) |
| Child characteristics | |||
| Sex | |||
| Female | 471 (48) | 419 (48) | 305 (48) |
| Male | 515 (52) | 446 (52) | 337 (52) |
| Duration of breastfeeding (months up to 12) | 6.3 ± 4.5 | 6.5 ± 4.6 | 6.4 ± 4.5 |
| Missing data | 51 (5) | 58 (7) | 52 (8) |
| Age at early childhood assessment (years) | 3.3 ± 0.4 | - | - |
| Age at mid-childhood assessment (years) | - | 8.0 ± 0.9 | 7.9 ± 0.8 |
| Year of mid-childhood blood draw | |||
| 2007 | - | - | 67 (10) |
| 2008 | - | - | 220 (34) |
| 2009 | - | - | 206 (32) |
| 2010 | - | - | 149 (23) |
| Household characteristics | |||
| HOME-SF scored at mid-childhood assessment | - | 18.4 ± 2.2 | 18.2 ± 2.3 |
| Missing data | - | 26 (3) | 13 (2) |
| Household annual income at enrollment | |||
| ≤$40 K | 115 (12) | - | - |
| >$40–≤$70 K | 217 (22) | - | - |
| >$70 K | 591 (60) | - | - |
| Missing data | 63 (6) | - | - |
| Household annual income at mid-childhood | |||
| ≤$40 K | - | 99 (11) | 90 (14) |
| >$40–≤$70 K | - | 110 (13) | 96 (15) |
| >$70–≤$150 K | - | 367 (42) | 272 (42) |
| >$150 K | - | 234 (27) | 148 (23) |
| Missing data | - | 55 (6) | 36 (6) |
PPVT-III, WRAVMA and KBIT-2 scores standardized to mean=100, SD=15.
WRAML2 scores standardized to mean=10, SD=3.
Estimated using maternal parity and breastfeeding data for the Project Viva child.
Home Observation for Measurement of the Environment (Short Form); scale: 0–22, with higher scores representing better support for cognitive development in home.
Participants excluded due to missing exposure or outcome data differed somewhat from included participants. For example, participants excluded from analyses of mid-childhood PFAS and mid-childhood cognitive outcomes had somewhat lower mean WRAVMA (90.7 vs. 92.2) and WRAML2 Picture Memory scores (7.9 vs. 8.9), slightly higher KBIT-2 (verbal: 112.9vs. 111.7; non-verbal: 107.1 vs. 106.2) and WRAML2 Design Memory scores (8.8 vs. 8.0) and slightly higher median prenatal PFOA (6.0 ng/mL vs. 5.4) and PFOS (26.2 ng/mL vs. 24.3) concentrations than included participants (Table S1). Excluded children also had shorter duration of breastfeeding, higher annual household income at mid-childhood, lower maternal parity, higher maternal IQ, and a higher proportion of white and lower proportion of black mothers than included children (Table S1).
Plasma concentrations of PFASs are presented in Table 3. We detected PFOS, PFOA, PFHxS, and PFNA in >98% of prenatal and childhood samples. EtFOSAA and MeFOSAA were detected in >99% of prenatal samples, but less often in childhood samples (5.1% and 65.2%, respectively), while PFDeA was detected more often in childhood samples (88.3%) versus prenatal samples (45.1%). FOSA was detected in 10.9% of prenatal samples and 0.1% of childhood samples. Given low detection frequencies, PFDeA and FOSA were excluded from analyses of prenatal PFASs and EtFOSAA, MeFOSAA, and FOSA were excluded from analyses of childhood PFASs. Correlations among PFASs quantified in the same sample were moderate to high (Spearman correlation coefficients 0.19–0.72 for prenatal samples, 0.14–0.78 for childhood), with PFOA and PFOS most highly correlated (Tables S2–S3). Correlations between prenatal and childhood concentrations of the same PFAS ranged from 0.09 for PFNA to 0.40 for PFHxS (Table S4).
Table 3.
Concentrations (in ng/mL) of per- and polyfluoroalkyl substances (PFASs) in maternal pregnancy plasma and child plasma (median age 7.7) for study participantsa
| PFAS | % Detect (≥LOD) | Median (25th %ile–75th %ile) | Minimum | Maximum |
|---|---|---|---|---|
| Prenatal concentrations (sampled 1999–2002, n=1095) | ||||
| PFOA | 100 | 5.6 (4.1–7.7) | 0.8 | 49.3 |
| PFOS | 99.9 | 24.9 (18.4–34.4) | <LOD | 185.0 |
| PFHxS | 99.3 | 2.4 (1.6–3.7) | <LOD | 46.4 |
| PFNA | 98.9 | 0.6 (0.5–0.9) | <LOD | 6.0 |
| EtFOSAA | 99.6 | 1.2 (0.7–1.9) | <LOD | 33.6 |
| MeFOSAA | 100 | 1.9 (1.2–3.0) | 0.1 | 29.7 |
| PFDeA | 45.1 | <LOD (<LOD–0.3) | <LOD | 3.0 |
| FOSA | 10.9 | All <LOD | <LOD | 1.8 |
| Childhood concentrations (sampled 2007–2010, n=642) | ||||
| PFOA | 99.5 | 4.4 (3.1–6.0) | <LOD | 14.3 |
| PFOS | 99.5 | 6.2 (4.2–9.7) | <LOD | 51.4 |
| PFHxS | 99.5 | 1.9 (1.2–3.4) | <LOD | 56.8 |
| PFNA | 99.5 | 1.5 (1.1–2.3) | <LOD | 25.7 |
| EtFOSAA | 5.1 | All <LOD | <LOD | 1.4 |
| MeFOSAA | 65.2 | 0.3 (<LOD–0.6) | <LOD | 6.7 |
| PFDeA | 88.3 | 0.3 (0.2–0.5) | <LOD | 1.9 |
| FOSA | 0.1 | All <LOD | <LOD | 0.5 |
Limit of detection (LOD) was 0.1 ng/mL except for prenatal PFOS measures (LOD=0.2 ng/mL)
3.2 PFAS exposures and cognitive outcomes
In covariate-adjusted models assessing quartiles of PFAS plasma concentrations (described below), associations varied across PFASs and cognitive outcomes, and differed depending on timing of PFAS measurement (prenatal or mid-childhood) and outcome assessment (early childhood or mid-childhood).
