Abstract
Purpose of Review
To review the current practices of nonoperative management of posterior cruciate ligament (PCL) injuries, the natural history of conservative care, and the latest PCL rehabilitation strategies.
Recent Findings
PCL injuries often occur as part of a multiligamentous knee injury and occasionally occur in isolation. Although patients may be able to tolerate or compensate for a PCL-deficient knee, long-term outcomes after conservative care demonstrate a high rate of arthrosis in the medial and patellofemoral compartments resulting from altered knee kinematics and loads. Good subjective outcomes and a high rate of return to sport have been reported after nonoperative treatment of isolated PCL injuries. However, PCL laxity grade on objective exam does not typically correlate with subjective outcomes, nor does it correlate with the risk of developing osteoarthritis. Although more research is needed on the optimal PCL rehabilitation strategies, general principles include avoiding posterior tibial translation in the initial period to optimize ligament healing, followed by progressive range of motion and strengthening of the quadriceps and core musculature. At 12 weeks, patients may begin an interval running program, followed by agility work and progressive sports-specific training to allow for return to sports.
Summary
Nonoperative treatment of isolated PCL injuries results in good subjective outcomes and high rate of return to sport.
Keywords: Posterior cruciate ligament, PCL, Nonoperative, Conservative, Rehabilitation, Return to sport
Introduction
Posterior cruciate ligament (PCL) injuries often present as part of a multiligamentous knee injury; however, they can occasionally occur in isolation depending upon the mechanism of injury. Conservative treatment of isolated PCL injuries has demonstrated good prognoses due to the intrinsic ability of the PCL to heal. However, chronic PCL deficiency leads to altered knee kinematics and loads, resulting in a prevalence of moderate to severe arthrosis of approximately 10% in the long-term [1]. Although nonoperative treatment is commonly used for isolated PCL injury, an ideal set of management and rehabilitation strategies remains undetermined.
Incidence, Mechanism of Injury, and Natural History of PCL Injury
Sanders et al. [2•] reported an age- and sex-adjusted annual incidence of isolated PCL tears of 1.8 per 100,000 persons. The incidence of PCL injury in acute knee injuries has been reported to be as high as 44% [3]. PCL injuries often present concomitantly with posterolateral corner (PLC) injury or multiligamentous injuries and occur less often in isolation [4]. Additionally, the severity of PCL injuries often corresponds with the presence of other ligamentous injury, as the majority of grade III PCL tears are associated with multiligament knee injuries [5]. PCL injuries most commonly occur from high-energy trauma (e.g., motor vehicle accidents) and sport-related injury [6]. Parolie and Bergfeld [7] reported a 2–3% incidence of chronic, asymptomatic PCL insufficiency in elite college football players.
Common mechanisms of PCL injury include the classic “dashboard” injury pattern, which involves a posteriorly directed force on the anterior aspect of the proximal tibia with the knee in a flexed position. In athletics, the typical mechanism of isolated PCL injury is a direct blow to the anterior tibia or a fall onto the knee with the foot in a plantarflexed position. When these mechanisms are combined with rotational or coronal plane forces, the medial and lateral knee structures are at risk for concurrent injury.
Although many patients may clinically tolerate a PCL-deficient knee, more recent studies have described altered kinematics and loads during functional activities [8–13]. Tibiofemoral kinematic changes in the PCL-deficient knee consist of increased passive sagittal laxity of the medial compartment, persistent posterior subluxation of the medial tibia, increased lateral tibial translation, and decreased varus rotation during weightbearing flexion [8, 11, 12]. Van de Velde et al. [10] reported a shift of the peak tibiofemoral contact point to a more anterior and medial location on the tibial plateau and increased cartilage deformation in the medial compartment of patients with PCL deficiency. In contrast, PCL deficiency did not appear to change the location or magnitude of peak cartilage deformation in the lateral compartment. Additionally, patellofemoral kinematic changes in the PCL-deficient knee consist of increased patellar flexion angles and decreased lateral shift, patellar tilt, and valgus rotation during high flexion angles, resulting in a distal and medial shift of patellofemoral cartilage contact points [13]. This data is consistent with the results of long-term studies of nonoperatively treated PCL injury showing a high prevalence of arthrosis ranging from 11 to 53% in the medial and patellofemoral compartments, as well as an increased risk of injury to the meniscus and posterolateral structures [2, 9, 14–16]. In a population-based comparative study, individuals with isolated PCL tears had a higher risk of symptomatic arthritis and subsequent treatment with total knee arthroplasty than individuals without PCL tears [2].
