Abstract
The goal of this work was to improve the lycopene storage capacity of the E. coli membrane by engineering both morphological and biosynthetic aspects. First, Almgs, a protein from Acholeplasma laidlawii that is involved in membrane bending is overexpressed to expand the storage space for lycopene, which resulted in a 12% increase of specific lycopene production. Second, several genes related to the membrane-synthesis pathway in E. coli, including plsb, plsc, and dgka, were also overexpressed, which led to a further 13% increase. In addition, membrane separation and component analysis confirmed that the increased amount of lycopene was mainly accumulated within the cell membranes. Finally, by integrating both aforementioned modification strategies, a synergistic effect could be observed which caused a 1.32-fold increase of specific lycopene production, from the 27.5 mg/g of the parent to 36.4 mg/g DCW in the engineered strain. This work demonstrates that membrane engineering is a feasible strategy for increasing the production and accumulation of lycopene in E. coli.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1298-8) contains supplementary material, which is available to authorized users.
Keywords: Membrane morphology, Membrane synthesis, Lycopene, Production, Escherichia coli
Introduction
Lycopene is one of the major precursors of downstream carotenoids produced by many plants and microorganisms. It is of great economic interest due to its attractive anti-oxidative, anti-cancer, and anti-inflammatory activities, and has been widely used in the pharmaceutical, nutraceutical, cosmetics, and food industries (Nagao and Yoshikawa 2009).
Lycopene has been produced by extraction (Clinton 1998), chemical synthesis (Michael and Bausch 2003), and fermentation (Mantzouridou and Tsimidou 2008). Because the lycopene content of tomatoes is low (approx. 0.02 g/kg) and because of the presence of many other carotenoids (Clinton 1998), the extraction method is relatively expensive. Engineering microorganisms to produce lycopene to meet the increasing demand has consequently attracted much attention, with a particular focus on Escherichia coli (Ajikumar et al. 2008; Bhataya et al. 2009).This is not only due to the potential benefits of lycopene for human health as an antioxidant (Rao and Agarwal 2000), but more importantly because lycopene has been extensively investigated as a model target for metabolic engineering (Farmer and Liao 2000; Wang et al. 2009; Alper et al. 2005). Up to now, several strategies have been employed to improve lycopene production in E. coli: first, overexpressing the native 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway (Choi et al. 2010; Jin and Stephanopoulos 2007) or introducing a heterologous mevalonate (MVA) pathway into E. coli to increase the supply of isoprenoid synthesis precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) (Yoon et al. 2007; Martin et al. 2003); second, identification of gene knockouts or amplification targets for improving lycopene production through systematic and combinatorial methods (Kang et al. 2005; Jin and Stephanopoulos 2007; Choi et al. 2010; Alper et al. 2005); third, the optimization of fermentation processes, for example, by adding auxiliary carbon sources (Kim et al. 2011) as well as maintaining high O2 levels and appropriate pH values was used to improve lycopene production (Yoon et al. 2006). In addition, optimization of the NADPH and ATP levels is an effective approach for enhancing lycopene production (Tao et al. 2014).
Hydrophobic terpene products are considered to accumulate in the membrane compartment of E. coli (Ahrazem et al. 2016). In the previous work (Wu et al. 2017), we engineered the membrane by overexpressing membrane-bending proteins, which increased the β-carotene specific production. Lycopene is a representative hydrophobic molecule with a long straight chain composed entirely of carbon and hydrogen atoms. In fact, the molecular structure of lycopene is quite similar to alkanes, oils and fats, but different from that of β-carotene, which contains hydrocarbon rings.
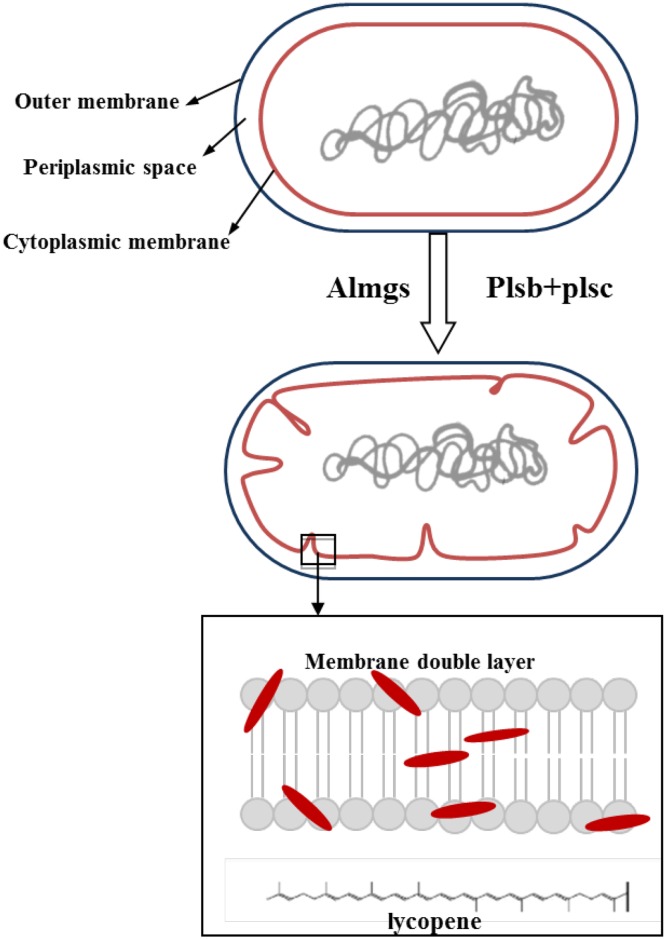
In this work, we aimed to apply the membrane engineering strategy to a lycopene hyper-producing strain of E. coli by introducing membrane-bending proteins and enhancing the membrane-synthesis pathway, to study how this strategy would affect the production and accumulation of lycopene (Fig. 1).
