Abstract
Purpose of Review
To discuss the potentially significant complications associated with medial patellofemoral ligament (MPFL) reconstruction. Additionally, to review the most current and relevant literature with an emphasis on avoiding these potential complications.
Recent Findings
Multiple cadaveric studies have characterized the anatomy of the MPFL and the related morphologic abnormalities that contribute to recurrent lateral patellar instability. Such abnormalities include patella alta, excessive tibial tubercle to trochlear grove (TT-TG) distance, trochlear dysplasia, and malalignment. Recent studies have evaluated the clinical outcomes associated with the treatment of concomitant pathology in combination with MPFL reconstruction, which is critical in avoiding recurrent instability and complications.
Summary
Although there remains a lack of consensus regarding various critical aspects of MPFL reconstruction, certain concepts remain imperative. Our preferred methods and rationales for surgical techniques are described. These include appropriate work up, a combination of procedures to address abnormal morphology, anatomical femoral insertion, safe and secure patellar fixation, appropriate graft length fixation, and thoughtful knee flexion during fixation.
Keywords: Medial patellofemoral ligament, MPFL, Patella instability, Recurrent patellar instability, Complications, MPFL reconstruction
Introduction
Patellar instability is a common orthopedic complaint representing up to 3% of clinical presentations involving the knee [1–3]. In the absence of intraarticular pathology, patients presenting with first-time dislocations are primarily treated nonoperatively [4–8]. Recurrent instability ranges from 15 to 71% of patients initially treated nonoperatively and requires operative intervention [1, 7, 9–12]. The medial patellofemoral ligament (MPFL) is the primary restraint to lateral displacement of the patella from 0° to 30° of knee flexion and is compromised in at least 80% of dislocations [13–16]. Thus, the treatment of MPFL incompetency is an essential element in the surgical management of recurrent patellar instability. However, there are multiple associated factors to consider and numerous technical aspects that bring challenges to a successful ligamentous reconstruction.
Steensen and colleagues studied the prevalence of associated anatomic factors in patellar dislocations and found statistically significant differences in Insall-Salvati ratio, tibial tubercle to trochlear groove (TT-TG) distance, and rotational alignment when comparing recurrent instability patients to controls, with anomalies most prevalent in the instability group [17]. Such disparities highlight the importance of understanding, identifying, and appropriately treating the anatomic factors that contribute to lateral patellar instability. This concept is particularly valid in cases occurring atraumatically, in which the baseline anatomy is the driving force for instability. In acute traumatic cases, MPFL rupture may exist in the presence of otherwise normal anatomy and be the solitary source of recurrent patellar instability.
Modern MPFL reconstruction is a well-described operative intervention which is successful in the vast majority of chronic patellar instability patients. Excellent functional results have been reported as high as 95%, and numerous studies have documented significant reduction in postoperative re-dislocation [18, 19]. Despite a high rate of success, the combined complication and failure rate of MPFL reconstruction remains substantial, reportedly 26% in one meta-analysis [20]. Recurrent postoperative instability does occur in a subset of patients. Other complications include anterior knee pain and decreased knee range of motion. Technical errors continue to be a major cause of complications and failures in patellar stabilization procedures. A thorough understanding of the technical aspects of MPFL reconstruction will allow surgeons to avoid complications and maximize clinical outcomes. We discuss the current literature on recurrent patellar instability, outline the evaluation and treatment of instability including associated factors, and describe our preferred operative techniques for MPFL reconstruction.
Associated Anatomic Factors: Diagnosis and Treatment
Patella Alta
The increase in patellar height due to patella alta places the patella superior to the deepest portion of the trochlea. Thus, patella alta contributes to recurrent patellar instability by placing the patella outside of the normal osteoarticular constraints of the trochlear groove. This concept is demonstrated clinically in patients with a positive J sign. On physical exam, a J sign is observed when the patella is displaced laterally out of the trochlear groove as the knee is extended from the flexed position. The patella shifts laterally on the round femoral shaft above the trochlear groove. Trochlear dysplasia can also result in a J sign on exam as the patella encounters the convex superior trochlear or supratrochlear spur in extension. The combination of patella alta plus trochlear dysplasia can result in a pronounced J sign with a dramatic lateral shift of the patella on active knee extension and very symptomatic patella instability.
Steensen et al. reported a 60.0% prevalence of patella alta in patients with recurrent instability compared to 20.8% in patients without [17]. Multiple methods have been described to quantify patella alta and to direct treatment. Widely accepted methods include the Insall-Salvati ratio, Blackburne-Peel ratio, Caton-Deschamps (CDI) index, and Plateau-patella angle [1, 21]. While choosing a single method is recommended to maintain consistency, an awareness and understanding of each of these methods is important as aberrant anatomy may preclude the use of a single modality. We prefer the CDI method as it measures the articulating patellar length rather than including the nonarticular patella nose, obviates the need for a 30° flexion lateral radiograph, and is highly reproducible. A CDI > 1.2 qualifies as patella alta, although the indication for tibial tubercle distalization is less rigid due to potential morbidity associated with the procedure and time for the osteotomy to heal. The combination of a J sign on physical examination and a high CDI classically necessitates a tibial tubercle osteotomy (TTO) with distalization. The tibial cut can be tapered and slid distally over the front of the distal tibia up to about 7 mm for smaller corrections. However, a step-cut TTO is generally needed for CDI > 1.4 and distalization approaching 1 cm or more. These step cuts can take a long time for bony union given the cut is across the tibial cortex. The goal is to achieve a CDI of approximately 1.1 as greater corrections can lead to stiffness or more pronounced changes in the patellofemoral articulation. Appropriate distalization restores the osseous constraints of the patellofemoral articulation by facilitating early patellar engagement.
Another consideration is the patella-trochlear index (PTI), which is the percent overlap of the patella and trochlear articular surfaces in extension on the MRI or CT scan. While this can reflect patella alta, it can also be influenced by a short trochlea. The combination of a PTI < 20% and CDI greater than 1.4 and a J sign on exam indicates clinically important patella alta that we feel should be corrected along with the MPFL reconstruction to ensure success.
Tibial Tubercle-Trochlear Groove Distance
Increased tibial tubercle-trochlear groove (TT-TG) distance is indicative of tibial tubercle (TT) lateralization and an increased Q-angle. Patients with increased TT-TG are susceptible to lateral patellar dislocation as the lateral component of the quadriceps contraction vector increases as the Q-angle increases. Thus, increased TT-TG is fairly common with a prevalence as high as 42.0% in recurrent instability cases [17]. Conventionally, clinical evidences of lateral patella tracking in combination with values of 20 mm and greater have been classified as excessive TT-TG with a recommendation to include an anteromedialization (AMZ) TTO in the treatment of recurrent instability [17, 22, 23]. Several authors have advocated for the use of TT-posterior cruciate ligament (TT-PCL) with a distance > 24 mm as the threshold for distal realignment as TT-PCL relies exclusively on tibial landmarks, eliminating the influence of femoral rotation [24–26]. Although TT-TG remains the more widely accepted method, there may be considerable benefit to the use of TT-PCL in the setting of Dejour type B, C, or D trochlear dysplasia due to the inherent challenges in identifying an abnormal or absent trochlear groove. The classic description and threshold of TT-TG by Dejour et al. were based on computed tomography (CT) scans. Currently, axial magnetic resonance imaging (MRI) is commonly utilized as the imaging modality of choice as it allows evaluation of the MPFL and other soft tissue anatomy in conjunction with TT-TG. Readers are cautioned as several studies have revealed that MRI-based TT-TG values are up to 4 mm less than CT based measurements [27, 28]. Moreover, it is important to consider increased TT-TG in the context of physical examination findings of lateral patellar tracking, and lateral translation of the patella on 45 degree flexed axial X-rays, as the TT-TG should not be evaluated as a lone absolute indication.
