Abstract
Tumor metastasis involves many stage-specific adhesive interactions. The expression of several cell adhesion molecules, notably the integrin αvβ3, has been associated with the metastatic potential of tumor cells. In this study, we used a novel in vitro assay to examine the role of αvβ3 in the transmigration of melanoma cells through a monolayer of human lung microvascular endothelial cells. Confocal microscopy revealed the presence of the integrin αvβ3 on melanoma membrane protrusions and pseudopods penetrating the endothelial junction. αvβ3 was also enriched in heterotypic contacts between endothelial cells and melanoma cells. Transendothelial migration of melanoma cells was inhibited by either a cyclic Arg-Gly-Asp peptide or the anti-αvβ3 monoclonal antibody LM609. Although both platelet endothelial cell adhesion molecule-1 and L1 are known to bind integrin αvβ3, only L1 serves as a potential ligand for αvβ3 during melanoma transendothelial migration. Also, polyclonal antibodies against L1 partially inhibited the transendothelial migration of melanoma cells. However, addition of both L1 and αvβ3 antibodies did not show additive effects, suggesting that they are components of the same adhesion system. Together, the data suggest that interactions between the integrin αvβ3 on melanoma cells and L1 on endothelial cells play an important role in the transendothelial migration of melanoma cells.
INTRODUCTION
The process of tumor metastasis consists of a complex cascade of adhesive interactions between tumor cells and host tissues (Nicolson, 1988; Stetler-Stevenson et al., 1993; Orr et al., 2000). The endothelium of blood vessels constitutes a physical barrier to cells in the circulatory system and metastatic cells must penetrate the interendothelial junctions to invade the underlying tissue. Although much is known about the adhesive interactions during the invasion of the basement membrane, relatively little is known about the mechanism by which tumor cells pass through the endothelial junction. We have developed a novel in vitro coculture assay to investigate the molecular interactions and morphological changes during cancer cell extravasation (Sandig et al., 1997; Voura et al., 1998a). Our previous work has shown that although platelet-endothelial cell adhesion molecule-1 (PECAM-1) and vascular endothelial-cadherin are not required for melanoma transendothelial migration, heterotypic interactions with classic cadherins may play a role in this process (Sandig et al., 1997). However, inclusion of anti-cadherin antibodies in the coculture assay results in only a low level of inhibition of the transmigration process, implicating the involvement of other cell adhesion molecules in this process.
In this report, we examine the role of αvβ3 during melanoma transendothelial migration in our in vitro assay system. The integrin αvβ3 was first identified as the “vitronectin receptor” but will adhere to a host of other extracellular matrix (ECM) proteins, including fibronectin, laminin, collagen, and osteopontin (Smith and Cheresh, 1990; Horton, 1997). αvβ3 is known to promote cell attachment and spreading, as well as cell locomotion (Seftor et al., 1992; Danen et al., 1994). Expression of αvβ3 by melanoma cells has been linked to the progression of disease (Albelda et al., 1990; Felding-Habermann et al., 1992; Danen et al., 1994; Weterman et al., 1994; Natali et al., 1997; Johnson, 1999). In addition to melanoma, the αv integrins have been implicated in invasion and metastasis of other forms of cancers (Lafrenie et al., 1994; Yun et al., 1996).
αvβ3 undergoes heterophilic binding with PECAM-1 and L1 (Buckley et al., 1996; Montgomery et al., 1996). Both PECAM-1 and L1 are members of the immunoglobulin (Ig) superfamily of cell adhesion molecules. PECAM-1 is expressed in high levels on endothelial cells and is concentrated in the endothelial junctions (Albelda et al., 1991). It has been shown to play a role in the transendothelial migration of leukocytes (Muller et al., 1993; Muller, 1995). L1 is expressed primarily in the nervous system. However, a nonneuronal form of L1 is found on leukocytes, epithelial cells, and various cancer cells (Kowitz et al., 1992; Kujat et al., 1995; Pancook et al., 1997). L1 contains six Ig-like domains and five fibronectin type III-like repeats (Hortsch, 1996). In addition to homophilic binding (Miura et al., 1992; Zhao and Siu, 1995, 1996), L1 interacts with αvβ3 and other integrins through an RGD sequence in its Ig-6 domain (Ruppert et al., 1995; Montgomery et al., 1996; Felding-Habermann et al., 1997; Yip et al., 1998, Yip and Siu, 2001). L1 also interacts heterophilically with laminin (Hall et al., 1997) and other Ig-like molecules (Kuhn et al., 1991; Horstkorte et al., 1993).
