Abstract
In diabetes, hyperamylinemia contributes to cardiac dysfunction. The interplay between hIAPP, blood glucose and other plasma components is, however, not understood. We show that glucose and LDL interact with hIAPP, resulting in β-sheet rich oligomers with increased β-cell toxicity and hemolytic activity, providing mechanistic insights for a direct link between diabetes and cardiovascular diseases.
Diabetes is a widespread disease affecting more than 415 million people and claiming more than 5 million lives worldwide only in 2015.1 Type 2 diabetes (T2D) is the most common form of the disease and accounts for approximately 90% of all cases.2 While the exact cellular pathomechanism of T2D remains elusive, at least six principal factors have been identify to contribute to T2D, which include insulin resistance, lipotoxicity, endoplasmic reticulum oxidative stress, tissue inflammation response, amyloid deposition and β-cell failure.3, 4 Pancreatic amyloid deposits are composed of the 37 residue peptide hormone human Islet Amyloid Polypeptide (hIAPP).5, 6 hIAPP is thought to be involved in the slowdown of post-meal increase of plasma glucose concentration.7 The mechanism of its cytotoxicity in T2D is, however, only partially understood.8 Recent studies have shown that soluble oligomers of hIAPP are responsible for cell toxicity and cell death.9–11 There is only little knowledge about the factors that lead to hIAPP aggregation in vivo. hIAPP is stored in a functional and soluble form in insulin granules in β-cells at concentrations up to 1–4 mM.12 The cellular environment has thus a strong impact on the rate of hIAPP aggregation. Factors affecting aggregation include pH,13 divalent cations such as Zn2+,14 insulin,15, 16 and the redox environment.17, 18 It is known that the serum of diabetic patients contains low concentrations of high-density lipoproteins (HDL) and high concentrations of very low-density lipoproteins (VLDL), low-density lipoproteins (LDL) and plasma triglycerides (TG).19 In addition, the concentration of sugars and insulin is increased. Whereas the blood glucose concentration in healthy individuals is on the order of 6 mM, it ranges between 10 mM and >14 mM in people affected by T2D.20 T2D is linked with other diseases such as Alzheimer’s disease,21, 22 cardiac dysfunction in obesity,23 kidney disease and other renal failures.24 The aim of this study is to investigate the impact of major plasma components known to be disturbed in T2D on the kinetic and structural aspects of hIAPP oligomerization and fiber formation. We find that LDL stabilizes high molecular weight hIAPP species and effects hIAPP aggregation. We observe further that sugars induce liquid-liquid phase separation and yield increased cellular toxicity. We propose that these oligomers may be the link between T2D and associated complications such as cardiac dysfunction in obesity.
In the past, we have studied the aggregation behaviour of hIAPP in vitro.18 We now want to understand how hIAPP behaves under physiological conditions. To address this question, we employ blood plasma of diabetic transgenic mice. The mouse models harbor either one (+/TG mice) or two copies (TG/TG mice) of human IAPP.25 As a control, we used plasma of +/+ wild type mice. Although pancreatic amyloid fibrils are present in both the +/TG and TG/TG mice, only TG/TG mice are affected by diabetes.26 It is known that the plasma of diabetic patients becomes lactescent due to an increase of dispersed lipids27 (increasing lactescence +/+ < +/TG < TG/TG) (Fig. 1A). In addition, previous results showed decreased HDL-cholesterol and insulin levels and high levels of plasma glucose, urea and LDL-cholesterol in hIAPP transgenic mice.26
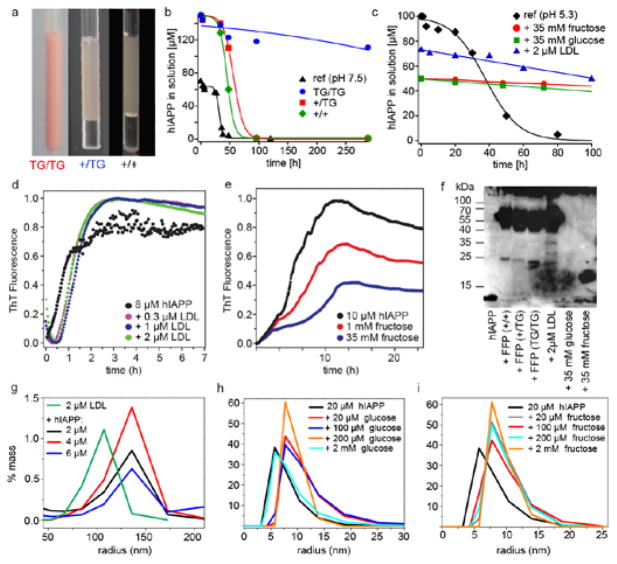
Fig. 1. Plasma induces retardation of hIAPP aggregation and induces formation of oligomers.
