Abstract
Background
U.S. unintended pregnancy rates remain high, and contraceptive providers are not universally trained to offer IUDs and implants to women who wish to use these methods.
Objective
To measure the impact of a provider training intervention on integration of intrauterine devices (IUDs) and implants into contraceptive care.
Methods
We measured the impact of a continuing medical education-accredited provider training intervention on provider attitudes, knowledge, and practices in a cluster randomized trial in 40 U.S. health centers from 2011–2013. Twenty clinics were randomly assigned to the intervention arm; 20 offered routine care. Clinic staff participated in baseline and one-year surveys assessing IUD and implant knowledge, attitudes and practices. We used a difference-in-differences approach to compare changes that occurred in the intervention sites to changes in the control sites one year later. Pre-specified outcome measures included: knowledge of patient eligibility for IUDs and implants; attitudes about method safety; and counseling practices. We used multivariable regression with generalized estimating equations to account for clustering by clinic to examine intervention effects on provider outcomes one year later.
Results
Overall, we surveyed 576 clinic staff (314 intervention, 262 control) at baseline and/or one-year follow-up. The change in proportion of providers who believed that the IUD was safe was greater in intervention (60% at baseline to 76% at follow-up) than control sites (66% at both times) (aOR=2.48 [1.13–5.4]). Likewise, for the implant, the proportion increased from 57% to 77% in intervention, compared to 61% to 65% in control sites (aOR=2.57 [1.44–4.59]). The proportion of providers who believed they were experienced to counsel on IUDs also increased in intervention (53% to 67%) and remained the same in control sites (60%) (aOR=1.89 [1.04–3.44]), and for the implant increased more in intervention (41% to 62%) compared to control sites (48% to 50%) (aOR=2.30 [1.28–4.12]). Knowledge scores of patient eligibility for IUDs increased at intervention sites (from 0.77 to 0.86) 6% more over time compared to control sites (from 0.78 to 0.80) (adjusted coefficient=0.058 [0.003–0.113]). Knowledge scores of eligibility for IUD and implant use with common medical conditions increased 15% more in intervention (0.65 to 0.79) compared to control sites (0.67 to 0.66) (adjusted coefficient=0.15 [0.09–0.21). Routine discussion of IUDs and implants by providers in intervention sites increased significantly, 71% to 87%, compared to in control sites, 76% to 82% (aOR=1.97 [1.02–3.80]).
Conclusion
Professional guidelines encourage IUD and implant competency for all contraceptive care providers. Integrating these methods into routine care is important for access. This replicable training intervention translating evidence into care had a sustained impact on provider attitudes, knowledge and counseling practices, demonstrating significant changes in clinical care a full year after the training intervention.
Keywords: continuing education, contraception, contraceptive implant, intrauterine device, provider training intervention
Introduction
Contraceptive use is a preference-sensitive decision that a woman often makes in collaboration with a healthcare provider.1,2 Information received from a trusted provider can impact women’s contraceptive decision-making.3 Yet providers often have misinformation about contraceptive methods, with relatively low knowledge about intrauterine devices (IUDs) and implants, well-documented among a variety of US contraceptive care providers.4–10 In settings where contraceptive care is provided by teams that include non-licensed health educators and medical assistants, it is also important to consider knowledge on long-acting reversible contraceptives (LARC) among these staff members.11
Obstetrician-gynecologists and women’s health nurse practitioners are more familiar with IUDs and implants compared to other clinicians,6–8,10 but even expert providers have some misinformation, resulting in restricted access for eligible patients. For example, some providers believe IUDs are inappropriate for nulliparous women, adolescents and immediately following abortion; or that IUDs and implants have the same contraindications as combined hormonal contraceptive methods.4–11 Providers with more recent training in IUDs or implants tend to have increased knowledge about medical eligibility of these methods for a wide range of women.5–8,12
Knowledge about IUDs and implants directly influences whether clinicians and health educators include them in routine contraceptive counseling.4–11 Because IUDs and implants are placed only by trained clinicians, women’s access to these methods depends on clinicians’ ability and willingness to provide them. Beyond provider knowledge and practices, there are other important barriers to access cited by providers, including high costs, clinic flow issues, and protocols disallowing same-visit placement.13–15 As changes in clinical care over time are necessary for improvement in patient health outcomes and access to these methods, it is important to demonstrate successful approaches to changing care.
