Abstract
Background
Hepatocellular carcinoma (HCC) is one of the major causes of tumor death; thus, the identification of markers related to its diagnosis and prognosis is critical. Previous studies have revealed that epithelial-to-mesenchymal transition (EMT) is involved in tumor invasion and metastasis, and the forkhead box protein C2 (FOXC2) has been shown to promote tumor cell proliferation, invasion, and EMT. In the present study, we examined the clinicopathological significance of FOXC2 and EMT-related markers in clinical HCC specimens and identified factors related to the diagnosis and prognosis of HCC.
Methods
The expression of FOXC2 and EMT-related markers was evaluated by immunohistochemistry in 84 cases of hepatocellular carcinoma.
Results
A high expression of FOXC2 was observed in 26 of 84 cases, and expression was significantly correlated with background liver cirrhosis, poor tumor differentiation, high serum AFP, and elevated cell proliferation markers. In addition, this high expression was related to the induction of the Cadherin switch and vimentin expression and was an independent predictor for poor prognosis.
Conclusion
The high expression of FOXC2 in HCC is correlated with tumor malignancy and poor prognosis, suggesting that FOXC2 may be an important prognostic factor for HCC.
Keywords: FOXC2, HCC, Epithelial-to-mesenchymal transition
Background
Hepatocellular carcinoma (HCC) is a cancer with a poor prognosis [1]. Recently, advances in molecular target therapies towards advanced HCC have significantly improved the prognosis of patients with HCC [2]. However, patients with HCC with metastasis or refractory disease often require more effective and intensive therapeutic strategies. Therefore, to improve the prognosis of patients with HCC, novel therapeutic targets related to the malignant potential of HCC need to be identified.
Epithelial-to-mesenchymal transition (EMT) is an important cellular process which related to a developmental switch from an epithelial to a mesenchymal phenotypes [3, 4]. This process is essential for the embryonic development, moreover, EMT is also thought one of the vital molecular mechanisms inducing tumor invasion and metastasis. TGF-β is a pleiotropic factor that has a physiological function in regulating cell proliferation, differentiation, development, wound healing, and angiogenesis [5, 6]. In addition, TGF-β induces EMT, which has been well established as an important mechanism of cancer progression. Several down-stream transcription factors of TGF-β, such as the basic helix-loop-helix protein Twist, the zinc-finger proteins Snail and Slug, the E-box-binding protein ZEB1, and the forkhead box protein C2 (FOXC2), have been reported to induce EMT through the repression of E-cadherin expression, thereby playing pivotal roles in tumor metastasis [4, 7].
Functionally, FOXC2, also known as mesenchyme forkhead 1, is known as an important regulator of lymphangiogenesis [8], and FOXC2-knockout mice display a lymphedema-distichiasis syndrome [9]. FOXC2 was previously reported to function as an EMT-related gene during not only tumor progression but also organ repair [7, 10]. We previously reported the significance of FOXC2 expression in patients with esophageal cancer and cholangiocarcinoma, and a high expression of FOXC2 in tumor tissues was found to be related to cancer progression and a poor prognosis [11, 12]. These data were consistent with other cancers, including breast, colorectal, nasopharyngeal, esophageal, lung, ovarian, cholangiocarcinoma, and osteosarcoma [11–17]. Yang et al. have shown that the up-regulation of FOXC2 is associated with tumor size, vascular invasion, advanced TNM stage, promoting proliferation, and invasion in HCC [18]. However, few studies have examined the expressional relationship of FOXC2 and other EMT-related genes in HCC.
The purpose of this study was to clarify the clinical significance of FOXC2 and the relationship between FOXC2 and EMT-related proteins, such as E-cadherin, N-cadherin, and vimentin, in HCC. For this purpose, we carried out immunohistochemistry analysis to evaluate the relationships between FOXC2 expression, clinicopathological factors, and EMT-related proteins in clinical HCC samples.
Methods
Patient and samples
Eighty four patients with HCC who had undergone surgical resections at Gunma University Hospital between 1996 and 2014 were included in the study. The ages of the patients ranged from 48 to 89 years old. The tumor stage was classified according to the 6th Japanese tumor-node-metastasis (TNM) classification of Liver Cancer Study Group of Japan. Written informed consent for the collection of specimens was obtained from all participating patients with HCC, and the study protocol was approved by the local Ethics Committee.
Tissue microarrays (TMAs)
Clinical formalin-fixed, paraffin-embedded (FFPE) samples were stored in the archives of the Clinical Department of Pathology, Gunma University Hospital. After reviewing the H&E-stained slides, two representative tumor area were marked on FFPE tissue blocks. These tumor areas were extracted as tissue core by using a cylinder. The diameter of tissue core was 2.0 mm. A manual arraying instrument (Beecher Instruments, Silver Spring, MD, USA) was used to assemble the paraffin blocks into TMAs, as previously described [19].
