Abstract
Background
Accumulating evidence supports the view that an imbalance of gut bacteria contributes to IBS, and that increasing the mass of beneficial species may reduce the numbers of pathogenic bacteria and help alleviate symptoms.
Methods
In this double-blind trial 400 adult patients with moderate-to-severe symptomatic diarrhea-predominant IBS (IBS-D) were randomized to treatment with the multi-strain probiotic Bio-Kult® (14 different bacterial strains) or placebo for 16 weeks. The change in severity and frequency of abdominal pain was the primary outcome measure.
Results
Probiotic treatment significantly improved the severity of abdominal pain in patients with IBS-D. A 69% reduction for probiotic versus 47% for placebo (p < 0.001) equates to a 145 point reduction on the IBS-severity scoring system (IBS-SSS). The proportion of patients who rated their symptoms as moderate-to-severe was reduced from 100% at baseline to 14% for the multi-strain probiotic at follow-up (month 5) versus 48% for placebo (p < 0.001). Also, the number of bowel motions per day from month 2 onwards was significantly reduced in the probiotic group compared with the placebo group (p < 0.05). In addition to relieving symptoms, the probiotic markedly improved all dimensions of quality of life in the 34-item IBS-Quality of Life (IBS-QoL) questionnaire. No serious adverse events were reported.
Conclusions
The multi-strain probiotic was associated with significant improvement in symptoms in patients with IBS-D and was well-tolerated. These results suggest that probiotics confer a benefit in IBS-D patients which deserves further investigation.
Trial registration
[Clinicaltrials.gov NCT03251625; retrospectively registered on August 9, 2017].
Electronic supplementary material
The online version of this article (doi:10.1186/s12876-018-0788-9) contains supplementary material, which is available to authorized users.
Keywords: BioKult, Diarrhea, Gastrointestinal well-being, IBS, Multi-strain probiotic, Probiotic, Randomized controlled trial, Quality of life
Background
Irritable bowel syndrome (IBS) is a common highly prevalent functional gastrointestinal (GI) disorder that places an enormous burden on resource-challenged healthcare systems [1, 2]. It has a heterogeneous clinical phenotype with various symptom combinations including abdominal pain, bloating and altered stool frequency, in the absence of any detectable organic disease with the available clinical tests and examinations [3]. Rome III criteria defines IBS as recurrent abdominal pain or discomfort at least 3 days/month in the last 3 months (symptom onset at least 6 months prior to diagnosis) associated with two or more of the following: improvement with defecation, onset associated with a change in frequency of stool, or onset associated with a change in form (appearance) of stool. The prevalence of IBS varies between geographic regions and populations, and is also dependent upon the diagnostic criteria used [4]. A worldwide prevalence of approximately 10–20% of the adult population has been reported [5] and in the largest analysis to date (41 countries, 288,103 individuals), the mean prevalence among different countries ranged from 1.1% (France and Iran) to 35.5% (Mexico) [1]. Three surveys in Bangladesh have reported IBS prevalence rates of 24.4% (Rome I criteria), 7.7% (Rome II criteria) and 12.9% (Rome III criteria) with a slightly higher rate in female patients [6–8]. Despite being highly symptomatic and negatively impacting the individual’s quality of life (QoL), it has been noted that only about one-third of patients present to their general practitioners [9].
The pathogenesis of IBS is multifactorial, including factors such as genetics, dietary intolerance, alterations in the GI microbiota, small intestinal overgrowth (SIBO), intestinal immune activation, increased intestinal permeability, visceral hypersensitivity, abnormal pain processing, disruption of the gut-brain axis, behavioral pathways and altered GI motility [10–12]. Diagnosis of IBS remains a challenge with no acceptable biochemical, histopathological or radiological tests available. Currently, it is diagnosed using symptom-based criteria initially proposed by Manning which were subsequently modified and incorporated into various iterations of the Rome criteria [13–16]. The role of inflammation and immunological mechanisms in the pathogenesis of IBS symptomatology may be particularly important since, during follow-up, IBS patients tend to have greater mucosal cellularity and other signs of an increased inflammatory response [17–20].
Many drugs have been advocated in the treatment of IBS, including antispasmodics, bulking agents, psychotropic agents, and 5-HT receptor antagonists. However, in the majority of cases these agents have proven to be disappointing for the relief of symptoms, possibly as a result of the heterogeneous pathogenesis of the disease. Probiotics are ‘live microorganisms which when administered in adequate amounts confer a health benefit on the host’ [21]. The rationale for the use of probiotics in the management of IBS is their potential to correct dysbiosis (qualitative and quantitative changes in the microbiota) or to stabilise the host microbiota. A decreased abundance of Bifidobacterium and Lactobacillus species [22], and an increase in Gammaproteobacteria species (a family comprised of numerous pathogens) [23] is frequently reported in IBS studies. Furthermore, PCR-denaturing gradient gel electrophoresis (PCR-DGGE) analysis of fecal samples from IBS patients reveals greater temporal instability of the microbiota compared to healthy controls [24]. The observation of dysbiosis, altered mucosal barrier function, activated immune responses and SIBO all support a potential role for bacteria in both the pathogenesis and treatment of IBS [25–27]. There is a growing body of opinion that an imbalance of gut bacteria contributes to IBS symptoms. Consequently, increasing the mass of beneficial species may help reduce the negative effects of pathogenic bacteria and help alleviate symptoms [28]. The majority of studies to date have been pilot studies [29–34], and this provided the stimulus for the current clinical trial to be undertaken in order to assess whether administration of a multi-strain probiotic (Bio-Kult®; 14 different bacterial strains; 2 billion colony-forming units per capsule) was more effective than placebo at reducing IBS symptoms (especially abdominal pain and frequency) and improving QoL in a large number of patients with diarrhea-predominant IBS (IBS-D) diagnosed in accordance with Rome III criteria.
Methods
Study design
The study was a randomized, double-blind, placebo-controlled, equal allocation ratio, parallel-group, clinical trial performed at the BSMMU Gastroenterology department between April 2014 and August 2016. The study was approved by the ethical board of BSMMU, IRB (Institutional Review Board) BSMMU, Dhaka prior to commencement of the study; reference number BSMMU/2015/1011. All participants were informed about the objectives, methodology and purpose of the study in an easily understandable way, and those who agreed to participate were required to provide verbal and written consent prior to entry. The study was conducted in accordance with the ethical principles set out in the Declaration of Helsinki and the ICH Harmonized Tripartite Guideline on Good Clinical Practice. [Clinicaltrials.gov NCT03251625; retrospectively registered August 9, 2017].
