Abstract
Rationale
Restoration of coronary artery blood flow is the most effective means of ameliorating myocardial damage triggered by ischemic heart disease. However, coronary reperfusion elicits an increment of additional injury to the myocardium. Accumulating evidence indicates that the unfolded protein response (UPR) in cardiomyocytes is activated by ischemia/reperfusion (I/R) injury. Xbp1s, the most highly conserved branch of the UPR, is protective in response to cardiac I/R injury. GRP78, a master regulator of the UPR and an Xbp1s target, is up-regulated after I/R. However, its role in the protective response of Xbp1s during I/R remains largely undefined.
Objective
To elucidate the role of GRP78 in the cardiomyocyte response to I/R using both in vitro and in vivo approaches.
Methods and Results
Simulated I/R (sI/R) injury to cultured neonatal rat ventricular myocytes (NRVM) induced apoptotic cell death and strong induction of the UPR and GRP78. Over-expression of GRP78 in NRVM significantly protected myocytes from I/R-induced cell death. Furthermore, cardiomyocyte-specific over-expression of GRP78 ameliorated I/R damage to the heart in vivo. Exploration of underlying mechanisms revealed that GRP78 mitigates cellular damage by suppressing the accumulation of reactive oxygen species. We go on to show that the GR78-mediated cytoprotective response involves plasma membrane translocation of GRP78 and interaction with PI3 kinase, culminating in stimulation of Akt. This response is required as inhibition of the Akt pathway significantly blunted the anti-oxidant activity and cardioprotective effects of GRP78.
Conclusions
I/R induction of GRP78 in cardiomyocytes stimulates Akt signaling and protects against oxidative stress, which together protect cells from I/R damage.
Keywords: GRP78, UPR, ischemia/reperfusion injury, heart disease, Akt, reactive oxygen species
Subject Terms: Ischemia
INTRODUCTION
Myocardial infarction is a leading cause of death worldwide accounting for tremendous burden to individuals and society.1 Partial or complete occlusion of a coronary artery deprives the downstream tissue of oxygen and nutrients, and restoration of blood supply aborts the ischemic insult. At the same time, resumption of oxygen delivery triggers a second wave of insult, termed reperfusion injury.2–5 Therapies targeting ischemic heart disease focus exclusively on the ischemic phase of ischemia/reperfusion (I/R) injury owing to a decades-long inability to translate to the human case clinically meaningful insights derived from animal models. Thus, there is great interest in defining mechanisms of reperfusion injury in hopes of mitigating its deleterious effects.
Multiple events participate in the pathogenesis of I/R, including accumulation of reactive oxygen species (ROS), inflammation, perturbation of calcium handling, and metabolic derangements.6–10 Most, if not all, of these processes are potent inducers of the unfolded protein response (UPR), a cellular mechanism evolved to cope with protein-folding stress.11–14 Indeed, accumulating evidence has shown that the UPR is activated in myocardium by I/R.15–19
The UPR comprises three discrete signaling branches: PERK, IRE1/Xbp1s and ATF6. These signaling cascades, in turn, are maintained at quiescent, basal levels by the 78 kDa glucose-regulated protein (GRP78), a master ER-resident protein chaperone.20 GRP78 interacts with the luminal domains of these three transmembrane molecules and retains them on the ER membrane surface. However, when misfolded proteins accumulate, GRP78 preferentially interacts with hydrophobic patches on the ER-resident misfolded proteins, thereby releasing the three signaling arms of the UPR. Activation of the UPR leads to transient suppression of protein translation, elevation of ER protein-folding chaperones, and up-regulation of ER-associated protein degradation. However, if the protein-folding stress is severe or persistent, apoptotic cell death is triggered for the benefit of the whole organism.
Our earlier studies have demonstrated that activation of the IRE1/Xbp1s pathway in I/R is cardioprotective in part through the activation of the hexosamine biosynthetic pathway (HBP) and resulting increases in O-GlcNAc protein modification.21 Whether other downstream targets of Xbp1s also participate in myocardial protection from I/R damage is unknown. Here, we set out to investigate whether and how cardiomyocyte GRP78, an Xbp1s target, is protective of I/R stress.
METHODS
The data, methods, and study materials are available on request by contacting the corresponding authors. An expanded methods section is provided in the Online Data Supplement.
Animals
All mice were of C57BL/6 background. They were maintained on a 12 hour dark/light cycle (6 AM to 6 PM) and housed in groups of ≤ 5 with unlimited access to water and chow (2916, Teklad). The Institutional Animal Care and the Use Committee of University of Texas Southwestern Medical Center approved all animal experiments. All PCR primers are provided in Online Table I.
RESULTS
I/R injury induces GRP78
We have shown previously that I/R injury to the myocardium is a potent inducer of the UPR.22 Xbp1s, the most highly conserved branch of the UPR is strongly augmented.22 As GRP78 is a bona fide target of Xbp1s,15, 21, 23–26 we set out to determine whether GRP78 is stimulated in I/R-stressed cardiomyocytes.
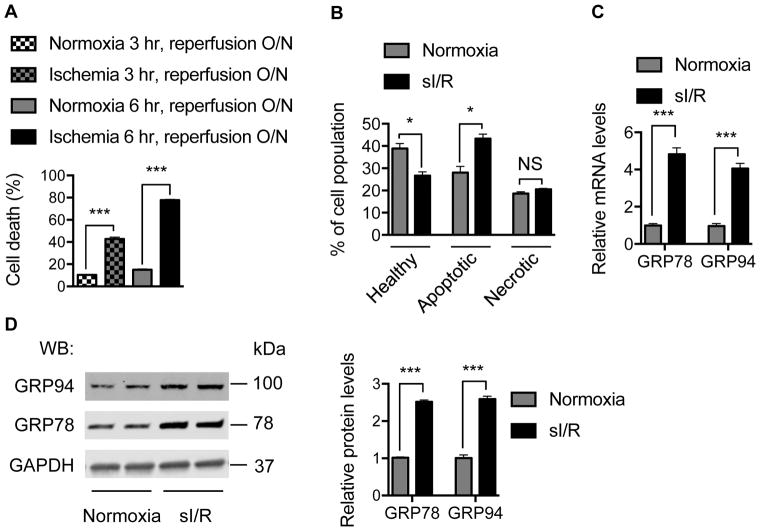
To do this, we subjected NRVM to sI/R by replacing the culture medium with Esumi ischemic buffer and incubating the cells in a 95% N2/5% CO2 hypoxia chamber (Online Figure IA).27, 28 After 3–6 hours, normal culture medium was restored. First, we confirmed that sI/R elicited time-dependent cell death as assessed by LDH release (Figure 1A). To determine the mode of cell death, we performed annexin V-FITC and propidium iodide (PI) double staining followed by flow cytometry (Online Figure IB). Consistent with the LDH release findings, sI/R triggered a significant increase in cell death, which was mainly accounted for by annexin V-positive apoptotic cells (Figure 1B). By contrast, the pool of cells harboring markers of necrosis was not affected by sI/R.
Figure 1. I/R injury induces GRP78.
A. sI/R elicited significant cell death. NRVM were treated for either 3 hours or 6 hours of ischemia before reperfusion. Lactate dehydrogenase (LDH) was determined in culture medium and in cell lysates. Relative cell death was calculated based on the ratio of released LDH into the medium. N = 6 per group.