This study is a pre-specified analysis of the provider data from a cluster randomized trial of a provider training intervention. Primary analyses from this trial measured the intervention impact on patient-level outcomes; findings showed the intervention increased women’s selection of LARC, without compromising autonomy in decision-making, and significantly reduced unintended pregnancy in family planning clinics.16 This study addresses a gap in the scientific literature on effective provider education for clinical practice change. While continuing education is routinely used to update provider skills and knowledge, its impact on clinical care is rarely tested in randomized trials. We conducted a randomized study to test the hypothesis that a training intervention can help clinics to integrate IUDs and implants into patient care. To our knowledge, this is the first randomized trial to measure provider outcomes and assess the translation of evidence to care for IUDs and contraceptive implants.
Materials and Methods
We conducted a cluster randomized trial of a clinic-wide training intervention from February 2011 to May 2013. Forty Planned Parenthood health centers in 15 statesa participated, with randomization at the clinic level. Cluster designs are appropriate for testing educational interventions because they prevent contamination that can occur between study arms with individual randomization of providers. We designed and tested a training intervention to address low knowledge about IUDs and implants among clinic staff, as well as other modifiable barriers to access. This study focuses on identifying the changes in provider practices that led to improved patient outcomes. This pre-specified secondary analysis estimated the intervention impact on the provider outcomes of attitudes, knowledge and counseling practices.
Study methods have been described in detail elsewhere.16 Sample size was determined for the study’s primary patient outcome, choice of LARC. We conducted the trial at 40 clinics collectively serving over 100,000 annual contraceptive patients, and followed 1,500 women for one year. Eligible clinics provided care for at least 400 patients annually, provided less than 20% of contraceptive patients with IUDs or implants, did not have other concurrent LARC interventions, and did not share staff with other study clinics. There were 544 eligible clinic staff from participating sites at baseline and 509 at follow-up. Clinic staff were eligible to participate, including clinicians and non-licensed staff, if they were employed by a participating clinic site.
Clinics were randomly assigned by an independent statistician at the UCSF Clinical and Translational Science Institute and stratified by clinic size (≤4,000 annual patients, >4,000). Allocation was concealed until study initiation. It was apparent whether clinics received the intervention training, so providers were not blinded after the study began. We collected baseline data prior to the trainings at intervention sites and prior to patient enrollment at all of the sites (February 2011–July 2012), and follow-up data one year later (February 2012–July 2013).
To assess the impact of the training on providers, we conducted surveys of all clinic staff at baseline and follow-up. Clinic staff who joined the health center during the study were included in the one-year follow-up, as the study measured changes in clinical practice at the clinic level under real world conditions. The self-administered surveys could be completed online or on paper. Survey invitations contained an information sheet with the elements of informed consent; staff confirmed consent by responding to the surveys. Respondents were entered into a random drawing for two tablet computers.
The study was registered with ClinicalTrials.gov (NCT01360216). The University of California, San Francisco, Committee on Human Research and the Allendale Investigational Review Board gave approval for the study.
Intervention
The intervention training was a 4-hour Continuing Education (CE)-accredited course. We designed the training intervention after conducting formative research to identify gaps in knowledge among US providers and the best educational approach. Results showed it was important to include the full clinic team in training in order to transform practice.5,6,8,13,14 The training included a didactic session with updated evidence on IUDs and implants, a hands-on practicum for clinicians on IUD insertion, and a practicum for health educators on contraceptive counseling. The training explained how to use the U.S. Medical Eligibility Criteria for Contraceptive Use, a tiered contraceptive effectiveness chart, and open ended questions about women’s pregnancy intentions and contraceptive preferences.18–20
The training emphasized ethical issues specific to IUDs and implants, including the importance of patient-centered counseling and removal upon patient request. We showed two videos: one of providers who had successfully integrated IUDs and implants into practice and one of young women who had used these contraceptive methods. For interested clinicians, we facilitated hands-on training for implant insertion. We provided a packet for new staff about six months after the training, highlighting its main principles.