3.2.1 Prenatal PFASs and early childhood WRAVMA and PPVT-III (Figure 2)
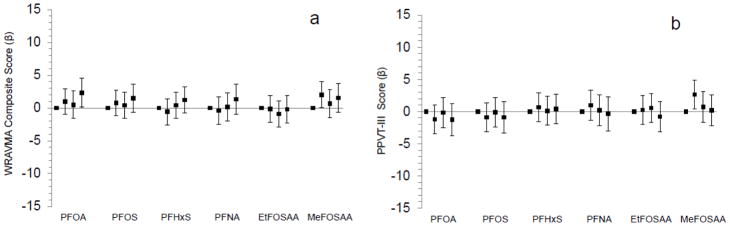
Figure 2.
Associations of maternal pregnancy plasma PFAS concentrations in quartiles with mean differences (+95% confidence intervals) in children’s early childhood (median age 3.2 years) Wide Range Assessment of Visual Motor Abilities (WRAVMA) Composite score (n=919) [a] and Peabody Picture Vocabulary Test (PPVT-III) (n=948) [b]. Standard mean (SD) for WRAVMA and PPVT-III=100 (15); higher scores indicate better performance. Models adjusted for: year of blood collection, gestational age at blood collection, estimated glomerular filtration rate, maternal race/ethnicity, age, education, IQ, pre-pregnancy body mass index, and smoking status, paternal education, household income, child’s sex and age at assessment, and proxy for breastfeeding of a prior child (incorporating parity).
Early childhood WRAVMA total scores were higher (representing better performance) among children born to mothers in the highest quartile of prenatal PFOA plasma concentrations (2.3 points, 95% confidence interval (CI): 0.1, 4.5) compared to those in the lowest quartile (Figure 2; Table S5). We also observed trends suggesting higher early childhood WRAVMA scores among children born to mothers with the highest quartile prenatal concentrations of PFOS, PFHxS, PFNA, and MeFOSAA. Early childhood visual motor and receptive vocabulary (PPVT-III) scores were higher among those born to mothers in the second (though not third or fourth) versus first quartile of MeFOSAA concentrations; other prenatal PFASs did not appear associated with PPVT-III scores.
3.2.2 Prenatal and mid-childhood PFASs and mid-childhood WRAVMA (Figure 3)
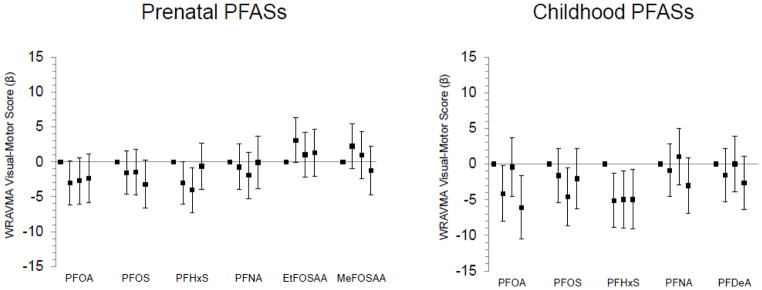
Figure 3.
Associations of prenatal and mid-childhood plasma PFAS concentrations (in quartiles) with mean differences (+95% confidence intervals) in mid-childhood (median age 7.7 years) Wide Range Assessment of Visual Motor Abilities (WRAVMA) Visual-Motor scores, adjusted for relevant covariates (n=853 for prenatal PFASs, 635 for childhood PFASs). Standard mean (SD) for WRAVMA=100 (15); higher scores indicate better performance. All models adjusted for year of blood collection, maternal race/ethnicity, age, education, and IQ, paternal education, household income, home environment, and child’s sex and age at assessment. Prenatal PFAS models additionally adjusted for: gestational age at blood collection, estimated glomerular filtration rate, maternal pre-pregnancy body mass index and smoking in pregnancy, and proxy for breastfeeding of a prior child (incorporating parity). Childhood PFAS models additionally adjusted for: breastfeeding duration (months up to 12) and maternal parity.
Mid-childhood visual-motor scores were somewhat lower among those with upper quartile prenatal exposure to PFOA and PFOS, and among children in the second and third, but not fourth, quartile of prenatal PFHxS concentration (vs. first quartile) (Figure 3; Table S6). Conversely, mid-childhood visual-motor scores were higher among those born to mothers in the second (though not third or fourth) quartile of EtFOSAA concentrations. Prenatal concentrations of other PFASs were not associated with mid-childhood WRAVMA scores. Mid-childhood visual-motor scores (WRAVMA Drawing subtest) were lower among children in the highest three quartiles of mid-childhood PFHxS concentration (Q2: −5.1, 95% CI: −8.9, −1.3; Q3: −5.0, 95% CI: −9.0., −0.9; Q4: −5.0, 95% CI: −9.1, −0.8), in the fourth quartile of mid-childhood PFOA concentration (−6.1, 95% CI: −10.5, −1.6) and in upper quartiles of mid-childhood PFOS concentration (Q3: −4.6, 95% CI: −8.7, −0.5; Q4: −2.0, 95% CI: −6.3, 2.2); mid-childhood PFNA and PFDeA were not associated with WRAVMA scores (Figure 3; Table S7).