Immunohistochemistry (IHC)
A 4-μm section was cut from paraffin blocks of samples. Each mounted sections were deparaffinized, rehydrated, and incubated with fresh 0.3% hydrogen peroxide in methanol for 30 min at room temperature to block endogenous peroxidase activity. The sections were then heated in boiled 10 mM citrate buffer (pH 6.0) at 98 °C for 30 min. Nonspecific binding sites were blocked by incubating with 0.25% Casein/1% BSA for 30 min at room temperature. Anti-FOXC2 primary monoclonal antibody (Abnova, Taipei, Taiwan) was used at a dilution of 1:100 at 4 °C overnight, as previously described [12]. The sections were washed in PBS, and the primary antibody was visualized using the Histofine Simple Stain MAX-PO (Multi) Kit (Nichirei, Tokyo, Japan) according to the instruction manual. The chromogen 3,3-diaminobenzidine tetrahydrochloride (Dojindo Laboratories, Kumamoto, Japan) was applied as a 0.02% solution containing 0.005% H2O2 in 50 mM Tris-HCl buffer (pH 7.6). The sections were lightly counterstained with Mayer’s haematoxylin and mounted. Negative controls were established by omitting the primary antibody. Other IHC was performed using the following primary anti-bodies: anti-E-cadherin (36; Ventana Medical Systems, Tucson, AZ, USA), anti-N-cadherin (6G11, Dako, Glostrup, Dermark), anti-Vimentin (V9, Dako), anti-ZEB1 (D91854, Atlas Antibodies, Stockholm, Sweden) and anti-Ki67 (30–9, Ventana).
Immunohistochemical evaluation
The immunohistochemical FOXC2 expression was evaluated independently by two observers. A staining was primarily cytoplasmic in positive cases. The intensity of FOXC2 staining was scored as 0, 1+, 2+, and 3+. Grade 0, 1+, and 2+ staining was considered to be negative for FOXC2 expression, while grades 3 was considered to be positive. E-cadherin was evaluated by proportion score and intensity score. The proportion score are as follows: Score 0: < 10%; Score 1: 10–40%; and Score 2: > 40%. The intensity score are follows: Score 0: negative; Score 1: weak; Score 2: intermediate; and Score 3: strong. Finally, the two scores are combined, and a total score of 3 or more is regarded as positive. The expression of N-cadherin were defined as negative if membrane staining was detected in < 20% of tumor cells. Then we defined Cadherin switch as the samples showing both E-cadherin negative and N-cadherin positive. The expression of Vimentin in the cytoplasm of > 1% of tumor cells was defined as positive, regardless of staining intensity. The expression of ZEB1 in the nuclei of > 1% of tumor cells was defined as positive. The Ki67 labeling index was evaluated as the percentage of positive tumor cell nuclei based of > 500 tumor cells, regardless of staining intensity. The percentage > 1% was considered as high Ki67 labeling index. The IHC slides were evaluated by two independent pathologists in a blind manner.
Statistical analysis
We used EZR (Saitama Medical Center Jichi Medical University; http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmed.html) for statistical analysis. The χ2 test and Fisher’s exact test were used to evaluate associations between clinicopathologic characteristics and FOXC2 expression intensities. To calculate and analyze overall survival and disease-free survival, we used the Kaplan–Meier method and the Log-rank test. Independent prognostic factors were tested by Univariate and multivariate analyses, which were based on the Cox proportional hazards regression model. A statistically significant p-value was considered to be less than 0.05.
Results
Immunohistochemical staining of FOXC2 in HCC tissues
The expression of FOXC2 was evaluated in 84 HCC samples by immunohistochemistry. Cytoplasmic staining of FOXC2 was primarily observed (Fig. 1a). In total, 26 of 84 HCC samples (31%) were considered high for FOXC2 expression, whereas 58 HCC samples (69%) were considered low for FOXC2 expression.
Fig. 1.