Measures
Study outcomes included provider attitudes, knowledge and practices regarding IUDs and implants. We measured provider attitudes about method safety with two items asking whether the provider believed that IUDs and the implant are safe for women to use (strongly agree, agree, disagree, strongly disagree, don’t know), and dichotomized responses strongly agree vs. other. We measured provider attitudes about whether they had enough experience to counsel on IUDs and implants using similar response categories. We assessed counseling practice with the frequency with which providers discussed contraceptives, including IUDs and the implant, as well as other methods, with contraceptive patients (never, sometimes, usually, always), and combined response categories for “routine counseling” to usually/always vs. never/sometimes.
To measure provider knowledge, we used two scales adapted from prior research:5,6,8 First, a patient IUD eligibility scale, with six items, measured whether the provider would consider an IUD for nulliparas, unmarried women, teens, immediately post-abortion, or for those with a history pelvic inflammatory disease (PID) or sexually transmitted infection (STI) in last two years.20 The scale had an internal consistency reliability of 0.72 in this study. Second, a patient eligibility scale for IUD or implant use with common medical conditions with 12 items measured whether the provider would consider a copper IUD, levonorgestrel IUD, or etonogestrel implant for a woman with obesity, diabetes, or hypertension, or a smoker. These methods can be used with these conditions.20 This scale had a reliability coefficient of 0.94 in this study. Scales were scored as the proportion of correct responses (range 0–1). We considered the few participants who responded to fewer than half of the items on either scale as having invalid scores (missing).
The variables to measure intervention impact on outcomes were study arm and time (baseline, one-year follow-up). We included covariates that might affect provider practice, such as provider age, race/ethnicity, and position (clinician, health educator).8 On the clinic level, we included variables for practice setting (abortion, family planning) and region.
Analyses
The study included all clinic staff who conducted contraceptive counseling, including clinicians and health educators. The analysis population comprised staff working at study sites who completed the baseline or follow-up surveys. We compared differences in characteristics between providers by study arm for the full analytic sample. We also compared baseline provider knowledge, attitudes, and counseling to establish similarity of providers by arm prior to the intervention. We used generalized estimating equation (GEE) models, to account for clustering of providers within clinics.
To examine the impact of the training on outcomes, we used a repeated cross-sections approach, including data from all providers completing a baseline or follow-up survey. We measured differences over time by study arm in attitudes, knowledge scales and counseling practices. This difference-in-differences approach compares the changes in intervention to the changes in control sites. This approach is the most appropriate for the data, allowing us to account for differences in respondent characteristics by arm and staff turnover over time.21 It also allows for a measure of all staff at the clinic at follow-up, including new hires, reflecting overall clinical practice. For each GEE model, we regressed the study outcome on arm, time period, and an arm-by-time interaction term. We estimated the intervention effect using the interaction term, which reflected the relative change in the outcome in intervention versus control, from baseline to follow-up. Multivariable models included covariables, selected a priori, associated with IUD and implant knowledge and practices.5,11 We derived predicted means and coefficients (for continuous knowledge scales) and predicted proportions and odds ratios (for dichotomous outcomes) by study arm.
We conducted sensitivity analyses by comparing changes in outcomes for the longitudinal cohort of providers who were present at both baseline and follow-up to assess whether results by study arm were consistent with repeated cross-sectional analyses.
Intent-to-treat analyses were conducted, with the provider data analyzed by the study arm assigned to their clinic (cluster), in Stata 14 (College Station, TX). Significance is reported at the p≤0.05 level.
Results
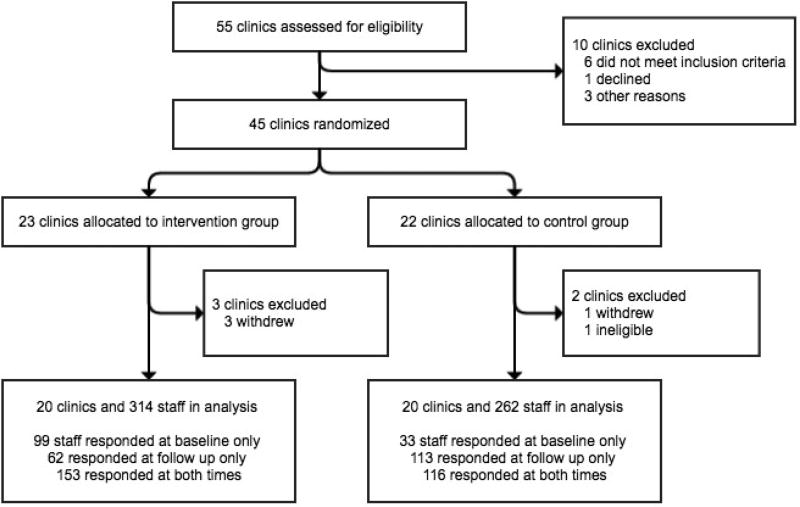
There were 401 respondents (74% of the 544 eligible staff) at baseline, with most surveys completed online. By arm, there were 252 respondents in intervention (89% of 284 staff) and 149 in control sites (57% of 260). At one-year, there were 444 respondents (87%), with 215 (88% of 244 staff) in intervention and 229 (86% of 265) in control sites. There was appreciable staff turnover during the year-long study: of the 401 baseline respondents, 126 staff were no longer working at that site one year later (31%). Despite this turnover, attrition analysis showed no differences between those remaining in versus exiting the study in any sociodemographic or baseline outcome variables. Repeated cross-sectional analyses included 576 respondents (314 intervention, 262 control) (Figure 1).