3.2.3 Prenatal and mid-childhood PFASs and mid-childhood KBIT-2 (Figure 4)
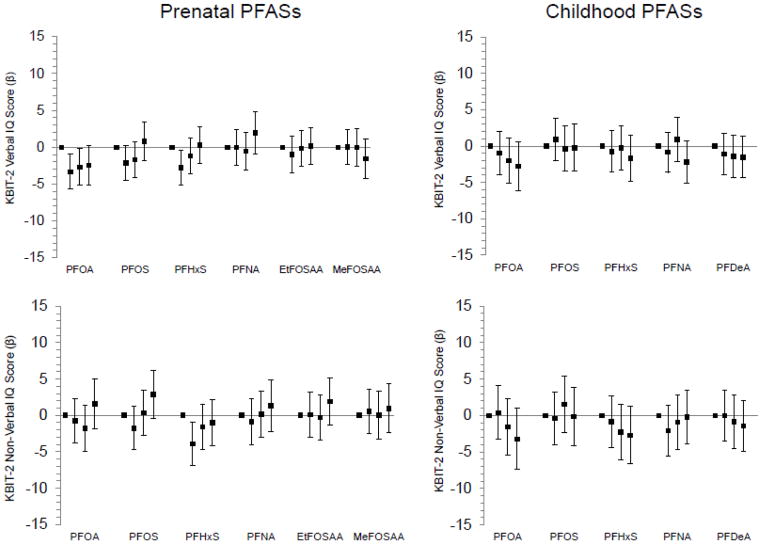
Figure 4.
Associations of prenatal and mid-childhood plasma PFAS concentrations (in quartiles) with mean differences (+95% confidence intervals) in mid-childhood (median age 7.7 years) Kaufman Brief Intelligence Test (KBIT-2) Verbal IQ and Non-Verbal IQ scores, adjusted for relevant covariates (n=851 for prenatal PFASs/Verbal IQ, 862 for prenatal PFASs/Non-Verbal IQ, 631 for childhood PFASs/Verbal IQ, 640 for childhood PFASs/Non-Verbal IQ). Standard mean (SD) for KBIT-2=100 (15); higher scores indicate better performance. All models adjusted for year of blood collection, maternal race/ethnicity, age, education, and IQ, paternal education, household income, home environment, and child’s sex and age at assessment. Prenatal PFAS models additionally adjusted for: gestational age at blood collection, estimated glomerular filtration rate, maternal pre-pregnancy body mass index and smoking in pregnancy, and proxy for breastfeeding of a prior child (incorporating parity). Childhood PFAS models additionally adjusted for: breastfeeding duration (months up to 12) and maternal parity.
KBIT-2 verbal IQ scores were lower among children with higher prenatal concentrations of PFOA, although the dose-response pattern appeared non-linear, with weaker associations observed for the third and fourth quartiles (Figure 4). Similarly non-linear patterns were observed for prenatal PFOA and KBIT-2 non-verbal IQ, as well as prenatal PFOS and verbal and non-verbal IQ; children born to women with top quartile PFOS concentrations had higher non-verbal IQ (Figure 4; Table S6). We observed potential trends of lower mid-childhood KBIT-2 IQ scores with higher mid-childhood concentrations of PFOA (verbal IQ and non-verbal IQ) and PFHxS (non-verbal IQ); other mid-childhood PFASs were not associated with KBIT scores (Figure 4; Table S7).
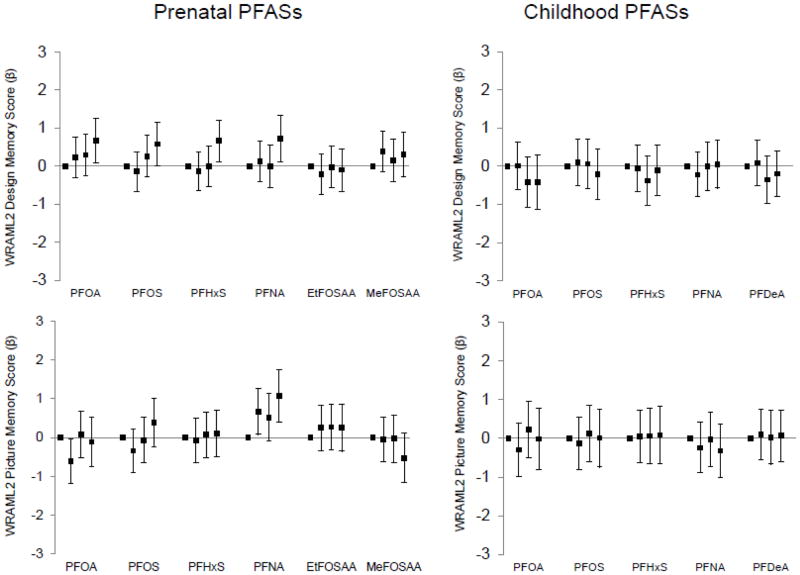
3.2.4 Prenatal and mid-childhood PFASs and mid-childhood WRAML2 (Figure 5)
Figure 5.
Associations of prenatal and mid-childhood plasma PFAS concentrations (in quartiles) with mean differences (+95% confidence intervals) in mid-childhood (median age 7.7 years) Visual Memory Index of the Wide Range Assessment of Memory and Learning (WRAML2) Design Memory and Picture Memory scores associated with prenatal and mid-childhood plasma PFAS concentrations in quartiles, adjusted for relevant covariates (n=853 for prenatal PFASs/Design Memory, 855 for prenatal PFASs/Picture Memory, 636 for childhood PFASs/Design Memory, 635 for childhood PFASs/Picture Memory). Standard mean (SD) for WRAML2 scores=10 (3); higher scores indicate better performance. All models adjusted for year of blood collection, maternal race/ethnicity, age, education, and IQ, paternal education, household income, home environment, and child’s sex and age at assessment. Prenatal PFAS models additionally adjusted for: gestational age at blood collection, estimated glomerular filtration rate, maternal pre-pregnancy body mass index and smoking in pregnancy, and proxy for breastfeeding of a prior child (incorporating parity). Childhood PFAS models additionally adjusted for: breastfeeding duration (months up to 12) and maternal parity.
Mid-childhood WRAML2 design memory scores were higher among children born to women in the top quartiles of PFOA, PFOS, PFHxS, and PFNA and WRAML2 picture memory scores were higher among children born to women with higher PFNA concentrations (Figure 5; Table S6). Mid-childhood PFASs did not appear associated with mid-childhood WRAML2 design memory or picture memory scores (Figure 5; Table S7).