a Plasma of +/+, +/TG and TG/TG mice. NMR hIAPP aggregation kinetics of hIAPP in b +/+, +/TG and TG/TG plasma, and c in the presence of LDL (green), fructose (grey), glucose (red). hIAPP (pH 7.5) is shown as a reference (blue). Thioflavin T (ThT) aggregation kinetics after incubation of hIAPP with d LDL and e fructose. f Immunoblot analysis of hIAPP oligomers in fresh frozen plasma (FFP), LDL and fructose. g–i Dynamic light scattering (DLS) of hIAPP complexes in the presence of LDL, glucose and fructose.
To understand the impact of plasma composition on the conformation and behavior of hIAPP, we dissolved isotopically labeled hIAPP in plasma. As a control, the peptide was measured in a standard buffer at pH 7.5. To estimate the stability of hIAPP in solution, we followed the kinetics by monitoring the solution-state NMR signal intensities over time. We find that hIAPP is most stable in plasma of TG/TG animals (Fig. 1B). In general, the NMR signal intensities decrease more slowly if the peptide is dissolved in plasma than in reference buffer, in agreement with previous studies which showed that crowding agents can induce a retardation of aggregation.28 Diabetic (TG/TG) and non-diabetic (+/+, +/TG) plasma differs mostly in the concentration of LDL and sugars.29 We therefore analyzed the effect of these two components on the aggregation kinetics, as well as on the conformation of hIAPP. Aggregation of hIAPP was monitored by NMR. As illustrated in Fig. 1C, LDL and sugar solutions (fructose or glucose) had a similar stabilizing effect as TG/TG plasma. To obtain a better understanding of the aggregation kinetics, we performed Thioflavin T (ThT) assays (Fig. 1D,E). In presence of LDL, we observe an increase of the lag time. The maximum fluorescence intensity is, however, unchanged. By contrast for fructose, the maximum fluorescence is significantly reduced. The results suggest that LDL, glucose and fructose have a direct impact on the aggregation state of hIAPP. This is in agreement with results from Kedia et al. who showed that sugars favor Aβ42 oligomer formation.30 To characterize the oligomerization state in more detail, we performed Dynamic Light Scattering (DLS) experiments. DLS reveals an increase of the hydrodynamic size of the hIAPP assemblies formed in the presence of LDL, glucose and fructose (Fig. 1G–I). In addition, we performed Western blot experiments using the hIAPP specific antibody A133 (Fig. 1F).31 As shown previously,18,32 hIAPP yields bands in the molecular weight range from 15 kDa to approximately 100 kDa. We find that +/+, +/TG, and TG/TG plasma induced oligomers have a similar molecular weight as the oligomers that are formed in the presence of 2 μM LDL. By contrast, glucose or fructose induced oligomers yield a band corresponding to a molecular weight on the order of 15 kDa, suggesting that hIAPP interacts differently with sugars and LDL.