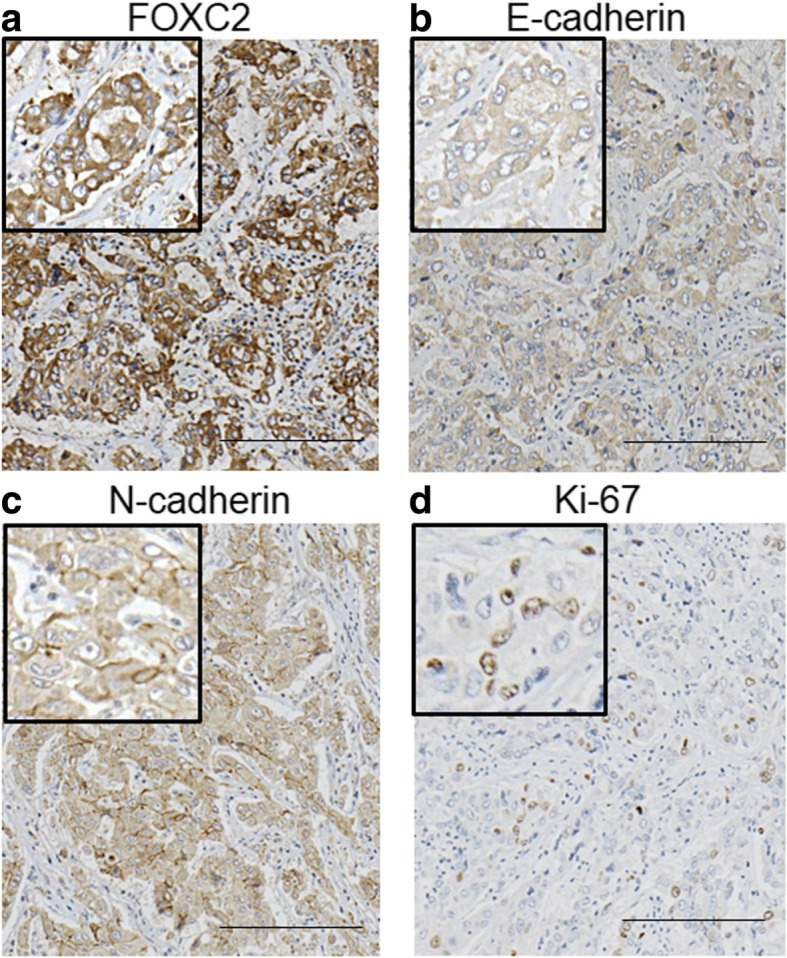
Immunohistochemical analysis of FOXC2, E-cadherin, N-cadherin, and Ki-67 in representative HCC tissues from an identical patient. a High FOXC2 expression in an HCC tissue; b Low E-cadherin expression in an HCC tissue; c High N-cadherin expression in an HCC tissue; d High Ki-67 expression in an HCC tissue. Scale bar, 200 μm
Expression of FOXC2 in HCC tissues and its correlation with clinicopathological factors
Correlations between FOXC2 expression in HCC samples and patient age, gender, liver cirrhosis, T classification, differentiation, tumor size, pattern of tumor growth, Fc-inf, IM, Vv, Vp, Va, serum AFP level, serum PIVKAII level, and the Ki-67 labeling index are shown in Table 1. High FOXC2 expression was correlated with liver cirrhosis (P = 0.0296), poor differentiation (P = 0.0302), high serum AFP levels (P = 0.00078), and the Ki-67 labeling index (P = 0.0348).
Table 1.
Clinicopathological characteristics of patients with hepatocellular carcinoma (HCC) according to forkhead box protein C2 (FOXC2) expression
| Parameters | FOXC2 | P value | |
|---|---|---|---|
| Low expressionn = 58 (%) | High expressionn = 26 (%) | ||
| Age (years) | |||
| < 65 | 18 (31.0) | 6 (23.1) | 0.603 |
| ≥ 65 | 40 (69.0) | 20 (76.9) | |
| Gender | |||
| Male | 46 (79.3) | 19 (73.1) | 0.578 |
| Female | 12 (20.7) | 7 (26.9) | |
| Liver cirrhosis | |||
| Negative | 40 (69.0) | 11 (42.3) | 0.0296a |
| Positive | 18 (31.0) | 15 (57.7) | |
| T classification | |||
| 1 | 5 (8.6) | 3 (11.6) | 0.333 |
| 2 | 20 (34.5) | 5 (19.2) | |
| 3 | 28 (48.3) | 13 (50.0) | |
| 4 | 5 (8.6) | 5 (19.2) | |
| Differentiation | |||
| Well or Moderate | 57 (98.3) | 22 (84.7) | 0.0302a |
| Poor | 1 (1.7) | 4 (15.3) | |
| Tumor size (mm) | |||
| ≤ 20 | 5 (8.6) | 4 (18.2) | 0.449 |
| > 20 | 53 (91.4) | 22 (81.8) | |
| Pattern of tumor growth | |||
| Eg | 49 (84.5) | 18 (55.6) | 0.143 |
| Ig | 9 (15.5) | 8 (44.4) | |
| Fc-Inf | |||
| Negative | 37 (63.8) | 11 (42.3) | 0.0948 |
| Positive | 21 (36.2) | 15 (57.7) | |
| IM | |||
| Negative | 50 (86.2) | 20 (76.9) | 0.347 |
| Positive | 8 (13.8) | 6 (23.1) | |
| Vv | |||
| Negative | 46 (79.3) | 22 (81.8) | 0.766 |
| Positive | 12 (20.7) | 4 (18.2) | |
| Vp | |||
| Negative | 38 (65.5) | 17 (65.4) | 1 |
| Positive | 20 (34.5) | 9 (34.6) | |
| Va | |||
| Negative | 55 (94.8) | 25 (96.2) | 1 |
| Positive | 3 (5.2) | 1 (3.8) | |
| AFP level (ng/ml) (n = 73) | |||
| Normal (≤10) | 27 (55.1) | 3 (12.5) | 0.000777a |
| High (> 10) | 22 (44.9) | 21 (87.5) | |
| PIVKA II level (AU/ml) (n = 69) | |||
| Normal (≤40) | 19 (39.6) | 11 (52.4) | 0.43 |
| High (> 40) | 29 (60.4) | 10 (47.6) | |