B. Cell death by sI/R was determined using flow cytometry to detect annexin V- and propidium iodide (PI)-positive cells. Significant increases in Annexin V-positive apoptotic cells were observed, which accounted for the preponderance of cell death. N = 3 for each group.
C. sI/R led to strong induction of ER resident chaperones at mRNA levels. NRVM were subjected to ischemia for 6 hours, followed by overnight reperfusion. Real-time PCR was conducted to measure mRNA levels with 18s as an internal control. N = 3 per group.
D. sI/R induced expression of the ER chaperones at protein levels. Immunoblotting showed that GRP78 and GRP94 were significantly increased at protein levels (left). GAPDH was used as a loading control. Quantification is shown at right. N = 3. NS; not significant; *, p < 0.05; ***, p < 0.001.
Simulated I/R led to robust induction of GRP78 at both mRNA and protein levels (Figure 1C and 1D), consistent with previous findings.29 Expression of GRP94, another ER-resident chaperone, was also strongly increased. These results confirm that sI/R in cardiomyocytes leads to potent activation of the UPR, as evidenced by induction of the ER chaperones GRP78 and GRP94.
Over-expression of GRP78 protects cardiomyocytes from I/R-induced cell death
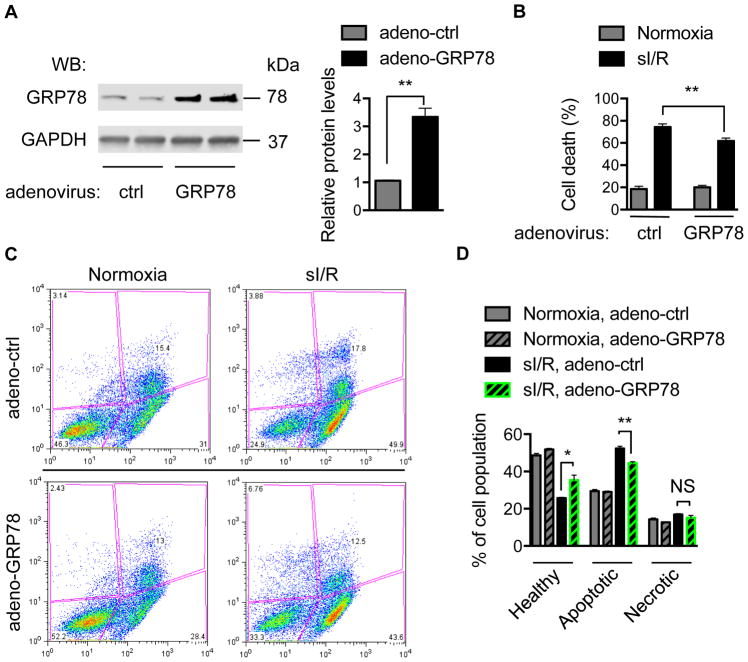
To define the downstream effects of I/R-triggered expression of GRP78, we initially pursued a gain-of-function approach. First, we over-expressed GRP78 in NRVM by adenoviral infection30 (Figure 2A) and then subjected the cells to sI/R. Under these conditions, over-expression of GRP78 induced modest but reproducible and statistically significant decreases in cell death as assayed by LDH release (Figure 2B). Additionally, flow cytometry analysis corroborated that GRP78 over-expression protected NRVM from sI/R-induced cell death, which was largely a result of decreased abundance of annexin V-positive apoptotic cells (Figure 2C and 2D). Together, these in vitro results demonstrate that GRP78 over-expression is sufficient to protect cardiomyocytes from I/R injury and suggest that a component of the protection afforded by UPR induction during I/R derives from increases in GRP78.
Figure 2. Over-expression of GRP78 protects cardiomyocytes from I/R-induced cell death.
A. Adenoviral over-expression increased GRP78 protein levels. A representative immunoblot is depicted on the left, and quantitation with GAPDH as loading control is shown on the right. N = 3.
B. Over-expression of GRP78 blunts I/R-induced cell death. After adenoviral infection, NRVM were subjected to ischemia for 6 hours followed by overnight reperfusion. LDH levels were measured to determine relative cell death. N = 7 per group.
C. Over-expression of GRP78 conferred significant protection against I/R-induced cell death as determined by flow cytometry for Annexin V- and PI-positive cells. A representative histogram is shown.
D. Quantification of data depicted in panel C revealed that over-expression of GRP78 protected NRVM from I/R injury. N = 3 per group. NS, not significant; *, p < 0.05; **, p < 0.01.
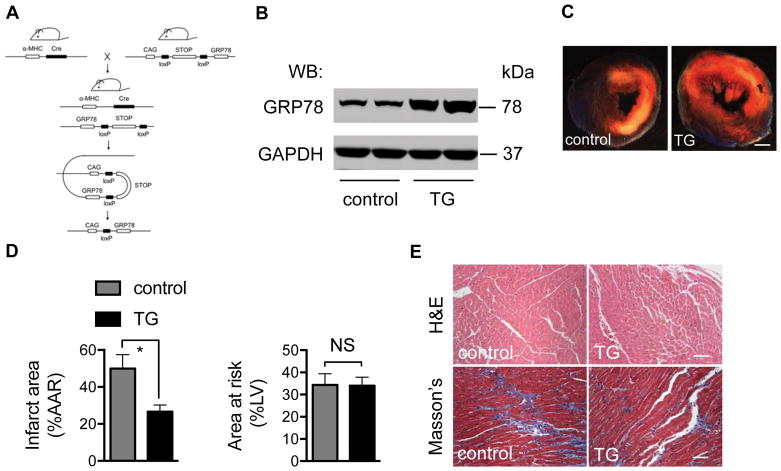
To test more directly the cardioprotective role of GRP78 in I/R injury, we turned to an in vivo mouse model. We engineered mice with cardiomyocyte-specific over-expression of GRP78. To do this, we generated a transgenic mouse model in which GRP78 is expressed under the control of a universal CAG (CMV, β-actin and β-globin) promoter (Figure 3A). A “floxed” transcriptional/translational “stop” cassette31 was inserted between the CAG promoter and GRP78 cDNA, and the animals were crossed with mice harboring a cardiomyocyte-specific Cre recombinase transgene (αMHC-Cre). First, we confirmed in the double transgenic mice that Cre-mediated excision of the stop region triggered expression of GRP78 in cardiomyocytes (Figure 3B). Prior studies have shown that over-expression in liver of GRP78, a master regulator of the UPR, leads to decreases in UPR downstream targets, such as Xbp1s.32 Consistent with this, we found that over-expression of GRP78 induced a trend toward decreases in the level of transcripts coding for multiple UPR markers (Online Figure IIA), but significant differences were not seen in UPR marker protein (Online Figure IIB). Furthermore, control and transgenic mice manifested similar body weight (Online Figure IIC), heart rate (Online Figure IID), ventricular size and contractile function (Online Figure IIE), and myocardial tissue histology (Online Figure IIF) at baseline.
Figure 3. Over-expression of GRP78 protects heart from I/R injury.
A. Strategy of cardiomyocyte-restricted over-expression of GRP78. A mouse model harboring a CAG-loxP-STOP-loxP-GRP78 transgene was engineered and bred to a cardiomyocyte-specific Cre mouse line. In the double transgenic mouse, Cre recombines and cleaves the STOP region, which leads to GRP78 expression exclusively in cardiomyocytes.
B. GRP78 expression is increased in the transgenic hearts. Protein lysates were extracted in control or GRP78-expressing transgenic (TG) hearts and Western blotting was performed to detect GRP78 protein levels. GAPDH was used as a loading control.