Patients
Male and female patients aged 18 to 55 years with moderate to severe IBS-D diagnosed according to Rome III criteria [recurrent abdominal pain or discomfort (an uncomfortable sensation not described as pain) at least three days a month in the past three months, associated with two or more of the following: improvement with defecation; onset associated with a change in frequency of stool; and onset associated with a change in form (appearance) of stool. The criteria should be fulfilled for the past three months with symptom onset at least six months before diagnosis]. Patients classified as IBS-D (diarrhea predominant) had > 25% loose/wet motions (Bristol stool scale 6–7) and < 25% firm/hard motions (Bristol stool scale 1–2). The severity of IBS was determined by the IBS Severity Scoring System (IBS-SSS) as described below.
The following alarm features were required to be absent as part of screening to minimize the risk of missing important organic diseases; rectal bleeding, anemia, unexplained weight loss, nocturnal diarrhea, and a family history of organic GI diseases (e.g. colon cancer or inflammatory bowel disease). All patients agreed not to start any other drug treatment unless clinically indicated. Exclusion criteria included: treatment with probiotics within last three months; concurrent severe illness (cancer, uncontrolled diabetes mellitus, hepatic, renal or cardiac dysfunction, and hyper- or hypothyroidism); previous GI surgery; chronic organic bowel disorders (e.g. inflammatory bowel disease, tuberculosis, diverticular disease, etc); treatment with antibiotics in the two months prior to enrolment; pregnancy or lactation. To exclude other diagnoses, patients fulfilling the inclusion criteria for IBS were screened using the following tests: full blood count (FBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), antibody testing for coeliac disease (endomysial antibodies or tissue transglutaminase).
Procedures
Patients fulfilling the inclusion criteria in the absence of exclusion criteria or an alternative diagnosis, and who provided a written informed consent form, were included in the study and comprised the randomization group (Fig. 1). All participants were advised to maintain their usual dietary practices throughout the study.
Fig. 1.
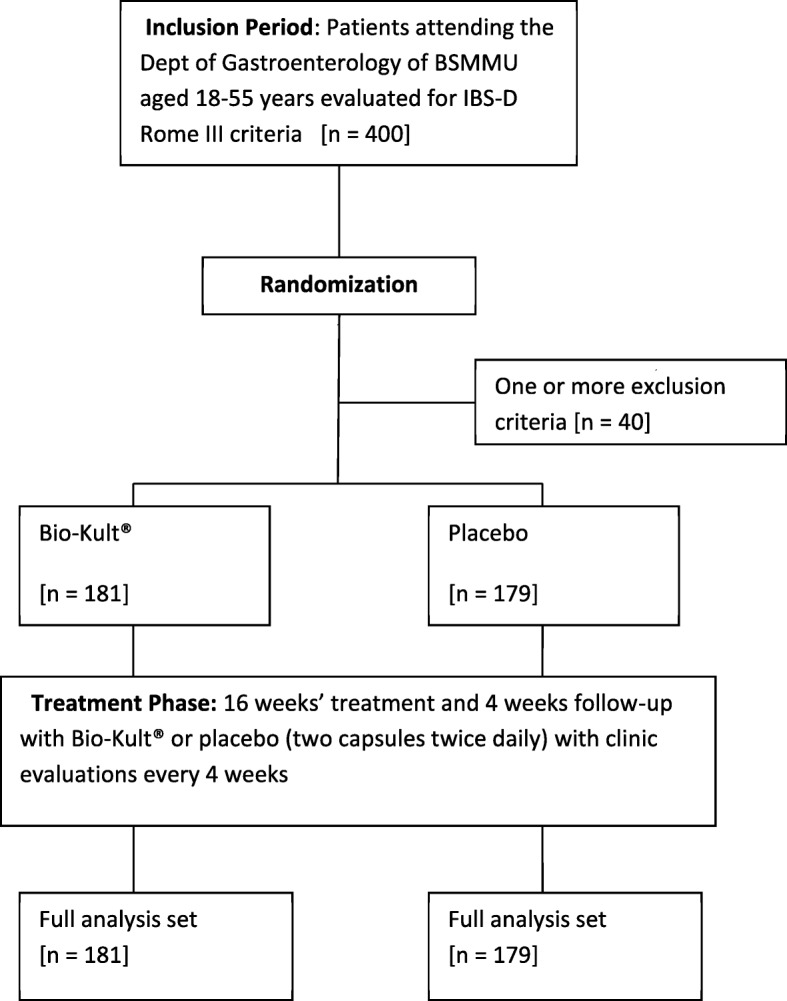
Study protocol
The aim was to recruit approximately 400 IBS-D patients (see Statistical Analyses section). Randomization into two groups (equal ratios) was performed by an independent statistician using the randomizer software (www.randomizer.org). One group received the multi-strain probiotic Bio-Kult® (Probiotics International Ltd. (Protexin), Somerset, UK), two capsules twice daily (manufacturer-recommended daily dosage), while the other group received identical placebo capsules (the filler was microcrystalline cellulose in a vegetable capsule made of hydroxypropyl methylcellulose), two capsules twice daily. Bio-Kult® is a capsule formulation containing 14 different bacterial strains (2 billion CFUs per capsule) and a dosage of two capsules twice a day is equivalent to 8 billion CFUs / day. The 14 different bacterial strains in Bio-Kult® are: Bacillus subtilis PXN 21, Bifidobacterium spp. (B. bifidum PXN 23, B. breve PXN 25, B. infantis PXN 27, B. longum PXN 30), Lactobacillus spp. (L. acidophilus PXN 35, L. delbrueckii spp. Bulgaricus PXN39, L. casei PXN 37, L. plantarum PXN 47, L. rhamnosus PXN 54, L.helveticus PXN 45, L. salivarius PXN 57), Lactococcus lactis PXN 63, and Streptococcus thermophilus PXN 66]. The capsules were administered before or during a meal for a total of 16 weeks; patients were followed-up one month after this time.
An independent data monitor maintained treatment codes and allocation, which was locked until all analyses had been completed. Thus, the clinical trial was performed double-blind with all patients and clinical staff unaware of which treatment had been allocated.
Before starting treatment each individual underwent a baseline assessment during which demographic data, IBS symptoms and QoL data were recorded. During treatment patients were required to return to the clinic once a month for reassessment of IBS symptoms and QoL, and to report any adverse events (AE) that had occurred.
The IBS-Severity Scoring System (IBS-SSS) questionnaire was completed at baseline, at each monthly clinic visit and after one month’s follow-up. The IBS-SSS is a 5-item instrument used to measure severity of abdominal pain, frequency of abdominal pain (number of days with abdominal pain over the last 10 days), severity of abdominal distension, dissatisfaction with bowel habits, and interference with quality of life, each on a 100-point scale [35]. The items are summed and thus the total score can range from 0 to 500 points. IBS severity has the following defined ranges: mild 75–174, moderate 175–300, and severe > 300. The IBS-SSS was completed by the physician at each clinic visit.