L1 is shed from melanoma cells and has been suggested to provide an adhesive matrix for these cells via cell bound αvβ3 (Montgomery et al., 1996). Ligation of αvβ3 with L1 also promotes haptotaxis of melanoma cells. Likewise, soluble L1 has been shown to provide an adhesive matrix for glioma cells (Izumoto et al., 1996). However, the precise roles of αvβ3 and L1 during cancer metastasis are not known. We have, therefore, used our in vitro model of melanoma cell transendothelial migration to determine whether melanoma αvβ3 integrin-mediated interactions with L1 are involved in the process. Our results show that αvβ3 is enriched in the heterotypic contacts between melanoma cells and endothelial cells. Transendothelial migration of melanoma cells is inhibited by Arg-Gly-Asp peptides and antibodies against αvβ3 and L1.
MATERIALS AND METHODS
Cells and Culture Conditions
Human microvascular endothelial cells (HMVECs) were purchased from Clonetics (San Diego, CA). HMVECs were cultured in EGM-2MV medium (Clonetics), containing 100 U of penicillin and 100 μg of streptomycin (Life Technologies, Gaithersburg, MD) per milliliter of EGM medium. The human melanoma cell line WM239 was a gift from Dr. Meenhard Herlyn (Wistar Institute, Philadelphia, PA). WM239 cells, as well as the M21 melanoma cell lines (M21, M21-L, M21-L12, and M21-L4) (Felding-Habermann et al., 1992; Montgomery et al., 1996), were cultured in RPMI-1640 medium prepared by the Ontario Cancer Institute Media Kitchen (Toronto, Ontario, Canada). The RPMI-1640 medium was supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml) and 10% fetal bovine serum (Life Technologies). All cells were maintained in a humidified 37°C atmosphere containing 5% CO2.
Antibodies and Peptides
The function-blocking LM609 monoclonal antibody (mAb) against αvβ3 was kindly provided by Dr. David Cheresh (Scripps Research Institute, La Jolla, CA) (Cheresh and Spiro, 1987). The rabbit antibodies recognizing L1 Ig-1–3, Ig-4–6, and fibronectin repeats were generated against recombinant L1 proteins (Zhao and Siu, 1995). The mAb P2B1 against CD31 (Ashman et al., 1991) was obtained from the Developmental Studies Hybridoma Bank, University of Iowa (Iowa City, IA). This antibody was used as a nonblocking control mAb in the antibody inhibition studies.
The linear RGD peptide (PSITWRGDGRDLQEL) and the control RAD peptide (PSITWRADGRDLQEL) were synthesized based on the human L1 RGD sequence in the sixth Ig-like domain (Ig6) (Yip et al., 1998). The cyclic RGD (cyclo-RGDfV) peptide and the cyclic RAD (cyclo-RADfV) peptide were purchased from Peptides International (Louisville, KY).
Transendothelial Migration Assays
The in vitro transendothelial migration assay was carried out as previously described (Sandig et al., 1997; Voura et al., 1998b). Round glass coverslips (12 mm in diameter and 0.1 mm in thickness) (Fisher Scientific, Fair Lawn, NJ) were coated Matrigel (Becton Dickinson, Bedford, MA). The Matrigel was diluted 1:8 in ice-cold water and applied to prechilled coverslips in 24-well plates (Flow Laboratories, McLean, VA). Matrigel (200 μl) was added to each well. Exactly 100 μl was subsequently removed and the remaining volume was air-dried overnight in a sterile flow hood at room temperature. Coverslips were rehydrated in Hanks' buffered saline solution. The Matrigel formed a thin layer of ECM to support HMVEC attachment and the formation of a monolayer of endothelial cells that mimics the endothelium. Coverslips were transferred to 35-mm-diameter dishes. Medium (∼200 μl) containing 1–1.5 × 105 HMVECs (passage 5–9) was placed on each coated coverslip. Cells were allowed to settle for 3–4 h. Coverslips were then carefully transferred to a new 24-well plate and incubated in the EGM medium with 10 ng/m1 tumor necrosis factor-α (Life Technologies). After 12 h, melanoma cells were added to the HMVEC monolayer.