Clinical Evaluation
A thorough history of the patient’s complaints and the mechanism of injury can aid in identification of PCL injuries and distinguishing them from other intra-articular injuries. Often, the patient has sustained a traumatic injury or a sport-related injury [17]. In the setting of high-energy trauma, such as a motor vehicle accident, patients typically report an inability to bear weight, instability, and decreased knee range of motion. The classic “dashboard” injury pattern results from a posteriorly directed force on the anterior aspect of the proximal tibia with the knee in a flexed position. Associated capsuloligamentous injury should be suspected in these patients due to the possibility of a transient knee dislocation. High-energy trauma patients may present with life-threatening injuries; and therefore, PCL or multiligamentous injuries can be easily overlooked.
In athletics, the typical mechanism of isolated PCL injury is a direct blow to the anterior tibia or a fall onto the knee with the foot in a plantarflexed position. In contrast to acute ACL injuries, patients seldom report feeling or hearing a “pop” and may be able to continue play. Isolated PCL injuries may have more subtle presentations, with patients reporting stiffness, swelling, and pain localized to the posterior knee or pain with deep knee flexion. For chronic isolated PCL injuries, complaints of anterior knee pain, difficulty ascending stairs, and instability are typical [18].
Physical examination should begin with assessment of the patient’s gait and overall limb alignment. Varus alignment, external rotation recurvatum, and varus thrust during the stance phase of gait may be present in patients with concomitant PLC injury. An effusion is usually present after an acute injury. A complete neurovascular examination of the lower extremity should be performed on all patients with suspected ligamentous injuries.
The integrity of the PCL is most accurately assessed with the posterior drawer test. With the patient lying supine and the knee flexed to 90°, a posteriorly directed force is placed on the proximal tibia. In a PCL-deficient knee, the tibia may be posteriorly subluxated; therefore, an anteriorly directed force is usually needed to reduce the tibia to neutral position before applying the posteriorly directed force. In cases of isolated PCL tears, posterior tibial translation is decreased with internal rotation of the tibia as a result of tightening of the superficial medial collateral ligament and posterior oblique ligament, which act as secondary restraints to posterior tibial translation. The grading system for PCL injuries is based on the amount of posterior tibial translation observed during the posterior drawer test. Grade I injuries are defined as those with 0 to 5 mm of increased posterior tibial translation compared to the contralateral knee. Grade II injuries are defined as those with 6 to 10 mm of increased posterior tibial translation. Grade III injuries are defined as those with more than 10 mm of increased posterior tibial translation. The presence or lack of a firm end point should also be noted. PCL insufficiency can also be graded with a more simplified clinical grading system based on the position of the medial tibial plateau relative to the medial femoral condyle during the posterior drawer test. In grade A injuries, the plateau remains anterior to the medial femoral condyle. In grade B injuries, the plateau is flush with the medial femoral condyle. In grade C injuries, the plateau displaces posterior to the medial femoral condyle [19].
The quadriceps active test can aid in the diagnosis of complete PCL tears. With this test, the patient lies supine and the knee is flexed to 90°. While the examiner stabilizes the foot, the patient is asked to contract the quadriceps isometrically. In a complete PCL tear, the posteriorly subluxed tibia will dynamically reduce during quadriceps contraction.
Combined PCL and PLC injuries can be diagnosed with the “dial” test or external rotation test. This test is performed with the patient positioned prone or supine. An external rotation force is applied to both feet with the knees at 30°and then 90° of flexion. A side-to-side difference of 10° or more is considered abnormal. Increased external rotation at 30° only indicates an isolated PLC injury, while increased external rotation at both 30 and 90° suggests a combined PCL and PLC injury. Varus laxity should also be evaluated.
Imaging
Plain radiographs of the knee should be obtained, including bilateral standing anteroposterior, 45° flexion weightbearing posteroanterior, Merchant, and a lateral view of the affected extremity. These views allow for assessment of fractures, pre-existing arthritis, and tibial slope. Any posterior tibial subluxation, avulsion fractures, and tibial plateau fractures should be noted. Medial or patellofemoral compartment arthritic changes may be indicative of the chronicity of the PCL injury. If coronal malalignment is suspected, full-length hip-to-ankle films are helpful in determining overall limb alignment.