Fig. 1.
Membrane engineering strategies for increasing lycopene production and accumulation. Membrane morphology and the membrane-synthesis pathway were engineered to improve the production and accumulation of lycopene in E. coli
Materials and methods
Strains, medium, and culture conditions
All bacterial strains used for DNA manipulation and lycopene production in this study are listed in Table 1. The lycopene-producing strain LYC101 was used as the parent strain for the membrane engineering practice. For strain construction, cultures were grown aerobically at 30 or 37 °C in Luria broth (per liter: 10 g tryptone, 5 g yeast extract, and 10 g NaCl). For lycopene production, single colonies were picked from LB plates and transferred into 15 mm × 100 mm tubes containing 4 mL of LB with or without 34 mg/L of chloramphenicol, and cultured at 37 °C and 250 rpm overnight. The resulting seed cultures were used to inoculate 100 mL flasks containing 10 mL of fermentation medium to an initial OD600 of 0.05, and grown at 37 °C and 250 rpm. For strains bearing the Ptrc promoter, 1 mM IPTG was added 3 h after inoculation, which was followed by 93 h of growth. The fermentation medium contained (per liter): 15 g glycerol, 1.7 g citric acid, 10.5 g KH2PO4·3H2O, 6 g (NH4)2HPO4, 3.44 g MgSO4·7H2O, and 10 mL trace metal solution. The trace metal solution contained (per liter): 10 gFeSO4·7H2O, 5.25 g ZnSO4·7H2O, 3.0 g CuSO4·5H2O, 0.5 g MnSO4·4H2O, 0.23 g Na2B4O7·10H2O, 2.0 g CaC12, and 0.1 g (NH4)6Mo7O24. After 48 h of growth, cells were collected for measurement of lycopene production and after which 1.5% (w/v) glycerol was added into the culture medium for another 48 h continued fermentation. After 96 h of fermentation, the cells were collected for the measurement of lycopene production.
Table 1.
Strains and plasmids used in this work
| Strains and plasmids | Relative characteristics | Resources |
|---|---|---|
| Strains | ||
| CAR005 | ATCC 8739, M1-37::dxs, M1-46::idi M1-93::Crt, M1-46::SucAB, M1-46::sdh, M1-46::talB |
Lab collection |
| CAR015 | CAR005, ispG-mRSL-4, ispH-mRSL-14 | Lab collection |
| CAR025 | CAR015, replacing the promoter of crtEYIB with Ptrc promoter | Lab collection |
| LYC101 | CAR025, △crtY | Lab collection |
| LYC101-37Almgs LYC101-46Almgs |
LYC101, M1-37::Almgs LYC101, M1-46::Almgs |
This work This work |
| Plasmids | ||
| pACYC184-M | cat; replace tet with lacI and Ptrc of pTrc99A-M | Lab collection |
| pCas9 | Cas9 | (Zhao et al. 2016) |
| pAlmgs | pACYC184-M with Ptrc controlled almgs | This work |
| pMtlA | pACYC184-M with Ptrc controlled mtla | This work |
| pTsr | pACYC184-M with Ptrc controlled tsr | This work |
| pPlsb | pACYC184-M with Ptrc controlled plsb | This work |
| pPlsb-plsc | pACYC184-M with Ptrc controlled plsb and plsc | This work |
| pPlsb-plsc-dgka | pACYC184-M with Ptrc controlled plsb, plsc, and dgka | This work |
Plasmids construction
All plasmids used in this study are listed in Table 1. Plasmids were assembled using the Golden Gate method (Hillson et al. 2012). We applied several plasmids used in article (Wu et al. 2017) in this study. All primers were synthesized by Genewiz (Beijing, China) and are listed in Supplementary Table 1. Gene sequencing was also carried out by Genewiz.