Anteromedialization TTO is a well-described technique recommended as a first-line treatment in combination with MPFL reconstruction for patients with excessive TT lateralization [29, 30]. Additionally, the anteriorization component has proven to be beneficial in patients with concomitant patellar chondromalacia. The goal of AMZ TTO is to normalize the TT-TG to 10–12 mm and thus prevent overloading the MPFL graft. When indicated, TTO is a critical component in the surgical management of recurrent patellar instability and avoiding failure of an MPFL reconstruction.
TTO performed for the treatment of patellar instability has resulted in less dislocation episodes and good to excellent Lysholm outcome scores in 73% of patients [31, 32]. A recent study reported a reduction in the rate of return to sport (RTS) and a delay in return to high-level competitive sports for TTO patients [32]. We have experienced similar RTS outcomes, however, observed return at 3 months for isolated MPFL reconstruction and 6 months for the combined procedure. Complications associated with TTO include painful hardware most commonly (36.7%), tibial fractures (1.0%), and nonunions (0.8%) with an overall complication rate of 4.6% for patients undergoing concomitant soft tissue stabilization for patellar instability [33]. TTO is a reproducible and often necessary procedure in the treatment of recurrent instability. Due to the proven differences compared to isolated MPFL reconstruction in regard to recovery, RTS, and complications, patient education and expectation management are paramount.
Trochlear Dysplasia
Trochlear dysplasia has been reported as the most prevalent associated anatomic factor in recurrent patellar instability, seen in 68.3% of patients compared to 5.8% of nondislocators [17]. The Dejour trochlear dysplasia classification describes a shallow trochlear with crossing sign as a type A. Type B represents a flat trochlea and a supratrochlear spur. The combination of lateral convexity, medial hypoplasia, and an anterior femoral double contour is designated by type C. Lastly, type D is characterized by a crossing sign, supratrochlear spur, and double contour sign (Fig. 1) [34]. Types B and D are considered the most problematic due to the supratrochlear spur which displaces the patella laterally, preventing distal trochlear groove engagement particularly in higher flexion angles. While types A and C are considered milder forms, such morphology may exhibit synergism with rotational malalignments resulting in recurrent dislocations.
Fig. 1.
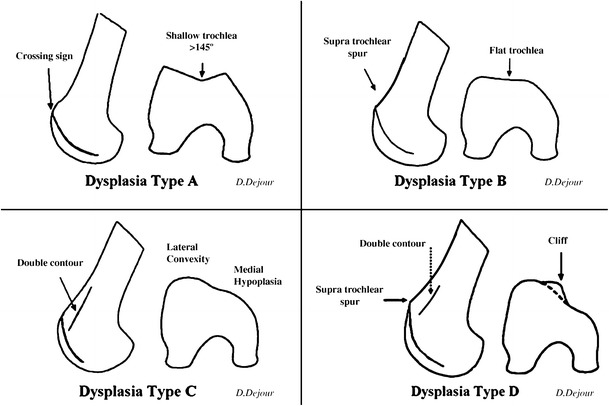
Trochlear dysplasia classification according to D. Dejour. In type A, the crossing sign is present. Type B features include the crossing sign and trochlear spur. In type C, there is a crossing sign and the double contour sign. Type D combines the crossing sign, supratrochlear spur, and double contour sign (with permission of Springer: Dejour D, Saggin P. The sulcus deepening trochleoplasty—the Lyon’s procedure. Int Orthop 2010;34:313; Fig. 2)
Both true lateral and axial knee radiographs are utilized in evaluating for trochlear dysplasia. Dysplastic changes primarily affect the proximal trochlea resulting in flattening or convexity. Of note, axial views obtained in greater knee flexion angles are often less helpful as the patella is frequently well centered within the trochlear distal groove despite the proximal groove dysplastic changes. Advanced cross sectional diagnostic imaging is recommended to better define trochlear morphology. With CT and/or MRI, the classification and severity of the dysplasia is identified and supratrochlear spur height is determined. A sulcus deepening trochleoplasty can be considered for dysplastic patients with recurrent patellar instability in combination with reconstruction of the MPFL. The presence of a convex proximal trochlea with a supratrochlear spur height greater than or equal to 5 mm, a DeJour type B or D trochlea, and a J sign on physical exam all indicate clinically important dysplasia that may benefit from treatment with deepening trochleoplasty. With appropriate patient selection, trochleoplasty has been shown to decrease patellar instability and normalize patellofemoral biomechanics [35, 36]. Favorable outcomes for the procedure have been observed in patients with supratrochlear spur height of 5–6 mm or greater [37, 38]. Contraindications for trochleoplasty include open physes and patellofemoral arthrosis. Arthrofibrosis and subsequent loss of knee range of motion are the most common associated complications and often require return to the operating room for lysis of adhesions (LOA) and manipulation under anesthesia (MUA) [39, 40]. Arthrofibrosis rates have been reported as high as 46%; however, our unpublished data is consistent with more recent literature reporting a prevalence of 0–20% [38, 41, 42]. We have found that early and aggressive range of motion is a key factor to reduce arthrofibrosis. Other complications include trochlear shingle fracture, particularly in arthritic patients, articular step off, and degenerative joint disease. While some authors have recommended trochleoplasty solely as a salvage due to concerning complication rates, others have reported adequate stabilization and improved function outcomes and offer a much stronger recommendation in the setting of combined MPFL insufficiency and trochlear dysplasia [29, 39, 41, 43–45].
Lateral Retinacular Tightness
Lateral retinacular tightness can occur in isolation, producing lateral patellar hypercompression, or in combination with MPFL insufficiency, further contributing to lateral patellar instability. In either scenario, both lateral retinacular release (LRR) and lateral retinacular lengthening (LRL) procedures have been described. In the setting of instability, thorough clinical, radiographic, and intraoperative assessments are critical. On examination, the patellar glide test in full knee extension isolates the soft tissue restraints. Medial patellar glide < 1 quadrant is consistent with lateral tightness. With normal physiologic patellar tilt ranging from 0° to 20°, the inability to evert the patella to neutral is indicative of tight lateral structures. A J sign can be associated with lateral retinacular tightness. Radiographically, a 30° flexed axial (Merchant) view is typically used to evaluate tilt; however, some authors recommend imaging with progressive flexion to evaluate tilt reduction in early knee flexion [46].
Once the appropriate proximal and/or distal realignment procedures for the treatment of lateral patellar instability have been completed and lateral retinacular tightness has been established, a release or lengthening must be performed in order to restore the neutral position of the patella within the trochlear groove. Neither LRR nor LRL should be performed in isolation for patellar instability as this would further destabilize the patellofemoral joint. Lateral release methods have fallen out of favor due to complications including iatrogenic medial instability [47–49]. The more commonly used LRL techniques obviate this complication by avoiding excessive release and muscle injury, improving overall soft tissue balance, and are associated with superior functional outcomes and rates of return to athletic activities [46, 50, 51]. We prefer a z-plasty LRL technique with reapproximation of the deep and superficial lateral retinacular layers without tension, allowing the patella to rest in a neutral position (Fig. 2). A more anatomic patellofemoral articulation likely reduces overload of the MPFL graft in higher (> 90°) knee flexion angles.