The IBS-QoL questionnaire is a 34-item measure constructed specifically to assess QoL impairment due to IBS symptoms [36]. Each item is scored on a five-point scale (1 = not at all, 5 = a great deal) that represents one of eight dimensions (dysphoria, interference with activity, body image, health-related worries, food avoidance, social reactions, sexual dysfunction, and relationships). Items are scored to derive an overall total score of IBS related QoL. To facilitate score interpretation, the summed total score is transformed to a 0–100 scale ranging from zero (poor QoL) to 100 (maximum QoL). The QoL instrument was translated into Bengali and given to each patient before treatment was started; the patient completed the form each month and all data were recorded and entered onto a data sheet.
Efficacy assessments and endpoints
The aim of this study was to determine whether administration of a multi-strain probiotic was more effective than placebo at reducing GI symptoms and improving QoL in patients with moderate to severe IBS-D.
Primary endpoint
The change in severity and frequency of abdominal pain on the IBS-SSS during treatment with a multi-strain probiotic or placebo, and compared with baseline.
Secondary endpoints
The change in other GI symptom severity scores (including stool consistency, frequency, and bloating) on the IBS-SSS during treatment with a multi-strain probiotic and placebo, and compared with baseline.
The change in QoL parameters (using a validated IBS-QoL questionnaire) during treatment with a multi-strain probiotic and placebo, and compared with baseline.
To assess any AEs reported during treatment with a multi-strain probiotic and placebo.
Sample size
The sample size for this trial was determined using the formula:
Where:
μ1 – μ2 represents the minimum clinically important difference which was set at 30% (this is advocated as the minimal clinically significant reduction for probiotics). A standard deviation (σ) of 87.77 for IBS-SSS from a similarly designed study was reported by Sisson and colleagues and was used in the sample size calculation [29]. Based on these assumptions the sample size was calculated to be 135 per treatment group (270 in total). Allowing for dropouts and non-adherence we estimated that the sample size should be increased to a total of approximately 384 patients (192 per group). In practice the first 400 patients with IBS-D attending the BSMMU Gastroenterology department between April 2014 and August 2016 and who provided written informed consent entered the study and were randomized to treatment.
Statistical analyses
All analyses were performed in the per-protocol (PP) set, i.e. treated patients that had no major protocol violations, met the minimum protocol requirements, and who were able to be evaluated for the primary endpoint. IBS-SSS symptom scores and IBS-QoL instrument scores were expressed as means ± standard deviation (SD) and analysed using the Student’s unpaired t-test. Categorical data are presented as frequencies/percentages and were analysed using the chi-square test. The relationship between different variables was investigated using Pearson’s correlation coefficient. P values < 0.05 were considered to be statistically significant. All analyses were performed using a computer based SPSS program (version 13.0).
Results
A total of 400 patients with IBS-D diagnosed by Rome III criteria and who met the inclusion criteria, were randomized to 16 weeks’ treatment. A total of 360 patients completed the study and comprised the PP analysis set. Of these, 181 patients received multi-strain probiotic treatment and 179 received placebo (Fig. 1).
Demographics and baseline characteristics
The treatment groups were comparable with respect to mean (±SD) age (32.2 ± 10.1 and 31.7 ± 9.7 years for probiotic and placebo,respectively) and gender (male / female ratio 3.7/1 with a slightly higher rate in the placebo group: 4.3/1 vs. 3.2/1 in the multi-strain probiotic group (p = 0.179) (Table 1). The two groups were comparable for the proportion of patients with moderate/severe IBS-D: 21.5/78.5% in the probiotic group and 29.1/70.9% in the placebo group (p = 0.101).
Table 1.
Patient demographics
| Variable | Probiotic (n = 181) | Placebo (n = 179) | P-value |
|---|---|---|---|
| Age (years) [mean ± SD] | 32.2 ± 10.1 | 31.7 ± 9.7 | 0.642 |
| Gender (males/ females) | 136/45 | 145/34 | 0.179 |
| IBS-D (Rome III criteria) | 181 (100) | 179 (100) | NS |
| Moderate | 39 (21.5) | 52 (29.1) | 0.101 |
| Severe | 142 (78.5) | 127 (70.9) | |
| Occupation: | |||
| Service industry | 59 | 50 | 0.043 |
| Student | 51 | 38 | |
| Business person | 19 | 23 | |
| Housewife | 22 | 25 | |
| Worker (painter, tailor, driver, farmer) | 30 | 43 | |
Changes in IBS symptom scores
Results pertaining to changes in IBS symptom scores are presented in Table 2. For the 5-item IBS-SSS endpoints (abdominal pain, frequency of abdominal pain (number of days with abdominal pain over the last 10 days), severity of abdominal distension, dissatisfaction with bowel habits, and interference with life), as well as the overall IBS-SSS score, the differences between the multi-strain probiotic and placebo groups were statistically significant at all time points. In the probiotic group the overall IBS-SSS was reduced by 145 points within 30 days (117 points in the placebo group) and by 223 points by month 5 (157 points in the placebo group). This is highlighted by a highly significant reduction in abdominal pain levels (primary outcome measure) during four months’ treatment and at follow-up (Fig. 2). At follow-up the abdominal pain level had decreased by 69% (decrease of 40 points) in the multi-strain probiotic group versus 47% (decrease of 27 points) in the placebo group (58.5 ± 11.1 to 18.1 ± 15.2 vs. 57.2 ± 10.6 to 30.2 ± 19.9; p < 0.001).
Table 2.