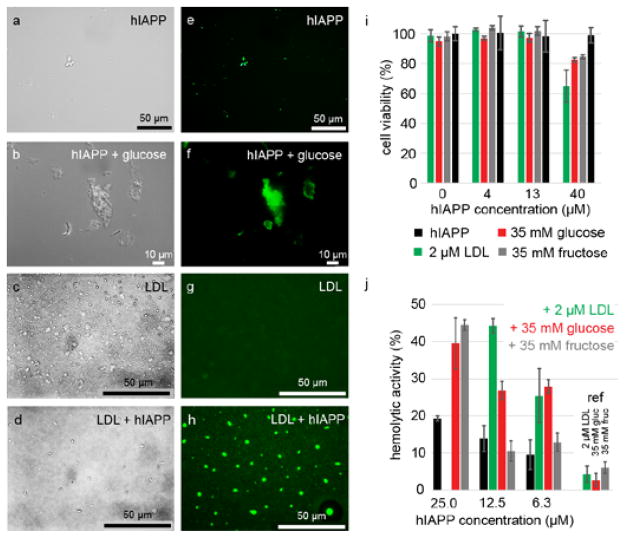
To test the hypothesis whether LDL, glucose and fructose are able to induce hIAPP oligomeric structures, we carried out fluorescence and Differential Interference Contrast (DIC) microscopy experiments. We find that sugar and LDL induced hIAPP aggregates yield an intrinsic fluorescence (Fig. 2), which has been observed previously for amyloid fibrils (Fig. S3).33,34 hIAPP alone yields only few colloidal structures. In the presence of sugars, however, larger and more aggregate structures are observed. In DIC experiments, LDL particles appear spherical and non-fluorescent. In the presence of hIAPP, however, LDL particles show an intrinsic fluorescence indicating that LDL interacts with hIAPP. To find out if these soluble oligomers are toxic in vivo, we carried out cellular toxicity assays using the pancreatic β-cell line b-tc3. B-tc3 cells were incubated with hIAPP in combination with glucose, LDL or fructose. Increasing concentrations of hIAPP yield a decrease of cellular viability in all cases (Fig. 2I), suggesting that LDL and glucose/fructose increase cellular toxicity. In addition, we performed a hemolytic assay to investigate whether hIAPP is able to lyse red blood cells (Fig. 2J). We find that the presence of LDL or sugars such as glucose or fructose increases the hemolytic activity strongly.
Fig. 2. Glucose and LDL induced hIAPP yield intrinsically fluorescent oligomers with increased neurotoxicity.
(a–d) DIC and (e–h) GFP-filtered fluorescence microscopy images of hIAPP in the absence and presence of glucose and LDL. i b-tc3 cellular toxicity of hIAPP in the presence of 2 μM LDL, 35 mM glucose and 35 mM fructose. j Hemolytic activity of hIAPP in the absence (black) and presence of 2 μM LDL (green), 35 mM glucose (red) and 35 mM fructose (gray).
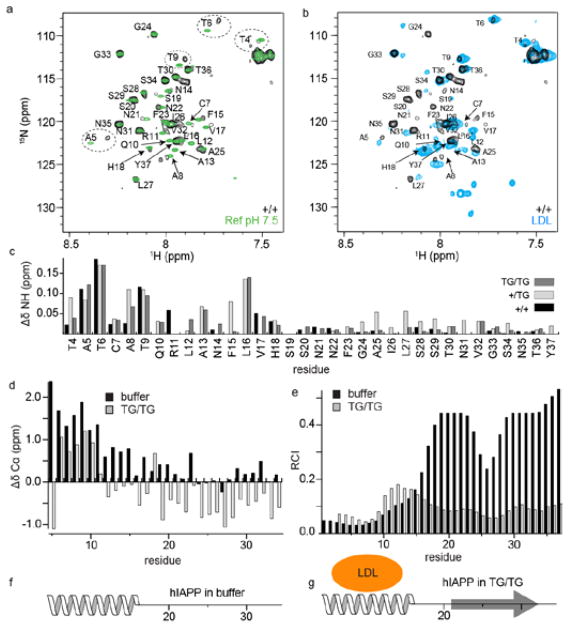
To obtain deeper insight into the interaction mechanism, we carried out solution-state NMR experiments. First, we dissolved isotopically labeled hIAPP35 in plasma (+/+, +/TG and TG/TG) and recorded HMQC experiments (Fig. 3A). As a control, spectra of hIAPP dissolved into phosphate buffer (pH 7.5) are recorded. Fig. 3C represents the chemical shift changes observed for the three different plasma samples with respect to the control sample. We find that chemical shifts of residues located in the N-terminal region of hIAPP are perturbed. To identify the molecules that are responsible for these shift changes, we recorded experiments for hIAPP dissolved in a 2 μM LDL solution (Fig. 3B). Many of the chemical shift changes in the N-terminal region of hIAPP observed in plasma are also seen in the presence of LDL, suggesting that lipid containing particles are responsible for these spectral changes. This observation is consistent with a previous study where it was shown that the N-terminal part of hIAPP interacts with membrane nanodiscs.36 To characterize the structural changes in hIAPP which are induced by TG/TG plasma, we performed 3D HNCA-experiments. The analysis of the hIAPP Δδ(13Cα) chemical shift differences (Fig. 3D) suggests that the peptide is converted from a random coil structure with an α-helical propensity in phosphate buffer18 to a conformer which is rich in β-sheet structure. The Random Coil Index (RCI) supports this finding (Fig. 3E). Based on the Δδ( 13Cα) data and the RCI, we propose a secondary structure model for hIAPP in buffer and TG/TG plasma (Fig. 3F,G).