Magnetic resonance imaging (MRI) is the imaging modality of choice to confirm the presence of a PCL tear and any associated ligamentous or cartilage injury. On T1- and T2-weighted sagittal MRI images, the normal PCL appears dark in nature and is curvilinear in appearance. With an acute PCL injury, the MRI will reveal increased signal within the PCL or disruption in the continuity of the ligament fibers (Fig. 1). Chronic PCL tears resulting in posterior tibial subluxation of less than 8 mm have the potential to heal with restoration of ligament fiber continuity on MRI [20].
Fig. 1.
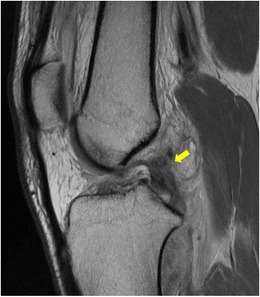
Sagittal magnetic resonance imaging of a posterior cruciate ligament tear showing discontinuity of fibers (yellow arrow). An anterior cruciate ligament tear is also present
Nonoperative Treatment
Historically, isolated PCL injury has been initially managed with a trial of conservative treatment, regardless of severity. This was in part due to inconsistent efficacy of PCL reconstruction in restoring normal function and kinematics, with the most commonly reported complication being residual posterior laxity [21–23]. Recent arthroscopic techniques for PCL reconstruction may result in improved reliability of the procedure, although there is a paucity of long-term outcome studies using these new techniques. Good subjective functional outcome scores and a healed appearance of the PCL on MRI are often noted after nonoperative treatment of isolated PCL injuries, even though less than satisfactory objective findings may be detected on physical exam [16, 24, 25]. Therefore, patients may be able to clinically tolerate or compensate for a PCL-deficient knee.
In general, nonsurgical treatment has been advocated for patients with isolated grade I or II PCL injuries or those with grade III injuries but have mild symptoms or only participate in low-demand activities [26•]. Factors that contribute to the secondary stability of the knee, including the posterior tibial slope, chondral topography, inherent knee laxity, and quadriceps strength, should also be considered in the management of these injuries. The clinical outcomes after nonoperative treatment of isolated PCL injuries have demonstrated good long-term subjective outcomes, a high rate of return to sport, and evidence of successful healing on MRI, although many of these studies may be influenced by the inclusion of mostly low-grade PCL injuries. The outcomes of clinical studies from the last 10 years of patients treated nonoperatively are summarized in Table 1. Shelbourne et al. [1] reported on 68 patients with an acute, isolated PCL injury treated nonoperatively and followed prospectively for a minimum of 10 years. In these patients, quadriceps strength was near equivalent to the uninvolved leg, and all had full knee range of motion. Moderate to severe osteoarthritis was present in 11% of knees, although the grade of osteoarthritis on radiographs was not correlated with PCL laxity grade. Agolley et al. [27•] examined the outcomes of nonoperative treatment of high-grade PCL injuries in 46 professional or semi-professional athletes with a mean age of 26.2 years. Sixty-one percent of patients played rugby, and 26% of patients played soccer. All patients were treated with initial bracing, followed by an individualized rehabilitation program determined by symptoms and physical signs. The mean time to return to sports-specific training was 10.6 weeks, and the mean time to return to full competitive sports was 16.4 weeks. At 2 years after injury, 91.3% of patients were playing at the same or higher level of sport.
Table 1.
Clinical outcomes and return to sport after nonoperative management of isolated PCL injuries
| Study | No. of patients | Mean age (range), years | Mean Follow-up, months | Outcomes and return to play |
|---|---|---|---|---|
| Agolley et al. | 46 | 26.2 (18–35) | 62 | Return to sports-specific training, 10.6 weeks; return to full competitive sport, 16.4 weeks; Tegner score, 9 |
| Ahn et al. | 38 | 30 (12–68) | 51 | Lysholm score, 88; HSS score, 91; IKDC subjective score, 83; 93% normal or nearly normal PCL continuity on follow-up |
| Jacobi et al. | 17 | 29.2 (17–60) | 24 | Lysholm score, 94; Tegner score, 7.2; IKDC subjective score, 95; 94% normal PCL continuity on follow-up MRI |
| Kocher et al. | 11 | 14.4 (7–18) | 44 | Pedi-IKDC, 87.4; Lysholm, 89.0; Tegner, 7.5; 100% return to play rate |
| Patel et al. | 57 | 27.9 (13–49) | 83 | Lysholm score, 85.2; Tegner score, 6.6; degenerative changes of the medial compartment in 17% of knees and of the patellofemoral joint in 7% of knees |
| Shelbourne et al. | 68 | 26.2 (10–60) | 211 | IKDC subjective score, 79.4; modified CKRS subjective score, 81.3; 11% prevalence of moderate to severe osteoarthritis |
CKRS Cincinnati Knee Rating System, HSS Hospital for Special Surgery, IKDC International Knee Documentation Committee, PCL posterior cruciate ligament
Goals of Rehabilitation
This section will discuss components of an evidence-based rehabilitation program based on physiology and reported outcomes, as well as details from the author’s clinical practice. The rehabilitation program consists of phases with generalized timeframes, and the patient’s progress depends on severity of injury and patient presentation.