3.3 Sensitivity analyses
Results for mid-childhood PFASs and mid-childhood cognitive assessment scores did not change appreciably in models additionally adjusted for maternal plasma PFAS concentrations (Table S8). Results of complete case analyses were similar to those of primary models (data not shown).
Results of models including imputed exposures and outcomes differed from primary models for some analyses (Tables S9–S11); most notably, associations of childhood PFHxS and mid-childhood WRAVMA visual motor scores were attenuated in models including imputed exposures and outcomes (e.g. Q4 vs. Q1: −2.8, 95% CI: −6.2, 0.9 compared to −5.0, 95% CI: −9.1, −0.8 in primary models).
Sensitivity analyses adjusting for assessor and excluding assessments from assessors who conducted <20 assessments (n=54 for early childhood, 28 for mid-childhood), those excluding individual cognitive assessment scores in which assessors had lower confidence (2–10% of scores, depending on assessment), and those with no adjustment for prior breastfeeding (prenatal PFAS models) or breastfeeding duration (childhood PFAS models) also yielded similar results (data not shown). Log-likelihood ratio tests and sex-stratified models did not suggest consistent patterns of effect measure modification by sex (data not shown). Results of models adjusted for plasma albumin were similar to those of primary models (data not shown).
4. DISCUSSION
In a prospective pre-birth cohort of Boston-area mothers and children, we observed lower visual motor abilities in mid-childhood (median age 7.7) among children with higher plasma PFHxS measured cross-sectionally; mean WRAVMA visual-motor scores were approximately five points, or a third of a standard deviation, lower among children in each of the top three quartiles versus the first quartile. Results suggested that higher plasma PFOA and PFOS in mid-childhood and PFOA, PFOS and PFHxS in maternal plasma from pregnancy might also be related to lower visual motor abilities in mid-childhood, though dose-response relationships appeared non-linear. We also observed trends suggesting lower mid-childhood intelligence test scores among those with higher mid-childhood PFOA (verbal IQ and non-verbal IQ) and PFHxS (non-verbal IQ).
Conversely, and contrary to our hypotheses, children born to women with the highest concentrations of PFOA, PFOS, PFHxS, and PFNA performed better on selected mid-childhood cognitive assessments (design memory for PFOA, non-verbal IQ and design memory for PFOS, design memory for PFHxS, and design memory and picture memory for PFNA). Children born to mothers with the highest concentrations of PFOA also scored higher on early childhood (median age 3.2) assessments of visual motor abilities, and we observed higher visual motor and receptive vocabulary scores among those in the second quartile of MeFOSAA. Prenatal PFAS concentrations were otherwise not associated with assessments of cognition conducted in early childhood. Adjustment for prenatal plasma PFASs had little effect on associations of mid-childhood plasma PFASs and cognitive outcomes, suggesting that gestational exposure to PFASs did not explain the observed relationships of mid-childhood PFASs and cognitive performance.
PFAS concentrations of mothers in our study (sampled 1999–2002) were similar to those of women in the 1999–2000 cycle of the nationally-representative U.S. NHANES (e.g. geometric mean (GM) PFOA=5.7 ng/mL in Project Viva and 4.8 ng/mL in NHANES women),45,48 and concentrations among children (sampled 2007–2010) were similar to those of adolescent participants in the 2007–2008 and 2009–2010 NHANES (e.g. GM PFOA=4.2 ng/mL in Project Viva and 3.9 ng/L in 2007–8 NHANES adolescents).49,50 Concentrations of the studied PFASs mostly decreased in NHANES over the 1999–2012 period following phase-outs by major U.S. manufactures of PFOS and PFOA, along with precursor chemicals that break down to PFOS and PFOA.50–53 Our analyses were adjusted for year of blood sampling to reduce potential confounding by temporal trends related to these changes. Prenatal and child PFAS concentrations in Project Viva were also broadly comparable to those measured in European birth cohorts from similar time periods (e.g. GM PFOA=3.2 ng/mL for mothers, 4.5 ng/mL in 7-year-old children in a Faroe Islands cohort enrolled 1996–2000;24 median maternal PFOA=5.4 ng/mL in Danish National Birth Cohort enrolled 1998–200219).
There are multiple mechanisms through which PFAS exposure may adversely affect neurodevelopment. In vitro models suggest that PFASs directly influence neuronal differentiation.54 In mice, neonatal exposure to PFOA, PFOS, or PFHxS was associated with alterations in levels of proteins related to synaptogenesis in the hippocampus and cerebral cortex in adulthood;12,55 exposed mice also had irregular nicotinic responses, suggesting alterations to the cholinergic system, and changes in adult behaviors potentially related to cognitive function.13,56 PFASs are known to activate the peroxisome proliferator-activated receptor alpha (PPAR-α), a nuclear receptor involved in regulation of metabolism and cell growth1,2,57 that may play a role in regulating the cholinergic activation of dopaminergic neurons.58 Finally, epidemiologic evidence suggests that, in pregnant women, PFASs may affect thyroid hormone levels, which are important for fetal brain development.11,59
Prior investigators have also noted mechanisms through which PFAS exposure could exert neuroprotective effects.60,61 PFASs appear to be partial agonists of peroxisome proliferator-activated receptor gamma (PPAR-γ),62 and PPAR-γ-agonists have been observed to induce anti-inflammatory effects in the central nervous system that may be neuroprotective.63
We observed evidence that PFAS exposure is associated with both better and worse cognitive performance in children, with findings varying across PFAS, cognitive endpoint, dose, and timing of exposure (prenatal versus postnatal). Prior cohort studies assessing PFAS exposure at multiple time points have also reported inconsistent associations with brain development across PFASs and exposure periods. In a Faroe Islands birth cohort (n=539), childhood (age 5) PFOA, PFNA, and PFDeA concentrations were associated with greater behavior problems at age 7, but no associations were observed with age 5 PFOS or PFHxS or prenatal PFAS measures (in that study, PFAS measures at age 7 were associated with greater behavior problems in girls, but with fewer behavioral problems in boys).24 In a Cincinnati, Ohio-based birth cohort (n=167), prenatal, age 3, and age 8 childhood PFNA, PFOA and PFOS (but not PFHxS) were associated with better reading skills at ages 5 and 8.27 Other studies in prospective cohorts have reported negative,15–17 null,18–23 and positive60,64–66 associations of prenatal or perinatal PFAS exposure with childhood neurodevelopmental outcomes. Childhood and adolescent exposures to certain PFASs have been linked to ADHD diagnoses6,25 and greater impulsivity26 in cross-sectional analyses, but these studies do not allow for comparison of prenatal and postnatal windows of exposure. Our results require confirmation in additional longitudinal studies examining cognitive development in relation to PFAS exposure at multiple time points.