C. Over-expression of GRP78 in cardiomyocytes protected hearts from I/R damage. Control and TG mice 12–14 weeks of age were subjected to myocardial ischemia for 45 minutes, followed by overnight reperfusion. TTC staining was conducted to detect, localize, and quantify the injured tissue. White region depicts ischemic infarct zone; pink/red area is the border zone; blue region represents the remote zone. Scale bar: 1mm.
D. The relative ratios of infarct region (IF) to area at risk (AAR, ischemic and border) were compared between control and TG hearts. The relative ratios of AAR to left ventricle did not differ between control and TG groups, suggesting the I/R surgery was performed similarly across genotypes. N = 5 per group.
E. TG mice manifested a decrease in fibrosis after I/R. Hearts from control and TG mice were harvested 21 days after I/R. Trichrome C staining was conducted to compare fibrosis between the two groups. Scale bar: 100 μm. NS, not significant; *, p < 0.05.
Double transgenic, GRP78-expressing mice 12–14 weeks of age were subjected to 45 minutes of myocardial ischemia followed by reperfusion for 24 hours. Single transgenic mice (either αMHC-Cre or CAG-STOP-GRP78) were used throughout as controls. Histological staining with 2,3,5-triphenyltetrazolium chloride (TTC) revealed that cardiomyocyte over-expression of GRP78 significantly protected GRP78-over-expressing hearts from I/R damage as compared with single transgene controls (Figure 3C). Quantitative analysis of the TTC staining revealed a significant decrease in the volume of the infarct, even though the extent of ischemic insult was similar, as evidenced by similar areas at risk (Figure 3D). Consistent with less infarction in the double transgenic hearts, I/R-elicited fibrosis was reduced by GRP78 over-expression (Figure 3E). Taken together, these findings of robust protection against I/R injury elicited by GRP78 over-expression under both in vitro and in vivo conditions suggest that important aspects of UPR-Xbp1s-driven cardioprotection are mediated by GRP78.
GRP78 blunts accumulation of reactive oxygen species
Accumulation of reactive oxygen species (ROS) is a central mechanism of I/R injury in heart.33–35 Oxygen free radicals and other reactive species modify proteins by post-translational modifications, including carbonylation. This event is a prevalent post-translational modification occurring on lysine, proline or threonine residues and culminating in effects on target protein localization, stability, and function.36
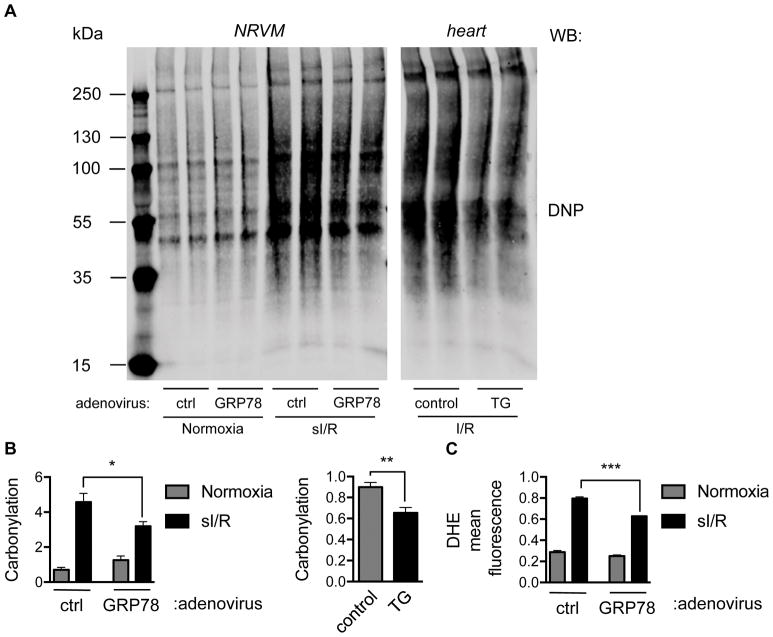
To determine whether GRP78 expression modulates ROS production, we first assayed protein carbonyl modifications. We infected NRVM with either control or GRP78 adenovirus and then subjected the cells to sI/R. After protein isolation, the lysates were incubated with 2.4-dinitrophenylhydrazine (DNPH), which is covalently conjugated to carbonyl groups, followed by immunoblotting using DNP-specific antibodies. As expected, sI/R in NRVM strongly induced protein carbonylation (Figure 4A and 4B). Furthermore, over-expression of GRP78 significantly ameliorated this modification, suggesting that GRP78 expression in NRVM suppresses ROS toxicity. Whereas, cardiomyocyte-specific over-expression of GRP78 did not alter carbonylation levels at baseline (Online Figure IIG), tissue lysates from control animals subjected to I/R manifested robust protein carbonylation, which was significantly reduced in GRP78 cardiomyocyte-specific transgenic mice (Figure 4A, 4B, and Online Figure IIH).
Figure 4. Over-expression of GRP78 ameliorates ROS production by I/R.
A. Over-expression of GRP78 in vitro and in vivo led to decreases in I/R-induced ROS levels. Protein lysates from NRVM or cardiac tissues were incubated with DNPH, which interacts with carbonyl groups in proteins. Immunoblotting was then conducted to detect carbonylation using anti-DNP antibodies. sI/R increased carbonyl modifications in NRVM, which was significantly reduced by GRP78 over-expression. Likewise, over-expression of GRP78 in heart suppressed I/R-induced protein carbonylation.
B. Quantification of data depicted in panel A revealed significant decreases in protein carbonylation triggered by GRP78 expression in NRVM or hearts. N = 4 per group.
C. Flow cytometry for dihydroethidium, a superoxide indicator, showed that over-expression of GRP78 led to strong reduction of I/R-elicited ROS levels. N = 3 for each group. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To confirm that the increase in protein carbonylation was indicative of increased ROS levels, we quantified those levels using dihydroethidium (DHE), a dye that emits blue fluorescence in the cytosol and which stains the nucleus red when oxidized. Using flow cytometry, we observed that sI/R elicited a significant increase in the mean red fluorescence signal which was reduced by GRP78 over-expression (Figure 4C).
Multiple intracellular pathways contribute to the production of ROS, and we set out to dissect the sources of ROS regulated by GRP78. Diphenyleneiodonium (DPI) and MitoTEMPO were used to suppress ROS production by NADPH oxidase and mitochondria, respectively. The increases in DHE fluorescence triggered by sI/R were significantly reduced individually by DPI, by MitoTEMPO, and by GRP78 over-expression (Online Figure II-I). In contrast to MitoTEMPO, however, the combination of GRP78 adenovirus and DPI did not yield an additive effect, suggesting that ROS regulation by GRP78 is largely mediated by NADPH oxidase (Online Figure II-I). In aggregate, these data indicate that I/R potently induces cardiomyocyte ROS levels and consequent protein oxidation, both of which are diminished by GRP78 over-expression.