Phase 1: Protective Phase (Weeks 1–6)
During the first few weeks, the goals of treatment should focus on effusion control, knee range of motion within prescribed limits, normalization of gait, and reactivation of the quadriceps musculature. Hyperextension of the knee and posterior tibial translation should be avoided during this initial phase. Immediately after injury, it is common to have swelling, generalized knee pain, and loss of motion. Joint effusion has been shown to inhibit the quadriceps, resulting in loss of musculature and subsequent “knee buckling” [28] (Fig. 2). Treatment strategies to address effusion consist of cryotherapy, elevation, joint compression, transcutaneous electrical stimulation, and manual therapy techniques. Once the effusion is controlled and the patient can perform a straight leg raise without a quadriceps lag, they can begin to progress off of crutches. Weight bearing may be limited the first 2 weeks to partial weight bearing (PWB) or weight bearing as tolerated (WBAT) depending on the grade of injury.
Fig. 2.
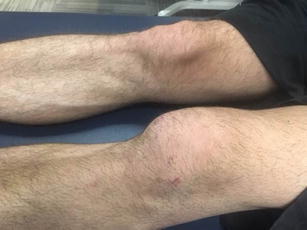
Joint effusion of the left knee during acute phase of injury
The knee is typically immobilized for 2–4 weeks to prevent tibial subluxation [29–32]. Currently, there is limited information evaluating the specific efficacy of PCL knee braces. One would expect a properly designed PCL brace to apply correct anatomic joint forces that vary with knee flexion angle, prevent excessive posterior tibial translation, and also provide adjustability to satisfy the demands of various activities [29–33]. It has been reported that healing of the ligament in an elongated position can lead to chronic instability [30, 34, 35]. As a result, bracing with an anterior drawer force has demonstrated that placing the PCL in a reduced position, with less posterior sag allows for improved healing [30, 34, 35]. Jacobi et al. [36] utilized a dynamic anterior drawer brace (PCL Jack brace) to treat patients with an acute, isolated PCL injury and reported reduced mean posterior sag from 7.1 mm at the time of injury to 2.3 mm at 12 months and 3.2 mm at 24 months. The brace was worn for 4 months and applied an anteriorly directed force on the posterior proximal tibia while allowing full weight bearing through a ROM from 0 to 110°.
Range of motion exercises initially should be performed in the prone position to avoid stressing the healing ligament from hamstring activation causing posterior translation of the tibia [30, 35]. Knee flexion may be limited to 90° for 2 weeks, then progressed to full motion. Biomechanical studies have demonstrated the least amount of stress on the PCL between 40 to 90° of flexion [30, 31, 37, 38]. Patella mobilizations are performed to minimize decreased ROM and quadriceps inhibition [37]. Restoration of soft tissue balance can be achieved with ROM exercises and addressing gastrocnemius-soleus and hamstring flexibility.
Strengthening for the quadriceps and proximal hip is crucial for stability as well as reducing loads to the knee [39–41]. Quadriceps setting is performed to address quadriceps activation, and straight leg raises (SLR) are performed once the quadriceps has sufficient strength to lock the joint into terminal knee extension without a quadriceps lag. Quadriceps inhibition can be addressed with electrical muscle stimulation and biofeedback with either quadriceps setting and/or straight leg raises [41]. Once 115° is achieved, a stationary bike may be performed with no resistance [30]. Weight shifting and proprioception exercises are progressed from 2 limbs to 1 limb (Fig. 3a–c). Double-limb strengthening, such as squats and leg press, are limited to no more than 70° of flexion to avoid stress to the healing PCL [30].
Fig. 3.