Integration of almgs into the E. coli chromosome
The plasmids pM1-37Almgs and pM1-46Almgs were constructed to integrate almgs (monoglucosyldiacylglycerol synthase from A. laidlawii) into the E. coli chromosome under the control of the M1-37 and M1-46 promoter, respectively. Each integration plasmid also contained homologous arms for integration and gRNA with an N20 sequence. The plasmid pCas9 was co-electroporated with each of the plasmids into the lycopene-producing strain LYC101 and the resulting strains were processed using the Cas9 genome editing protocol as described previously (Zhao et al. 2016), yielding the almgs integration strains.
Analysis of lycopene production
The lycopene titers were quantified by measuring the absorption at 480 nm of acetone extracts of the cells as described previously (Yuan et al. 2006) with some modifications as follows: cells were harvested by centrifugation at 16,200×g for 3 min, resuspended in 1 mL of acetone, incubated at 55 °C for 15 min in the dark, and centrifuged at 16,200×g for 10 min, and the acetone supernatant containing lycopene transferred to a new tube. The lycopene content was analyzed using HPLC (Agilent Technologies Series 1200 system, Agilent, USA) with a variable wavelength detector set to 480 nm and a Symmetry C18 column (250 mm × 4.6 mm, 5 µm, Waters, Ireland). Methanol/acetonitrile/dichloromethane (21:21:8, by vol) was used as the mobile phase at 1 mL/min and the column was kept at 30 °C (Yoon et al. 2009). The results represent the means ± S.D. of three independent experiments. Dry cell weight (DCW) was calculated according to the empirical formula: 1 OD600 = 0.323 g DCW/L.
Extraction of cell membranes from strains derived from LYC101
Cell membranes of the strains derived from LYC101 were extracted as described previously (Herskovits et al. 2002; Lu et al. 2014) with some modifications as follows: After induction at 37 °C for 48 h, the cells were collected and resuspended in buffer (50 mM Tris/HCl pH 7.5 and 150 mM NaCl). Following cell disruption by passing the suspension four times through a French Press (JN-3000plus, China) at 8000 psi, cell debris was removed by centrifugation at 8500g for 40 min. The supernatant was collected and centrifuged at 210,000×g for 1 h using a Beckman Optima L-100XP ultracentrifuge equipped with a Beckman-Coulter 41Ti rotor (Beckman-Coulter, Germany). The membrane fraction was collected at the bottom of the centrifuge tube as a pellet and stored at − 80 °C for further analysis.
Analysis of lycopene content in the cell membranes
The harvested cell membranes were resuspended in 10 mL of acetone, incubated at 55 °C for 15 min in the dark, and centrifuged at 16,200×g for 10 min. The acetone supernatant fraction containing lycopene was collected and quantified as described above. The lycopene content in the cell debris was analyzed using the same method.
Results and discussion
Engineering membrane morphology to improve lycopene production
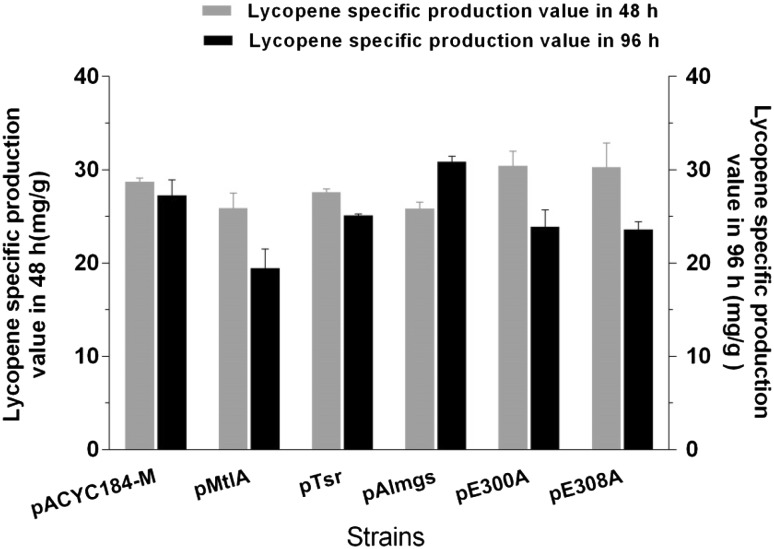
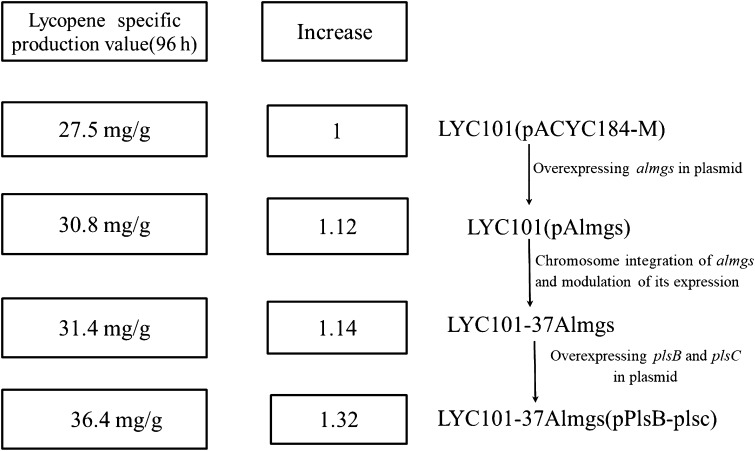