Figure 1. Flow chart of clinic sites and staff in a cluster randomized trial of an IUD and implant training (n=576).
There were no differences in providers’ baseline characteristics by study arm, nor for the full study sample (Table 1). Providers were mainly female (97%), and about half were white (52%), a third Hispanic (31%) and 17% Black or another race/ethnicity. Thirty-one percent were clinicians and 69% health educators. Among clinicians, 15% were physicians (9% obstetrician-gynecologists) and 85% advanced practice clinicians (nurse practitioners, nurse midwives, or physician assistants) or nurses. All obstetrician-gynecologists reported that they inserted IUDs during medical training. Overall, 31% of clinicians reported they had not inserted IUDs during training, 51% had inserted up to 20, and 17% over 20.
Table 1.
Sociodemographic characteristics of providers in a cluster randomized trial of an IUD and implant training, by arm (n=576)
| Intervention (n=314) |
Control (n=262) |
Total (n=576) |
|
|---|---|---|---|
| Age, mean years ± SD | 34.2 ± 11.2 | 35.6 ± 11.7 | 34.8 ± 11.4 |
| Sex, n (%) | |||
| Female | 308 (98) | 248 (95) | 556 (97) |
| Male | 6 (2) | 14 (5) | 20 (3) |
| Race/ethnicity, n (%) | |||
| White | 165 (53) | 133 (51) | 298 (52) |
| Hispanic | 88 (28) | 88 (34) | 176 (31) |
| Black or other | 58 (19) | 40 (15) | 98 (17) |
| Position, n (%) | |||
| Clinician | 106 (34) | 73 (28) | 179 (31) |
| Health educator | 208 (66) | 189 (72) | 397 (69) |
| Clinic practice, n (%) | |||
| Abortion | 142 (45) | 141 (54) | 283 (49) |
| Family planning | 172 (55) | 121 (46) | 293 (51) |
| Clinic region, n (%) | |||
| West | 236 (75) | 159 (61) | 395 (69) |
| Other regions | 78 (25) | 103 (39) | 181 (31) |
| Surveys completed, n (%) | |||
| Both | 153 (49) | 116 (44) | 269 (47) |
| Baseline only | 99 (32) | 33 (13) | 132 (23) |
| One year only | 62 (20) | 113 (43) | 175 (30) |
At baseline, there were no significant differences in IUD and implant attitudes, knowledge or practices by arm (Table 2). Most respondents reported that their patients would be receptive to learning about IUDs (92%) and the implant (83%), and that they had sufficient time to counsel on contraceptive options (80%). Many staff considered the implant (64%) and the IUD (37%) underused by their patients compared to the injectable (9%) and oral contraception (8%; data not shown). Knowledge of patient eligibility for IUDs and implants varied across items: only 67% was willing to offer a levonorgestrel-releasing IUD (LNG-IUS) to a patient with obesity, diabetes (58%), hypertension (61%), or a smoker (64%), and 56–64% were willing to offer an implant for patients with these conditions. There was somewhat higher awareness of the suitability of a copper IUD with these conditions, with 68–82% willing to offer this method. Follow-up data in Table 2 showed increases over time in the measures, and the intraclass correlation coefficients, showing the correlation between providers within clinic site, ranged from 0.06 to 0.16.
Table 2.