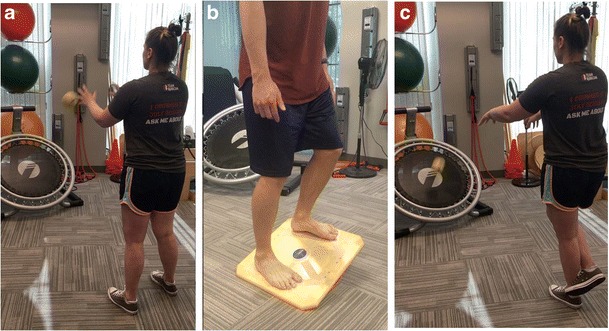
a Patient standing evenly on both limbs while tossing and catching a ball bouncing off a plyoback rebounder. b Patient performing weight shifts side to side on a balance board with cues to perform them slow and controlled. c Patient challenging her balance by tossing and catching a ball off the plyoback rebounder while standing on one leg
Transitional Phase: Phase II (Weeks 6 to 12)
The goals of phase II are to enhance proprioception and strengthen the lower extremities in order to perform light low-impact activities pain free and without effusion. Patients are full weight bearing and should achieve full knee ROM. Knee hyperextension and posterior tibial translation should continue to be avoided. Double-leg strengthening may still be limited to no more than 70° of knee flexion [42]. Strengthening with closed kinetic chain exercises (squats and leg press) are performed and limited to 0 to 70° of flexion. With squats, the patient and therapist must ensure that compensatory movements are not performed, such as shifting away from the injured limb (leg dominance) and genu valgus (Fig. 4a–c). Leg dominance relates to side-to-side symmetry, balance, and muscular strength, which may place both limbs at risk [34–36]. This will cause the weaker limb to be compromised in its ability to dissipate forces while the stronger limb is subjected to high forces secondary to increased dependence and excessive loading [39, 40]. Proprioceptive exercises are advanced from double to single limb, and on varying unstable surfaces.
Fig. 4.

a Patient performing a squat with proper form, no genu valgum, and weight evenly distributed between both limbs. b Patient performing a squat and shifting to her left limb (non-injured limb). c Patient performing a squat with genu valgum to her right limb
Functional Phase (Weeks 12–16)
In phase 3, any bracing should be discontinued and the goal is to return to light activities such as a jogging program [42]. Closed-chain exercises may advance past 70° and can progress to single-limb pending strength and quality of movement. Isolated hamstring exercises can be performed and advanced as tolerated (Fig. 5a–c). Core strengthening, proprioception, and neuromuscular control are advanced to restore dynamic stabilization of the knee joint (Fig. 6a, b). In order to advance to running, the patient must demonstrate sufficient strength and stability on one limb, as well as be pain free and without effusion with functional activities (Fig. 7a, b). Agility work may begin with a focus on quality of movement and coordination that is specific to the patient’s goals or sport.
Fig. 5.

a Patient advanced to single-limb bridge with leg curl. Patient lies on mat with heel of one limb on the physioball and other limb in the air. Patient engages core with neutral spine then pushes downward through the left heel. b Patient maintains neutral spine and pushes heel downward through ball lifting lower back and gluteals off of the ground. (c) Patient maintains neutral spine and core engagement while rolling ball out and then back in with hamstrings prior to returning to start position
Fig. 6.

a Patient performing a plank and advancing to perform with hip extension repetitions while maintaining neutral spine. b A side plank being performed and advanced with hip abduction while maintaining a neutral spine
Fig. 7.
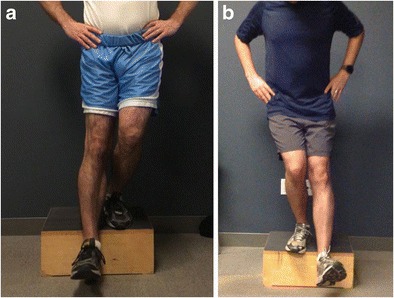
a Patient performing step down with good control, no genu valgus or hip drop and proper trunk alignment demonstrating appropriate strength for running progression, b Patient performing step down with genu valgum, trunk shift, and hip drop demonstrating inadequate strength base and lack of readiness to return to running at this time
Return to Play Phases (Weeks 16–24)
Further phases are dedicated to establishing a full-strength base for advanced movements and functional exercises, endurance, sports-specific agility, neuromuscular control, and ensure quality of movement to avoid re-injury [29, 42–44]. Compensatory movements should be assessed and addressed to avoid re-injury as well as future injury. Dynamic stabilization drills and plyometrics are advanced from double limb to single limb. Plyometric exercises are explosive and meant to build power, strength, and speed. These activities involve jumping, landing, and cutting maneuvers in varying planes of motion at varying intensity levels. Return to sport-related activities has been demonstrated at 6 months [4, 39].