To study the relationship between membrane surface area and lycopene production in E. coli, proteins reported to induce the formation of membrane stacks or vesicles were overexpressed in the previously engineered lycopene-producing strain LYC101 (Table 1). The membrane-bending proteins used in this study include the mannitol permease MtlA (van Weeghel et al. 1990) and the chemotaxis receptor Tsr (Herskovits et al. 2002), as well as Almgs and its two functionally-defective mutants, E300A and E308A (Eriksson et al. 2009). The medium-copy plasmid pACYC184-M was used to express these genes under the control of the Ptrc promoter. Among the membrane-bending proteins, Almgs had the highest impact (Fig. 2). The strain LYC101(pAlmgs) produced 327.2 mg/L lycopene with a specific production of 30.8 mg/g DCW after 96 h of growth, which was 20.7% and 12% higher than the corresponding values of the parent strain, respectively (Fig. 2, Supplementary Fig. 1).
Fig. 2.
Lycopene-specific production of strain LYC101 expressing various membrane-bending proteins in 48 and 96 h. The overexpressed genes included mtlA, tsr, almgs, almgs-E300A, and almgs-E308A. Three repeats were performed for each strain, and the error bars represent standard deviation
Thus, expression of proteins with a membrane-bending function actually improved lycopene production in E. coli, suggesting that membrane engineering is broadly applicable for increasing the production of hydrophobic terpenes, and even other products with similar storage mechanisms. Among all the tested proteins, Eriksson et al. (2009) observed the most extensive formation of intracellular vesicles with the expression of Almgs. In agreement with this, we achieved the highest lycopene production with the same protein after 96 h of growth (Fig. 2, Supplementary Fig. 1). Furthermore, with the supplementation of glycerol after 48 h of culture, all strains grew further, whereby the strain LYC101(pAlmgs) maintained a higher specific production of lycopene, which suggested that the Almgs protein helped the cells produce sufficient intracellular membrane vesicles in the lycopene accumulation period, and thus provided more space for lycopene accumulation.
Integrating almgs into the chromosome and modulating its expression
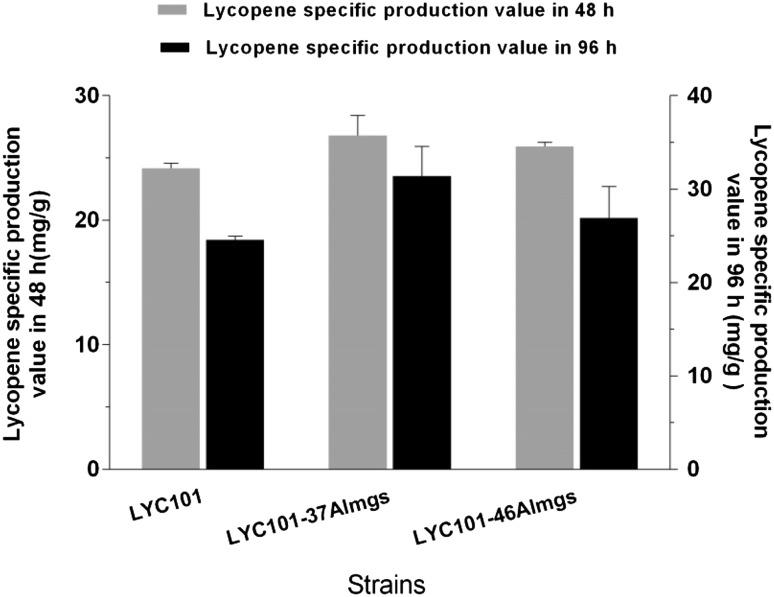
Since the plasmid-based expression of almgs showed the most promising results after 96 h of growth, it was integrated into the chromosome of strain LYC101 to yield two strains, each using one of the two different regulatory parts M1-46 and M1-37 (Lu et al. 2012). The strengths of these regulatory parts were 1.7 and 2.5 times the strength of a fully induced E. coli lacZ promoter (Lu et al. 2012). Both engineered strains had higher specific lycopene production values than the parent strain LYC101 with and without the almgs expression plasmid, among which almgs expression under the control of M1-37 gave the highest specific production and titer (29.4 mg/g DCW and 302.5 mg/L, respectively) (Fig. 3, Supplementary Fig. 2). The results suggested that an intermediate expression level was the most appropriate for this specific application.