IBS symptom scores at baseline, during 16 weeks’ treatment and after one month’s follow-up
| Probiotic (Bio-Kult®) (n = 181) | Placebo (n = 179) | P-value | |
|---|---|---|---|
| Overall IBS-SSS scores | |||
| Before treatment | 333.0 ± 40.4 | 332.9 ± 42.0 | 0.992 |
| Month 1 | 187.9 ± 61.3 | 215.4 ± 75.0 | < 0.001 |
| Month 2 | 146.5 ± 76.4 | 188.0 ± 92.0 | < 0.001 |
| Month 3 | 122.0 ± 78.3 | 199.5 ± 104.1 | < 0.001 |
| Month 4 | 115.2 ± 75.0 | 179.7 ± 100.2 | < 0.001 |
| Month 5 | 110.0 ± 71.8 | 176.0 ± 100.0 | < 0.001 |
| IBS-SSS: Severity score of abdominal pain | |||
| Before treatment | 58.5 ± 11.1 | 57.2 ± 10.6 | 0.264 |
| Month 1 | 30.3 ± 14.8 | 35.3 ± 15.9 | 0.002 |
| Month 2 | 23.8 ± 16.2 | 31.1 ± 18.8 | < 0.001 |
| Month 3 | 20.3 ± 15.8 | 33.1 ± 19.7 | < 0.001 |
| Month 4 | 18.5 ± 16.2 | 30.4 ± 20.3 | < 0.001 |
| Month 5 | 18.1 ± 15.2 | 30.2 ± 19.9 | < 0.001 |
| IBS-SSS: Number of days in the last 10 days with pain | |||
| Before treatment | 7.7 ± 2.3 | 8.1 ± 2.3 | 0.056 |
| Month 1 | 3.6 ± 2.1 | 4.4 ± 2.5 | 0.001 |
| Month 2 | 2.9 ± 2.3 | 3.8 ± 2.7 | 0.001 |
| Month 3 | 2.5 ± 2.2 | 4.2 ± 2.8 | < 0.001 |
| Month 4 | 2.4 ± 2.1 | 4.1 ± 3.2 | < 0.001 |
| Month 5 | 2.2 ± 1.9 | 3.9 ± 3.0 | < 0.001 |
| IBS-SSS: Severity score of abdominal distension | |||
| Before treatment | 58.5 ± 11.5 | 58.9 ± 12.0 | 0.695 |
| Month 1 | 34.2 ± 16.2 | 38.4 ± 19.3 | 0.028 |
| Month 2 | 25.7 ± 16.9 | 35.6 ± 20.2 | < 0.001 |
| Month 3 | 21.1 ± 16.4 | 37.5 ± 22.3 | < 0.001 |
| Month 4 | 20.1 ± 16.6 | 36.3 ± 23.3 | < 0.001 |
| Month 5 | 19.6 ± 15.8 | 35.9 ± 23.5 | < 0.001 |
| IBS-SSS: Satisfaction score for bowel symptoms | |||
| Before treatment | 71.0 ± 9.7 | 69.6 ± 13.1 | 0.256 |
| Month 1 | 44.9 ± 14.2 | 50.3 ± 16.9 | 0.001 |
| Month 2 | 34.3 ± 17.2 | 43.0 ± 19.6 | < 0.001 |
| Month 3 | 29.6 ± 18.3 | 45.5 ± 22.5 | < 0.001 |
| Month 4 | 28.0 ± 18.9 | 43.3 ± 23.8 | < 0.001 |
| Month 5 | 26.5 ± 19.1 | 42.2 ± 25.0 | < 0.001 |
| Highest number of bowel motions per day | |||
| Before treatment | 6.1 ± 2.6 | 5.6 ± 1.8 | 0.024 |
| Month 1 | 3.3 ± 1.4 | 3.3 ± 1.4 | 0.891 |
| Month 2 | 2.9 ± 1.2 | 3.2 ± 1.3 | 0.043 |
| Month 3 | 3.0 ± 1.5 | 3.6 ± 1.3 | < 0.001 |
| Month 4 | 2.7 ± 1.5 | 3.5 ± 1.3 | < 0.001 |
| Month 5 | 2.5 ± 1.4 | 3.4 ± 1.4 | < 0.001 |
| Passing excess mucus [no. pts. (%)] | |||
| Before treatment | 181 (100.0) | 179 (100.0) | |
| Month 1 | 170 (93.9) | 177 (98.9) | 0.012 |
| Month 2 | 175 (96.7) | 175 (97.8) | 0.748 |
| Month 3 | 175 (98.9) | 177 (98.9) | 0.157 |
| Month 4 | 171 (94.5) | 174 (97.2) | 0.195 |
| Month 5 | 168 (92.8) | 171 (95.5) | 0.272 |
| IBS-SSS: Score of IBS affecting or interfering with life | |||
| Before treatment | 68.6 ± 12.6 | 66.1 ± 11.1 | 0.049 |
| Month 1 | 42.5 ± 15.0 | 47.4 ± 16.6 | 0.004 |
| Month 2 | 33.4 ± 17.2 | 40.6 ± 19.7 | < 0.001 |
| Month 3 | 26.0 ± 17.9 | 41.8 ± 23.4 | < 0.001 |
| Month 4 | 24.9 ± 16.5 | 29.1 ± 19.6 | 0.028 |
| Month 5 | 23.4 ± 17.4 | 28.2 ± 20.1 | 0.015 |
The unpaired t-test was used to determine the level of statistical significance
Fig. 2.
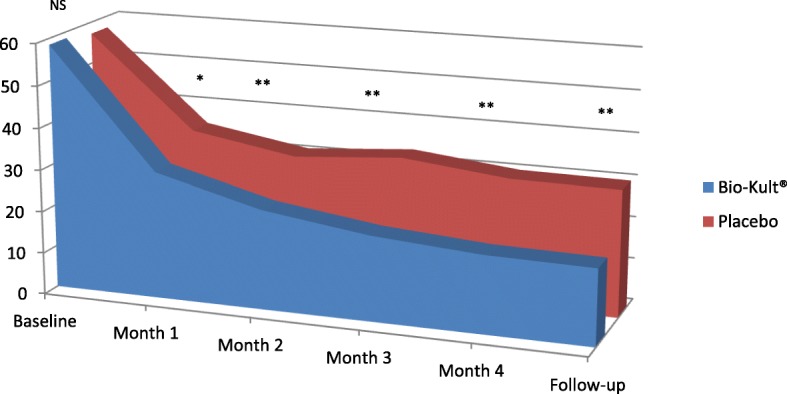
IBS-SSS abdominal pain ratings with probiotic (Bio-Kult®) or placebo (16 weeks’ treatment and 1-month follow-up). The lower the score the less the pain: * p = 0.002; ** p < 0.001; NS = not significant
In addition to improvements in IBS-SSS ratings, the number of bowel motions/day was significantly reduced from month 2 onwards in the multi-strain probiotic group compared with the placebo group (Table 2). In contrast the passing of excess mucus was similar in the two treatment groups.
At baseline all patients rated their symptoms as moderate to severe (Table 3). However, at the end of the follow-up period 52.5% of patients in the multi-strain probiotic group rated their symptoms as mild compared with 39.1% in the placebo group (p < 0.001). Moreover, the number of patients symptom free at the end of the study was 33.7% in the multi-strain probiotic group compared with only 12.8% in the placebo group (p < 0.001).
Table 3.