The varied and inconsistent relationships between prenatal PFAS exposure and cognitive outcomes in our study mirror the inconsistency in the existing literature, and could reflect that biological mechanisms of action vary across PFASs or differ depending on plasma PFAS concentration. For instance, a potential mechanism of PFAS neurotoxicty is competition with thyroxine (T4) in binding with human thyroid hormone transport protein transthyretin (TTR), resulting in reduced concentrations of free T4, which could alter fetal neurodevelopment. The studied PFASs have been shown to vary in TTR binding potency, with PFHxS more potent than PFOS and PFOA, and PFNA less potent, while EtFOSAA and MeFOSAA were conversely associated with a slight increase in T4-TTR binding at high concentrations.67 Additional research into the potentially complex mechanisms through which PFASs may influence the developing brain is needed.
Limitations of our study should be noted. Cross-sectional associations of mid-childhood PFAS concentrations and cognitive outcomes could be impacted by reverse causation if children’s cognitive abilities were related to behaviors that might influence PFAS exposure. Only a subset of originally enrolled Project Viva participants with available exposure and outcome data were included in each of our analyses (30–46% of the original cohort, depending on analysis), and excluded participants differed somewhat from included participants. In order to minimize potential selection bias due to differential loss to follow-up, we adjusted our models for covariates observed to predict drop-out, but bias is still possible. The observed attenuation in some effect estimates, including associations of mid-childhood PFHxS and mid-childhood WRAVMA visual motor scores, in models including imputed exposures and outcomes may indicate that selection bias influenced these results, though this attenuation could also stem from misclassification of imputed exposure and outcome values.
We relied on a proxy measure of prior breastfeeding, meaning that residual confounding resulting from incomplete adjustment for prior breastfeeding could have biased observed associations with prenatal PFASs. Sensitivity analyses excluding this proxy variable yielded very similar results to those of primary models, however, indicating that confounding related to prior breastfeeding was likely minimal. There were moderate to high correlations between studied PFASs, limiting our capacity to fully differentiate the effects of individual chemicals; reported associations with individual PFASs could therefore reflect combined effects of concurrent exposure to multiple PFASs.
We based estimates of prenatal PFAS exposure on a single measurement of maternal plasma PFAS concentration in the first or second trimester of pregnancy (5.6–20.9 weeks of gestation), rather than a more direct measure of fetal exposure, such as cord blood. Maternal gestational PFAS concentrations, however, likely represent a good estimate of PFAS fetal exposure throughout gestation because half-lives of PFASs in humans are relatively long (e.g. 2.3 years for PFOA, 5.4 years for PFOS, 8.5 years for PFHxS)1 and concentrations of PFASs in maternal peripheral blood correlate highly with levels in cord blood.9–11,68 Similarly, concentrations of PFASs in mid-childhood plasma likely reflect PFAS exposure over multiple preceding years.
Cognitive outcomes were measured using well-validated assessments, and assessor training and double-scoring protocols were employed to ensure consistent scoring across assessors, but outcome misclassification related to challenges inherent in assessing cognitive function in young children is possible. Results of sensitivity analyses adjusting for assessor were similar to those of primary models, suggesting that inter-rater variability is unlikely to have introduced substantial bias.
The study also has a number of strengths. PFAS concentrations in the study cohort were similar to concentrations observed in NHANES during the same time periods, and therefore representative of exposures experienced by the general U.S. population. Our study was one of the first to examine multiple windows of PFAS exposure (prenatal and childhood) in relation to cognitive development. The study was conducted in a relatively large, prospective cohort with rich data on maternal and child health and behaviors, which enabled adjustment for important potential confounders including physiologic measures in pregnancy, maternal IQ, support for cognitive development in the home, breastfeeding, and sociodemographic factors.
5. CONCLUSIONS
Our study suggests that exposure to PFASs (including PFOA, PFOS, and PFHxS) during gestation and childhood may be associated with lower childhood visual motor abilities. Other results were inconsistent, with higher prenatal PFASs associated in some cases with better cognitive outcomes. These findings indicate that PFASs may influence children’s cognitive development, though magnitude and direction of effects may vary depending on PFAS, dose, cognitive endpoint, and timing of exposure (prenatal versus postnatal). As PFAS exposures are ubiquitous among pregnant women and children, even modest adverse impacts of PFASs on cognitive development could cause a substantial neurodevelopmental burden at the population level.69 These findings highlight the need for further research on mechanisms through which PFASs may act on the developing brain.
Supplementary Material
Highlights.
Per- and polyfluoroalkyl substances (PFASs) are suspected developmental toxicants.
We examined prenatal and childhood PFAS exposure in relation to child cognition.
Prenatal PFASs were associated with both better and worse cognitive scores.
Childhood PFASs were associated cross-sectionally with lower visual motor abilities.
Acknowledgments
Sources of funding:
The results reported herein correspond to specific aims of grant R01 ES021447 to Dr. Sharon Sagiv from the National Institutes of Health (NIEHS). This work was also supported by grants R01 HD034568, K24 HD069408, P30 DK092924, and T32ES014562 from the National Institutes of Health. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or any other body.