| Ki67 labeling index | |||
| < 1% | 21 (36.2) | 3 (11.5) | 0.0348a |
| ≥ 1% | 37 (63.8) | 23 (88.5) | |
aStatistical significance is indicated by P < 0.05. Eg (expansive growth); Boundary between cancer and surrounding liver tissue is clear. Ig (infiltrative growth); Boundary between cancer and surrounding liver tissue is unclear
Relationship between FOXC2 expression and EMT-related markers
We examined the relationship between FOXC2 expression and IHC staining of the EMT-related markers E-cadherin, N-cadherin, vimentin, and ZEB1. High FOXC2 expression had a strong association with the induction of the Cadherin switch and a high expression of vimentin (Table 2; P = 0.0414 and P = 0.0273) (Fig. 1).
Table 2.
The relationship of FOXC2 expression and EMT-related markers in 84 HCC samples
| Parameters | FOXC2 | P value | |
|---|---|---|---|
| Low expression n = 58 (%) | High expression n = 26 (%) | ||
| E-cadherin | |||
| Negative | 23 (39.7) | 13 (50) | 0.476 |
| Positive | 35 (60.3) | 13 (50) | |
| N-cadherin | |||
| Negative | 31 (53.4) | 16 (61.5) | 0.635 |
| Positive | 27 (46.6) | 10 (38.5) | |
| Cadherin switch | |||
| Negative | 53 (91.4) | 19 (73.1) | 0.0414a |
| Positive | 5 (8.6) | 7 (26.9) | |
| Vimentin | |||
| Negative | 58 (100) | 23 (88.5) | 0.0273a |
| Positive | 0 (0) | 3 (11.5) | |
| ZEB1 | |||
| Negative | 32 (55.2) | 16 (61.3) | 0.639 |
| Positive | 26 (44.8) | 10 (38.7) | |
aStatistical significance is indicated by P < 0.05
Prognostic significance of FOXC2 expression in HCC
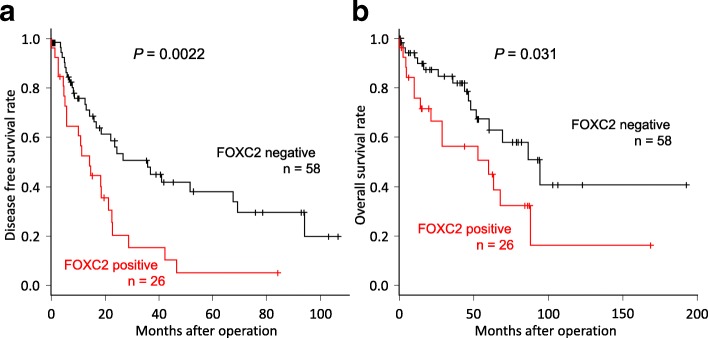
Disease-free survival rates (DFS) and the overall survival rates (OS) of patients with HCC are shown in Fig. 2. Patients with high FOXC2 expression had significantly poorer prognoses than patients with low expression in both DFS (P = 0.0022) and OS (P = 0.031). For OS, FOXC2 expression was a prognostic factor for poor survival in a univariate analysis (Table 3; RR 2.14, 95% CI 1.05–4.34, P = 0.035). Additionally, the pattern of tumor growth, T classification, and portal vein invasion were also prognostic factors in the univariate analysis. In the multivariate analysis, FOXC2 expression was an independent prognostic factor for poor survival (Table 3; RR 2.21, 95% CI 1.06–4.57, P = 0.033).
Fig. 2.
Relationship between postoperative survival and FOXC2 expression in 84 patients with HCC. Kaplan–Meier curves of the low expression of FOXC2 and high expression of FOXC2 groups are shown. a A high expression of FOXC2 indicated a poor prognosis for the disease-free survival rate (P = 0.0022). b A high expression of FOXC2 indicated a poor prognosis for the overall survival rate (P = 0.031)
Table 3.