Fig. 2.
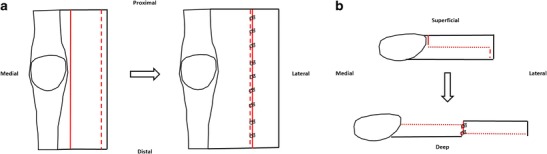
Lateral retinacular lengthening technique with reapproximation of the deep and superficial lateral retinacular layers without tension, allowing the patella to rest in a neutral position. Adapted from Shubin Stein/Strickland/Redler; Patellofemoral Arthroplasty chapter, Masters Techniques: Reconstructive Knee Surgery 4th Edition
Malalignment
The aforementioned TTO, trochleoplasty, and lateral lengthening procedures are among those more commonly performed in combination with MPFL reconstruction. Less frequent abnormal osseous anatomy, often but not exclusively seen in the pediatric population, must not be overlooked. Genu valgum can contribute to lateral patellar instability, particularly in severe cases. Distal femoral opening wedge osteotomy combined with MPFL reconstruction has been shown to decrease recurrence in patients with closed physes [52]. Similarly, hemiepiphysiodesis has been utilized in skeletally immature patients with success in limiting further dislocations [6, 53]. Although the parameters for rotational malalignment have not been clearly defined, excessive femoral anteversion in the setting of an incompetent MPFL has been postulated as a contributing factor to recurrent patellar dislocation [17]. Outcome data has been favorable for improved symptomatology without further instability in patients with femoral anteversion greater than 25° undergoing MPFL reconstruction in combination with supracondylar distal femoral derotation osteotomies [54].
Overall, the appropriate work up of recurrent lateral patellar instability must routinely include a systematic assessment of patella height, TT-TG, trochlear dysplasia, lateral retinacular tightness, patellar chondromalacia, as well as coronal and axial/rotational malalignment. Recurrent instability with clinical apprehension from 0 to 30° of flexion, TT-TG < 20 mm, CDI < 1.2, and the absence of significant trochlear dysplasia (i.e., normal or Dejour grade A) may be successfully treated with isolated MPFL reconstruction. Otherwise, the resulting evaluation of the above associated factors serves to direct the appropriate combination of surgical interventions to address the specific anatomy of each individual patient (Fig. 10).
Fig. 10.
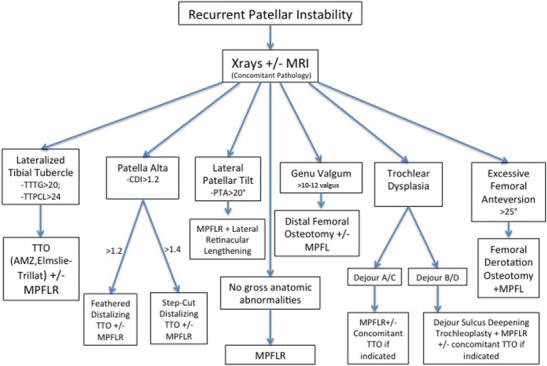
Derived from Laidlaw MS, Diduch DR. Current concepts in the management of patellar instability. Indian Journal of Orthopaedics. 2017;51 [5]:493–504
MPFL Reconstruction
Repair vs. Reconstruction
While both MPFL repair and reconstruction have been described as viable treatment options, the current standard continues to trend towards reconstruction. This paradigm shift has occurred over the past 5–10 years due to a combination of studies reporting high repair failure rates and greater than 80% good to excellent results with reconstruction [55–59]. Recently, Dragoo and colleagues reported similar clinical outcomes at 2 years for MPFL reconstruction and patients undergoing repairs of single-sided, femoral or patellar, insertional tears [60]. While this may represent a subset of chronic patellar instability patients for whom ligament repair is a viable option, reconstruction remains the most widely accepted technique as further research on selective MPFL repair is warranted.
Femoral Placement and Fixation: “High and Tight” vs. “Low and Loose”
Perhaps the most critical technical aspect of MPFL reconstruction is anatomic femoral graft placement. Restoration of the true femoral insertion maintains isometry of the graft which is essential to achieving favorable patella tracking throughout knee range of motion. Nonanatomic femoral insertion and the resulting graft anisometry have been associated with increased patellofemoral contact pressures in cadaveric studies as well as graft failure in reconstructions [61–63].
Several methods exist for identifying the anatomic femoral insertion site intraoperatively based on radiographic measurements and palpation of osseous landmarks. The radiographic techniques described by Stephen et al. and Schöttle et al. are reproducible and represent the most commonly utilized methods. Stephen’s percentage is a method by which the anterior to posterior distance of the medial femoral condyle is measured and taken to be 100%. The anatomic insertion site is identified 40% from the posterior, 50% from the distal, and 60% from the anterior condylar margin (Fig. 3) [62, 64]. Caution must be taken in utilizing this method as obtaining accurate percentages while maintaining sterility may be problematic. Schöttle’s point is our preferred method as studies have shown consistent placement of the femoral tunnel within 5 mm the femoral anatomic site of isometry [65–68]. First and foremost, a true lateral radiograph of the knee with complete superimposition of the medial and lateral condyles is essential. Even minimal rotational or translational deviations can result in inaccuracies and ultimately anisometry (Fig. 4) [69]. According to Schöttle’s method, the isometric point lies between the two intersections created by an extension of the posterior cortex and one perpendicular line crossing the posterior aspect of Blumensaat’s line and another perpendicular line crossing the posterior condylar-cortical transition (Fig. 5) [65]. In pediatric MPFL femoral tunnel placement, the starting point is slightly distal to conventional methods to ensure that drilling is contained to the epiphysis. The safest trajectory is approximately 15°–20° both anterior and distal as this angle minimizes the risk of iatrogenic damage to the physis, intercondylar notch, and the distal femoral cartilage. There is an increased emphasis on the intraoperative anteroposterior view to facilitate obtaining the appropriate angles [70].
Fig. 3.
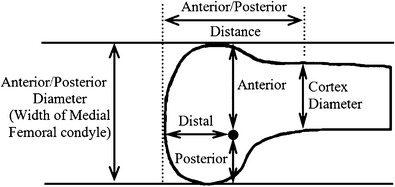
The medial patellofemoral ligament (MPFL) attachment is defined in relation to the anterior-posterior size of the medial femoral condyle: If the anterior-posterior size is referred to as 100%, then the MPFL attachment is 40% from the posterior and 50% from the distal outline. (Reprinted with permission from Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40:1871–1879. #2012, The American Orthopaedic Society for Sports Medicine)
Fig. 4.