Severity of symptoms at baseline, during 16 weeks’ treatment and after one month’s follow-up)
| Severity of IBS-D | Probiotic (Bio-Kult®) (n = 181) | Placebo (n = 179) | P-value |
|---|---|---|---|
| Baseline | |||
| Moderate | 39 (21.5) | 52 (29.1) | 0.101 |
| Severe | 142 (78.5) | 127 (70.9) | |
| Month 1 | |||
| Symptoms free period | 2 (1.1) | 2 (1.1) | 0.086 |
| Mild | 78 (43.1) | 58 (32.4) | |
| Moderate | 91 (50.3) | 99 (55.3) | |
| Severe | 10 (5.5) | 20 (11.2) | |
| Month 2 | |||
| Symptoms free period | 16 (8.8) | 18 (10.1) | < 0.001 |
| Mild | 112 (61.9) | 61 (34.1) | |
| Moderate | 42 (23.2) | 82 (45.8) | |
| Severe | 11 (6.1) | 18 (10.1) | |
| Month 3 | |||
| Symptoms free period | 54 (29.8) | 20 (11.2) | < 0.001 |
| Mild | 98 (54.1) | 62 (34.6) | |
| Moderate | 23 (12.7) | 57 (31.8) | |
| Severe | 6 (3.3) | 40 (22.3) | |
| Month 4 | |||
| Symptoms free period | 56 (30.4) | 22 (11.2) | < 0.001 |
| Mild | 99 (54.7) | 68 (38.0) | |
| Moderate | 21 (11.6) | 66 (36.9) | |
| Severe | 6 (3.3) | 23 (12.8) | |
| Follow-up: Month 5 | |||
| Symptoms free period | 61 (33.7) | 23 (12.8) | < 0.001 |
| Mild | 95 (52.5) | 70 (39.1) | |
| Moderate | 21 (11.6) | 65 (36.3) | |
| Severe | 4 (2.2) | 21 (11.7) | |
The unpaired Chi-square test was used to determine the level of statistical significance
Comparison of IBS symptom score improvements by age and gender in the multi-strain probiotic group revealed no statistically significant differences by month 5. In contrast, in the placebo group, patients aged 30 years and over had significantly improved pain (p < 0.001) and abdominal bloating (p = 0.041) scores compared with patient aged < 30 years.
Changes in IBS-QoL scores
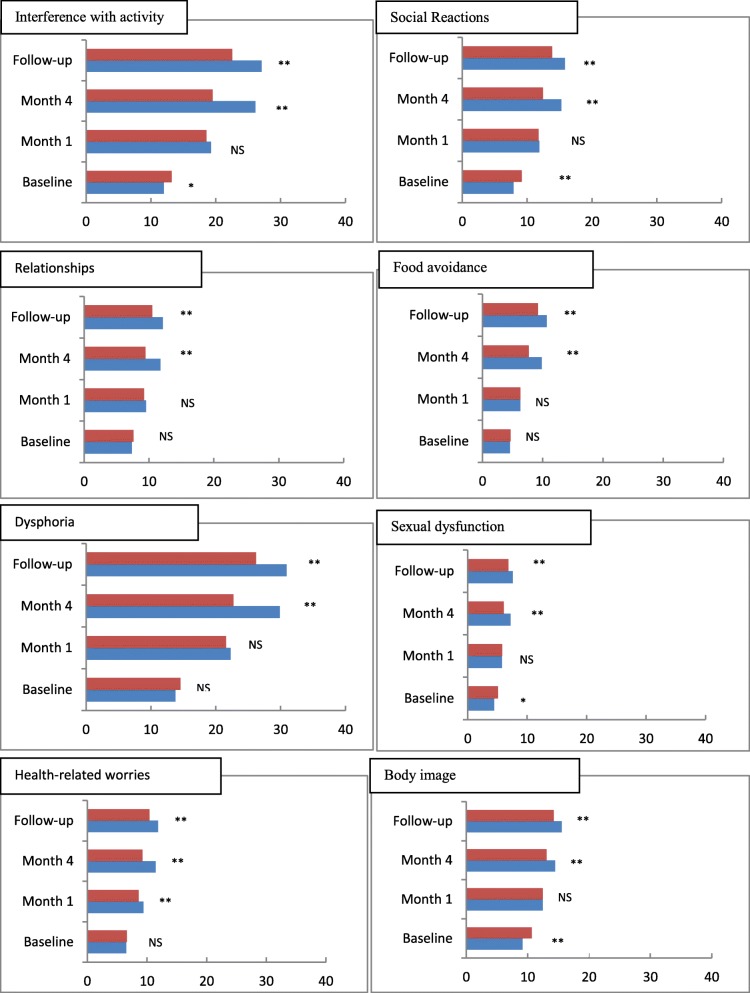
Changes in overall QoL as assessed by the IBS-QoL questionnaire are shown in Table 4. From month 2 onwards there was a statistically significant improvement in QoL in the multi-strain probiotic group compared with the placebo group. Figure 3 details the results for the eight individual dimensions on the IBS QoL questionnaire. Food avoidance, sexual dysfunction and health-related worries had the most negative impact on QoL at baseline in both groups of patients. The benefits of multi-strain probiotic therapy were consistent across all eight dimensions with a steady increase over time. After 4 months’ treatment, and at follow-up, the ratings in the probiotic group were statistically significantly higher than in the placebo group (p < 0.001 in all cases). Neither age nor gender had any statistically significant effects on IBS-QoL scores at month 5.
Table 4.
IBS-QoL scores at baseline, during 16 weeks’ treatment and after one month’s follow-up)
| Probiotic (Bio-Kult®) (n = 181) | Placebo (n = 179) | P-value | |
|---|---|---|---|
| Before treatment | 22.6 ± 10.5 | 27.5 ± 13.0 | < 0.001 |
| At 1st month | 46.5 ± 13.6 | 44.8 ± 15.8 | 0.270 |
| At 2nd month | 59.0 ± 18.9 | 48.7 ± 20.3 | < 0.001 |
| At 3rd month | 66.4 ± 21.6 | 47.6 ± 22.9 | < 0.001 |
| At 4th month | 68.3 ± 21.8 | 48.4 ± 24.5 | < 0.001 |
| At 5th month | 72.0 ± 16.5 | 58.5 ± 16.8 | < 0.001 |
The unpaired t-test was used to determine the level of statistical significance
Note: In this scoring system, higher scores indicate better QoL
Fig. 3.
Individual dimension scores for IBS-QoL during 16 weeks’ treatment with multi-strain probiotic (Bio-Kult®; blue square) or placebo (red square). * p < 0.05; ** p < 0.001; NS, not significant. Note: In this scoring system, higher scores indicate better QoL
Tolerability and safety
Both multi-strain probiotic treatment and placebo were well-tolerated with no treatment-related adverse events (AEs) reported during the study.