Results of univariate and multivariate analyses of clinicopathological factors affecting the overall survival rate following surgery
| Clinicopathologic variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| RR | 95%CI | P value | RR | 95%CI | P value | |
| FOXC2 expression (low/high) | 2.14 | 1.05–4.34 | 0.035a | 2.21 | 1.06–4.57 | 0.033a |
| Age (≤65/> 65) | 1.17 | 0.518–2.66 | 0.70 | – | – | – |
| Gender (male/female) | 1.13 | 0.485–2.62 | 0.78 | – | – | – |
| Liver cirrhosis (negative/positive) | 1.86 | 0.913–3.77 | 0.087 | – | – | – |
| Differentiation (well or moderate/poor) | 1.30 | 0.307–5.49 | 0.72 | – | – | – |
| Pattern of tumor growth (Eg/Ig) | 3.29 | 1.53–7.07 | 0.0023a | 3.06 | 1.37–6.80 | 0.0062a |
| T classification (T1–3/T4) | 4.33 | 1.82–10.34 | < 0.001a | 3.82 | 1.18–12.37 | 0.025a |
| Portal vein invasion (negative/positive) | 2.19 | 1.03–4.65 | 0.042a | 1.31 | 0.477–3.59 | 0.6 |
| AFP level (normal/high) | 1.24 | 0.529–2.91 | 0.62 | – | – | – |
| PIVKA II level (normal/high) | 0.749 | 0.323–1.73 | 0.50 | – | – | – |
RR relative risk, CI confidence interval
aStatistical significance is indicated by P < 0.05
Discussion
In the present study, we demonstrated that a high expression of the EMT inducer, FOXC2, in primary HCC samples is associated with liver cirrhosis, malignant potential, high serum AFP, and poor prognosis. Moreover, FOXC2 accumulation was related to the induction of the Cadherin switch and increased expression of the mesenchymal marker vimentin.
Yang et al. have indicated that high expression of FOXC2 is related to tumor size, vascular invasion, advanced TNM stage, and promoting proliferation and invasion in HCC [18]. They focused on AKT-mediated MMP-2 and MMP-9 to explain FOXC2-related cancer aggressiveness. Conversely, by focusing on a correlation between high FOXC2 and Cadherin switch, we showed that high expression of FOXC2 in clinical HCC samples is involved in EMT-related tumor aggressiveness.
High expression levels of FOXC2 in HCC were significantly associated with the low expression of E-cadherin/high N-cadherin, termed the Cadherin switch, and accumulation of the mesenchymal marker vimentin. FOXC2 has been reported to be an important EMT inducer via TGF-β signaling in several cancers [7, 17, 20, 21]. Moreover, FOXC2 can induce the expression of cancer-related genes, including AKT, GSK3β, and Snail [22]. The activation of the AKT-GSK3β-Snail signaling pathway in colon cancer has been previously reported to induce EMT. From these observations, it is suggested that suppression by targeting FOXC2 may be important to overcome EMT in patients with clinical HCC.
High expression of FOXC2 in HCC was significantly associated with the progression of cirrhosis in the background liver. Hepatic cirrhosis is known to be induced by viral infection, alcohol, fatty liver, and non-alcoholic steatohepatitis [23, 24]. At that time, it was reported that the activation of the TGF-β/Smad signal is likely an important key regulator, and TGF-β inhibitors suppress hepatic fibrosis [25]. In breast cancer cell lines, FOXC 2 is known to be induced by the activation of TGF-β signaling [7]. These data suggest that TGF-β signaling may be one of the main FOXC2 regulation mechanisms and that hepatic cirrhosis, induced by TGF-β signaling, may cause HCC expressing high FOXC2 with aggressive phenotypes.
In the present study, we clarified the positive correlation between FOXC2 expression and Ki-67 accumulation. Cui et al. previously reported that FOXC2 can facilitate the proliferation ability in pancreatic cancer via the activation of β-catenin/TCF signaling, which is well known as a proliferation regulator in cancer cells [26]. Moreover, FOXC2 has been known to promote cell proliferation through the activation of MAPK and AKT pathways [27]. Moreover, we previously reported that FOXC2 suppression by RNA interference induces anti-proliferative activity in esophageal cancer cells and cholangiocarcinoma cells [11, 12]. Thus, FOXC2 may be an important regulator in cancer proliferation in not only HCC but also other cancers.
Many studies have reported that FOXC2 is related to chemotherapeutic resistance in several cancers [28–31]. Indeed, FOXC2 suppression may inhibit EMT induction and multidrug resistance in basal-like breast cancer and nasopharyngeal cancer [30, 32]. On the other hand, Zang et al. clarified that FOXC2 accumulation, induced by long non-coding RNA FOXC2-AS1, can increase the expression of the multidrug-related gene ATP binding cassette subfamily B member 1 (ABCB1) [33]. As mentioned above, FOXC2 appears to be related to cancer aggressiveness, including proliferative marker accumulation and EMT induction/Cadherin switch in HCC. Targeting FOXC2 may be effective to overcome aggressive phenotypes and therapeutic resistance in HCC.