Melanoma cells were labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) (Molecular Probes, Eugene, OR) by incubation in 12.5 μg/ml DiI for 10 min at 37°C. The DiI-labeled cells were washed three times in Hanks' buffered saline solution and resuspended at 2.4 × 106/ml HMVEC medium. Then, 25 μl of labeled cells was added to the monolayer. For inhibition experiments, antibodies, and peptides were added to the HMVEC monolayers for 30 min before the addition of melanoma cells. For those experiments examining the inhibitory effects of antibodies on each cell type individually, the cells were preincubated with the inhibitor and then washed before coculture. The mAb P2B1 against PECAM-1/CD31, which does not interfere with the function of PECAM-1, was used as the control in these experiments. Preincubations involving HMVECs were carried out by adding the reagent or phosphate-buffered saline (PBS) to the monolayer after the overnight culture. The unbound material was removed by washing. The coculture was carried out for 1, 3, and 5 h at 37°C before fixation and staining of F-actin for epifluorescence microscopy. In all inhibition studies, the total number of melanoma cells associated with the HMVEC monolayer was estimated for all coverslips to ensure that any reduction in the number of transmigrated cells was not due to an impairment of cell attachment.
Immunofluorescence Staining of Cells
To label F-actin, cells were fixed with the use of 3.5% (wt/vol) paraformaldehyde at room temperature for 5 min. These cells were washed three times for 3 min each in PBS, pH 7.4, and then extracted for 5 min in cytoskeleton-stabilizing buffer, pH 6.9, containing 0.1 M 1,4-piperazine-N,N′,-bis(2-ethanesulfonic acid), 1 mM EGTA, 4% (wt/vol) polyethylene glycol 8000, and 0.1% Triton-X 100. The extraction procedure was followed by another series of washes and a 5-min blocking step in 1% (wt/vol) bovine serum albumin (BSA). Cells were labeled in a 1:10 dilution of dipyrrometheneborondifluoride-fluorocein (BODIPY-FL) phallacidin (Molecular Probes) in blocking solution for 45 min at room temperature. Coverslips were then washed three times for 3 min each in PBS. Strips cut from plastic coverslips were used as spacers (stacked 2 high) when the coverslips were mounted on microscope slides in a mounting medium composed of 80% glycerol and 2.5% (wt/vol) 1,4-diazabicyclo-[2,2,2]-octane as an antibleaching agent (Sigma, St. Louis, MO) in PBS. The preparations were sealed with nail enamel and then subjected to epifluorescence microscopy.
To label the integrin αvβ3, cells on coverslips were fixed with 100% methanol, which was prechilled to −20°C. After three washes, cells were incubated in the blocking solution for 5 min. The anti-αvβ3 mAb LM609 was diluted 1:100 in blocking solution and added to coverslips. After 45 min of incubation at room temperature, cells were washed three times and then incubated with fluorescein isothiocyanate-conjugated goat anti-mouse secondary antibody (Sigma), which was diluted 1:300 in blocking solution. Incubation was carried out for 45 min at room temperature. The coverslips were washed and then mounted for confocal microscopy.
Staining of the L1 was carried out with the use of a rabbit antiserum increased against the recombinant protein that contained the five fibronectin type III-like repeats of L1 (Zhao and Siu, 1995). Cells were fixed with 3.5% paraformaldehyde. After blocking with 1% BSA, cells were incubated with the primary antiserum at a dilution of 1:100 for 45 min at room temperature. The coverslips were washed and then incubated for another 45 min with Texas Red-conjugated or fluorescein isothiocyanate-conjugated goat anti-rabbit antibodies at a dilution of 1:300. The coverslips were washed and then mounted for confocal microscopy.
For double staining of L1 and αvβ3 integrin, coverslips of cocultures were fixed in 3.5% (wt/vol) paraformaldehyde in PBS at room temperature for 15 min. The coverslips were blocked with 1% (wt/vol) BSA for 5 min and then incubated for 1.5 h with the primary antibodies as described above. After washing, coverslips were incubated with secondary antibodies with the use of a 1:200 dilution of Alexa 488 goat anti-mouse and Alexa 598 goat anti-rabbit (Molecular Probes) in 1% BSA in PBS for 1 h. Coverslips were washed and mounted for confocal microscopy.
Laser Scanning Confocal Microscopy
Laser scanning confocal microscopy was carried out with the use of an MRC 600 confocal imaging system (Bio-Rad, Richmond, CA) on a Nikon Optiphot microscope, equipped with a 60× objective. Alternatively, a Zeiss Axiovert 135 inverted microscope equipped with a 63× Neofluor objective and an LSM 410 confocal attachment was used. Serial optical sections were routinely taken at 1-μm thickness in an apical-to-basal direction.