Discussion
Summary of main findings
According to Rome III criteria, abdominal pain or discomfort are hallmark determinants for the initial diagnosis of IBS and changes in bowel movements define different subtypes [16]. Typically, the pain or discomfort is related to defecation, or its onset is associated with an increase or decrease in the frequency or form of stool, which can be further exacerbated by stressful life events. Therefore, changes in the severity of abdominal pain are a reliable measure of treatment outcome and were assessed in this cohort using IBS-SSS questionnaire, which incorporates two pain-related items [29, 35, 37]. A decrease of > 50 points in the IBS-SSS score is indicative of a clinical improvement [35], although it has been suggested that a minimum reduction of 95 points is needed to show a clinically relevant change in symptoms [37]. In the current study an average reduction in the overall IBS-SSS score was 145 points achieved within 30 days of intervention and this was increased to over 200 points after 16 weeks of treatment. These reductions with multi-strain probiotic are clinically meaningful and they were not significantly affected by age or gender. Furthermore, they are markedly greater than changes in IBS-SSS reported previously with a single strain probiotic after 12 weeks’ treatment (reduction of 50 points after 12 weeks) [38] and in multi-strain probiotic trial (reduction of 63 points after 12 weeks) [29]. With respect to the primary outcome, 16 weeks supplementation with a multi-strain probiotic, reduced the abdominal pain level by 69% versus 47% in the placebo group (58 points to 18 points vs. 57 points to 30 points; p < 0.001). Over 85% of patients in the probiotic group reported an improvement in their severity category, whereas nearly half (48%) of the placebo group did not see an improvement in their severity category. The relatively high response in the placebo group has been previously reported in functional bowel disorder studies, which was shown to have a negative correlation with study duration [30]. As per European Medicines Agency “Guidance on The Evaluation of Medicinal Products for the Treatment of IBS”, a responder has to demonstrate abdominal pain score which has improved at least 30% compared to baseline [39]. In the current study key symptoms of IBS were statistically significantly improved compared with placebo, with the change in most parameters exceeding 30%.
There is accumulating evidence showing that certain probiotics may be capable of significantly reducing abdominal pain, abdominal distension and flatulence while, at the same time, increasing health-related QoL in IBS patients [40]. Likewise in the current study, in addition to relieving symptoms, multi-strain probiotic treatment was shown to markedly improve all dimensions of QoL evaluated using the 34-item IBS-QoL questionnaire. Beneficial changes were noted within one month of starting treatment and, after 4 months’ treatment, the improvements were statistically significant (p < 0.001) for all eight measures included in this QoL instrument. During the course of this clinical trial, multi-strain probiotic treatment and placebo were equally well-tolerated with no treatment-related AEs reported.
Comparison with existing literature
A recently published systematic review of probiotics undertaken by the British Dietetic Association (BDA) found that of 35 probiotic RCTs analysed, almost three-quarters were potentially pilot studies with a sample size too small (< 50 patients/group) to develop any probiotic-specific clinical recommendations [41]. The sample size (N = 360) of the current study was based on robust statistical analyses to achieve adequate power and represents one of the largest probiotic IBS clinical trials to date. Moreover, the research recommendations from the BDA review called for high-quality RCTs of probiotics to consider IBS sub-type and to use validated symptom and QoL assessments [41]. In this regard our study only included patients with IBS-D, and assessments were performed using validated IBS-SSS and IBS-QoL instruments.
Another interesting finding in the BDA review was that studies which used multi-strain probiotics seemed to produce better clinical results than single-strain probiotics in terms of global symptoms (14 of 35 studies reported statistically significant benefit and 65% of these used multi-strain probiotics), abdominal pain (8 of 35 studies reported statistically significant benefit and 63% of these used multi-strain probiotics) and QoL (only 2 of 16 studies that measured QoL reported statistically significant benefit, and both of these studies used multi-strain probiotics) [41]. Research teams at our institution (BSMMU) have also seen variable responses dependent upon the bacterial strains used as probiotic. Results of a randomized, double-blind, placebo-controlled trial of the effects of a single strain probiotic, S. boulardii, in IBS-D patients were not very promising [32]. However, a follow-up study with a multi-strain probiotic yielded a beneficial outcome which was significant both clinically and statistically [33]. A number of studies have shown a decrease in the microbial diversity of IBS patients compared to healthy controls [42], and specifically in relation to Bifidobacteria and Lactobacilli spp. [12, 43]. The 14-strain probiotic used in this current study (including 7 Lactobacilli and 4 Bifidobacteria spp.) may have provided clinical benefits by increasing the microbial diversity in these patients, and may also help explain why single-strain probiotic studies have not been as successful in reducing symptoms in IBS patients.
It is undeniable that the gut microbiota has both direct and indirect effects on the immune system and inflammation [44, 45]. Current evidence suggests that IBS patients have greater mucosal cellularity and other signs of increased inflammatory activity which might contribute to the development of IBS [12, 46]. In this study markers of inflammation were not measured, however future studies would benefit from monitoring inflammation to explore the impact of probiotics on systemic and local inflammatory markers. Overall, and despite the growing interest in this field, our understanding of the role of the gut microbiota in functional GIs such as IBS remains limited [12]. The results of the current trial provide an insight into the benefits that may be obtained using a multi-strain probiotic, but the study was not designed to help elucidate the physiological mechanisms underpinning the observed clinical improvements.
Study strengths and limitations
The strengths of this clinical trial relate to its design involving a large number of IBS-D patients with strict controls to reduce factors which could influence bias (maintained double-blind by independent personnel) and variation (large number of patients with severe IBS-D in a placebo-controlled study). The vast majority of IBS probiotic trials undertaken so far suffer from small sample sizes, which renders their findings inconclusive. In contrast, this trial appears to be one of the largest published to date in a ‘relatively’ homogeneous group of patients with severe IBS-D. The trial had adequate statistical power to determine statistically/clinical relevant differences between the multi-strain probiotic and placebo in patients with IBS-D. This limits conclusions that can be drawn from findings to similar types of patients treated with the same multi-strain probiotic species.
For a trial of this type there are a number of important limitations that need to be outlined. Firstly, compliance was only checked qualitatively and reinforced at each client visit via verbal questioning and no metrics were maintained. Secondly, while all participants were advised to maintain their usual dietary practices throughout the study, and this was monitored informally at client visits, no nutritional assessments were undertaken to confirm dietary adherence. Thirdly, the reasons for patients withdrawing from the study were not always readily available. Because of this it was decided to perform a per-protocol analysis which presents a potential biased best-case view of the available data since the conclusions only apply to ‘ideal’ patients who are fully adherent to the treatment that they have been given. Another potential limitation of the study is that routine colonoscopy was not performed to rule out the presence of microscopic colitis (MC), as is the case for almost all studies in this therapeutic setting. However, the relatively young age of our cohort (approximate mean age 32 years) with a predominance of males (almost 80%) means that the relative incidence of MC would be low and unlikely to significantly impact on the reported findings.