Interestingly, Yu et al. reported a new FOXC2-targeting strategy using the natural compound resveratrol, which is known as a beneficial compound found in red wine, which suppressed FOXC2 expression in lung cancer cells via miR-520 h suppression [34]. Actually, resveratrol has been reported to suppress the viability of HCC cells [35–37]; therefore, the administration of resveratrol in patients with clinical HCC may be a good therapeutic candidate to overcome HCC aggressiveness via targeting FOXC2.
Conclusions
In conclusion, we have shown that FOXC2 expression in HCC is associated with several factors, including poor survival, poor differentiation, serum AFP levels, proliferation marker Ki67 expression, and the Cadherin switch. High FOXC2 expression levels may be a powerful marker of aggressive phenotypes and poor survival in patients with HCC.
Acknowledgements
The authors thank clinical department of pathology, Gunma university hospital for useful discussions and excellent technical assistance.
Funding
This work was supported in part by the following grants and foundations: Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS; grant numbers 15 K08339). The funding body was responsible for supplying experimental materials in this study.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ABCB1
ATP binding cassette subfamily B member 1
- DFS
Disease-free survival rates
- EMT
Epithelial-to-mesenchymal transition
- FFPE
Formalin-fixed, paraffin-embedded
- FOXC2
Forkhead box protein C2
- HCC
Hepatocellular carcinoma
- IHC
Immunohistochemistry
- OS
Overall survival rates
- TMAs
Tissue microarrays
- TNM
Tumor-node-metastasis
Authors’ contributions
YS and TO are the principal investigator of this study. YS, YU, TH, TY, AK and TH conducted most of the experiments and result analysis. TW, DG, KH, TY, MT, TI, AW, NK, KA and NH treated patients and collected clinical data and tissue samples. TS, KO, HK and KS participated in designing the study and wrote this manuscript. All authors participated in writing the manuscript and have read and approved the final version.
Ethics approval and consent to participate
The study protocol was approved by the Gunma University school of medicine ethical review board for medical research involving human subjects (Permit Number: 150044). Written informed consent for the collection of specimens and the use of specimens for this study was obtained from all participating patients.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuki Shimoda, Email: m15702031@gunma-u.ac.jp.
Yasunari Ubukata, Email: ubktysnr0710@gmail.com.
Tadashi Handa, Email: thanda@gunma-u.ac.jp.
Takehiko Yokobori, Phone: +81-27-220-8224, Email: bori45@gunma-u.ac.jp.
Takayoshi Watanabe, Email: m09112tw@jichi.ac.jp.
Dolgormaa Gantumur, Email: dolgormaa6275@gmail.com.
Kei Hagiwara, Email: asikaika.rt@gmail.com.
Takahiro Yamanaka, Email: takahiro.13.special@gmail.com.
Mariko Tsukagoshi, Email: scorpio27@hotmail.co.jp.
Takamichi Igarashi, Email: takamichi.iga@gmail.com.
Akira Watanabe, Email: akira_watanabe@gunma-u.ac.jp.
Norio Kubo, Email: nkubo@gunma-u.ac.jp.
Kenichiro Araki, Email: karaki@gunma-u.ac.jp.
Norifumi Harimoto, Email: nharimotoh1@gunma-u.ac.jp.
Ayaka Katayama, Email: m14702031@gunma-u.ac.jp.
Toshiaki Hikino, Email: ikuys1986@yahoo.co.jp.
Takaaki Sano, Email: sanot@gunma-u.ac.jp.
Kyoichi Ogata, Email: kogata@gunma-u.ac.jp.
Hiroyuki Kuwano, Email: hkuwano@gunma-u.ac.jp.
Ken Shirabe, Email: kshirabe@gunma-u.ac.jp.
Tetsunari Oyama, Email: oyama@gunma-u.ac.jp.