Provider attitudes, knowledge, and practices, by arm in a cluster randomized trial of an IUD and implant training
| Baseline (n=401) | Follow-up at one year (n=444) | |||||
|---|---|---|---|---|---|---|
| Intervention (n=252) |
Control (n=149) |
Intraclass correlation coefficients |
Intervention (n=215) |
Control (n=229) |
Intraclass correlation coefficients |
|
| Attitudes | ||||||
| IUDs are safe | 149 (60) | 96 (66) | 0.09 | 154 (76) | 142 (66) | 0.06 |
| Implant is safe | 141 (57) | 91 (61) | 0.10 | 164 (77) | 146 (65) | 0.08 |
| Experienced to counsel on IUDs | 129 (53) | 89 (60) | 0.10 | 139 (67) | 138 (60) | 0.05 |
| Experienced to counsel on implant | 99 (41) | 70 (48) | 0.06 | 130 (62) | 114 (50) | 0.09 |
| Knowledge scales (range 0–1) | ||||||
| IUD eligibility, mean ±SD | 0.77 ±0.25 | 0.78 ±0.24 | 0.22 | 0.86 ±0.20 | 0.80 ±0.23 | 0.11 |
| IUD/implant eligibility with medical conditions, mean ±SD | 0.65 ±0.38 | 0.67 ±0.35 | 0.13 | 0.79 ±0.29 | 0.66 ±0.36 | 0.16 |
| Practices | ||||||
| Routinely discusses LARC | 178 (71) | 112 (76) | 0.05 | 188 (87) | 183 (82) | <0.001 |
| Routinely discusses IUDs | 177 (71) | 112 (76) | 0.05 | 188 (87) | 184 (81) | 0.01 |
| Routinely discusses implant | 139 (56) | 86 (58) | 0.11 | 170 (79) | 157 (70) | 0.10 |
Notes: Numbers are n (%) unless otherwise noted
SD = standard deviation
Analyses of the intervention effect showed increases in intervention compared to control in the outcomes. At intervention sites, the proportion of providers who strongly agreed that IUDs were safe increased from 60% at baseline to 76% at follow-up, and in control sites, it remained at 66% at baseline and follow-up (Table 2). The intervention effect was highly significant (aOR=2.48 [1.13 – 5.44]) (Table 3). Providers at intervention sites also showed a similar increase in belief that the implant is safe (56% to 77% in intervention and 61% to 65% in control), with a significant intervention effect aOR=2.57 [1.44– 4.59]). There was a significant intervention effect in the change in the proportion of providers who strongly agreed they were experienced to counsel on IUDs (intervention effect aOR=1.89 [1.04–3.44]), as well as implants (intervention effect aOR=2.30 [1.28–4.12]). Results also showed significant increases in provider knowledge scores in intervention compared to control. The largest changes in providers’ knowledge were that teens, and women with a history of STI or PID could use an IUD. Overall knowledge of patient IUD eligibility increased at intervention from 0.77 to .86, and the change from the intervention training was 6% more in intervention compared to control (p=0.037). Scores on knowledge of eligibility for IUDs and implants for women with common medical conditions increased at intervention sites from a mean of 0.66 to 0.79. In a multivariable model, the change from the intervention training was 15% more compared to control (p<0.001). The largest changes in providers’ knowledge for this scale occurred for the implant.
Table 3.
Changes in provider IUD and implant attitudes, knowledge and practices, with intervention effect estimates from a cluster randomized trial (n=576)
| Intervention effect¥ | ||
|---|---|---|
| Attitudes | Adjusted OR | 95% CI |
| IUDs are safe | 2.48** | 1.13 – 5.44 |
| Implant is safe | 2.57*** | 1.44 – 4.59 |
| Experienced to counsel on IUDs | 1.89* | 1.04 – 3.44 |
| Experienced to counsel on implant | 2.30** | 1.28 – 4.12 |
| Knowledge scales | Adjusted coefficient | 95% CI |
| IUD eligibility | 0.058* | 0.003 – 0.113 |
| IUD/implant eligibility with medical conditions | 0.15*** | 0.09 – 0.21 |
| Practices | Adjusted OR | 95% CI |
| Routinely discusses LARC | 1.97* | 1.02 – 3.81 |
| Routinely discusses IUDs | 2.26* | 1.16 – 4.41 |
| Routinely discusses implant | 1.89 | 0.93 – 3.86 |
Notes: Models adjust for age, race/ethnicity, provider type, practice setting, and region.