Finally, the duration of the study (4 months’ treatment plus one month’s follow-up) is consistent with clinical trials in this therapeutic setting, but it is relatively short for a disease which is potentially life-long. Consequently, no conclusions regarding the durability of the response can be made.
Conclusions
In this large controlled clinical trial well-validated instruments to assess symptom severity (IBS-SSS) and QoL (IBS-QoL) were used in patients with IBS-D. We found that the multi-strain probiotic Bio-Kult® (14 different bacterial strains; 8 billion colony-forming units per day) was safe and superior to placebo in improving GI symptoms over a period of 4 months in patients with IBS-D. Furthermore, symptom improvement was paralleled by statistically significant benefits in all measures of QoL. Finally, it is important to note that the findings only apply to the multi-strain probiotic administered and should not be generalised to other probiotics or IBS patient subtypes.
Additional file
The consort checklist. (DOC 254 kb)
Acknowledgements
The authors would like to thank all support staff in the hospital that helped with the trial. They also thank Dr. Steve Clissold (Content Ed Net, UK) for assistance with medical writing that was funded by Probiotics International Ltd. (Protexin), Lopen Head, Somerset, UK.
Funding
Probiotics International Ltd. (Protexin), Lopen Head, Somerset, UK provided support in relation to supply of Bio-Kult® probiotic and placebo capsules, review of the draft manuscript and financial support for medical writing. The paper was reviewed by the sponsor and an expert nominated by them with some requested changes included in the final version.
Availability of data and materials
The datasets used and analysed during the study will be available from the corresponding author on reasonable request.
CONSORT guidelines
This study was conducted in adherence to the CONSORT guidelines.
CONSORT checklist
The completed CONSORT checklist is available as an Additional file 1.
Authors’ contributions
Study conception and design: SMI. Acquisition of data: SMK. Analysis and interpretation of data: SMI. Drafting of manuscript: MPS. Critical revision: DSA. The full trial protocol, and datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request. All the authors have read and approved the manuscript.
Ethics approval and consent to participate
Ethical approval was obtained from IRB (Institutional Review Board) BSMMU, Dhaka prior to commencement of the study; reference number BSMMU/2015/1011. Informed consent to participate in the study was obtained from all participants. All participants were informed about the objectives, methodology, and purpose of the study in an easily understandable way, and those who agreed to participate were required to provide verbal and written consent prior to entry.
Consent for publication
Verbal and written consent for publication was obtained from participate.
Competing interests
All authors declare that:
1. They have no non-financial interest that may be relevant to the submitted work.
2. All authors confirm no other relationship with Probiotics International Ltd. (Protexin) whose.
involvement in the study was confined to supply of Bio-Kult® probiotic and placebo capsules, review.
of the draft manuscript and financial support for medical writing.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12876-018-0788-9) contains supplementary material, which is available to authorized users.
Contributor Information
Shamsuddin M. Ishaque, Email: s.md.ishaque@gmail.com
S. M. Khosruzzaman, Email: drkhosru603@gmail.com
Dewan Saifuddin Ahmed, Email: saifk36@yahoo.com.
Mukesh Prasad Sah, Email: mukeshjnk@gmail.com.
References
- 1.Sperber AD, Dumitrascu D, Fukudo S, et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome foundation working team literature review. Gut. 2017;66:1075–1082. doi: 10.1136/gutjnl-2015-311240. [DOI] [PubMed] [Google Scholar]
- 2.Canavan C, West J, Card T. Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther. 2014;40:1023–1034. doi: 10.1111/apt.12938. [DOI] [PubMed] [Google Scholar]
- 3.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949–958. doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]
- 4.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Thompson WG, Heaton KW, Smyth GT, Smyth C. Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut. 2000;46:78–82. doi: 10.1136/gut.46.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masud MA, Hasan M, Azad Khan AK. Irritable bowel syndrome in a rural community in Bangladesh. Prevalence, symptom pattern and health seeking behavior. Am J Gastroenterol. 2001;96:1547–1552. doi: 10.1111/j.1572-0241.2001.03760.x. [DOI] [PubMed] [Google Scholar]
- 7.Perveen I, Hasan M, Masud MA, Bhuiyan MM, Rahman MM. Irritable bowel syndrome in a Bangladeshi urban community: prevalence and health care seeking pattern. Saudi J Gastroenterol. 2009;15:239–243. doi: 10.4103/1319-3767.56099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perveen I, Rahman MM, Saha M, Rahman MM, Hasan MQ. Prevalence of irritable bowel syndrome and functional dyspepsia, overlapping symptoms, and associated factors in a general population of Bangladesh. Indian J Gastroenterol. 2014;33:265–273. doi: 10.1007/s12664-014-0447-1. [DOI] [PubMed] [Google Scholar]
- 9.Jones R, Lydeard S. Irritable bowel syndrome in the general population. BMJ. 1992;304:87–90. doi: 10.1136/bmj.304.6819.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang JY, Talley NJ. An update on irritable bowel syndrome: from diagnosis to emerging therapies. Curr Opin Gastroenterol. 2011;27:72–78. doi: 10.1097/MOG.0b013e3283414065. [DOI] [PubMed] [Google Scholar]
- 11.Bolino CM, Bercik P. Pathogenic factors involved in the development of irritable bowel syndrome: focus on a microbial role. Infect Dis Clin N Am. 2010;24:961–975. doi: 10.1016/j.idc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Imperatore N, Tortora R, Morisco F, Caporaso N. Gut microbiota and functional diseases of the gastrointestinal tract. Minerva Gastroenterol Dietol. 2017;63:355–372. doi: 10.23736/S1121-421X.16.02336-9. [DOI] [PubMed] [Google Scholar]
- 13.Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. Br Med J. 1978;2:653–654. doi: 10.1136/bmj.2.6138.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drossman DA, Richter JE, Talley NJ. The functional gastrointestinal bowel disorders, pathophysiology and treatment: a multinational consensus. Boston: Little Brown; 1994. [Google Scholar]
- 15.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl 2):II43–II47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drossman DA, Chang L, Bellamy N, et al. Severity in irritable bowel syndrome: a Rome foundation working team report. Am J Gastroenterol. 2011;106:1749–1759. doi: 10.1038/ajg.2011.201. [DOI] [PubMed] [Google Scholar]
- 17.Gwee KA, Graham JC, McKendrick MW, et al. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet. 1996;347:150–153. doi: 10.1016/S0140-6736(96)90341-4. [DOI] [PubMed] [Google Scholar]
- 18.Gwee KA. Irritable bowel syndrome in developing countries--a disorder of civilization or colonization? Neurogastroenterol Motil. 2005;17:317–324. doi: 10.1111/j.1365-2982.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 19.Ghoshal UC, Park H, Gwee KA. Bugs and irritable bowel syndrome: the good, the bad and the ugly. J Gastroenterol Hepatol. 2010;25:244–251. doi: 10.1111/j.1440-1746.2009.06133.x. [DOI] [PubMed] [Google Scholar]
- 20.Gwee KA, Bak YT, Ghoshal UC, Asian Neurogastroenterology and Motility Association et al. Asian consensus on irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1189–1205. doi: 10.1111/j.1440-1746.2010.06353.x. [DOI] [PubMed] [Google Scholar]
- 21.Joint FAO/WHO Working Group. Guidelines for the Evaluation of Probiotics in Food London. Report of a joint FAO/WHO Working Group. Report on Drafting, Ontario, Canada, April 30 and May 1, 2002. Available at: http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf [last accessed August 2017].