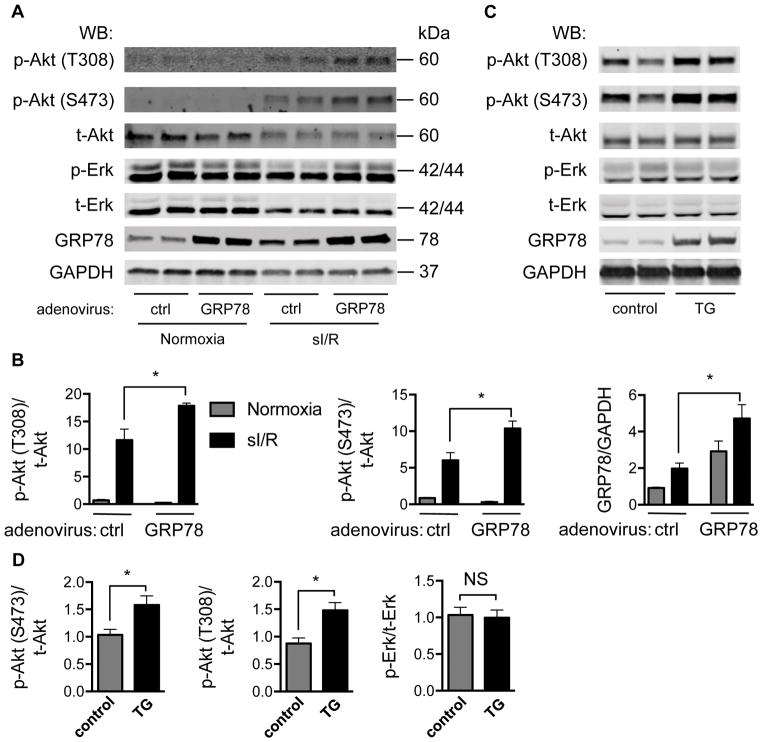
GRP78 expression stimulates Akt signaling
Akt, also named protein kinase B, is a signaling nexus governing cell survival, cell proliferation, and cell growth37, and is potently activated by I/R.38 Numerous studies have shown that Akt plays pivotal roles in protecting cardiac myocytes from I/R injury.38–40 To test whether Akt participates in the cardioprotective effects of GRP78, we first examined the relationship between increased GRP78 levels and Akt activation. Under basal conditions, over-expression of GRP78 in NRVM did not significantly alter the activation of Akt as determined indirectly by Akt phosphorylation at both Thr 308 and Ser 473 residues (Figure 5A and 5B). As expected, 6 hours of ischemia followed by 1 hour of reperfusion, however, stimulated Akt phosphorylation. Importantly, over-expression of GRP78 in the context of sI/R triggered yet further, statistically significant Akt activation (Figure 5A and 5B). Phosphorylation of Erk, on the other hand, showed no significant changes as a result of GRP78 over-expression.
Figure 5. Over-expression of GRP78 stimulates Akt signaling.
A. Akt phosphorylation was augmented by GRP78 over-expression. NRVM were infected with adenovirus expressing GFP or GRP78. After ischemia for 6 hours and reperfusion for 1 hour, NRVM were collected and protein lysates were prepared for immunoblotting. GAPDH was used as loading control.
B. Quantification revealed that GRP78 over-expression elicited significant increases in Akt phosphorylation at both Thr 308 and Ser 473 residues. However, Erk phosphorylation was not altered. N = 3 per group.
C. Over-expression of GRP78 in vivo in hearts stimulated Akt phosphorylation. Protein lysates from control or GRP78-expressing TG hearts were subjected to Western blotting. GAPDH was used as loading control.
D. Relative quantification showed that GRP78 over-expression in vivo significantly increased Akt phosphorylation. N = 3 for each group. NS, not significant; *, p < 0.05.
We have shown previously that augmentation of protein O-GlcNAcylation in the heart confers strong cardioprotection in response to I/R.16, 22 Here, no significant changes in O-GlcNAcylation were revealed by GRP78 over-expression (Online Figure IIIA), indicating that this post-translational modification may not participate in GRP78-mediated protection against reperfusion injury.
Similarly, over-expression of GRP78 in vivo was sufficient to activate Akt in GRP78-expressing transgenic hearts (Figure 5C and 5D). Again, no changes in Erk phosphorylation were observed. In aggregate, these data suggest that GRP78 over-expression leads to activation of Akt signaling in cardiomyocytes.
To unveil mechanisms whereby GRP78 stimulates Akt, we examined cell surface localization of GRP78. Previous studies in tumor cells have shown that GRP78 translocates to the cell surface membrane under stress conditions, and this specific localization promotes Akt stimulation.25, 41 To test for this in cardiomyocytes, we isolated cell membranes from cardiac tissues after I/R, and subjected them to sucrose gradient fractionation. We found that the plasma membrane could be readily separated from SR/ER membrane. More importantly, GRP78 was detected in the same fractions as plasma membrane (Online Figure IIIB).
To evaluate GRP78 cell surface localization further, we subjected NRVM to sI/R, followed by crosslinking with EZ-link Sulfo-NHS-LC-Biotin. After isolation of biotinylated surface proteins, immunoblotting was conducted to reveal GRP78.42 We found that GRP78 was localized to the cardiomyocyte surface under normoxic conditions, and this cell surface localization was significantly increased after sI/R (Online Figure IIIC).
Recent studies suggest that cell surface-localized GRP78 interacts with PI3K and activates its kinase activity, thereby generating PIP3 and stimulating Akt.43 Consistent with this, we observed that Flag-tagged GRP78 co-localized with p85, the regulatory subunit of PI3K (Online Figure IVA and IVB). Lee and colleagues have shown that an insertion mutant of GRP78 remains capable of cell surface translocation but binding with PI3K is disrupted.43 To evaluate this mechanism in NRVM, we engineered the insertion mutant species and compared its PI3K binding with that of wild-type GRP78. We found that colocalization of mutant GRP78 mutant with p85 was significantly diminished (Online Figure IVA and IVB), which correlated with impaired production of PIP3 (Online Figure IVC and IVD). Indeed, the biotinylation assay demonstrated that over-expression of wild-type GRP78 stimulated cell membrane association of p85 that was significantly decreased when mutant GRP78 was expressed (Online Figure IVE). To further evaluate for direct interaction between membrane GRP78 and p85, we eluted biotinylated cell membrane proteins and immunoprecipitated GRP78. We found the associated p85 was reduced in cells overexpressing mutant GRP78 (Online Figure IVF).
We next sought to determine the functional effects wild-type and mutant GRP78. Overexpression of GRP78 protected NRVM from sI/R-induced cell death as evidenced by LDH release assay, whereas mutant GRP78 was less protective (Online Figure VA through VC). At the cellular level, we found that the anti-apoptotic actions of GRP78 were significantly attenuated with the mutant (Online Figure VD and VE), correlating with less suppression of ROS accumulation (Online Figure VF). Collectively, these results indicate that GRP78, when upregulated, translocates to the cardiomyocyte plasma membrane, where it interacts with PI3K and stimulates Akt.