Conclusions
Although good subjective outcomes and high rate of return to sport have been reported after nonoperative treatment of isolated PCL injuries, the resultant aberrant knee kinematics and loads often leads to arthrosis of the medial and patellofemoral compartments. The ideal management of PCL injuries remains uncertain and controversial. More research is needed on the optimal rehabilitations strategies in order to reliably improve outcomes after nonoperative treatment.
Compliance with Ethical Standards
Conflict of Interest
All authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on PCL Update
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Shelbourne KD, Clark M, Gray T. Minimum 10-year follow-up of patients after an acute, isolated posterior cruciate ligament injury treated nonoperatively. Am J Sports Med. 2013;41(7):1526–1533. doi: 10.1177/0363546513486771. [DOI] [PubMed] [Google Scholar]
- 2.Sanders TL, Pareek A, Barrett IJ, Kremers HM, Bryan AJ, Stuart MJ, et al. Incidence and long-term follow-up of isolated posterior cruciate ligament tears. Knee Surg Sports Traumatol Arthrosc. 2017;25(10):3017–3023. doi: 10.1007/s00167-016-4052-y. [DOI] [PubMed] [Google Scholar]
- 3.Fanelli GC. Posterior cruciate ligament injuries in trauma patients. Arthroscopy. 1993;9(3):291–294. doi: 10.1016/S0749-8063(05)80424-4. [DOI] [PubMed] [Google Scholar]
- 4.Petrigliano FA, McAllister DR. Isolated posterior cruciate ligament injuries of the knee. Sports Med Arthrosc Rev. 2006;14(4):206–212. doi: 10.1097/01.jsa.0000212325.23560.d2. [DOI] [PubMed] [Google Scholar]
- 5.Becker EH, Watson JD, Dreese JC. Investigation of multiligamentous knee injury patterns with associated injuries presenting at a level I trauma center. J Orthop Trauma. 2013;27(4):226–231. doi: 10.1097/BOT.0b013e318270def4. [DOI] [PubMed] [Google Scholar]
- 6.Swenson DM, Collins CL, Best TM, Flanigan DC, Fields SK, Comstock RD. Epidemiology of knee injuries among U.S. high school athletes, 2005/2006-2010/2011. Med Sci Sports Exerc. 2013;45(3):462–469. doi: 10.1249/MSS.0b013e318277acca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14(1):35–38. doi: 10.1177/036354658601400107. [DOI] [PubMed] [Google Scholar]
- 8.Logan M, Williams A, Lavelle J, Gedroyc W, Freeman M. The effect of posterior cruciate ligament deficiency on knee kinematics. Am J Sports Med. 2004;32(8):1915–1922. doi: 10.1177/0363546504265005. [DOI] [PubMed] [Google Scholar]
- 9.Skyhar MJ, Warren RF, Ortiz GJ, Schwartz E, Otis JC. The effects of sectioning of the posterior cruciate ligament and the posterolateral complex on the articular contact pressures within the knee. J Bone Joint Surg Am. 1993;75(5):694–699. doi: 10.2106/00004623-199305000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Van de Velde SK, Bingham JT, Gill TJ, Li G. Analysis of tibiofemoral cartilage deformation in the posterior cruciate ligament-deficient knee. J Bone Joint Surg Am. 2009;91(1):167–175. doi: 10.2106/JBJS.H.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyal K, Tashman S, Wang JH, Li K, Zhang X, Harner C. In vivo analysis of the isolated posterior cruciate ligament-deficient knee during functional activities. Am J Sports Med. 2012;40(4):777–785. doi: 10.1177/0363546511435783. [DOI] [PubMed] [Google Scholar]
- 12.Li G, Papannagari R, Li M, Bingham J, Nha KW, Allred D, Gill T. Effect of posterior cruciate ligament deficiency on in vivo translation and rotation of the knee during weightbearing flexion. Am J Sports Med. 2008;36(3):474–479. doi: 10.1177/0363546507310075. [DOI] [PubMed] [Google Scholar]