References
- 1.Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis. 2010;31(1):100–110. doi: 10.1093/carcin/bgp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torrecilla S, Llovet JM. New molecular therapies for hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2015;39(Suppl 1):S80–S85. doi: 10.1016/j.clinre.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 3.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 4.Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, Baba H, Mori M. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101(2):293–299. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitisin K, Saha T, Blake T, Golestaneh N, Deng M, Kim C, Tang Y, Shetty K, Mishra B, Mishra L. Tgf-Beta signaling in development. Sci STKE. 2007;2007(399):cm1. doi: 10.1126/stke.3992007cm1. [DOI] [PubMed] [Google Scholar]
- 6.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8(12):970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 7.Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, Kutok JL, Hartwell K, Richardson AL, Weinberg RA. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci U S A. 2007;104(24):10069–10074. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kume T. Foxc2 transcription factor: a newly described regulator of angiogenesis. Trends Cardiovasc Med. 2008;18(6):224–228. doi: 10.1016/j.tcm.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kriederman BM, Myloyde TL, Witte MH, Dagenais SL, Witte CL, Rennels M, Bernas MJ, Lynch MT, Erickson RP, Caulder MS, et al. FOXC2 haploinsufficient mice are a model for human autosomal dominant lymphedema-distichiasis syndrome. Hum Mol Genet. 2003;12(10):1179–1185. doi: 10.1093/hmg/ddg123. [DOI] [PubMed] [Google Scholar]
- 10.Hader C, Marlier A, Cantley L. Mesenchymal-epithelial transition in epithelial response to injury: the role of Foxc2. Oncogene. 2010;29(7):1031–1040. doi: 10.1038/onc.2009.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishida N, Mimori K, Yokobori T, Sudo T, Tanaka F, Shibata K, Ishii H, Doki Y, Mori M. FOXC2 is a novel prognostic factor in human esophageal squamous cell carcinoma. Ann Surg Oncol. 2011;18(2):535–542. doi: 10.1245/s10434-010-1274-y. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe A, Suzuki H, Yokobori T, Altan B, Kubo N, Araki K, Wada S, Mochida Y, Sasaki S, Kashiwabara K, et al. Forkhead box protein C2 contributes to invasion and metastasis of extrahepatic cholangiocarcinoma, resulting in a poor prognosis. Cancer Sci. 2013;104(11):1427–1432. doi: 10.1111/cas.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollier BG, Tinnirello AA, Werden SJ, Evans KW, Taube JH, Sarkar TR, Sphyris N, Shariati M, Kumar SV, Battula VL, et al. FOXC2 expression links epithelial-mesenchymal transition and stem cell properties in breast cancer. Cancer Res. 2013;73(6):1981–1992. doi: 10.1158/0008-5472.CAN-12-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui YM, Jiao HL, Ye YP, Chen CM, Wang JX, Tang N, Li TT, Lin J, Qi L, Wu P, et al. FOXC2 promotes colorectal cancer metastasis by directly targeting MET. Oncogene. 2015;34(33):4379–4390. doi: 10.1038/onc.2014.368. [DOI] [PubMed] [Google Scholar]
- 15.Song L, Tang H, Liao W, Luo X, Li Y, Chen T, Zhang X. FOXC2 positively regulates YAP signaling and promotes the glycolysis of nasopharyngeal carcinoma. Exp Cell Res. 2017;357(1):17–24. doi: 10.1016/j.yexcr.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Jiang W, Fan H, Qian C, Ding J, Wang Q, Pang X. Prognostic value of high FoxC2 expression in resectable non-small cell lung cancer, alone or in combination with E-cadherin expression. BMC Cancer. 2016;16:16. doi: 10.1186/s12885-016-2056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu B, Han SM, Tang XY, Han L, Li CZ. Overexpressed FOXC2 in ovarian cancer enhances the epithelial-to-mesenchymal transition and invasion of ovarian cancer cells. Oncol Rep. 2014;31(6):2545–2554. doi: 10.3892/or.2014.3119. [DOI] [PubMed] [Google Scholar]
- 18.Yang F, Lv L, Zhang K, Cai Q, Liu J, Jiang Y. Elevated FOXC2 expression promotes invasion of HCC cell lines and is associated with poor prognosis in hepatocellular carcinoma. Cell Physiol Biochem. 2017;44(1):99–109. doi: 10.1159/000484586. [DOI] [PubMed] [Google Scholar]
- 19.Katayama A, Handa T, Komatsu K, Togo M, Horiguchi J, Nishiyama M, Oyama T. Expression patterns of claudins in patients with triple-negative breast cancer are associated with nodal metastasis and worse outcome. Pathol Int. 2017;67(8):404–413. doi: 10.1111/pin.12560. [DOI] [PubMed] [Google Scholar]