Akt signaling is required for GRP78-mediated cardioprotection against I/R
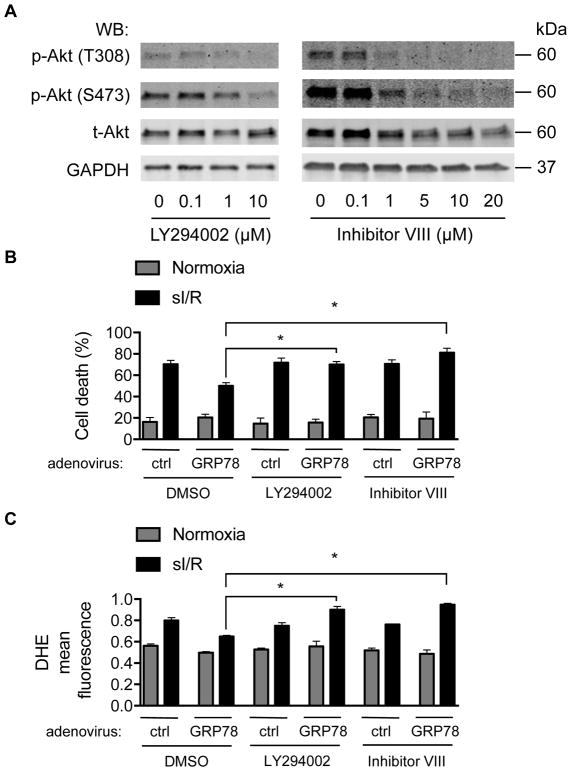
To determine whether GRP78-mediated Akt activation participates in the cardioprotective effects we have observed, we employed two structurally distinct small molecule inhibitors of Akt. LY294002 is a PI3K inhibitor commonly used to suppress the PI3K/Akt pathway.44 As expected, treatment of NRVM with LY294002 inhibited Akt phosphorylation in a dose-dependent manner (Figure 6A, left). Likewise, Akt inhibitor VIII, a cell-permeable inhibitor of Akt45, suppressed Akt phosphorylation in a dose-dependent manner (Figure 6A, right). We then utilized these tools to test the necessity of Akt activation in GRP78-mediated I/R cardioprotection. We treated NRVM (24h) expressing GRP78 with either LY294002 (10 μM) or Akt inhibitor VIII (20 μM). After sI/R, we collected both culture medium and cells to measure cell death, finding that GRP78-mediated protection from sI/R-induced cell death was abolished by either Akt repressor (Figure 6B). Additionally, measurements of DHE red fluorescence showed that Akt inhibition blunted GRP78-mediated protection against ROS accumulation (Figure 6C). Together, these results suggest strongly that GRP78 expression activates Akt which is, in turn, required for GRP78-mediated protection against I/R injury in cardiomyocytes.
Figure 6. Akt signaling is required for GRP78-mediated cardioprotection against I/R.
A. Akt inhibitors suppressed Akt signaling. Two structurally distinct Akt inhibitors, LY294002 and Akt inhibitor VIII, were used to treat NRVM for 24 hours. Both inhibitors elicited dose-dependent inhibition of Akt signaling as indicated by decreases in Akt phosphorylation.
B. Suppression of Akt signaling abolished GRP78-mediated protection against I/R damage. Control or GRP78 adenovirus was used to infect NRVM for 48 hours. Cells were then subjected to sI/R. Akt inhibitors were incubated during ischemia and reperfusion. Relative cell death was determined by LDH assay. N = 4 per group.
C. Akt signaling was required for GRP78-mediated suppression of ROS production. After treatment with Akt inhibitors during sI/R, flow cytometry for dihydroethidium, a superoxide indicator, was conducted to determine relative ROS levels. N = 3 for each group. *, p < 0.05.
DISCUSSION
Multiple lines of evidence point to critical roles of UPR activation in ischemic heart disease, including the major signaling cascades of ATF629, 46–48 and IRE1/Xbp1s22, 49. However, whereas UPR activation in I/R injury is cardioprotective7, 15, 17, underlying mechanisms remain poorly characterized. Here, we confirm that I/R injury triggers robust increases in the expression of GRP78.48, 49 We go on to demonstrate that over-expression of GRP78, both in vitro and in vivo, protects myocytes from I/R-induced cell death. Exploration of underlying mechanisms revealed that GRP78 mitigates cellular damage, and ultimate apoptosis, by its cell surface translocation, interaction with PI3K, activation of Akt, and suppression of reactive oxygen species accumulation. Finally, our findings point to a necessary role for GRP78-dependent activation of Akt, independent of GRP78-mediated protein chaperone activity, in the anti-oxidant and cardioprotective responses.
UPR activation in ischemic heart disease
Activation of the UPR in cardiomyocytes has been reported previously15, and evidence points to meaningful cardioprotective effects in the setting of disease-related stress.17 Those cardioprotective effects have been assigned to both the ATF6 arm of the UPR29, 46–48, as well as to the IRE1/Xbp1s arm22, 49. Protein O-GlcNAcylation is cardioprotective50–55, and we have reported that Xbp1s-dependent activation of the HBP drives this response. 23 However, whether other UPR effectors participate in the cardioprotective response, and if so by what mechanisms, remains unknown.
Some evidence points to GRP78, a molecule which functions as both UPR governor and UPR target, in this biology. Glembotski and colleagues have reported that myocardial ischemia stimulates GRP78 expression.48, 49 Furthermore, inducible over-expression in cardiomyocytes of the ATF6 arm of the UPR confers cardioprotection against I/R, which is associated with strong induction of GRP78.46, 48 In fact, evidence suggests that GRP78 is up-regulated by both hypoxic insult and by re-oxygenation. 23 As noted, we have shown previously that induction of the Xbp1s branch of the UPR also confers protection to the heart in response to I/R, a pathway which also up-regulates GRP78.22 In fact, ATF6 and Xbp1s are known to form a heterodimer that activates GRP78 transcription.26, 56 Despite these findings, the role of GRP78 per se in I/R has not been elucidated, and its downstream effectors have remained obscure.
GRP78
GRP78 is a direct transcriptional target of the UPR-induced transcription factors Xbp1s and ATF6.15, 21, 23–26 Expression of GRP78, which is itself an ER chaperone, augments protein-folding capacity of the ER, helps translocate aberrant protein out of the ER for degradation and can also act ultimately to shut down the UPR branches.26 Prior studies have shown that GRP78 is strongly induced by I/R at both mRNA and protein levels.22, 29, 48, 49 Moreover, over-expression of either Xbp1s or ATF6 in cardiomyocytes leads to increases in GRP78.22, 46, 48 Even brief stimulation by tunicamycin, an ER stress inducer, enhances GRP78 expression, which is associated with protection against subsequent I/R damage.57 Of note, most of these studies attribute GRP78-related cardioprotection to enhanced protein folding capacity within the ER. A direct role for GRP78 in protection against I/R injury has never yet been demonstrated.
GRP78 orchestrates the action of the three distinct branches of the UPR in an integrated manner to maintain cellular homeostasis.20, 58, 59 Indeed, silencing of GRP78 leads to widespread ER stress that is unresolvable and culminates in cell death.60 GRP78 acts not only to govern activation of the UPR as a sensor of protein-folding stress, it also functions as a chaperone that enhances ER protein folding capacity and facilitates disposal of unfolded protein by means of ER-associated degradation61; this protein chaperone activity likely plays some role in the cardioprotection we observe. Kitakaze and colleagues have shown that over-expression of GRP78 protects cardiac myocytes from proteasome inhibition-induced cell death.62 In addition, GRP78 may confer beneficial effects in reperfusion by reducing pro-apoptotic signaling of the UPR.17, 63, 64 Consistent with this, chemical chaperone treatment leads to significant protection against I/R injury.65, 66 Outside the ER, GRP78 has been shown to have a host of roles, from suppression of apoptotic signals to regulation of mitochondrial energy balance.25 GRP78 has also been reported to promote cell survival by the Akt and NF-κB signaling cascades.67 Data reported here in the context of cardiac I/R injury reveal that GRP78 mediates activation of the Akt pathway and inhibition of oxidative stress, which occurs in addition to its chaperone function.29, 48 Collectively, our results uncover a novel mechanism linking UPR activation, Akt signaling, and oxidative stress in I/R.