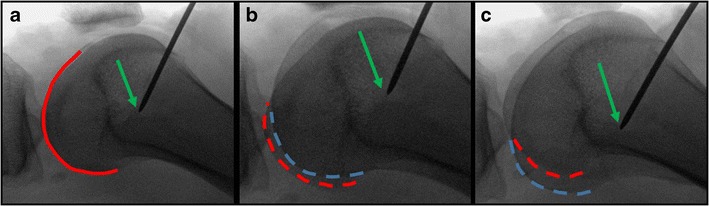
If a radiographic technique is used to locate the femoral MPFL insertion, it is imperative to obtain a perfect lateral radiograph. a A perfect lateral radiograph is confirmed with complete overlap of the posterior femoral condyles (red solid line). b If the femur is slightly internally rotated, then two condyles contours are now visualized with the medial condyle (red dashed line) appearing posterior to the lateral condyle (blue dashed line). c With external femoral rotation, now the lateral condyle (blue dashed line) appears posterior to the medial condyle (red dashed line). Importantly, the location of where the guide pin contacts the femur (green arrow) appears to change substantially based on rotation of the femur even though these are actually all the same location
Fig. 5.
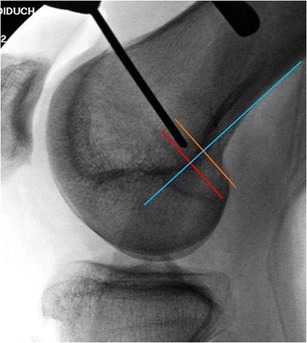
Intraoperative localization of Schöttle’s point. The blue line is drawn down the posterior femoral cortex. The orange line marks the transition of the curve of the posterior femoral condyle and is perpendicular to the blue line. The red line is at the posterior aspect of the Blumensaat line and is also perpendicular to the blue line. (Reproduced with permission from Burrus MT, Werner BC, Conte EJ, Diduch DR. Location, location, location—troubleshooting the femoral MPFL attachment. Orthop J of Sports Med, January 2015; 3 [1])
Identifying osseous landmarks by palpation is a helpful method by which preliminary location of the femoral insertion site may be obtained. In our experience, this step routinely lessens the number of corrections required under radiographic guidance. The apex of the adductor tubercle is a consistent landmark and is located 10.6 mm proximal to the MPFL insertion [71]. The palpable depression between the adductor tubercle and the medial epicondyle is the location of the femoral insertion. Final guide pin position must be confirmed radiographically prior to drilling. Additionally, the trajectory of the pin is also evaluated in order to avoid violation of the posterior cortex. Anterior and proximal angulation of the guide pin prevents posterior cortical and intercondylar notch penetration, respectively. Once the desired pin location is achieved, graft isometry is assessed through a full range of motion. Following patellar fixation, the graft is passed between the second and third medial soft tissue layers in the traditional fashion and the two graft limbs are wrapped around the pin. The knee is then taken through a full range of motion, and the graft is closely analyzed for changes in length (Fig. 6). Increased graft tension with flexion is indicative of proximal pin placement, i.e., “high and tight.” Graft laxity during flexion represents distal placement, i.e., “low and loose.” In either scenario, placement must be adjusted to achieve isometry and avoid complications of anterior knee pain and patellofemoral chondromalacia associated with graft tightness or recurrent instability due to graft laxity.
Fig. 6.
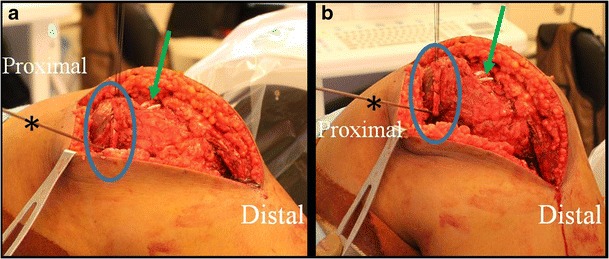
Intraoperative pictures of a MPFL reconstruction demonstrating isometry testing following placement of the guidewire (asterisk). Once the graft (blue circle) is looped around the femoral guidewire, the knee is taken through a range of motion to ensure that graft tension does not change. In these images, the two tails of the graft (green arrow) can be seen prior to their insertion on the patellar. Note that the MPFL was combined with additional procedures and thus the larger incision
Although the aforementioned femoral insertion location methods are routinely used and overall MPFL reconstruction outcomes remain favorable, an in-depth assessment of preoperative imaging is critical to understanding individual patient anatomy. High rates of anatomic abnormalities are pervasive in the recurrent patellar instability population and they frequently alter osseous landmarks causing inaccuracies when using the above methods [69, 72]. Thus, multiple methods must be utilized in order to most accurately identify the anatomic femoral insertion site. Ultimately, graft isometry must be demonstrated through full knee range of motion to ensure satisfactory outcomes.
The actual method of femoral fixation is much less critical once the proper location has been achieved and several techniques have been described. Common techniques include interference screw, suture anchor, all suture, suture onto medial condyle or proximal third of MCL, and bone tunnel fixation. Currently, there is a lack of high level evidence to support one technique over another [73–75]. We prefer interference screw fixation with an osteoconductive, fast absorbing biocomposite implant.
Patellar Placement and Fixation
The patellar insertion of the MPFL is a broad attachment along the proximal half of the medial patella and the distal most vastus medialis obliquus (VMO). Graft placement onto this location has been associated with reduced patellar instability and low complication rates [65, 76]. The onlay method secures the loop portion of the tendon graft to the insertion site with two suture anchors tied over the graft (Fig. 7). Other fixation techniques have been described including transpatellar sutures, suspensory devices, and interference screw fixation, without clear data suggesting a greater benefit of any one method [74, 77–79]. Alternatively, patellar fixation may be achieved by passing the graft limbs through two transosseous tunnels exiting medially (Fig. 8). A recent study reported a 3.6% fracture and 14.1% major complication rate using two 4.5 mm patellar tunnels [80]. Drilling smaller tunnels (< 4.5 mm in diameter) without convergence as well as avoiding transverse drilling will decrease the risk of such complications [6]. For those who choose to drill tunnels, we recommend drilling two provisional guide pins just off of the medial cartilage exiting anteriorly on the patella. This will allow confirmation of position and parallelism prior to final drilling. Once confirmed, the pins are overdrilled with a 3.5-mm drill to minimize the stress riser effect (Figs. 9 and 10). Mineral oil is used as needed to facilitate graft passage through the smaller tunnels.
Fig. 7.
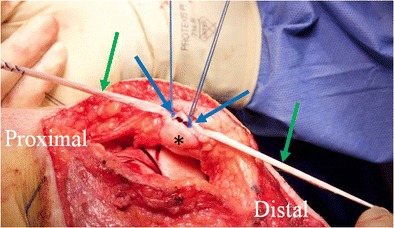
Intraoperative picture of a MPFL reconstruction using a suture anchor technique for patellar (asterisk) fixation. The gracilis autograft (green arrows) is laid over the top of the two suture anchors (blue arrows) which is then tied in place. The medial patellar cortical bone can be roughened up with a burr to encourage healing of the graft to the patella. Note that the MPFL was combined with additional procedures and thus the larger incision than would be required for only an MPFL reconstruction
Fig. 8.
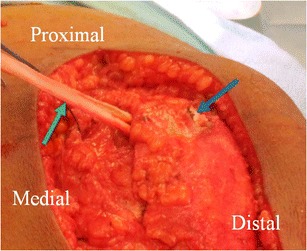
Intraoperative picture of a MPFL reconstruction which shows how the graft (green arrow) is looped through short, oblique drill holes over (blue arrow) the top of the patella (asterisk). Note that the MPFL was combined with additional procedures and thus the larger incision than would be required for only an MPFL reconstruction
Fig. 9.