OR = odds ratio; CI = confidence interval;
p≤0.05
p≤0.01
p≤0.001
Intervention effect is modeled as the interaction between the intervention and time. Statistically significant interaction effects indicate greater change in the outcome in the intervention than control group from baseline to follow-up (difference in differences).
While the practice of routinely discussing LARC increased in both study arms from baseline to follow-up (Table 2), there was a significantly greater effect in the intervention arm compared to control (intervention effect aOR=1.97 [1.02–3.81]) (Table 3). For routine discussion of IUDs, there was a significant increase in intervention compared to control (intervention effect aOR=2.26 [1.16 – 4.41]). Routine discussion of impls also increased in intervention compared to control, but the intervention effect did not reach statistical significance (aOR=1.89 [0.93 – 3.86]). For comparison, the proportion of providers reporting routine discussion of condoms (91% control, 87% intervention), oral contraceptive pills (95%, 94%), and the injectable (77%, 84%) at baseline had no differences by study arm and did not change significantly over time. Model results showed the intervention effect to be insignificant for condoms (p=0.125), oral contraceptives (p=0.624) and the injectable (p=0.411). Providers’ perception of whether they had sufficient time to counsel did not change over time, with no difference in the intervention arm. Sensitivity analyses for the longitudinal cohort of providers who were present at both baseline and follow-up showed consistent results.
Comment
This trial shows that a scalable intervention, a clinic training, can change provider attitudes, knowledge and counseling practices related to IUDs and implants. Importantly, data from a year after the training show that its impact endured over time and despite staff turnover. The study was conducted in real-world clinic environments, where frequent staff turnover can be a challenge for clinical practice improvements. Over the study year, almost one-third of staff turned over. Clinical practice change, however, did take hold. Results suggest that intervention clinic staff shifted toward greater inclusion of LARC methods in routine contraceptive care, which may have required changes in clinic systems such as scheduling, flow, and protocols. Integration of IUDs and implants into contraceptive counseling did not reduce the discussion of other methods, nor affect the perceived sufficiency of time available for contraceptive counseling.
To ensure equitable access, efforts must go beyond provider training to address the high costs of IUDs and implants, particularly for uninsured and post-abortion women.17,19 Interviews with providers have shown that when they are aware of patients’ health insurance coverage, they sometimes feel that discussing options a patient cannot afford is unethical.14 This study did not provide free IUDs or implants. Rather, it relied on the existing patchwork of contraceptive coverage in the 15 states where study sites were located. The range in state policies existing during the study allowed us to measure the intervention and demonstrate its impact over varied policy contexts. However, together with the intervention, Medicaid family planning expansion programs and state mandates for private insurance to cover contraception increase access even further.17,19
Results showed there were also improvements in IUD and implant knowledge, attitudes, and practices at control sites. During the study period, professional organizations introduced clinical bulletins supporting a broader range of IUD and implant candidates, and the CHOICE Project findings from St. Louis were in the national news.22–25 These initiatives likely contributed to improvements that we also observed at control sites, underscoring the utility of randomized designs to test the impact of an intervention and to account for temporal trends.
This study has limitations. It was conducted in Planned Parenthood health centers, and findings may not be generalizable to all settings providing contraceptive care. However, practices at these health centers can have a large impact, given that they provide contraceptive care for 2 million US women annually. Potential contamination could have occurred if staff left a clinic and started to work at another clinic site in the study during the year, although if it had occurred, it would serve to decrease any differences detected between intervention and control sites. Routine counseling on IUDs and the implant at study sites was already relatively high in both arms at baseline compared to other contraceptive providers.8,11 While knowledge retention was high at one year post-training, there may be a need for supplementary training in subsequent years.
This study also has strengths. It is the first with a randomized design to provide evidence that a training intervention helped providers to effectively integrate IUDs and implants into contraceptive care. The cluster design is ideal to measure the impact of a provider educational intervention because it addresses the potential for contamination were providers or patients randomized within sites. The trial was reviewed scientifically and evaluated as high-quality.26 The intervention was effective for all members of the healthcare team, including physicians, advance practice clinicians and health educators. The intervention was also effective across a range of contraceptive policy contexts, and it is replicable, with each training reaching a large patient population, thereby having a widespread impact.