- 22.Staudacher HM, Whelan K. Altered gastrointestinal microbiota in irritable bowel syndrome and its modification by diet: probiotics, prebiotics and the low FODMAP diet. Proc Nutr Soc. 2016;75:306–318. doi: 10.1017/S0029665116000021. [DOI] [PubMed] [Google Scholar]
- 23.Saulnier DM, Riehle K, Mistretta TA, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. 2011;141:1782–1791. doi: 10.1053/j.gastro.2011.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mättö J, Maunuksela L, Kajander K, Palva A, Korpela R, Kassinen A, Saarela M. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome--a longitudinal study in IBS and control subjects. FEMS Immunol Med Microbiol. 2005;43:213–222. doi: 10.1016/j.femsim.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Ghoshal UC, Shukla R, Ghoshal U, Gwee KA, Ng SC, Quigley EM. The gut microbiota and irritable bowel syndrome: friend or foe? Int J Inflam. 2012;2012:151085. doi: 10.1155/2012/151085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quigley EM. Probiotics in irritable bowel syndrome: the science and the evidence. J Clin Gastroenterol. 2015;49(Suppl 1):S60–S64. doi: 10.1097/MCG.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 27.Quigley EM, Shanahan F. The future of probiotics for disorders of the brain-gut axis. Adv Exp Med Biol. 2014;817:417–432. doi: 10.1007/978-1-4939-0897-4_19. [DOI] [PubMed] [Google Scholar]
- 28.Lee HR, Pimentel M. Bacteria and irritable bowel syndrome: the evidence for small intestinal bacterial overgrowth. Curr Gastroenterol Rep. 2006;8:305–311. doi: 10.1007/s11894-006-0051-3. [DOI] [PubMed] [Google Scholar]
- 29.Sisson G, Ayis S, Sherwood RA, Bjarnason I. Randomised clinical trial: a liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome-a 12 week double-blind study. Aliment Pharmacol Ther. 2014;40:51–62. doi: 10.1111/apt.12787. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Li L, Guo C, Mu D, Feng B, Zuo X, Li Y. Effects of probiotic type, dose and treatment duration on irritable bowel syndrome diagnosed by Rome III criteria: a meta-analysis. BMC Gastroenterol. 2016;16:62. doi: 10.1186/s12876-016-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng J, Li YQ, Zuo XL, Zhen YB, Yang J, Liu CH. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:994–1002. doi: 10.1111/j.1365-2036.2008.03818.x. [DOI] [PubMed] [Google Scholar]
- 32.Kabir MA, Ishaque SM, Ali MS, Mahmuduzzaman M, Hasan M. Role of Saccharomyces Boulardii in diarrhea predominant irritable bowel syndrome. Mymensingh Med J. 2011;20:397–401. [PubMed] [Google Scholar]
- 33.Rahman MZ, Chowdhury MS, Rahman MA. Efficacy of probiotics in irritable bowel syndrome-a randomized, double blind placebo controlled study. BSMMU J. 2013;6:21–28. [Google Scholar]
- 34.Roberts LM, McCahon D, Holder R, Wilson S, Hobbs FDR. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45. doi: 10.1186/1471-230X-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 36.Drossman DA, Patrick DL, Whitehead WE, Toner BB, Diamant NE, Hu Y, Jia H, Bangdiwala SI. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol. 2000;95:999–1007. doi: 10.1111/j.1572-0241.2000.01941.x. [DOI] [PubMed] [Google Scholar]
- 37.Spiegel B, Bolus R, Harris LA, Lucak S, Naliboff B, Esrailian E, Chey WD, Lembo A, Karsan H, Tillisch K, Talley J, Mayer E, Chang L. Measuring irritable bowel syndrome patient-reported outcomes with an abdominal pain numeric rating scale. Aliment Pharmacol Ther. 2009;30:1159–1170. doi: 10.1111/j.1365-2036.2009.04144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyra A, Hillilä M, Huttunen T, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22:10631–10642. doi: 10.3748/wjg.v22.i48.10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.European Medicines Agency. Guidance on the evaluation of medicinal products for the treatment of IBS. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/09/WC500173457.pdf [last accessed June, 2017].
- 40.Bixquet Jimenez M. Treatment of irritable bowel syndrome with probiotics. An etiopathogenetic approach at last? Rev Esp Dig. 2009;101:553–564. doi: 10.4321/s1130-01082009000800006. [DOI] [PubMed] [Google Scholar]
- 41.McKenzie YA, Thompson J, Gulia P, Lomer MC, (IBS Dietetic Guideline Review Group on behalf of Gastroenterology Specialist Group of the British Dietetic Association) British dietetic association systematic review of systematic reviews and evidence-based practice guidelines for the use of probiotics in the management of irritable bowel syndrome in adults (2016 update) J Hum Nutr Diet. 2016;29:576–592. doi: 10.1111/jhn.12386. [DOI] [PubMed] [Google Scholar]
- 42.Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J Gastroenterol. 2014;20:14105–14125. doi: 10.3748/wjg.v20.i39.14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staudacher HM, Irving PM, Lomer MC, Whelan K. Mechanisms and efficacy of dietary FODMAP restriction in IBS. Nat Rev Gastroenterol Hepatol. 2014;11:256–266. doi: 10.1038/nrgastro.2013.259. [DOI] [PubMed] [Google Scholar]
- 44.Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 45.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 46.Gwee KA. Post-infectious irritable bowel syndrome, an inflammation-immunological model with relevance for other IBS and functional dyspepsia. J Neurogastroenterol Motil. 2010;16:30–34. doi: 10.5056/jnm.2010.16.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The consort checklist. (DOC 254 kb)
Data Availability Statement
The datasets used and analysed during the study will be available from the corresponding author on reasonable request.