We thank the participants and staff of Project Viva. We also thank Kayoko Kato, Ayesha Patel, and Tao Jia for PFAS measurements.
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- CDC
Centers for Disease Control and Prevention
- DAG
Directed Acyclic Graph
- EtFOSAA
2-(N-ethyl-perfluorooctane sulfonamido) acetate
- FOSA
perfluorooctane sulfonamide
- GFR
glomerular filtration rate
- GM
geometric mean
- HOME-SF
Home Observation for Measurement of the Environment - Short Form
- IQ
intelligence quotient
- KBIT-2
Kaufman Brief Intelligence Test, Second Edition
- LOD
limit of detection
- MeFOSAA
2-(N-methyl-perfluorooctane sulfonamido) acetate
- NHANES
National Health and Nutrition Examination Survey
- PFAS
per- and polyfluoroalkyl substance
- PFDeA
perfluorodecanoate
- PFHxS
perfluorohexane sulfonate
- PFNA
perfluorononanoate
- PFOA
perfluorooctanoate
- PFOS
perfluorooctane sulfonate
- PPAR-α
peroxisome proliferator-activated receptor alpha
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- PPVT-III
Peabody Picture Vocabulary Test, 3rd edition
- SD
standard deviation
- T4
thyroxine
- TTR
transthyretin
- VIF
variance inflation factor
- WISC-III
Wechsler Intelligence Scale for Children-III
- WRAML2
Wide Range Assessment of Memory and Learning, Second Edition
- WRAVMA
Wide Range Assessment of Visual Motor Abilities
Footnotes
Conflicts of Interest:
Dr. Harris is employed part-time as an Environmental Epidemiologist for Environmental Defense Fund, an environmental non-profit organization with advocacy activities related to consumer product chemical safety. The authors have no other potential conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99(2):366–94. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 2.Mariussen E. Neurotoxic effects of perfluoroalkylated compounds: mechanisms of action and environmental relevance. Arch Toxicol. 2012;86(9):1349–67. doi: 10.1007/s00204-012-0822-6. [DOI] [PubMed] [Google Scholar]
- 3.Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect. 2007;115(11):1596–602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119(6):878–85. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain RB. Effect of pregnancy on the levels of selected perfluoroalkyl compounds for females aged 17–39 years: data from National Health and Nutrition Examination Survey 2003–2008. J Toxicol Environ Health A. 2013;76(7):409–21. doi: 10.1080/15287394.2013.771547. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman K, Webster TF, Weisskopf MG, Weinberg J, Vieira VM. Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in U.S. children 12–15 years of age. Environ Health Perspect. 2010;118(12):1762–7. doi: 10.1289/ehp.1001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinney SM, Biro FM, Windham GC, Herrick RL, Yaghjyan L, Calafat AM, Succop P, Sucharew H, Ball KM, Kato K, Kushi LH, Bornschein R. Serum biomarkers of polyfluoroalkyl compound exposure in young girls in Greater Cincinnati and the San Francisco Bay Area, USA. Environ Pollut. 2014;184:327–34. doi: 10.1016/j.envpol.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schecter A, Malik-Bass N, Calafat AM, Kato K, Colacino JA, Gent TL, Hynan LS, Harris TR, Malla S, Birnbaum L. Polyfluoroalkyl compounds in Texas children from birth through 12 years of age. Environ Health Perspect. 2012;120(4):590–4. doi: 10.1289/ehp.1104325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, Faber F, Hannibal I, Genzel-Boroviczeny O, Koletzko B, Volkel W. Pre- and postnatal exposure to perfluorinated compounds (PFCs) Environ Sci Technol. 2010;44(18):7123–9. doi: 10.1021/es101184f. [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Choi K, Ji K, Seo J, Kho Y, Park J, Kim S, Park S, Hwang I, Jeon J, Yang H, Giesy JP. Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ Sci Technol. 2011;45(17):7465–72. doi: 10.1021/es202408a. [DOI] [PubMed] [Google Scholar]
- 11.Kato K, Wong LY, Chen A, Dunbar C, Webster GM, Lanphear BP, Calafat AM. Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003–2006. Environ Sci Technol. 2014;48(16):9600–8. doi: 10.1021/es501811k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson N, Eriksson P, Viberg H. Neonatal exposure to PFOS and PFOA in mice results in changes in proteins which are important for neuronal growth and synaptogenesis in the developing brain. Toxicol Sci. 2009;108(2):412–8. doi: 10.1093/toxsci/kfp029. [DOI] [PubMed] [Google Scholar]
- 13.Viberg H, Lee I, Eriksson P. Adult dose-dependent behavioral and cognitive disturbances after a single neonatal PFHxS dose. Toxicology. 2013;304:185–91. doi: 10.1016/j.tox.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Onishchenko N, Fischer C, Wan Ibrahim WN, Negri S, Spulber S, Cottica D, Ceccatelli S. Prenatal exposure to PFOS or PFOA alters motor function in mice in a sex-related manner. Neurotox Res. 2011;19(3):452–61. doi: 10.1007/s12640-010-9200-4. [DOI] [PubMed] [Google Scholar]