This training improved knowledge and skills for contraceptive methods that a growing share of US women use, IUDs and implants, but are not yet universally accessible.27 Ensuring that providers have up-to-date and evidence-based information about all methods, including IUDs and implants, supports informed decision making and patient-centered contraceptive care.
Implications and Contributions.
To increase provider IUD and implant knowledge and skills, and to help to integrate LARC methods into clinical practice
The intervention successfully changed provider knowledge, attitudes and counseling on LARC methods
This randomized study showed that a CME-accredited LARC training course was effective in increasing provider competency in LARC methods, and that these improvements were sustained in the clinic a year after the half-day educational session, even in real world clinic conditions with high staff turnover.
Acknowledgments
We are grateful to Maya Blum and Rosalyn Schroeder for data management; to Dr. Philip Darney, Dr. Joe Speidel, Dr. Charles McCulloch and Dr. Tina Raine-Bennett for study design and clinical expertise. We would like to thank and Planned Parenthood investigators and research coordinators at these affiliates: Central and Greater Northern New Jersey; Columbia Willamette; Great Northwest; Greater Ohio; Greater Washington and North Idaho; Mar Monte; Mid & South Michigan; Minnesota, North Dakota, South Dakota; Mount Baker; Northern California; Pacific Southwest; Pasadena and San Gabriel Valley; Rocky Mountains; South Atlantic; Southeastern Pennsylvania; Southern New England; and Southwest and Central Florida.
Funding: Supported by grants from the William and Flora Hewlett Foundation, The JPB Foundationand the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD HD052163). Bayer and Teva Pharmaceuticals provided educational samples of intrauterine devices for the training. The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of interests: The authors report no conflict of interest.
Clinical trial registration: ClinicalTrials.gov NCT01360216
Paper presentation: Preliminary findings were presented at the annual meeting of the Association of Reproductive Health Professionals in Denver, CO (September 19–21, 2013).
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent views of Planned Parenthood Federation of America, Inc.
CA, CO, CT, FL, HI, ID, MI, MN, NC, NJ, NM, OH, OR, PA, WA
References
- 1.Dehlendorf C, Diedrich J, Drey E, Postone A, Steinauer J. Preferences for decision-making about contraception and general health care among reproductive age women at an abortion clinic. Patient Educ Couns. 2010;81:343–8. doi: 10.1016/j.pec.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnelly KZ, Foster TC, Thompson R. What matters most? The content and concordance of patients' and providers' information priorities for contraceptive decision making. Contraception. 2014;90:280–7. doi: 10.1016/j.contraception.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Dehlendorf C, Krajewski C, Borrero S. Contraceptive counseling: best practices to ensure quality communication and enable effective contraceptive use. Clin Obstet Gynecol. 2014;57:659–73. doi: 10.1097/GRF.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanwood NL, Garrett JM, Konrad TR. Obstetrician-gynecologists and the intrauterine device: a survey of attitudes and practice. Obstet Gynecol. 2002;99:275–80. doi: 10.1016/s0029-7844(01)01726-4. [DOI] [PubMed] [Google Scholar]
- 5.Harper CC, Blum M, de Bocanegra HT, et al. Challenges in translating evidence to practice: The provision of intrauterine contraception. Obstet Gynecol. 2008;111:1359–69. doi: 10.1097/AOG.0b013e318173fd83. [DOI] [PubMed] [Google Scholar]
- 6.Harper CC, Henderson JT, Raine TR, et al. Evidence-based IUD practice: family physicians and obstretrician-gynecologists. Fam Med. 2012;44:637–45. [PMC free article] [PubMed] [Google Scholar]
- 7.Tyler CP, Whiteman MK, Zapata LB, Curtis KM, Hillis SD, Marchbanks PA. Health care provider attitudes and practices related to intrauterine devices for nulliparous women. Obstet Gynecol. 2012;119:762–71. doi: 10.1097/AOG.0b013e31824aca39. [DOI] [PubMed] [Google Scholar]