- 13.Van de Velde SK, Gill TJ, Li G. Dual fluoroscopic analysis of the posterior cruciate ligament-deficient patellofemoral joint during lunge. Med Sci Sports Exerc. 2009;41(6):1198–1205. doi: 10.1249/MSS.0b013e3181981eb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozanek M, Fu EC, Van de Velde SK, Gill TJ, Li G. Posterolateral structures of the knee in posterior cruciate ligament deficiency. Am J Sports Med. 2009;37(3):534–541. doi: 10.1177/0363546508325664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strobel MJ, Weiler A, Schulz MS, Russe K, Eichhorn HJ. Arthroscopic evaluation of articular cartilage lesions in posterior-cruciate-ligament-deficient knees. Arthroscopy. 2003;19(3):262–268. doi: 10.1053/jars.2003.50037. [DOI] [PubMed] [Google Scholar]
- 16.Boynton MD, Tietjens BR. Long-term followup of the untreated isolated posterior cruciate ligament-deficient knee. Am J Sports Med. 1996;24(3):306–310. doi: 10.1177/036354659602400310. [DOI] [PubMed] [Google Scholar]
- 17.Schulz MS, Russe K, Weiler A, Eichhorn HJ, Strobel MJ. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186–191. doi: 10.1007/s00402-002-0471-y. [DOI] [PubMed] [Google Scholar]
- 18.Margheritini F, Mariani PP. Diagnostic evaluation of posterior cruciate ligament injuries. Knee Surg Sports Traumatol Arthrosc. 2003;11(5):282–288. doi: 10.1007/s00167-003-0409-0. [DOI] [PubMed] [Google Scholar]
- 19.MacGillivray JD, Stein BE, Park M, Allen AA, Wickiewicz TL, Warren RF. Comparison of tibial inlay versus transtibial techniques for isolated posterior cruciate ligament reconstruction: minimum 2-year follow-up. Arthroscopy. 2006;22(3):320–328. doi: 10.1016/j.arthro.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 20.Mariani PP, Margheritini F, Christel P, Bellelli A. Evaluation of posterior cruciate ligament healing: a study using magnetic resonance imaging and stress radiography. Arthroscopy. 2005;21(11):1354–1361. doi: 10.1016/j.arthro.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 21.Lipscomb AB, Jr, Anderson AF, Norwig ED, Hovis WD, Brown DL. Isolated posterior cruciate ligament reconstruction. Long-term results. Am J Sports Med. 1993;21(4):490–496. doi: 10.1177/036354659302100402. [DOI] [PubMed] [Google Scholar]
- 22.Hermans S, Corten K, Bellemans J. Long-term results of isolated anterolateral bundle reconstructions of the posterior cruciate ligament: a 6- to 12-year follow-up study. Am J Sports Med. 2009;37(8):1499–1507. doi: 10.1177/0363546509333479. [DOI] [PubMed] [Google Scholar]
- 23.Zawodny SR, Miller MD. Complications of posterior cruciate ligament surgery. Sports Med Arthrosc Rev. 2010;18(4):269–274. doi: 10.1097/JSA.0b013e3181f2f4c2. [DOI] [PubMed] [Google Scholar]
- 24.Shelbourne KD, Davis TJ, Patel DV. The natural history of acute, isolated, nonoperatively treated posterior cruciate ligament injuries. A prospective study. Am J Sports Med. 1999;27(3):276–283. doi: 10.1177/03635465990270030201. [DOI] [PubMed] [Google Scholar]
- 25.Tewes DP, Fritts HM, Fields RD, Quick DC, Buss DD. Chronically injured posterior cruciate ligament: magnetic resonance imaging. Clin Orthop Relat Res. 1997;335:224–232. [PubMed] [Google Scholar]
- 26.Bedi A, Musahl V, Cowan JB. Management of posterior cruciate ligament injuries: an evidence-based review. J Am Acad Orthop Surg. 2016;24(5):277–289. doi: 10.5435/JAAOS-D-14-00326. [DOI] [PubMed] [Google Scholar]
- 27.Agolley D, Gabr A, Benjamin-Laing H, Haddad FS. Successful return to sports in athletes following non-operative management of acute isolated posterior cruciate ligament injuries: medium-term follow-up. Bone Joint J. 2017;99-B(6):774–778. doi: 10.1302/0301-620X.99B6.37953. [DOI] [PubMed] [Google Scholar]
- 28.Spencer JD, Hayes KC, Alexander IJ. Knee joint effusion and quadriceps reflex inhibition in man. Arch Phys Med Rehabil. 1984;65(4):171–177. [PubMed] [Google Scholar]
- 29.Harner CD, Hoher J. Evaluation and treatment of posterior cruciate ligament injuries. Am J Sports Med. 1998;26(3):471–482. doi: 10.1177/03635465980260032301. [DOI] [PubMed] [Google Scholar]