Fig. 3. NMR analysis of hIAPP dissolved in plasma and LDL.
a Superposition of 1H,15N-HMQC spectra of 100 μM hIAPP in +/+ plasma and buffer (pH 7.5) and b +/+ plasma and LDL. c Residue specific amide chemical shift differences. d Δδ(Cα) chemical shift differences between hIAPP in buffer (pH 7.5) and hIAPP in plasma. e Random Coil Index (RCI) obtained from the backbone chemical shifts for hIAPP dissolved in buffer and for hIAPP dissolved in TG/TG plasma. f, g Secondary structural model of hIAPP in buffer and in TG/TG plasma, respectively.
After intake of food, the blood glucose level rises, stimulating the pancreatic secretion of insulin and hIAPP. In diabetes, these two peptide hormones become overexpressed, which can drive aggregation and in turn cellular toxicity.10 So far, aggregation of hIAPP has been observed to occur only in pancreatic beta-cells, without affecting significantly other organs. We find that key molecules in plasma which are known to be elevated in T2D, such as LDL and glucose induce a stabilization of a non-aggregation prone state of the peptide. hIAPP solubilized in plasma and LDL yields high molecular weight oligomer complexes with an apparent molecular weight in the range of ~15 kDa to ≥ 100 kDa. LDL and sugar induced hIAPP oligomers yield an increased hemolytic activity as well as cellular toxicity. Interestingly, the LDL/sugar induced hIAPP oligomeric assemblies display an intrinsic fluorescence. This might indicate that β-sheets are already preformed in these oligomeric structures, as otherwise no intrinsic fluorescence would be observed. Intrinsic fluorescence of oligomeric Aβ assemblies has been observed previously by super-resolution microscopy.37 By NMR, we have observed that the peaks originating from residues located in the N-terminal region of the peptide are changing in chemical shift in the presence of diabetic plasma and LDL, suggesting that this part of hIAPP is involved in interactions with the respective plasma components. At the same time, the C-terminal part of the peptide adopts a certain propensity for β-sheet structure. For the hIAPP - glucose/fructose mixtures, no chemical shift changes are observed after mixing. We find, however, that aggregation of hIAPP is significantly retarded in the presence of sugars. Under these conditions, hIAPP populates oligomers that yield a reduced ThT fluorescence signal, but a very pronounced intrinsic fluorescence. In EM, hIAPP adopts very thin and fragile protofibrillar structures in presence of fructose and glucose (Fig. S2). We speculate that sugars might induce formation of hIAPP-rich colloidal structures. In fact, glucose is a cosmotropic osmolyte,38,39 coordinates water and gets excluded from interactions with the hIAPP backbone, thereby inducing structure. At the same time, hIAPP might exchange between a high molecular weight and a monomeric state which would allow to explain the relatively high intensities observed in the NMR experiments. In case of LDL, EM shows that hIAPP aggregates are lined up along a series of LDL particles (Fig. S2). The presence of a lipid particle might catalyze the transition between a monomeric and a high molecular weight state of hIAPP, which might facilitate the detecton of a hIAPP monomeric state. The plasma concentration of hIAPP has been reported to be in the pM range.40, 41 Nevertheless, antibodies which specifically recognize oligomeric hIAPP assemblies were identified in the serum of diabetic patients,10 suggesting that hIAPP oligomers can assemble even at these very low concentrations.
In conclusion, we have shown that hIAPP, LDL, and sugars mutually interact, suggesting a direct link between TD2 and cardiovascular diseases.
Supplementary Material
Acknowledgments
This work was performed in the framework of the SFB-1035 (project B07 and -B11, Deutsche Forschungsgemeinschaft, DFG, to B.R. and M.J.F.). We acknowledge support from the Technische Universitaet Muenchen, Institute for Advanced Study (IAS), funded by the German Excellence Initiative and the European Union Seventh Framework Program, grant no. 291763. M.J.F. is an IAS Rudolf Mößbauer Tenure Track Professor. A.R. is supported by an IAS Hans Fischer Senior Fellowship. Y.M. and M.J.F. gratefully acknowledge funding by a DAAD PhD scholarship. A.R. was partly supported by funds from NIH (AG048934). K.T. acknowledges support from the MEYS of the Czech Republic under the project CEITEC 2020 (LQ1601). We are grateful to Gunilla Westermark for providing us with a sample of the antibody A133. Furthermore, we would like to thank Dr. Christoph Goebl, Dr. Vanessa Morris, Dr. Sam Asami and Dr. Carina Motz for stimulating discussions.