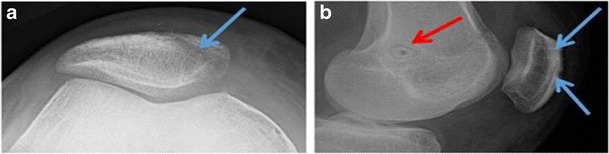
Postoperative sunrise (a) and lateral (b) radiographs demonstrating the appropriately placed MPFL tunnels. The blue arrows represent the two short, oblique patellar tunnels, and the red arrow shows the femoral tunnel located at Schöttle’s point with secure fixation using a bioabsorbable interference screw
Knee Flexion Angle for Fixation
Currently, there is a lack of consensus regarding the ideal knee flexion angle during MPFL fixation. Literature recommendations provide a wide range of descriptions which presents a challenge to surgeons seeking evidence based guidance [55, 61, 81–85]. Lorbach and colleagues conducted a cadaveric study in which MPFL femoral fixation at 60° of flexion achieved patellofemoral contact pressure most comparable to that of the intact knee [86]. In our cadaveric study, lower flexion angles proved to minimize the deleterious effects of femoral insertion malpositioning [81]. Lower flexion angles appear to be safer, as higher angles could potentially amplify femoral tunnel location errors. Thus, we recommend graft fixation in 30°–45° of flexion and postfixation confirmation of graft isometry throughout knee range of motion.
Graft Length
Graft tensioning for MPFL reconstruction is somewhat counterintuitive to other soft tissue reconstructions in which the objective is to maximize tightening. The MPFL serves as a checkrein to lateral patellar displacement and is not under tension during normal patellofemoral alignment. The appropriate tension required to reestablish native biomechanics has been demonstrated as 2 N or 0.5 lbs. [62, 87] Interference screw fixation must be done with caution, as insertion often increases tension as the graft is advanced into the tunnel. We recommend visual inspection and palpation of the graft during insertion as well as turning the screw back one half turn following full insertion. This technique serves to alleviate excess tension and avoid the complications of anterior knee pain and patellar chondromalacia due to increased contact pressures.
Graft Selection
There are many options for MPFL graft selection which include autograft and allograft. Although a double-tailed hamstring tendon graft is the most common configuration, there remains a paucity of evidence favoring one particular graft. Greater and more anatomic coverage of the native footprint is a potential benefit of utilizing a double-tailed graft [88]. Prior surgery, such as cruciate reconstruction wherein the hamstring tendons were harvested for the ipsilateral knee or to augment a contralateral reconstruction, must be noted preoperatively as this will limit autograft options. Skeletally immature patients may benefit from a medial quadriceps turndown, which functions as a local autograft with suture anchor fixation. This technique limits femoral bone tunneling in patients with open physes and is discussed in detail in a separate chapter.
Observations and Preferred Methods
When performing multiple procedures in combination with MPFL reconstruction, it is important to establish a clear order of operations. We recommend early semitendinosus graft harvest if autograft is chosen. This allows for simultaneous graft preparation during arthroscopy, provided that an assistant is available, and efficient use of tourniquet time. A thorough arthroscopic exam is essential to remove any loose bodies associated with patellofemoral chondral injuries and allows for precise chondroplasty as indicated. The TTO is performed next; however, fixation is deferred as the TTO provides excellent trochleoplasty exposure, if needed. Following the trochleoplasty, TTO fixation is completed restoring patellofemoral alignment and anatomic articulation. Patellar fixation of the graft is performed and the femoral guide pin is placed. Both the femoral insertion site and TTO screw fixation are verified radiographically and adjustments made as needed. Once location and isometry are confirmed, low tension femoral MPFL fixation is completed at lower flexion angles. Patellar tilt is evaluated and a LRL is performed as indicated. Common clinical pearls and pitfalls are summarized and must be considered for each MPFL reconstruction (Table 1).
Table 1.
Pearls and pitfalls of MPFL reconstruction
| Critical portions | Pitfalls | Pearls |
|---|---|---|
| Femoral insertion site | Poor or inconsistent technique | • Use radiographic or anatomic landmarks for accurate location • Same methods each case • Obtain perfect lateral X-ray prior to obtaining insertion site; frequently reassess • Avoid “high and tight” and “low and loose” |
| Relying on one technique | ||
| Imperfect lateral knee X-ray | ||
| Nonanatomic insertion of the MPFL | ||
| Tightening during knee flexion is the cardinal error | ||
| Patellar fixation | Drilling transverse tunnels | • Oblique, parallel tunnels • 3.2 mm tunnels to reduce fracture risk • Avoid interference screw(s) • Patellar ORIF set available |
| Convergent tunnels | ||
| ≥ 4.5 mm tunnels | ||
| Interference screw(s) | ||
| Graft tensioning | Applying maximum tension | • 2 N (or 0.5 lbs) of tension • MPFL is a checkrein • Reverse screw one half turn after final insertion |
| Inference screw graft tensioning | ||
| Knee flexion during fixation | Lack of consensus in literature | • 30°–45° of knee flexion • Allows the trochlear groove to capture the patella and set the graft length • Minimizes effect of femoral tunnel inaccuracies |
| Failure to consider implications of varying knee flexion angles |
Conclusion
All recurrent lateral patellar instability is not created equal. Moreover, it is an umbrella term which encompasses various etiologies such as patella alta, increased TT-TG, trochlear dysplasia, lateral retinacular tightness, and malalignment. In patients who have failed conservative treatments, appropriate work up, diagnosis, and surgical management of concomitant factors in combination with MPFL reconstruction is essential to avoiding complications and poor clinical outcomes. Numerous viable techniques have been described for MPFL reconstruction. We emphasize anatomic femoral placement via palpable osseous and radiographic landmarks, location confirmation with graft length analysis through range of motion, 30°–45° knee flexion during fixation, and graft tensioning at 2 N. Additionally, preferred methods are offered based on clinical experience and unpublished data. The multifactorial nature of patellar instability and the complexity of MFPL reconstruction present challenges to surgeons treating this condition. The importance of attention to proper surgical technique, appreciation of associated nuances, as well as an awareness of potential pitfalls cannot be overstated. Despite the inherent challenges employing a consistent, stepwise algorithm for both the diagnosis and the surgical treatment of recurrent patellar instability will aid in mitigating avoidable complications.