Fig. 3.
Lycopene-specific production of engineered E. coli strains obtained by integrating almgs into the chromosome of strain LYC101, followed by gene expression modulation. The expression of the Almgs gene was modulated using two different promoters (M1-37 and M1-46). Three repeats were performed for each strain, and the error bars represent standard deviation
Engineering the membrane-synthesis pathway to improve lycopene production
The inner membrane of E. coli is a glycerophospholipid bilayer, which contains three major phospholipids: phosphatidylethanolamine (PE), phosphatidylglycerol(PG), and cardiolipin (CL) (also called diphosphatidyl glycerol) (Cronan and Rock 2008). Plsb(glycerol-3-phosphate-acyltransferase), Plsc (1-acylglycerol-3-phosphate-acyltransferase), and Dgka (diacylglycerol kinase) are the key enzymes of the diglyceride-3-phosphate synthesis pathway, the overexpression of which was reported to increase the amount of glycerophospholipids in E.coli cells (Janßen and Steinbüchel 2014). In this work, we overexpressed plsb alone, plsb with plsc, or plsb with both plsc and dgka to engineer membrane synthesis to enhance lycopene accumulation.
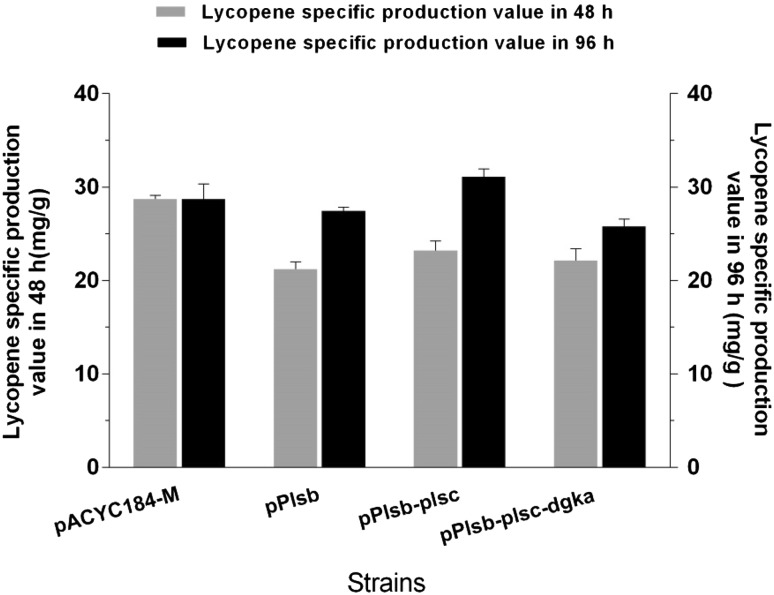
As illustrated in Fig. 4 and Supplementary Fig. 3, the specific production and titer of lycopene of the engineered strains were, indeed, improved compared with the parent strain. The strain LYC101(pPlsb-plsc) was the best performer, with a production of 31.1 mg/g DCW and 297.5 mg/L lycopene. The simultaneous overexpression of plsb and plsc enhanced the pathway flux towards diglyceride-3-phosphate synthesis, which also increased the amount of membrane structures and provided more space to store lycopene.
Fig. 4.
Lycopene-specific production of strains obtained from LYC101 by overexpressing membrane-synthesis genes in 48 and 96 h. Overexpression plasmids included pPlsb, pPlsb-plsc, and pPlsb-plsc-dgka. Three repeats were performed for each strain, and the error bars represent standard deviation
The combined engineering of membrane synthesis and membrane morphology further improved lycopene production
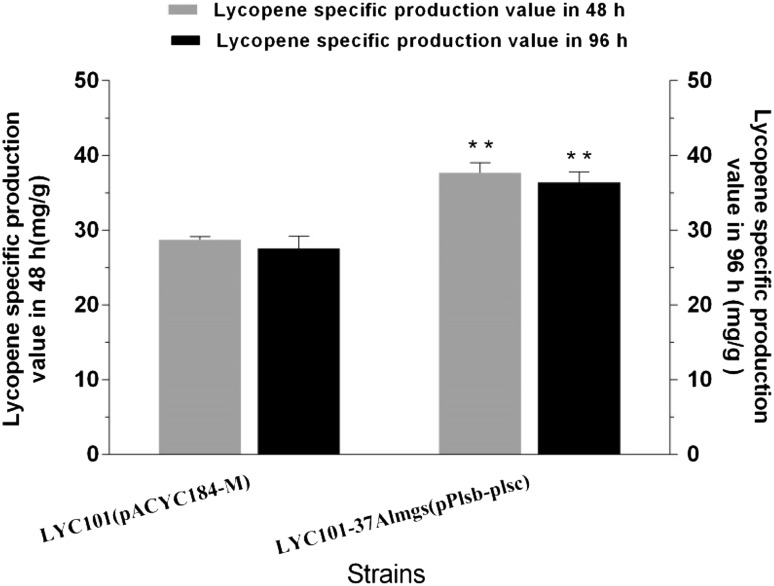
To combine membrane synthesis and membrane morphology engineering strategies in one strain, the plasmid pPlsb-plsc was introduced into LYC101-37Almgs to study possible synergistic effects of their combined expression. As illustrated in Fig. 5 and Supplementary Fig. 4, the strain LYC101-37Almgs(pPlsb-plsc) had a more pronounced increase of lycopene production which reached 216.3 mg/L lycopene with a specific production of 37.7 mg/g DCW at 48 h, and 358.9 mg/L lycopene with 36.4 mg/g DCW at 96 h. After 96 h of growth, the titer of all strains was increased, but, since more carbon source was used for cell growth, it was reasonable that specific production of some strains was decreased slightly.