Footnotes
Electronic Supplementary Information (ESI) available.
Notes and references
- 1.Ogurtsova K, da Rocha Fernandes J, Huang Y, Linnenkamp U, Guariguata L, Cho N, Cavan D, Shaw J, Makaroff L. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Scully T. Nature. 2012;492:S2–S3. doi: 10.1038/492S2a. [DOI] [PubMed] [Google Scholar]
- 3.Amos AF, McCarty DJ, Zimmet P. Diabetic Med. 1997;14(Suppl 5):S1–S85. [PubMed] [Google Scholar]
- 4.Donath MY, Shoelson SE. Nature Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 5.Westermark P, Wernstedt C, Wilander E, Hayden DW, Obrien TD, Johnson KH. Proc Natl Acad Sci USA. 1987;84:3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper GJS, Willis AC, Clark A, Turner RC, Sim RB, Reid KBM. Proc Natl Acad Sci USA. 1987;84:8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Percy AJ, Trainor DA, Rittenhouse J, Phelps J, Koda JE. Clin Chem. 1996;42:576–585. [PubMed] [Google Scholar]
- 8.Cao P, Marek P, Noor H, Patsalo V, Tu LH, Wang H, Abedini A, Raleigh DP. FEBS Lett. 2013;587:1106–1118. doi: 10.1016/j.febslet.2013.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin CY, Gurlo T, Kayed R, Butler AE, Haataja L, Glabe CG, Butler PC. Diabetes. 2007;56:1324–1332. doi: 10.2337/db06-1579. [DOI] [PubMed] [Google Scholar]
- 10.Bram Y, Frydman-Marom A, Yanai I, Gilead S, Shaltiel-Karyo R, Amdursky N, Gazit E. Sci Rep. 2014;4:04267. doi: 10.1038/srep04267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abedini A, Plesner A, Cao P, Ridgway Z, Zhang JH, Tu LH, Middleton CT, Chao B, Sartori DJ, Meng FL, Wang H, Wong AG, Zanni MT, Verchere CB, Raleigh DP, Schmidt AM. Elife. 2016:5. doi: 10.7554/eLife.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutton JC. Diabetologia. 1989;32:271–281. doi: 10.1007/BF00265542. [DOI] [PubMed] [Google Scholar]
- 13.Khemtémourian L, Doménech E, Doux JP, Koorengevel MC, Killian JA. J Am Chem Soc. 2011;133:15598–15604. doi: 10.1021/ja205007j. [DOI] [PubMed] [Google Scholar]
- 14.Brender JR, Hartman K, Nanga RPR, Popovych N, Bea RD, Vivekanandan S, Marsh ENG, Ramamoorthy A. J Am Chem Soc. 2010;132:8973–8983. doi: 10.1021/ja1007867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson JL, Miranker AD. J Mol Biol. 2004;335:221–231. doi: 10.1016/j.jmb.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 16.Wei L, Jiang P, Yau YH, Summer H, Shochat SG, Mu YG, Pervushin K. Biochemistry. 2009;48:2368–2376. doi: 10.1021/bi802097b. [DOI] [PubMed] [Google Scholar]
- 17.Koo BW, Miranker AD. Prot Sci. 2005;14:231–239. doi: 10.1110/ps.041051205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez Camargo DC, Tripsianes K, Buday K, Franko A, Göbl C, Hartlmüller C, Sarkar R, Aichler M, Mettenleiter G, Schulz M, Böddrich A, Erck C, Martens H, Walch AK, Madl T, Wanker EE, Conrad M, Hrabě de Angelis M, Reif B. Sci Rep. 2017;7:44041. doi: 10.1038/srep44041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guérin M, Le Goff W, Lassel TS, Van Tol A, Steiner G, Chapman MJ. Arterioscler Thromb Vasc Biol. 2001;21:282–288. doi: 10.1161/01.atv.21.2.282. [DOI] [PubMed] [Google Scholar]
- 20.van Dijk JW, van Loon LJ. Diabetes Spectr. 2015;28:24–31. doi: 10.2337/diaspect.28.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, Obrien PC, Palumbo PJ. Am J Epidemiol. 1997;145:301–308. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- 22.Ott A, Stolk RP, van Harskamp F, Pols HAP, Hofman A, Breteler MMB. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 23.Boudina S, Abel ED. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 24.Gong W, Liu Z, Zeng C, Peng A, Chen H, Zhou H, Li L. Kidney Int. 2007;72:213–218. doi: 10.1038/sj.ki.5002305. [DOI] [PubMed] [Google Scholar]