Compliance with Ethical Standards
Conflict of Interest
David R. Diduch reports personal fees from Depuy Mitek and grants from Zimmer outside of the submitted work. Brian C. Werner reports research support from Arthrex, Inc. and Biomet, Inc., outside of the submitted work, and is a committee member for AOSSM. Marvin K. Smith has no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Advances in Patellofemoral Surgery
References
- 1.Fithian DC, Paxton EW, Stone ML, Silva P, Davis DK, Elias DA, White LM. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114–1121. doi: 10.1177/0363546503260788. [DOI] [PubMed] [Google Scholar]
- 2.Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499–519. doi: 10.1016/S0278-5919(02)00031-5. [DOI] [PubMed] [Google Scholar]
- 3.Atkin DM, Fithian DC, Marangi KS, Stone ML, Dobson BE, Mendelsohn C. Characteristics of patients with primary acute lateral patellar dislocation and their recovery within the first 6 months of injury. Am J Sports Med. 2000;28(4):472–479. doi: 10.1177/03635465000280040601. [DOI] [PubMed] [Google Scholar]
- 4.Biedert R, Friederich N. Femoropatellar pain syndrome: which operation is still sensible? Ther Umsch. 1996;53(10):775–779. [PubMed] [Google Scholar]
- 5.Oestern S, Varoga D, Lippross S, et al. Patella dislocation. Unfallchirurg. 2011;114(4):345–358. doi: 10.1007/s00113-011-2012-z. [DOI] [PubMed] [Google Scholar]
- 6.Laidlaw MS, Diduch DR. Current concepts in the management of patellar instability. Indian J Orthop. 2017;51(5):493–504. doi: 10.4103/ortho.IJOrtho_164_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmu S, Kallio PE, Donell ST, Helenius I, Nietosvaara Y. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am. 2008;90(3):463–470. doi: 10.2106/JBJS.G.00072. [DOI] [PubMed] [Google Scholar]
- 8.Ries Z, Bollier M. Patellofemoral instability in active adolescents. J Knee Surg. 2015;28(4):265–277. doi: 10.1055/s-0035-1549017. [DOI] [PubMed] [Google Scholar]
- 9.Bitar AC, Demange MK, D'Elia CO, Camanho GL. Traumatic patellar dislocation: nonoperative treatment compared with MPFL reconstruction using patellar tendon. Am J Sports Med. 2012;40(1):114–122. doi: 10.1177/0363546511423742. [DOI] [PubMed] [Google Scholar]
- 10.Camanho GL, Viegas Ade C, Bitar AC, Demange MK, Hernandez AJ. Conservative versus surgical treatment for repair of the medial patellofemoral ligament in acute dislocations of the patella. Arthroscopy. 2009;25(6):620–625. doi: 10.1016/j.arthro.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Maenpaa H, Huhtala H, Lehto MU. Recurrence after patellar dislocation. Redislocation in 37/75 patients followed for 6-24 years. Acta Orthop Scand. 1997;68(5):424–426. doi: 10.3109/17453679708996255. [DOI] [PubMed] [Google Scholar]
- 12.Smith TO. Song F, Donell ST, Hing CB. Operative versus non-operative management of patellar dislocation. A meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2011;19(6):988–998. doi: 10.1007/s00167-010-1355-2. [DOI] [PubMed] [Google Scholar]
- 13.Conlan T, Garth WP, Jr LJE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682–693. doi: 10.2106/00004623-199305000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 1998;26(1):59–65. doi: 10.1177/03635465980260012701. [DOI] [PubMed] [Google Scholar]
- 15.Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;349(349):174–182. doi: 10.1097/00003086-199804000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Elias DA, White LM, Fithian DC. Acute lateral patellar dislocation at MR imaging: injury patterns of medial patellar soft-tissue restraints and osteochondral injuries of the inferomedial patella. Radiology. 2002;225(3):736–743. doi: 10.1148/radiol.2253011578. [DOI] [PubMed] [Google Scholar]
- 17.Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921–927. doi: 10.1177/0363546514563904. [DOI] [PubMed] [Google Scholar]
- 18.Hinterwimmer S, Imhoff AB, Minzlaff P, Saier T, Rosenstiel N, Hawe W, Feucht MJ. Anatomical two-bundle medial patellofemoral ligament reconstruction with hardware-free patellar graft fixation: technical note and preliminary results. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2147–2154. doi: 10.1007/s00167-013-2498-8. [DOI] [PubMed] [Google Scholar]
- 19.Kita K, Tanaka Y, Toritsuka Y, Amano H, Uchida R, Takao R, Horibe S. Factors affecting the outcomes of double-bundle medial patellofemoral ligament reconstruction for recurrent patellar dislocations evaluated by multivariate analysis. Am J Sports Med. 2015;43(12):2988–2996. doi: 10.1177/0363546515606102. [DOI] [PubMed] [Google Scholar]
- 20.Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40(8):1916–1923. doi: 10.1177/0363546512442330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Insall J, Salvati E. Patella position in the normal knee joint. Radiology. 1971;101(1):101–104. doi: 10.1148/101.1.101. [DOI] [PubMed] [Google Scholar]
- 22.Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19–26. doi: 10.1007/BF01552649. [DOI] [PubMed] [Google Scholar]
- 23.Sanchis-Alfonso V. How to deal with chronic patellar instability: what does the literature tell us? Sports Health. 2016;8(1):86–90. doi: 10.1177/1941738115604156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seitlinger G, Scheurecker G, Hogler R, Labey L, Innocenti B, Hofmann S. Tibial tubercle-posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med. 2012;40(5):1119–1125. doi: 10.1177/0363546512438762. [DOI] [PubMed] [Google Scholar]
- 25.Heidenreich MJ, Camp CL, Dahm DL, Stuart MJ, Levy BA, Krych AJ. The contribution of the tibial tubercle to patellar instability: analysis of tibial tubercle-trochlear groove (TT-TG) and tibial tubercle-posterior cruciate ligament (TT-PCL) distances. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2347–2351. doi: 10.1007/s00167-015-3715-4. [DOI] [PubMed] [Google Scholar]
- 26.Camp CL, Heidenreich MJ, Dahm DL, Stuart MJ, Levy BA, Krych AJ. Individualizing the tibial tubercle-trochlear groove distance: patellar instability ratios that predict recurrent instability. Am J Sports Med. 2016;44(2):393–399. doi: 10.1177/0363546515602483. [DOI] [PubMed] [Google Scholar]
- 27.Camp CL, Stuart MJ, Krych AJ, Levy BA, Bond JR, Collins MS, Dahm DL. CT and MRI measurements of tibial tubercle-trochlear groove distances are not equivalent in patients with patellar instability. Am J Sports Med. 2013;41(8):1835–1840. doi: 10.1177/0363546513484895. [DOI] [PubMed] [Google Scholar]
- 28.Anley CM, Morris GV, Saithna A, James SL, Snow M. Defining the role of the tibial tubercle-trochlear groove and tibial tubercle-posterior cruciate ligament distances in the work-up of patients with patellofemoral disorders. Am J Sports Med. 2015;43(6):1348–1353. doi: 10.1177/0363546515576128. [DOI] [PubMed] [Google Scholar]
- 29.Bollier M, Fulkerson JP. The role of trochlear dysplasia in patellofemoral instability. J Am Acad Orthop Surg. 2011;19(1):8–16. doi: 10.5435/00124635-201101000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Wagner D, Pfalzer F, Hingelbaum S, Huth J, Mauch F, Bauer G. The influence of risk factors on clinical outcomes following anatomical medial patellofemoral ligament (MPFL) reconstruction using the gracilis tendon. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):318–324. doi: 10.1007/s00167-012-2015-5. [DOI] [PubMed] [Google Scholar]