- 30.Pierce CM, O’Brien L, Griffin LW, Laprade RF. Posterior cruciate ligament tears: functional and postoperative rehabilitation. Knee Surg Sports Traumatol Arthrosc. 2013;21(5):1071–1084. doi: 10.1007/s00167-012-1970-1. [DOI] [PubMed] [Google Scholar]
- 31.Grood ES, Stowers SF, Noyes FR. Limits of movement in the human knee. Effect of sectioning the posterior cruciate ligament and posterolateral structures. J Bone Joint Surg Am. 1988;70(1):88–97. doi: 10.2106/00004623-198870010-00014. [DOI] [PubMed] [Google Scholar]
- 32.Margheritini F, Rihn J, Musahl V, Mariani PP, Harner C. Posterior cruciate ligament injuries in the athlete: an anatomical, biomechanical and clinical review. Sports Med. 2002;32(6):393–408. doi: 10.2165/00007256-200232060-00004. [DOI] [PubMed] [Google Scholar]
- 33.Shelbourne KD, Jennings RW, Vahey TN. Magnetic resonance imaging of posterior cruciate ligament injuries: assessment of healing. Am J Knee Surg. 1999;12(4):209–213. [PubMed] [Google Scholar]
- 34.Jung YB, Tae SK, Lee YS, Jung HJ, Nam CH, Park SJ. Active non-operative treatment of acute isolated posterior cruciate ligament injury with cylinder cast immobilization. Knee Surg Sports Traumatol Arthrosc. 2008;16(8):729–733. doi: 10.1007/s00167-008-0531-0. [DOI] [PubMed] [Google Scholar]
- 35.Veltri DM, Warren RF. Isolated and combined posterior cruciate ligament injuries. J Am Acad Orthop Surg. 1993;1(2):67–75. doi: 10.5435/00124635-199311000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Jacobi M, Reischl N, Wahl P, Gautier E, Jakob RP. Acute isolated injury of the posterior cruciate ligament treated by a dynamic anterior drawer brace: a preliminary report. J Bone Joint Surg Br. 2010;92(10):1381–1384. doi: 10.1302/0301-620X.92B10.24807. [DOI] [PubMed] [Google Scholar]
- 37.Fox RJ, Harner CD, Sakane M, Carlin GJ, Woo SL. Determination of the in situ forces in the human posterior cruciate ligament using robotic technology. A cadaveric study. Am J Sports Med. 1998;26(3):395–401. doi: 10.1177/03635465980260030901. [DOI] [PubMed] [Google Scholar]
- 38.Pandy MG, Shelburne KB. Dependence of cruciate-ligament loading on muscle forces and external load. J Biomech. 1997;30(10):1015–1024. doi: 10.1016/S0021-9290(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 39.Hewett TE, Myer GD, Ford KR, Paterno MV, Quatman CE. The 2012 ABJS Nicolas Andry Award: the sequence of prevention: a systematic approach to prevent anterior cruciate ligament injury. Clin Orthop Relat Res. 2012;470(10):2930–2940. doi: 10.1007/s11999-012-2440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myer GD, Paterno MV, Ford KR, Quatman CE, Hewett TE. Rehabilitation after anterior cruciate ligament reconstruction: criteria-based progression through the return-to-sport phase. J Orthop Sports Phys Ther. 2006;36(6):385–402. doi: 10.2519/jospt.2006.2222. [DOI] [PubMed] [Google Scholar]
- 41.Snyder-Mackler L, Delitto A, Bailey SL, Stralka SW. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. A prospective, randomized clinical trial of electrical stimulation. J Bone Joint Surg Am. 1995;77(8):1166–1173. doi: 10.2106/00004623-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Peccin MS, Almeida GJ, Amaro J, Cohen M, Soares BG, Atallah AN. Interventions for treating posterior cruciate ligament injuries of the knee in adults. Cochrane Database Syst Rev. 2005;2:CD002939. doi: 10.1002/14651858.CD002939.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Edson CJ, Fanelli GC, Beck JD. Postoperative rehabilitation of the posterior cruciate ligament. Sports Med Arthrosc Rev. 2010;18(4):275–279. doi: 10.1097/JSA.0b013e3181f2f23d. [DOI] [PubMed] [Google Scholar]
- 44.Petrigliano FA, Suero EM, Voos JE, Pearle AD, Allen AA. The effect of proximal tibial slope on dynamic stability testing of the posterior cruciate ligament- and posterolateral corner-deficient knee. Am J Sports Med. 2012;40(6):1322–1328. doi: 10.1177/0363546512439180. [DOI] [PubMed] [Google Scholar]