- 20.Katoh M, Katoh M. Integrative genomic analyses of CXCR4: transcriptional regulation of CXCR4 based on TGFbeta, nodal, Activin signaling and POU5F1, FOXA2, FOXC2, FOXH1, SOX17, and GFI1 transcription factors. Int J Oncol. 2010;36(2):415–420. doi: 10.3892/ijo_00000514. [DOI] [PubMed] [Google Scholar]
- 21.Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci U S A. 2010;107(35):15449–15454. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q, Wu J, Wei P, Xu Y, Zhuo C, Wang Y, Li D, Cai S. Overexpression of forkhead box C2 promotes tumor metastasis and indicates poor prognosis in colon cancer via regulating epithelial-mesenchymal transition. Am J Cancer Res. 2015;5(6):2022–2034. [PMC free article] [PubMed] [Google Scholar]
- 23.D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, Valsecchi MG. Clinical states of cirrhosis and competing risks. J Hepatol 2018;68(3):563–76. [DOI] [PubMed]
- 24.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66(5):1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 25.Xu F, Liu C, Zhou D, Zhang L. TGF-beta/SMAD pathway and its regulation in hepatic fibrosis. J Histochem Cytochem. 2016;64(3):157–167. doi: 10.1369/0022155415627681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui L, Dang S, Qu J, Mao Z, Wang X, Zhang J, Chen J. FOXC2 is up-regulated in pancreatic ductal adenocarcinoma and promotes the growth and migration of cancer cells. Tumour Biol. 2016;37(7):8579–8585. doi: 10.1007/s13277-015-4607-4. [DOI] [PubMed] [Google Scholar]
- 27.Cui YM, Jiang D, Zhang SH, Wu P, Ye YP, Chen CM, Tang N, Liang L, Li TT, Qi L, et al. FOXC2 promotes colorectal cancer proliferation through inhibition of FOXO3a and activation of MAPK and AKT signaling pathways. Cancer Lett. 2014;353(1):87–94. doi: 10.1016/j.canlet.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Ding H, Tian J, Wu L, Wang Y, Xing Y, Chen M. Forkhead box protein C2 (FOXC2) promotes the resistance of human ovarian Cancer cells to cisplatin in vitro and in vivo. Cell Physiol Biochem. 2016;39(1):242–252. doi: 10.1159/000445620. [DOI] [PubMed] [Google Scholar]
- 29.Yang C, Cui X, Dai X, Liao W. Downregulation of Foxc2 enhances apoptosis induced by 5-fluorouracil through activation of MAPK and AKT pathways in colorectal cancer. Oncol Lett. 2016;11(2):1549–1554. doi: 10.3892/ol.2016.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Z, Zhang L, Xie B, Wang X, Yang X, Ding N, Zhang J, Liu Q, Tan G, Feng D, et al. FOXC2 promotes chemoresistance in nasopharyngeal carcinomas via induction of epithelial mesenchymal transition. Cancer Lett. 2015;363(2):137–145. doi: 10.1016/j.canlet.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Paranjape AN, Soundararajan R, Werden SJ, Joseph R, Taube JH, Liu H, Rodriguez-Canales J, Sphyris N, Wistuba I, Miura N, et al. Inhibition of FOXC2 restores epithelial phenotype and drug sensitivity in prostate cancer cells with stem-cell properties. Oncogene. 2016;35(46):5963–5976. doi: 10.1038/onc.2015.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai J, Tian AX, Wang QS, Kong PZ, Du X, Li XQ, Feng YM. FOXF2 suppresses the FOXC2-mediated epithelial-mesenchymal transition and multidrug resistance of basal-like breast cancer. Cancer Lett. 2015;367(2):129–137. doi: 10.1016/j.canlet.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Zhang CL, Zhu KP, Ma XL. Antisense lncRNA FOXC2-AS1 promotes doxorubicin resistance in osteosarcoma by increasing the expression of FOXC2. Cancer Lett. 2017;396:66–75. doi: 10.1016/j.canlet.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 34.Yu YH, Chen HA, Chen PS, Cheng YJ, Hsu WH, Chang YW, Chen YH, Jan Y, Hsiao M, Chang TY, et al. MiR-520h-mediated FOXC2 regulation is critical for inhibition of lung cancer progression by resveratrol. Oncogene. 2013;32(4):431–443. doi: 10.1038/onc.2012.74. [DOI] [PubMed] [Google Scholar]
- 35.Chai R, Fu H, Zheng Z, Liu T, Ji S, Li G. Resveratrol inhibits proliferation and migration through SIRT1 mediated posttranslational modification of PI3K/AKT signaling in hepatocellular carcinoma cells. Mol Med Rep. 2017;16(6):8037–8044. doi: 10.3892/mmr.2017.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao F, Deng G, Liu W, Zhou K, Li M. Resveratrol suppresses human hepatocellular carcinoma via targeting HGF-c-met signaling pathway. Oncol Rep. 2017;37(2):1203–1211. doi: 10.3892/or.2017.5347. [DOI] [PubMed] [Google Scholar]
- 37.Dai W, Wang F, Lu J, Xia Y, He L, Chen K, Li J, Li S, Liu T, Zheng Y, et al. By reducing hexokinase 2, resveratrol induces apoptosis in HCC cells addicted to aerobic glycolysis and inhibits tumor growth in mice. Oncotarget. 2015;6(15):13703–13717. doi: 10.18632/oncotarget.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.