- 31.Pritsch T, Haim A, Arbel R, Snir N, Shasha N, Dekel S. Tailored tibial tubercle transfer for patellofemoral malalignment: analysis of clinical outcomes. Knee Surg Sports Traumatol Arthrosc. 2007;15(8):994–1002. doi: 10.1007/s00167-007-0325-9. [DOI] [PubMed] [Google Scholar]
- 32.Krych AJ, O'Malley MP, Johnson NR, et al. Functional testing and return to sport following stabilization surgery for recurrent lateral patellar instability in competitive athletes. Knee Surg Sports Traumatol Arthrosc. 2016;26:711–718. doi: 10.1007/s00167-016-4409-2. [DOI] [PubMed] [Google Scholar]
- 33.Payne J, Rimmke N, Schmitt LC, Flanigan DC, Magnussen RA. The incidence of complications of tibial tubercle osteotomy: a systematic review. Arthroscopy. 2015;31(9):1819–1825. doi: 10.1016/j.arthro.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 34.Dejour D, Saggin P. The sulcus deepening trochleoplasty—the Lyon’s procedure. Int Orthop. 2010;34(2):311–316. doi: 10.1007/s00264-009-0933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amis AA, Oguz C, Bull AM, Senavongse W, Dejour D. The effect of trochleoplasty on patellar stability and kinematics: a biomechanical study in vitro. J Bone Joint Surg Br. 2008;90(7):864–869. doi: 10.1302/0301-620X.90B7.20447. [DOI] [PubMed] [Google Scholar]
- 36.Van Haver A, De Roo K, De Beule M, et al. The effect of trochlear dysplasia on patellofemoral biomechanics: a cadaveric study with simulated trochlear deformities. Am J Sports Med. 2015;43(6):1354–1361. doi: 10.1177/0363546515572143. [DOI] [PubMed] [Google Scholar]
- 37.Pfirrmann CW, Zanetti M, Romero J, Hodler J. Femoral trochlear dysplasia: MR findings. Radiology. 2000;216(3):858–864. doi: 10.1148/radiology.216.3.r00se38858. [DOI] [PubMed] [Google Scholar]
- 38.Donell ST, Joseph G, Hing CB, Marshall TJ. Modified dejour trochleoplasty for severe dysplasia: operative technique and early clinical results. Knee. 2006;13(4):266–273. doi: 10.1016/j.knee.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 39.McNamara I, Bua N, Smith TO. Ali K, Donell ST. Deepening trochleoplasty with a thick osteochondral flap for patellar instability: clinical and functional outcomes at a mean 6-year follow-up. Am J Sports Med. 2015;43(11):2706–2713. doi: 10.1177/0363546515597679. [DOI] [PubMed] [Google Scholar]
- 40.Verdonk R, Jansegers E, Stuyts B. Trochleoplasty in dysplastic knee trochlea. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):529–533. doi: 10.1007/s00167-004-0570-0. [DOI] [PubMed] [Google Scholar]
- 41.Ntagiopoulos PG, Byn P, Dejour D. Midterm results of comprehensive surgical reconstruction including sulcus-deepening trochleoplasty in recurrent patellar dislocations with high-grade trochlear dysplasia. Am J Sports Med. 2013;41(5):998–1004. doi: 10.1177/0363546513482302. [DOI] [PubMed] [Google Scholar]
- 42.von Knoch F, Bohm T, Burgi ML, von Knoch M, Bereiter H. Trochleaplasty for recurrent patellar dislocation in association with trochlear dysplasia. A 4- to 14-year follow-up study. J Bone Joint Surg Br. 2006;88(10):1331–1335. doi: 10.1302/0301-620X.88B10.17834. [DOI] [PubMed] [Google Scholar]
- 43.Longo UG, Berton A, Salvatore G, Migliorini F, Ciuffreda M, Nazarian A, Denaro V. Medial patellofemoral ligament reconstruction combined with bony procedures for patellar instability: current indications, outcomes, and complications. Arthroscopy. 2016;32(7):1421–1427. doi: 10.1016/j.arthro.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 44.Camathias C, Studer K, Kiapour A, Rutz E, Vavken P. Trochleoplasty as a solitary treatment for recurrent patellar dislocation results in good clinical outcome in adolescents. Am J Sports Med. 2016;44(11):2855–2863. doi: 10.1177/0363546516652894. [DOI] [PubMed] [Google Scholar]
- 45.Buchanan G, Torres L, Czarkowski B, Giangarra CE. Current concepts in the treatment of gross patellofemoral instability. Int J Sports Phys Ther. 2016;11(6):867–876. [PMC free article] [PubMed] [Google Scholar]
- 46.Hinckel BB, Arendt EA. Lateral retinaculum lengthening or release. Oper Tech Sports Med. 2015;23(2):100–106. doi: 10.1053/j.otsm.2015.02.012. [DOI] [Google Scholar]
- 47.Hughston JC, Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med. 1988;16(4):383–388. doi: 10.1177/036354658801600413. [DOI] [PubMed] [Google Scholar]
- 48.McCarthy MA, Bollier MJ. Medial patella subluxation: diagnosis and treatment. Iowa Orthop J. 2015;35:26–33. [PMC free article] [PubMed] [Google Scholar]
- 49.Sanchis-Alfonso V, Merchant AC. Iatrogenic medial patellar instability: an avoidable injury. Arthroscopy. 2015;31(8):1628–1632. doi: 10.1016/j.arthro.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 50.Pagenstert G, Wolf N, Bachmann M, Gravius S, Barg A, Hintermann B, Wirtz DC, Valderrabano V, Leumann AG. Open lateral patellar retinacular lengthening versus open retinacular release in lateral patellar hypercompression syndrome: a prospective double-blinded comparative study on complications and outcome. Arthroscopy. 2012;28(6):788–797. doi: 10.1016/j.arthro.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 51.O’Neill DB. Open lateral retinacular lengthening compared with arthroscopic release. A prospective, randomized outcome study. J Bone Joint Surg Am. 1997;79(12):1759–1769. doi: 10.2106/00004623-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Swarup I, Elattar O, Rozbruch SR. Patellar instability treated with distal femoral osteotomy. Knee. 2017;24(3):608–614. doi: 10.1016/j.knee.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 53.Kearney SP, Mosca VS. Selective hemiepiphyseodesis for patellar instability with associated genu valgum. J Orthop. 2015;12(1):17–22. doi: 10.1016/j.jor.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelitz M, Dreyhaupt J, Williams SR, Dornacher D. Combined supracondylar femoral derotation osteotomy and patellofemoral ligament reconstruction for recurrent patellar dislocation and severe femoral anteversion syndrome: surgical technique and clinical outcome. Int Orthop. 2015;39(12):2355–2362. doi: 10.1007/s00264-015-2859-7. [DOI] [PubMed] [Google Scholar]
- 55.Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909–1914. doi: 10.1007/s00167-011-1516-y. [DOI] [PubMed] [Google Scholar]
- 56.Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248–2254. doi: 10.1177/0363546510376230. [DOI] [PubMed] [Google Scholar]
- 57.Drez D, Jr ETB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthroscopy. 2001;17(3):298–306. doi: 10.1053/jars.2001.21490. [DOI] [PubMed] [Google Scholar]
- 58.Ellera Gomes JL. Medial patellofemoral ligament reconstruction for recurrent dislocation of the patella: a preliminary report. Arthroscopy. 1992;8(3):335–340. doi: 10.1016/0749-8063(92)90064-I. [DOI] [PubMed] [Google Scholar]