- 15.Chen MH, Ha EH, Liao HF, Jeng SF, Su YN, Wen TW, Lien GW, Chen CY, Hsieh WS, Chen PC. Perfluorinated compound levels in cord blood and neurodevelopment at 2 years of age. Epidemiology. 2013;24(6):800–8. doi: 10.1097/EDE.0b013e3182a6dd46. [DOI] [PubMed] [Google Scholar]
- 16.Høyer BB, Ramlau-Hansen CH, Obel C, Pedersen HS, Hernik A, Ogniev V, Jonsson BA, Lindh CH, Rylander L, Rignell-Hydbom A, Bonde JP, Toft G. Pregnancy serum concentrations of perfluorinated alkyl substances and offspring behaviour and motor development at age 5–9 years--a prospective study. Environ Health. 2015;14:2. doi: 10.1186/1476-069X-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vuong AM, Yolton K, Webster GM, Sjodin A, Calafat AM, Braun JM, Dietrich KN, Lanphear BP, Chen A. Prenatal polybrominated diphenyl ether and perfluoroalkyl substance exposures and executive function in school-age children. Environ Res. 2016;147:556–64. doi: 10.1016/j.envres.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fei C, McLaughlin JK, Lipworth L, Olsen J. Prenatal exposure to perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) and maternally reported developmental milestones in infancy. Environ Health Perspect. 2008;116(10):1391–5. doi: 10.1289/ehp.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fei C, Olsen J. Prenatal exposure to perfluorinated chemicals and behavioral or coordination problems at age 7 years. Environ Health Perspect. 2011;119(4):573–8. doi: 10.1289/ehp.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forns J, Iszatt N, White RA, Mandal S, Sabaredzovic A, Lamoree M, Thomsen C, Haug LS, Stigum H, Eggesbo M. Perfluoroalkyl substances measured in breast milk and child neuropsychological development in a Norwegian birth cohort study. Environ Int. 2015;83:176–82. doi: 10.1016/j.envint.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Liew Z, Ritz B, von Ehrenstein OS, Bech BH, Nohr EA, Fei C, Bossi R, Henriksen TB, Bonefeld-Jorgensen EC, Olsen J. Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: a nested case-control study in the Danish National Birth Cohort. Environ Health Perspect. 2015;123(4):367–73. doi: 10.1289/ehp.1408412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ode A, Kallen K, Gustafsson P, Rylander L, Jonsson BA, Olofsson P, Ivarsson SA, Lindh CH, Rignell-Hydbom A. Fetal exposure to perfluorinated compounds and attention deficit hyperactivity disorder in childhood. PLoS One. 2014;9(4):e95891. doi: 10.1371/journal.pone.0095891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strøm M, Hansen S, Olsen SF, Haug LS, Rantakokko P, Kiviranta H, Halldorsson TI. Persistent organic pollutants measured in maternal serum and offspring neurodevelopmental outcomes--a prospective study with long-term follow-up. Environ Int. 2014;68:41–8. doi: 10.1016/j.envint.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Oulhote Y, Steuerwald U, Debes F, Weihe P, Grandjean P. Behavioral difficulties in 7-year old children in relation to developmental exposure to perfluorinated alkyl substances. Environ Int. 2016;97:237–245. doi: 10.1016/j.envint.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein CR, Savitz DA. Serum perfluorinated compound concentration and attention deficit/hyperactivity disorder in children 5–18 years of age. Environ Health Perspect. 2011;119(10):1466–71. doi: 10.1289/ehp.1003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gump BB, Wu Q, Dumas AK, Kannan K. Perfluorochemical (PFC) exposure in children: associations with impaired response inhibition. Environ Sci Technol. 2011;45(19):8151–9. doi: 10.1021/es103712g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Yolton K, Webster GM, Ye X, Calafat AM, Dietrich KN, Xu Y, Xie C, Braun JM, Lanphear BP, Chen A. Prenatal and childhood perfluoroalkyl substances exposures and children’s reading skills at ages 5 and 8 years. Environ Int. 2017;111:224–231. doi: 10.1016/j.envint.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, Rich-Edwards JW, Rifas-Shiman SL, Sagiv S, Taveras EM, Weiss ST, Belfort MB, Burris HH, Camargo CA, Jr, Huh SY, Mantzoros C, Parker MG, Gillman MW. Cohort profile: Project Viva. Int J Epidemiol. 2015;44(1):37–48. doi: 10.1093/ije/dyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. 3. Circle Pines, MN: American Guidance Services, Inc; 1997. [Google Scholar]
- 30.Adams W, Sheslow D. WRAVMA (Wide Range Assessment of Visual Motor Abilities) Wilmington, DE: Wide Range, Inc; 1995. [Google Scholar]
- 31.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test Second Edition (KBIT-2) Bloomington, MN: Pearson, Inc; 2004. [Google Scholar]
- 32.Adams W, Sheslow D. Wide Range Assessment of Memory and Learning Administration and Technical Manual. 2. Lutz, FL: Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- 33.Viva P. Measuring WRAVMA Drawing Designs with Scoring Templates—Revision. 02 Jul 2; https://www.hms.harvard.edu/viva/protocol-wravma-drawing-scoring.pdf.
- 34.Centers for Disease Control and Prevention (CDC) Laboratory Procedure Manual for Polyfluorinated Compounds in Serum (NHANES 2011–2012) (Method No. 6304.04) 2013 https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/pfc_g_met.pdf.