Fig. 5.
Lycopene-specific production of strain LYC101 -37Almgs overexpressing plsb + plsc. Three repeats were performed for each strain, and the error bars represent standard deviation. The significance of differences was calculated by one-way ANOVA; asterisks indicate a significant difference compared with the control (**P < 0.01)
Compared with the original strain LYC101(pACYC184-M), the lycopene titer and specific production increased 26.7 and 26% after 48 h of growth, respectively. Furthermore, the lycopene titer and specific production increased 27 and 30% after 96 h of growth, respectively (Fig. 5 and Supplementary Fig. 4). The significant lycopene production improvement (P < 0.01) in strain LYC101-37Almgs(pPlsb-plsc) suggested a synergistic effect of membrane synthesis and membrane morphology engineering. As we have stated in the previous paper, at the level of membrane morphology engineering, Almgs induced the formation of membrane stacks or tubules and increased the membrane surface area, which probably increased the demand for membrane building blocks needed to fulfill such an increase. Thus, the overexpression of plsb and plsc enhanced membrane synthesis, accommodated the demand for membrane building blocks, and achieved a synergetic effect on lycopene production.
Lycopene was mainly accumulated within the cell membranes
To further determine whether the accumulated lycopene was localized within the cell membranes, the membrane fraction was extracted as described previously (Herskovits et al. 2002; Lu et al. 2014) with some modifications, and acetone was used to extract the lycopene from the collected membranes for analysis. As shown in Table 2, the lycopene titers in the cell debris was low, and the majority of the lycopene content was associated with the cell membrane fraction in all strains. In the original lycopene-producing strains LYC101(pACYC184-M), lycopene in the cell membranes accounted for 55% of the total lycopene content. By contrast, in the engineered strain LYC101-37Almgs(pPlsb-plsc), the membrane-associated lycopene fraction increased markedly, reaching to 73.2% of the total content. It should be noted that the apparent total content of lycopene decreased in the process of membrane extraction. For example, LYC101-37Almgs(pPlsb-plsc) produced 187.6 mg/L lycopene. After extraction, the total lycopene from the two portions only added up to 103 mg/L, indicating a more than 45% loss. Nevertheless, considering the complex cell manipulation and lycopene extraction procedure, we considered the loss reasonable.
Table 2.
Lycopene contents in different portions of engineered E. coli strains
| Strains | Cell debris (mg/L) | Cell membrane (mg/L) | Ratio (lycopene in cell membrane/total lycopene content) |
|---|---|---|---|
| LYC101(pACYC184-M) | 36.33 ± 0.99 | 45.10 ± 4.79 | 55.4% |
| LYC101-37Almgs(pPlsb-plsc) | 27.84 ± 1.14 | 75.89 ± 5.26 | 73.2% |
The results proved that lycopene, a representative hydrophobic molecule with straight long hydrocarbon chain, was also mainly accumulated in the membrane compartment of E. coli cell factories, which is probably true for other compounds with such characteristics. The experiments also demonstrated that engineering the cell membrane increased the accumulation of lycopene within it, and led to an overall increase of lycopene production, similar to our previous work has shown for β-carotene. implying that this strategy may, indeed, be broadly applicable.
Conclusion
In this work, the membrane of E. coli was engineered in both morphological and biosynthetic aspects, which improved its storage capacity for lycopene. Through membrane engineering, a lycopene-producing strain was obtained which exhibited a 1.32-fold-specific production increase over the parent strain (Fig. 6). It appears increasingly likely that microbial cell factories for a wide range of hydrophobic products, such as alkanes, oils, fats, and a range of important hydrophobic natural products, can be further improved using this novel engineering strategy.
Fig. 6.