Oxidative stress in myocardial I/R injury
I/R stress to the heart involves a phase of oxygen and nutrient deprivation followed by abrupt restoration of blood flow in the previously occluded artery; both phases elicit a wide range of molecular events in cardiac myocytes.5 During hypoxic conditions, cardiomyocytes respond with alterations in metabolic pathways, intracellular signaling, and functionality.4 Metabolically, oxidation of nutrients is severely suppressed due to the lack of oxygen.68 As a consequence, glycolysis, occurring in the absence of oxygen consumption, becomes the predominant means of energy production. To accommodate these dramatic changes in metabolic events, the cellular metabolic machinery adjusts accordingly.
During reperfusion, sudden influx of nutrients, such as free fatty acids and glucose, as well as restoration of oxygen, together replenish components necessary for oxidative phosphorylation and more efficient ATP production. However, ischemia-induced changes to the electron transport chain result in increases in mitochondrial generation of ROS.4 Indeed, over-production and accumulation of ROS or other free radicals plays a central role in I/R injury to the heart.35, 69 Suppression of ROS by genetic or pharmacological approaches strongly protects the heart from I/R damage.62
We report that GRP78 inhibits ROS accumulation through activation of Akt. Interestingly, GRP78’s function as an ER-resident chaperone seems at first difficult to reconcile with a role in Akt activation and ROS regulation. However, recent studies from the cancer field provide pertinent clues.41 GRP78 can translocate to the cell surface under certain conditions.25, 42, 70 This translocation within tumor cells is critical to GRP78-dependent protection against several stressors.65 Here, we tested this in NRVM; under conditions of sI/R or adenovirus-mediated over-expression of GRP78, we find that, indeed, a fraction of GRP78 is translocated to the cell surface, and we demonstrate that this leads to GRP78 interaction with PI3K and consequent Akt activation. Our results also suggest that some GRP78 may be localized at the cell surface even under normoxic conditions, and upon induction by I/R, cell membrane translocation is significantly increased. These data are consistent with reports that an anti-GRP78 monoclonal antibody inhibits signaling mediated by cell surface-localized GRP78 and thereby suppresses downstream PI3K/Akt signaling to block tumor growth and metastasis.71 Other studies have shown that GRP78 itself is a suppressor of apoptosis by interacting with caspase 772 or Bcl-2.73 In addition, GRP78 is also associated with Raf-1 in mitochondria, protecting cells from ER stress-induced apoptosis.74 Thus, GRP78 may protect cardiomyocytes from I/R injury via multiple mechanisms, and the relative importance of each warrants further investigation.
Conclusion and perspective
It is established that myocardial I/R injury activates the UPR and promotes GRP78 expression. Here, we report that one critical downstream effector of the UPR, GRP78, directly mitigates I/R injury. This beneficial effect stems largely from Akt-dependent inhibition of ROS accumulation. Thus, modulation of GRP78 signaling represents a novel target in myocardial I/R injury and one with potential therapeutic relevance.
Supplementary Material
Novelty and Significance.
What Is Known?
Coronary reperfusion following ischemia (I/R) triggers a second wave of injury to the heart.
Glucose regulated protein of 78 kDa (GRP78) is strongly induced by I/R in the heart.
What New Information Does This Article Contribute?
Cardiomyocyte-specific overexpression of GRP78 confers cardioprotection against I/R damage.
When activated, GRP78 translocates to cardiomyocyte cell surface membrane, stimulates pro-survival Akt signaling, and diminishes accumulation of reactive oxygen species.
Myocardial infarction is a leading cause of death worldwide. Restoration of blood flow in the infarct-related artery is a major approach to mitigate cardiac damage and improve clinical outcomes. Reperfusion per se, however, triggers additional injury. Indeed, reperfusion injury may account for up to 40% of final infarct size. GRP78, a master endoplasmic reticulum-resident chaperone, is potently induced by I/R in the heart. In this study, we show that overexpression of GRP78 in cardiac myocytes strongly protects the heart against I/R injury. At a mechanistic level, GRP78 migrates to the cell surface membrane, directly stimulates the Akt pathway and ameliorates the production of reactive oxygen species. These findings uncover a previously unrecognized role of GRP78 in protecting cardiomyocytes against I/R injury, thereby highlighting a novel pathway with potential therapeutic relevance.
Acknowledgments
We thank members from the Wang lab and the Hill labs for valuable discussions. We thank the Molecular Pathology Core (John Shelton) for help with histology. We also thank the Transgenic Core facility and Animal Resource Center for help with transgenic mouse generation and maintenance.
SOURCES OF FUNDING
Work in the Wang lab was supported by grants from the American Heart Association (Scientist Development Grant 14SDG18440002 and Innovative Research Grant 17IRG33460191), the American Diabetes Association (Innovative Basic Science Award 1-17-IBS-120), and the National Institutes of Health (HL-137723). Work in the Hill lab was supported by grants from the National Institutes of Health (HL-120732; HL-126012; HL-128215), American Heart Association (14SFRN20510023; 14SFRN20670003), Fondation Leducq (11CVD04), and Cancer Prevention and Research Institute of Texas (RP110486P3). This work was also supported, in part, by research grants to Richard C. Austin from the Heart and Stroke Foundation of Canada (G-15-0009389), the Canadian Institutes of Health Research (MOP-286787), and a Heart and Stroke Foundation of Ontario Program Grant (PRG6502). Financial support from St. Joseph’s Healthcare Hamilton is acknowledged. Richard C. Austin is a Career Investigator of the Heart and Stroke Foundation of Ontario and holds the Amgen Canada Research Chair in the Division of Nephrology at St. Joseph’s Healthcare and McMaster University.