- 35.Centers for Disease Control and Prevention (CDC) Laboratory Procedure Manual for Perfluoroalkyl and Polyfluoroalkyl Substances (NHANES 2013–2014) (Method No. 6304.06) 2013. [Google Scholar]
- 36.Kato K, Basden BJ, Needham LL, Calafat AM. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A. 2011;1218(15):2133–7. doi: 10.1016/j.chroma.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 37.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Applied Occupational and Environmental Hygiene. 1990;5(1):46–51. [Google Scholar]
- 38.Frankenburg WK, Coons CE. Home Screening Questionnaire: its validity in assessing home environment. J Pediatr. 1986;108(4):624–6. doi: 10.1016/s0022-3476(86)80853-8. [DOI] [PubMed] [Google Scholar]
- 39.Morken NH, Travlos GS, Wilson RE, Eggesbo M, Longnecker MP. Maternal glomerular filtration rate in pregnancy and fetal size. PLoS One. 2014;9(7):e101897. doi: 10.1371/journal.pone.0101897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30(4):377–99. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 41.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: Wiley-Interscience; 2004. [Google Scholar]
- 42.Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155(2):176–84. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 43.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22(5):745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- 44.Savitz DA. Invited commentary: interpreting associations between exposure biomarkers and pregnancy outcome. Am J Epidemiol. 2014;179(5):545–7. doi: 10.1093/aje/kwt314. [DOI] [PubMed] [Google Scholar]
- 45.Sagiv SK, Rifas-Shiman SL, Webster TF, Mora AM, Harris MH, Calafat AM, Ye X, Gillman MW, Oken E. Sociodemographic and Perinatal Predictors of Early Pregnancy Per- and Polyfluoroalkyl Substance (PFAS) Concentrations. Environ Sci Technol. 2015;49(19):11849–58. doi: 10.1021/acs.est.5b02489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mogensen UB, Grandjean P, Nielsen F, Weihe P, Budtz-Jorgensen E. Breastfeeding as an Exposure Pathway for Perfluorinated Alkylates. Environ Sci Technol. 2015;49(17):10466–73. doi: 10.1021/acs.est.5b02237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mondal D, Weldon RH, Armstrong BG, Gibson LJ, Lopez-Espinosa MJ, Shin HM, Fletcher T. Breastfeeding: a potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environ Health Perspect. 2014;122(2):187–92. doi: 10.1289/ehp.1306613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. Serum concentrations of 11 polyfluoroalkyl compounds in the u.s. population: data from the national health and nutrition examination survey (NHANES) Environ Sci Technol. 2007;41(7):2237–42. doi: 10.1021/es062686m. [DOI] [PubMed] [Google Scholar]
- 49.Harris MH, Rifas-Shiman SL, Calafat AM, Ye X, Mora AM, Webster TF, Oken E, Sagiv SK. Predictors of Per- and Polyfluoroalkyl Substance (PFAS) Plasma Concentrations in 6–10 Year Old American Children. Environ Sci Technol. 2017;51(9):5193–5204. doi: 10.1021/acs.est.6b05811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention (CDC) Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, February 2015. 2015. [Google Scholar]
- 51.Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SP. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. 2011;7(4):513–41. doi: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D’Eon JC, Mabury SA. Is indirect exposure a significant contributor to the burden of perfluorinated acids observed in humans? Environ Sci Technol. 2011;45(19):7974–84. doi: 10.1021/es200171y. [DOI] [PubMed] [Google Scholar]
- 53.Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ Sci Technol. 2011;45(19):8037–45. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- 54.Slotkin TA, MacKillop EA, Melnick RL, Thayer KA, Seidler FJ. Developmental neurotoxicity of perfluorinated chemicals modeled in vitro. Environ Health Perspect. 2008;116(6):716–22. doi: 10.1289/ehp.11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee I, Viberg H. A single neonatal exposure to perfluorohexane sulfonate (PFHxS) affects the levels of important neuroproteins in the developing mouse brain. Neurotoxicology. 2013;37:190–6. doi: 10.1016/j.neuro.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Johansson N, Fredriksson A, Eriksson P. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology. 2008;29(1):160–9. doi: 10.1016/j.neuro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 57.Rosen MB, Lau C, Corton JC. Does exposure to perfluoroalkyl acids present a risk to human health? Toxicol Sci. 2009;111(1):1–3. doi: 10.1093/toxsci/kfp142. [DOI] [PubMed] [Google Scholar]
- 58.Melis M, Scheggi S, Carta G, Madeddu C, Lecca S, Luchicchi A, Cadeddu F, Frau R, Fattore L, Fadda P, Ennas MG, Castelli MP, Fratta W, Schilstrom B, Banni S, De Montis MG, Pistis M. PPARalpha regulates cholinergic-driven activity of midbrain dopamine neurons via a novel mechanism involving alpha7 nicotinic acetylcholine receptors. J Neurosci. 2013;33(14):6203–11. doi: 10.1523/JNEUROSCI.4647-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Rogan WJ, Chen PC, Lien GW, Chen HY, Tseng YC, Longnecker MP, Wang SL. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study. Environ Health Perspect. 2014;122(5):529–34. doi: 10.1289/ehp.1306925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stein CR, Savitz DA, Bellinger DC. Perfluorooctanoate and neuropsychological outcomes in children. Epidemiology. 2013;24(4):590–9. doi: 10.1097/EDE.0b013e3182944432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Power MC, Webster TF, Baccarelli AA, Weisskopf MG. Cross-sectional association between polyfluoroalkyl chemicals and cognitive limitation in the National Health and Nutrition Examination Survey. Neuroepidemiology. 2013;40(2):125–32. doi: 10.1159/000342310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol Sci. 2006;92(2):476–89. doi: 10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- 63.Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008;13:1813–26. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjodin A, Hauser R, Webster GM, Chen A, Lanphear BP. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environ Health Perspect. 2014;122(5):513–20. doi: 10.1289/ehp.1307261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lien GW, Huang CC, Shiu JS, Chen MH, Hsieh WS, Guo YL, Chen PC. Perfluoroalkyl substances in cord blood and attention deficit/hyperactivity disorder symptoms in seven-year-old children. Chemosphere. 2016;156:118–27. doi: 10.1016/j.chemosphere.2016.04.102. [DOI] [PubMed] [Google Scholar]
- 66.Quaak I, de Cock M, de Boer M, Lamoree M, Leonards P, van de Bor M. Prenatal Exposure to Perfluoroalkyl Substances and Behavioral Development in Children. Int J Environ Res Public Health. 2016;13(5) doi: 10.3390/ijerph13050511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weiss JM, Andersson PL, Lamoree MH, Leonards PE, van Leeuwen SP, Hamers T. Competitive binding of poly- and perfluorinated compounds to the thyroid hormone transport protein transthyretin. Toxicol Sci. 2009;109(2):206–16. doi: 10.1093/toxsci/kfp055. [DOI] [PubMed] [Google Scholar]
- 68.Liu J, Li J, Liu Y, Chan HM, Zhao Y, Cai Z, Wu Y. Comparison on gestation and lactation exposure of perfluorinated compounds for newborns. Environ Int. 2011;37(7):1206–12. doi: 10.1016/j.envint.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 69.Bellinger DC. Comparing the population neurodevelopmental burdens associated with children’s exposures to environmental chemicals and other risk factors. Neurotoxicology. 2012;33(4):641–3. doi: 10.1016/j.neuro.2012.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.