Diagram summarizing the increase of lycopene-specific production obtained through membrane engineering
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (31522002), National High Technology Research and Development Program of China (2015AA020202), Tianjin Key Technology R&D program of Tianjin Municipal Science and Technology Commission (14ZCZDSY00067), and Novo Nordisk-Chinese Academy of Sciences (NN-CAS) Research Fund (NN-CAS-2015-2).
Author Contributions
WT performed research, analyzed data, designed research, and wrote the paper; YL, ZD, and LS designed research and analyzed data; LQ provided the bacteria; BC and ZB designed research, analyzed data, and wrote the paper. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1298-8) contains supplementary material, which is available to authorized users.
Contributor Information
Tao Wu, Email: wutao@tib.cas.cn.
Lijun Ye, Email: ye_lj@tib.cas.cn.
Dongdong Zhao, Email: zhao_dd@tib.cas.cn.
Siwei Li, Email: li_sw@tib.cas.cn.
Qingyan Li, Email: li_qy@tib.cas.cn.
Bolin Zhang, Email: Zhangbolin888@sina.com.cn.
Changhao Bi, Email: bi_ch@tib.cas.cn.
References
- Ahrazem O, Rubio-Moraga A, Berman J, Capell T, Christou P, Zhu C, Gomez-Gomez L. The carotenoid cleavage dioxygenase CCD2 catalysing the synthesis of crocetin in spring crocuses and saffron is a plastidial enzyme. New Phytol. 2016;209(2):650–663. doi: 10.1111/nph.13609. [DOI] [PubMed] [Google Scholar]
- Ajikumar PK, Tyo K, Carlsen S, Mucha O, Phon TH, Stephanopoulos G. Terpenoids: opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol Pharm. 2008;5(2):167. doi: 10.1021/mp700151b. [DOI] [PubMed] [Google Scholar]
- Alper H, Jin YS, Moxley JF, Stephanopoulos G. Identifying gene targets for the metabolic engineering of lycopene biosynthesis in Escherichia coli. Metab Eng. 2005;7(3):155–164. doi: 10.1016/j.ymben.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Bhataya A, Schmidt-Dannert C, Lee PC. Metabolic engineering of Pichia pastoris X-33 for lycopene production. Process Biochem. 2009;44(10):1095–1102. doi: 10.1016/j.procbio.2009.05.012. [DOI] [Google Scholar]
- Choi HS, Lee SY, Kim TY, Woo HM. In silico identification of gene amplification targets for improvement of lycopene production. Appl Environ Microbiol. 2010;76(10):3097–3105. doi: 10.1128/AEM.00115-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SK. Lycopene: chemistry, biology, and implications for human health and disease. Nutr Rev. 1998;56(2):35–51. doi: 10.1111/j.1753-4887.1998.tb01691.x. [DOI] [PubMed] [Google Scholar]
- Cronan JE, Jr, Rock CO. Biosynthesis of membrane lipids. EcoSal Plus. 2008 doi: 10.1128/ecosalplus.3.6.4. [DOI] [PubMed] [Google Scholar]
- Eriksson HM, Wessman P, Ge C, Edwards K, Wieslander A. Massive formation of intracellular membrane vesicles in Escherichia coli by a monotopic membrane-bound lipid glycosyltransferase. J Biol Chem. 2009;284(49):33904–33914. doi: 10.1074/jbc.M109.021618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer WR, Liao JC. Improving lycopene production in Escherichia coli by engineering metabolic control. Nat Biotechnol. 2000;18(5):533–537. doi: 10.1038/75398. [DOI] [PubMed] [Google Scholar]
- Herskovits AA, Shimoni E, Minsky A, Bibi E. Accumulation of endoplasmic membranes and novel membrane-bound ribosome-signal recognition particle receptor complexes in Escherichia coli. J Cell Biol. 2002;159(3):403–410. doi: 10.1083/jcb.200204144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillson NJ, Rosengarten RD, Keasling JD. j5 DNA assembly design automation software. ACS Synth Biol. 2012;1(1):14–21. doi: 10.1021/sb2000116. [DOI] [PubMed] [Google Scholar]
- Janßen HJ, Steinbüchel A. Fatty acid synthesis in Escherichia coli and its applications towards the production of fatty acid based biofuels. Biotechnol Biofuels. 2014;7(1):7. doi: 10.1186/1754-6834-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YS, Stephanopoulos G. Multi-dimensional gene target search for improving lycopene biosynthesis in Escherichia coli. Metab Eng. 2007;9(4):337–347. doi: 10.1016/j.ymben.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Kang MJ, Lee YM, Yoon SH, Kim JH, Ock SW, Jung KH, Shin YC, Keasling JD, Kim SW. Identification of genes affecting lycopene accumulation in Escherichia coli using a shot-gun method. Biotechnol Bioeng. 2005;91(5):636. doi: 10.1002/bit.20539. [DOI] [PubMed] [Google Scholar]