- 25.Verchere CB, Dalessio DA, Palmiter RD, Weir GC, BonnerWeir S, Baskin DG, Kahn SE. Proc Natl Acad Sci USA. 1996;93:3492–3496. doi: 10.1073/pnas.93.8.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franko A, Rodriguez Camargo DC, Böddrich A, Garg D, Rodriguez Camargo A, Rozman J, Rathkolb B, Janik D, Aichler M, Feuchtinger A, Neff F, Fuchs H, Wanker EE, Reif B, Häring H-U, Peter A, Hrabě de Angelis M. Sci Rep. 2018;8 doi: 10.1038/s41598-017-18807-8. article no. 1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tremblay K, Methot J, Brisson D, Gaudet D. J Clin Lipid. 2011;5:37–44. doi: 10.1016/j.jacl.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Seeliger J, Werkmuller A, Winter R. Plos One. 2013:8. doi: 10.1371/journal.pone.0069652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franko A, Neschen S, Rozman J, Rathkolb B, Aichler M, Feuchtinger A, Brachthauser L, Neff F, Kovarova M, Wolf E, Fuchs H, Haring HU, Peter A, de Angelis MH. Mol Metabol. 2017;6:256–266. doi: 10.1016/j.molmet.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kedia N, Almisry M, Bieschke J. Phys Chem Chem Phys. 2017;19:18036–18046. doi: 10.1039/c7cp02849k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulsson JF, Westermark GT. Diabetes. 2005;54:2117–2125. doi: 10.2337/diabetes.54.7.2117. [DOI] [PubMed] [Google Scholar]
- 32.Oskarsson ME, Paulsson JF, Schultz SW, Ingelsson M, Westermark P, Westermark GT. Am J Pathol. 2015;185:834–846. doi: 10.1016/j.ajpath.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Chan FTS, Schierle GSK, Kumita JR, Bertoncini CW, Dobson CM, Kaminski CF. Analyst. 2013;138:2156–2162. doi: 10.1039/c3an36798c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.del Mercato LL, Pompa PP, Maruccio G, Della Torre A, Sabella S, Tamburro AM, Cingolani R, Rinaldi R. Proc Natl Acad Sci USA. 2007;104:18019–18024. doi: 10.1073/pnas.0702843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez Camargo DC, Tripsianes K, Kapp TG, Mendes J, Schubert J, Cordes B, Reif B. Protein Expr Purif. 2015;106:49–56. doi: 10.1016/j.pep.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez Camargo DC, Korshavn KJ, Jussupow A, Raltchev K, Goricanec D, Fleisch M, Sarkar R, Xue K, Aichler M, Mettenleiter G, Walch AK, Camilloni C, Hagn F, Reif B, Ramamoorthy A. eLife. 2017;6:e31226. doi: 10.7554/eLife.31226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schierle GSK, van de Linde S, Erdelyi M, Esbjorner EK, Klein T, Rees E, Bertoncini CW, Dobson CM, Sauer M, Kaminski CF. J Am Chem Soc. 2011;133:12902–12905. doi: 10.1021/ja201651w. [DOI] [PubMed] [Google Scholar]
- 38.Street TO, Bolen DW, Rose GD. Proc Natl Acad Sci USA. 2006;103:13997–14002. doi: 10.1073/pnas.0606236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burg MB, Ferraris JD. J Biol Chem. 2008;283:7309–7313. doi: 10.1074/jbc.R700042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butler PC, Chou J, Carter WB, Wang YN, Bu BH, Chang D, Chang JK, Rizza RA. Diabetes. 1990;39:752–756. doi: 10.2337/diab.39.6.752. [DOI] [PubMed] [Google Scholar]
- 41.Pittner RA, Albrandt K, Beaumont K, Gaeta LSL, Koda JE, Moore CX, Rittenhouse J, Rink TJ. J Cell Biochem. 1994;55:19–28. doi: 10.1002/jcb.240550004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.