Nonstandard Abbreviations and Acronyms
- UPR
unfolded protein response
- GRP78
glucose regulated protein of 78 kDa
- I/R
ischemia/reperfusion
- sI/R
simulated I/R
- NRVM
neonatal rat ventricular myocytes
- HBP
hexosamine biosynthetic pathway
- PCR
polymerase chain reaction
- ROS
reactive oxygen species
- O-GlcNAc
O-linked β-N-acetylglucosamine
- Xbp1s
spliced x-box binding protein 1
Footnotes
DISCLOSURES
None.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017 doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. The New England journal of medicine. 2007;357:1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 3.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. The American journal of cardiology. 2010;106:360–8. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hausenloy DJ, Barrabes JA, Botker HE, Davidson SM, Di Lisa F, Downey J, Engstrom T, Ferdinandy P, Carbrera-Fuentes HA, Heusch G, Ibanez B, Iliodromitis EK, Inserte J, Jennings R, Kalia N, Kharbanda R, Lecour S, Marber M, Miura T, Ovize M, Perez-Pinzon MA, Piper HM, Przyklenk K, Schmidt MR, Redington A, Ruiz-Meana M, Vilahur G, Vinten-Johansen J, Yellon DM, Garcia-Dorado D. Ischaemic conditioning and targeting reperfusion injury: a 30 year voyage of discovery. Basic Res Cardiol. 2016;111:70. doi: 10.1007/s00395-016-0588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang ZV, Hill JA. Protein quality control and metabolism: bidirectional control in the heart. Cell metabolism. 2015;21:215–26. doi: 10.1016/j.cmet.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bravo R, Parra V, Gatica D, Rodriguez AE, Torrealba N, Paredes F, Wang ZV, Zorzano A, Hill JA, Jaimovich E, Quest AF, Lavandero S. Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int Rev Cell Mol Biol. 2013;301:215–90. doi: 10.1016/B978-0-12-407704-1.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altamirano F, Wang ZV, Hill JA. Cardioprotection in ischaemia-reperfusion injury: novel mechanisms and clinical translation. J Physiol. 2015;593:3773–88. doi: 10.1113/JP270953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hausenloy DJ, Yellon DM. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol. 2016;13:193–209. doi: 10.1038/nrcardio.2016.5. [DOI] [PubMed] [Google Scholar]
- 11.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer. 2014;14:581–97. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- 13.Doroudgar S, Glembotski CC. New concepts of endoplasmic reticulum function in the heart: programmed to conserve. J Mol Cell Cardiol. 2013;55:85–91. doi: 10.1016/j.yjmcc.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin RC. The unfolded protein response in health and disease. Antioxid Redox Signal. 2009;11:2279–87. doi: 10.1089/ars.2009.2686. [DOI] [PubMed] [Google Scholar]
- 15.Glembotski CC. Endoplasmic reticulum stress in the heart. Circ Res. 2007;101:975–84. doi: 10.1161/CIRCRESAHA.107.161273. [DOI] [PubMed] [Google Scholar]
- 16.Glembotski CC. Finding the missing link between the unfolded protein response and O-GlcNAcylation in the heart. Circ Res. 2014;115:546–8. doi: 10.1161/CIRCRESAHA.114.304855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010;107:1071–82. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 18.Qi X, Vallentin A, Churchill E, Mochly-Rosen D. deltaPKC participates in the endoplasmic reticulum stress-induced response in cultured cardiac myocytes and ischemic heart. Journal of molecular and cellular cardiology. 2007;43:420–8. doi: 10.1016/j.yjmcc.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Xu L, Gillette TG, Jiang X, Wang ZV. The unfolded protein response in ischemic heart disease. J Mol Cell Cardiol. 2018;117:19–25. doi: 10.1016/j.yjmcc.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 21.Groenendyk J, Sreenivasaiah PK, Kim do H, Agellon LB, Michalak M. Biology of endoplasmic reticulum stress in the heart. Circ Res. 2010;107:1185–97. doi: 10.1161/CIRCRESAHA.110.227033. [DOI] [PubMed] [Google Scholar]
- 22.Wang ZV, Deng Y, Gao N, Pedrozo Z, Li DL, Morales CR, Criollo A, Luo X, Tan W, Jiang N, Lehrman MA, Rothermel BA, Lee AH, Lavandero S, Mammen PP, Ferdous A, Gillette TG, Scherer PE, Hill JA. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell. 2014;156:1179–92. doi: 10.1016/j.cell.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–59. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glimcher LH. XBP1: the last two decades. Ann Rheum Dis. 2010;69(Suppl 1):i67–71. doi: 10.1136/ard.2009.119388. [DOI] [PubMed] [Google Scholar]
- 25.Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J. 2011;434:181–8. doi: 10.1042/BJ20101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K. Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem. 2004;136:343–50. doi: 10.1093/jb/mvh122. [DOI] [PubMed] [Google Scholar]
- 27.Townsend PA, Cutress RI, Carroll CJ, Lawrence KM, Scarabelli TM, Packham G, Stephanou A, Latchman DS. BAG-1 proteins protect cardiac myocytes from simulated ischemia/reperfusion-induced apoptosis via an alternate mechanism of cell survival independent of the proteasome. J Biol Chem. 2004;279:20723–8. doi: 10.1074/jbc.M400399200. [DOI] [PubMed] [Google Scholar]
- 28.Esumi K, Nishida M, Shaw D, Smith TW, Marsh JD. NADH measurements in adult rat myocytes during simulated ischemia. Am J Physiol. 1991;260:H1743–52. doi: 10.1152/ajpheart.1991.260.6.H1743. [DOI] [PubMed] [Google Scholar]
- 29.Doroudgar S, Thuerauf DJ, Marcinko MC, Belmont PJ, Glembotski CC. Ischemia activates the ATF6 branch of the endoplasmic reticulum stress response. J Biol Chem. 2009;284:29735–45. doi: 10.1074/jbc.M109.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson LM, Chan AK, Berry LR, Li J, Sood SK, Dickhout JG, Xu L, Werstuck GH, Bajzar L, Klamut HJ, Austin RC. Overexpression of the 78-kDa glucose-regulated protein/immunoglobulin-binding protein (GRP78/BiP) inhibits tissue factor procoagulant activity. J Biol Chem. 2003;278:17438–47. doi: 10.1074/jbc.M301006200. [DOI] [PubMed] [Google Scholar]
- 31.Dragatsis I, Zeitlin S. A method for the generation of conditional gene repair mutations in mice. Nucleic Acids Res. 2001;29:E10. doi: 10.1093/nar/29.3.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–15. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461–70. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 34.Goswami SK, Maulik N, Das DK. Ischemia-reperfusion and cardioprotection: a delicate balance between reactive oxygen species generation and redox homeostasis. Ann Med. 2007;39:275–89. doi: 10.1080/07853890701374677. [DOI] [PubMed] [Google Scholar]
- 35.Chouchani ET, Pell VR, James AM, Work LM, Saeb-Parsy K, Frezza C, Krieg T, Murphy MP. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell metabolism. 2016;23:254–63. doi: 10.1016/j.cmet.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki YJ, Carini M, Butterfield DA. Protein carbonylation. Antioxid Redox Signal. 2010;12:323–5. doi: 10.1089/ars.2009.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fayard E, Tintignac LA, Baudry A, Hemmings BA. Protein kinase B/Akt at a glance. J Cell Sci. 2005;118:5675–8. doi: 10.1242/jcs.02724. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita K, Kajstura J, Discher DJ, Wasserlauf BJ, Bishopric NH, Anversa P, Webster KA. Reperfusion-activated Akt kinase prevents apoptosis in transgenic mouse hearts overexpressing insulin-like growth factor-1. Circ Res. 2001;88:609–14. doi: 10.1161/01.res.88.6.609. [DOI] [PubMed] [Google Scholar]