- 8.Harper CC, Stratton L, Raine TR, et al. Counseling and provision of long-acting reversible contraception in the US: National survey of nurse practitioners. Prev Med. 2013;57:883–8. doi: 10.1016/j.ypmed.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biggs A, Malvin J, Harper CC, Brindis C. Factors influencing the provision of long-acting reversible contraception in California. Obstet Gynecol. 2014;123:593–601. doi: 10.1097/AOG.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 10.Luchowski AT, Anderson BL, Power ML, Raglan GB, Espey E, Schulkin J. Obstetrician-gynecologists and contraception: practice and opinions about the use of IUDs in nulliparous women, adolescents and other patient populations. Contraception. 2014b;89:572–7. doi: 10.1016/j.contraception.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Thompson KM, Stern L, Gelt M, Speidel JJ, Harper CC. Counseling for IUDs and implants: are health educators and clinicians on the same page? Perspect Sex Reprod Health. 2013;45:191–5. doi: 10.1363/4519113. [DOI] [PubMed] [Google Scholar]
- 12.Luchowski AT, Anderson BL, Power ML, Raglan GB, Espey E, Schulkin J. Obstetrician-gynecologists and contraception: long-acting reversible contraception practices and education. Contraception. 2014a;89:578–83. doi: 10.1016/j.contraception.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Thompson KMJ, Speidel JJ, Saporta V, Waxman NJ, Harper CC. Contraceptive policies affect post-abortion provision of long-acting reversible contraception. Contraception. 2011;83:41–7. doi: 10.1016/j.contraception.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Morse J, Freedman L, Speidel JJ, Thompson KMJ, Stratton L, Harper CC. Post-abortion contraception: Qualitative interviews on counseling and provision of long-acting reversible contraceptive methods. Perspect Sex Reprod Health. 2012;44:100–6. doi: 10.1363/4410012. [DOI] [PubMed] [Google Scholar]
- 15.Biggs A, Harper CC, Brindis C. California family planning health care providers' challenges to same-day long-acting reversible contraception provision. Obstet Gynecol. 2015;126:338–345. doi: 10.1097/AOG.0000000000000969. [DOI] [PubMed] [Google Scholar]
- 16.Harper CC, Rocca CH, Thompson KM, et al. Reductions in pregnancy rates in the USA with long-acting reversible contraception: a cluster randomised trial. Lancet. 2015;386:562–8. doi: 10.1016/S0140-6736(14)62460-0. [DOI] [PubMed] [Google Scholar]
- 17.Thompson KM, Rocca CH, Kohn JE, et al. Public funding for contraception, provider training, and use of highly effective contraceptives: a cluster randomized trial. AJPH. 2016;106:541–6. doi: 10.2105/AJPH.2015.303001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steiner MJ, Trussell J, Johnson S. Communicating contraceptive effectiveness: an updated counseling chart. Am J Obstet Gynecol. 2007;197:118. doi: 10.1016/j.ajog.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 19.Rocca CH, Thompson KM, Goodman S, Westhoff CL, Harper CC. Funding policies and postabortion long-acting reversible contraception: results from a cluster randomized trial. Am J Obstet Gynecol. 2016;214:716.e1–8. doi: 10.1016/j.ajog.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR. 2016;65:1–104. doi: 10.15585/mmwr.rr6503a1. [DOI] [PubMed] [Google Scholar]
- 21.Hayes RJ, Moulton LH. Cluster Randomised Trials. Chapman & Hall/CRC; Boca Raton, FL: 2009. [Google Scholar]
- 22.World Health Organization (WHO) Department of Reproductive Health and Research and Johns Hopkins Bloomberg School of Public Health/Center for Communication Programs (CCP) Family Planning: A global handbook for providers. Baltimore and Geneva: CCP and WHO; 2007. [Google Scholar]
- 23.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol. 2011;118:184–96. doi: 10.1097/AOG.0b013e318227f05e. [DOI] [PubMed] [Google Scholar]
- 24.Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group, The American College of Obstetricians and Gynecologists. Committee opinion no. 539: Adolescents and long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol. 2012;120:983–8. doi: 10.1097/AOG.0b013e3182723b7d. [DOI] [PubMed] [Google Scholar]
- 25.Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366:1998–2007. doi: 10.1056/NEJMoa1110855. [DOI] [PubMed] [Google Scholar]
- 26.Lopez LM, Grey TW, Tolley EE, Chen M. Brief educational strategies for improving contraception use in young people. Cochrane Database Syst Rev. 2016;3:CD012025. doi: 10.1002/14651858.CD012025.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among U.S. women, 2009–2012. Obstet Gynecol. 2015;126:917–27. doi: 10.1097/AOG.0000000000001094. [DOI] [PMC free article] [PubMed] [Google Scholar]