- 59.Ellera Gomes JL, Stigler Marczyk LR, Cesar de Cesar P, Jungblut CF. Medial patellofemoral ligament reconstruction with semitendinosus autograft for chronic patellar instability: a follow-up study. Arthroscopy. 2004;20(2):147–151. doi: 10.1016/j.arthro.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 60.Dragoo JL, Nguyen M, Gatewood CT, Taunton JD, Young S. Medial patellofemoral ligament repair versus reconstruction for recurrent patellar instability: two-year results of an algorithm-based approach. Orthop J Sports Med. 2017;5(3):2325967116689465. doi: 10.1177/2325967116689465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stephen JM, Kaider D, Lumpaopong P, Deehan DJ, Amis AA. The effect of femoral tunnel position and graft tension on patellar contact mechanics and kinematics after medial patellofemoral ligament reconstruction. Am J Sports Med. 2014;42(2):364–372. doi: 10.1177/0363546513509230. [DOI] [PubMed] [Google Scholar]
- 62.Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871–1879. doi: 10.1177/0363546512449998. [DOI] [PubMed] [Google Scholar]
- 63.Hopper GP, Leach WJ, Rooney BP, Walker CR, Blyth MJ. Does degree of trochlear dysplasia and position of femoral tunnel influence outcome after medial patellofemoral ligament reconstruction? Am J Sports Med. 2014;42(3):716–722. doi: 10.1177/0363546513518413. [DOI] [PubMed] [Google Scholar]
- 64.Burrus MT, Werner BC, Conte EJ, Diduch DR. Troubleshooting the femoral attachment during medial patellofemoral ligament reconstruction: location, location, location. Orthop J Sports Med. 2015;3(1):232596711556919. doi: 10.1177/2325967115569198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schottle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801–804. doi: 10.1177/0363546506296415. [DOI] [PubMed] [Google Scholar]
- 66.Redfern J, Kamath G, Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38(2):293–297. doi: 10.1177/0363546509347602. [DOI] [PubMed] [Google Scholar]
- 67.Jaecker V, Brozat B, Banerjee M, Otchwemah R, Bouillon B, Shafizadeh S. Fluoroscopic control allows for precise tunnel positioning in MPFL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25(9):2688–2694. doi: 10.1007/s00167-015-3613-9. [DOI] [PubMed] [Google Scholar]
- 68.Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221–227. doi: 10.1016/S0968-0160(03)00038-3. [DOI] [PubMed] [Google Scholar]
- 69.Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133–142. doi: 10.1177/0363546515611652. [DOI] [PubMed] [Google Scholar]
- 70.Nguyen CV, Farrow LD, Liu RW, Gilmore A. Safe drilling paths in the distal femoral epiphysis for pediatric medial patellofemoral ligament reconstruction. Am J Sports Med. 2017;45(5):1085–1089. doi: 10.1177/0363546516677795. [DOI] [PubMed] [Google Scholar]
- 71.Fujino K, Tajima G, Yan J, Kamei Y, Maruyama M, Takeda S, Kikuchi S, Shimamura T. Morphology of the femoral insertion site of the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):998–1003. doi: 10.1007/s00167-013-2797-0. [DOI] [PubMed] [Google Scholar]
- 72.Sanchis-Alfonso V, Ramirez-Fuentes C, Montesinos-Berry E, Aparisi-Rodriguez F, Marti-Bonmati L. Does radiographic location ensure precise anatomic location of the femoral fixation site in medial patellofemoral ligament surgery? Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2838–2844. doi: 10.1007/s00167-015-3523-x. [DOI] [PubMed] [Google Scholar]
- 73.Matthews JJ, Schranz P. Reconstruction of the medial patellofemoral ligament using a longitudinal patellar tunnel technique. Int Orthop. 2010;34(8):1321–1325. doi: 10.1007/s00264-009-0918-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schottle PB, Hensler D, Imhoff AB. Anatomical double-bundle MPFL reconstruction with an aperture fixation. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):147–151. doi: 10.1007/s00167-009-0868-z. [DOI] [PubMed] [Google Scholar]
- 75.Sillanpaa PJ, Maenpaa HM, Mattila VM, Visuri T, Pihlajamaki H. A mini-invasive adductor magnus tendon transfer technique for medial patellofemoral ligament reconstruction: a technical note. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):508–512. doi: 10.1007/s00167-008-0713-9. [DOI] [PubMed] [Google Scholar]
- 76.Carnesecchi O, Neri T, Di Iorio A, Farizon F, Philippot R. Results of anatomic gracilis MPFL reconstruction with precise tensioning. Knee. 2015;22(6):580–584. doi: 10.1016/j.knee.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 77.Mariani PP, Liguori L, Cerullo G, Iannella G, Floris L. Arthroscopic patellar reinsertion of the MPFL in acute patellar dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):628–633. doi: 10.1007/s00167-010-1315-x. [DOI] [PubMed] [Google Scholar]
- 78.Siebold R, Chikale S, Sartory N, Hariri N, Feil S, Passler HH. Hamstring graft fixation in MPFL reconstruction at the patella using a transosseous suture technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1542–1544. doi: 10.1007/s00167-010-1147-8. [DOI] [PubMed] [Google Scholar]
- 79.Song SY, Kim IS, Chang HG, Shin JH, Kim HJ, Seo YJ. Anatomic medial patellofemoral ligament reconstruction using patellar suture anchor fixation for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2431–2437. doi: 10.1007/s00167-013-2730-6. [DOI] [PubMed] [Google Scholar]
- 80.Schiphouwer L, Rood A, Tigchelaar S, Koeter S. Complications of medial patellofemoral ligament reconstruction using two transverse patellar tunnels. Knee Surg Sports Traumatol Arthrosc. 2017;25(1):245–250. doi: 10.1007/s00167-016-4245-4. [DOI] [PubMed] [Google Scholar]
- 81.Burrus MT, Werner BC, Cancienne JM, Gwathmey FW, Diduch DR. MPFL graft fixation in low degrees of knee flexion minimizes errors made in the femoral location. Knee Surg Sports Traumatol Arthrosc. 2017;25(10):3092–3098. doi: 10.1007/s00167-016-4111-4. [DOI] [PubMed] [Google Scholar]
- 82.Feller JA, Richmond AK, Wasiak J. Medial patellofemoral ligament reconstruction as an isolated or combined procedure for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2470–2476. doi: 10.1007/s00167-014-3132-0. [DOI] [PubMed] [Google Scholar]
- 83.Lippacher S, Dreyhaupt J, Williams SR, Reichel H, Nelitz M. Reconstruction of the medial patellofemoral ligament: clinical outcomes and return to sports. Am J Sports Med. 2014;42(7):1661–1668. doi: 10.1177/0363546514529640. [DOI] [PubMed] [Google Scholar]
- 84.Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58–63. doi: 10.1177/0363546512463683. [DOI] [PubMed] [Google Scholar]
- 85.Nomura E, Horiuchi Y, Kihara M. A mid-term follow-up of medial patellofemoral ligament reconstruction using an artificial ligament for recurrent patellar dislocation. Knee. 2000;7(4):211–215. doi: 10.1016/S0968-0160(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 86.Lorbach O, Zumbansen N, Kieb M, et al. Medial patellofemoral ligament reconstruction: Impact of knee flexion angle during graft fixation on dynamic patellofemoral contact pressure-A biomechanical study. Arthroscopy. 2018. [DOI] [PubMed]
- 87.Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(9):1557–1563. doi: 10.1177/0363546507300872. [DOI] [PubMed] [Google Scholar]
- 88.Sanchis-Alfonso V. Guidelines for medial patellofemoral ligament reconstruction in chronic lateral patellar instability. J Am Acad Orthop Surg. 2014;22(3):175–182. doi: 10.5435/JAAOS-22-03-175. [DOI] [PubMed] [Google Scholar]