- 39.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–5. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 40.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–7. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Hashimi AA, Caldwell J, Gonzalez-Gronow M, Pizzo SV, Aboumrad D, Pozza L, Al-Bayati H, Weitz JI, Stafford A, Chan H, Kapoor A, Jacobsen DW, Dickhout JG, Austin RC. Binding of anti-GRP78 autoantibodies to cell surface GRP78 increases tissue factor procoagulant activity via the release of calcium from endoplasmic reticulum stores. J Biol Chem. 2010;285:28912–23. doi: 10.1074/jbc.M110.119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Liu R, Ni M, Gill P, Lee AS. Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J Biol Chem. 2010;285:15065–75. doi: 10.1074/jbc.M109.087445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Tseng CC, Tsai YL, Fu X, Schiff R, Lee AS. Cancer cells resistant to therapy promote cell surface relocalization of GRP78 which complexes with PI3K and enhances PI(3,4,5)P3 production. PLoS One. 2013;8:e80071. doi: 10.1371/journal.pone.0080071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maira SM, Stauffer F, Schnell C, Garcia-Echeverria C. PI3K inhibitors for cancer treatment: where do we stand? Biochem Soc Trans. 2009;37:265–72. doi: 10.1042/BST0370265. [DOI] [PubMed] [Google Scholar]
- 45.Lindsley CW, Zhao Z, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, Jones RE, Hartman GD, Huff JR, Huber HE, Duggan ME. Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett. 2005;15:761–4. doi: 10.1016/j.bmcl.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Jin JK, Blackwood EA, Azizi K, Thuerauf DJ, Fahem AG, Hofmann C, Kaufman RJ, Doroudgar S, Glembotski CC. ATF6 Decreases Myocardial Ischemia/Reperfusion Damage and Links ER Stress and Oxidative Stress Signaling Pathways in the Heart. Circ Res. 2017;120:862–875. doi: 10.1161/CIRCRESAHA.116.310266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glembotski CC. Roles for ATF6 and the sarco/endoplasmic reticulum protein quality control system in the heart. J Mol Cell Cardiol. 2014;71:11–5. doi: 10.1016/j.yjmcc.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martindale JJ, Fernandez R, Thuerauf D, Whittaker R, Gude N, Sussman MA, Glembotski CC. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res. 2006;98:1186–93. doi: 10.1161/01.RES.0000220643.65941.8d. [DOI] [PubMed] [Google Scholar]
- 49.Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circulation research. 2006;99:275–82. doi: 10.1161/01.RES.0000233317.70421.03. [DOI] [PubMed] [Google Scholar]
- 50.Marsh SA, Collins HE, Chatham JC. Protein O-GlcNAcylation and cardiovascular (patho)physiology. The Journal of biological chemistry. 2014;289:34449–56. doi: 10.1074/jbc.R114.585984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Darley-Usmar VM, Ball LE, Chatham JC. Protein O-linked beta-N-acetylglucosamine: a novel effector of cardiomyocyte metabolism and function. J Mol Cell Cardiol. 2012;52:538–49. doi: 10.1016/j.yjmcc.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ngoh GA, Facundo HT, Zafir A, Jones SP. O-GlcNAc signaling in the cardiovascular system. Circ Res. 2010;107:171–85. doi: 10.1161/CIRCRESAHA.110.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dassanayaka S, Jones SP. O-GlcNAc and the cardiovascular system. Pharmacol Ther. 2014;142:62–71. doi: 10.1016/j.pharmthera.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zachara NE. The roles of O-linked beta-N-acetylglucosamine in cardiovascular physiology and disease. Am J Physiol Heart Circ Physiol. 2012;302:H1905–18. doi: 10.1152/ajpheart.00445.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wright JN, Collins HE, Wende AR, Chatham JC. O-GlcNAcylation and cardiovascular disease. Biochem Soc Trans. 2017;45:545–553. doi: 10.1042/BST20160164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem. 1998;273:33741–9. doi: 10.1074/jbc.273.50.33741. [DOI] [PubMed] [Google Scholar]
- 57.Petrovski G, Das S, Juhasz B, Kertesz A, Tosaki A, Das DK. Cardioprotection by endoplasmic reticulum stress-induced autophagy. Antioxid Redox Signal. 2011;14:2191–200. doi: 10.1089/ars.2010.3486. [DOI] [PubMed] [Google Scholar]
- 58.Little E, Ramakrishnan M, Roy B, Gazit G, Lee AS. The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit Rev Eukaryot Gene Expr. 1994;4:1–18. doi: 10.1615/critreveukargeneexpr.v4.i1.10. [DOI] [PubMed] [Google Scholar]
- 59.Zhu G, Lee AS. Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J Cell Physiol. 2015;230:1413–20. doi: 10.1002/jcp.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;26:5688–97. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee AS. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat Rev Cancer. 2014;14:263–76. doi: 10.1038/nrc3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fu HY, Minamino T, Tsukamoto O, Sawada T, Asai M, Kato H, Asano Y, Fujita M, Takashima S, Hori M, Kitakaze M. Overexpression of endoplasmic reticulum-resident chaperone attenuates cardiomyocyte death induced by proteasome inhibition. Cardiovasc Res. 2008;79:600–10. doi: 10.1093/cvr/cvn128. [DOI] [PubMed] [Google Scholar]
- 63.Arrieta A, Blackwood EA, Glembotski CC. ER Protein Quality Control and the Unfolded Protein Response in the Heart. Curr Top Microbiol Immunol. 2017 doi: 10.1007/82_2017_54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glembotski CC. The role of the unfolded protein response in the heart. J Mol Cell Cardiol. 2008;44:453–9. doi: 10.1016/j.yjmcc.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jian L, Lu Y, Lu S, Lu C. Chemical Chaperone 4-Phenylbutyric Acid Reduces Cardiac Ischemia/Reperfusion Injury by Alleviating Endoplasmic Reticulum Stress and Oxidative Stress. Med Sci Monit. 2016;22:5218–5227. doi: 10.12659/MSM.898623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu Y, Sun G, Luo Y, Wang M, Chen R, Zhang J, Ai Q, Xing N, Sun X. Cardioprotective effects of Notoginsenoside R1 against ischemia/reperfusion injuries by regulating oxidative stress- and endoplasmic reticulum stress- related signaling pathways. Sci Rep. 2016;6:21730. doi: 10.1038/srep21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Misra UK, Deedwania R, Pizzo SV. Activation and cross-talk between Akt, NF-kappaB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J Biol Chem. 2006;281:13694–707. doi: 10.1074/jbc.M511694200. [DOI] [PubMed] [Google Scholar]
- 68.Lopaschuk GD, Stanley WC. Glucose metabolism in the ischemic heart. Circulation. 1997;95:313–5. doi: 10.1161/01.cir.95.2.313. [DOI] [PubMed] [Google Scholar]
- 69.King AL, Lefer DJ. Cytoprotective actions of hydrogen sulfide in ischaemia-reperfusion injury. Exp Physiol. 2011;96:840–6. doi: 10.1113/expphysiol.2011.059725. [DOI] [PubMed] [Google Scholar]
- 70.Tsai YL, Zhang Y, Tseng CC, Stanciauskas R, Pinaud F, Lee AS. Characterization and mechanism of stress-induced translocation of 78-kilodalton glucose-regulated protein (GRP78) to the cell surface. J Biol Chem. 2015;290:8049–64. doi: 10.1074/jbc.M114.618736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu R, Li X, Gao W, Zhou Y, Wey S, Mitra SK, Krasnoperov V, Dong D, Liu S, Li D, Zhu G, Louie S, Conti PS, Li Z, Lee AS, Gill PS. Monoclonal antibody against cell surface GRP78 as a novel agent in suppressing PI3K/AKT signaling, tumor growth, and metastasis. Clin Cancer Res. 2013;19:6802–11. doi: 10.1158/1078-0432.CCR-13-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J Biol Chem. 2003;278:20915–24. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- 73.Zhou H, Zhang Y, Fu Y, Chan L, Lee AS. Novel mechanism of anti-apoptotic function of 78-kDa glucose-regulated protein (GRP78): endocrine resistance factor in breast cancer, through release of B-cell lymphoma 2 (BCL-2) from BCL-2-interacting killer (BIK) J Biol Chem. 2011;286:25687–96. doi: 10.1074/jbc.M110.212944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shu CW, Sun FC, Cho JH, Lin CC, Liu PF, Chen PY, Chang MD, Fu HW, Lai YK. GRP78 and Raf-1 cooperatively confer resistance to endoplasmic reticulum stress-induced apoptosis. J Cell Physiol. 2008;215:627–35. doi: 10.1002/jcp.21340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.