- Kim YS, Lee JH, Kim NH, Yeom SJ, Kim SW, Oh DK. Increase of lycopene production by supplementing auxiliary carbon sources in metabolically engineered Escherichia coli. Appl Microbiol Biotechnol. 2011;90(2):489–497. doi: 10.1007/s00253-011-3091-z. [DOI] [PubMed] [Google Scholar]
- Lu J, Tang J, Liu Y, Zhu X, Zhang T, Zhang X. Combinatorial modulation of galP and glk gene expression for improved alternative glucose utilization. Appl Microbiol Biotechnol. 2012;93(6):2455–2462. doi: 10.1007/s00253-011-3752-y. [DOI] [PubMed] [Google Scholar]
- Lu P, Ma D, Yan C, Gong X, Du M, Shi Y. Structure and mechanism of a eukaryotic transmembrane ascorbate-dependent oxidoreductase. Proc Natl Acad Sci USA. 2014;111(5):1813–1818. doi: 10.1073/pnas.1323931111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzouridou F, Tsimidou MZ. Lycopene formation in Blakeslea trispora. Chemical aspects of a bioprocess. Trends Food Sci Technol. 2008;19(7):363–371. doi: 10.1016/j.tifs.2008.01.003. [DOI] [Google Scholar]
- Martin VJ, Pitera DJ, Withers ST, Newman JD, Keasling JD. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat Biotechnol. 2003;21(7):796–802. doi: 10.1038/nbt833. [DOI] [PubMed] [Google Scholar]
- Michael MR, Bausch J. Summary of safety studies conducted with synthetic lycopene. Regul Toxicol Pharmacol. 2003;37(2):274–285. doi: 10.1016/S0273-2300(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Nagao A, Yoshikawa T. Absorption and function of dietary carotenoids. Forum Nutr. 2009;61:55. doi: 10.1159/000212738. [DOI] [PubMed] [Google Scholar]
- Rao AV, Agarwal S. Role of antioxidant lycopene in cancer and heart disease. J Am Coll Nutr. 2000;19(5):563–569. doi: 10.1080/07315724.2000.10718953. [DOI] [PubMed] [Google Scholar]
- Tao S, Miao L, Li Q, Dai G, Lu F, Tao L, Zhang X, Ma Y. Production of lycopene by metabolically-engineered Escherichia coli. Biotechnol Lett. 2014;36(7):1515–1522. doi: 10.1007/s10529-014-1543-0. [DOI] [PubMed] [Google Scholar]
- van Weeghel RP, Keck W, Robillard GT. Regulated high-level expression of the mannitol permease of the phosphoenolpyruvate-dependent sugar phosphotransferase system in Escherichia coli. Proc Natl Acad Sci USA. 1990;87(7):2613–2617. doi: 10.1073/pnas.87.7.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HH, Isaacs FJ, Carr PA, Sun ZZ, Xu G, Forest CR, Church GM. Programming cells by multiplex genome engineering and accelerated evolution. Nature. 2009;460(7257):894–898. doi: 10.1038/nature08187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Ye L, Zhao D, Li S, Li Q, Zhang B, Bi C, Zhang X. Membrane engineering—A novel strategy to enhance the production and accumulation of β-carotene in Escherichia coli. Metab Eng. 2017;43 Part A:85–91. doi: 10.1016/j.ymben.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Lee YM, Kim JE, Lee SH, Lee JH, Kim JY, Jung KH, Shin YC, Keasling JD, Kim SW. Enhanced lycopene production in Escherichia coli engineered to synthesize isopentenyl diphosphate and dimethylallyl diphosphate from mevalonate. Biotechnol Bioeng. 2006;94(6):1025–1032. doi: 10.1002/bit.20912. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Park HM, Kim JE, Lee SH, Choi MS, Kim JY, Oh DK, Keasling JD, Kim SW. Increased beta-carotene production in recombinant Escherichia coli harboring an engineered isoprenoid precursor pathway with mevalonate addition. Biotechnol Prog. 2007;23(3):599–605. doi: 10.1021/bp070012p. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Lee SH, Das A, Ryu HK, Jang HJ, Kim JY, Oh DK, Keasling JD, Kim SW. Combinatorial expression of bacterial whole mevalonate pathway for the production of beta-carotene in E. coli. J Biotechnol. 2009;140(3–4):218–226. doi: 10.1016/j.jbiotec.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Yuan L, Rouviere P, Larossa R, Suh W. Chromosomal promoter replacement of the isoprenoid pathway for enhancing carotenoid production in E. coli. Metab Eng. 2006;8(1):79–90. doi: 10.1016/j.ymben.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Zhao D, Yuan S, Xiong B, Sun H, Ye L, Li J, Zhang X, Bi C. Development of a fast and easy method for Escherichia coli genome editing with CRISPR/Cas9. Microb Cell Factories. 2016;15(1):205. doi: 10.1186/s12934-016-0605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.