Quantification of Transmigration by Melanoma Cells
Quantitative analysis of the melanoma cell transmigration was carried out by epifluorescence microscopy with the use of a Wild Leitz Orthoplan universal large-field microscope equipped with a 25× objective. All experiments were done in triplicates unless indicated otherwise. Melanoma cells associated with the endothelium were separated into three stages of transmigration according to the morphological criteria of Voura et al. (1998a): 1) round cells attached on the endothelium, 2) cells showing clear signs of penetration into the endothelial junctions and those intercalated between endothelial cells, and 3) cells spreading underneath the endothelium and those invading the Matrigel. Melanoma cells in category 3 were taken to be transmigrated cells.
Three sets of 15 fields were scored for each coverslip to account for any preferential accumulation of melanoma cells in certain areas of the coverslip. Each set of 15 fields usually contained >100 melanoma cells. In triplicate experiments, >1000 cells were examined and scored for any one time point. All cell counts were carried out on F-actin–stained preparations with the melanoma cells preloaded with DiI for identification. Selected coverslips were also examined by laser scanning confocal microscopy to confirm the relative distribution of melanoma cells in all three categories.
RESULTS
Enrichment of αvβ3 in Heterotypic Contacts between Melanoma Cells and Endothelial Cells
As a first step to examine the role of the integrin αvβ3 in the transendothelial migration of melanoma cells, we examined the distribution of αvβ3 on both HMVEC and WM239 melanoma cells. Immunofluorescence labeling experiments were carried out with the use of the anti-αvβ3 mAb LM609 (Figure 1). The overall αvβ3 staining was relatively weak in HMVECs and was mainly associated with the plasma membrane. WM239 melanoma cells also expressed αvβ3 primarily on the cell membrane and a higher concentration of αvβ3 was present in the cell-cell contact regions.
Figure 1.
Confocal images showing the distribution of αvβ3 in HMVEC and WM239 melanoma cells. Cells were fixed with cold methanol and immunofluorescence staining was carried out with the use of mAb LM609 directed against αvβ3 integrin. (a) αvβ3 staining of a monolayer of HMVECs cultured on Matrigel. (b) WM239 cells showing an enrichment of αvβ3 staining at the cell-cell contact region (arrowheads). Bars, 10 μm.
To examine the distribution of αvβ3 during extravasation of melanoma cells, cocultures of WM239 cells and HMVECs were labeled with the anti-αvβ3 mAb LM609 and series of optical images in the X/Y plane were taken for further analysis (Figure 2). To distinguish melanoma cells from endothelial cells, WM239 cells were preloaded with DiI before seeding on the HMVEC monolayer. Before extravasation, diffuse αvβ3 staining was observed on the entire melanoma cell membrane. The first sign of invasion through the endothelial junction was the formation of membrane blebs from the basolateral regions of the attached melanoma cells. These membrane protrusions eventually formed a pseudopod, which penetrated into the endothelial junction. Both blebs and pseudopods generally showed stronger αvβ3 staining, suggesting the presence of a higher concentration of αvβ3 on these membrane protrusions (Figure 2A). On the retraction of neighboring HMVECs, the transmigrating WM239 cell became intercalated between endothelial cells. αvβ3 staining was clearly associated with the heterotypic contacts between melanoma cells and the surrounding endothelial cells, whereas staining of the homotypic contact regions between endothelial cells was much weaker (Figure 2B). These images thus indicate an enrichment of αvβ3 in the contact regions between melanoma cells and endothelial cells. Also, endothelial cells spreading on top of a transmigrating melanoma cell often displayed strong αvβ3 staining in the leading edges. A higher concentration of αvβ3 persisted in the heterotypic contacts of melanoma cells spreading on the Matrigel (Figure 2C). These results suggest that the integrin αvβ3 plays an important role throughout the transmigration process of melanoma cells.
Figure 2.
Confocal series showing an enrichment of αvβ3 on membrane protrusions of melanoma cells and in heterotypic contacts during transendothelial migration. DiI-labeled melanoma cells were seeded on top of an HMVEC monolayer and fixed at different times of coculture. Coverslips were stained with the use of mAb LM609 and serial optical images were taken at 1-μm thickness. A schematic drawing is shown at the top of each series. Individual images are shown in an apical-to-basal direction and labeled with its distance from the bottom of the endothelium. (A) An optical series showing a melanoma cell at the initial stage of invasion through the endothelium. Membrane protrusions sent from the basolateral surfaces of the melanoma cells were labeled with αvβ3 (arrowheads). (B) An optical series showing a spindle-shaped melanoma cell intercalated between two endothelial cells. The heterotypic contacts were enriched in αvβ3, especially at the leading edge of an endothelial cell spreading on top of the melanoma cell (arrowheads). (C) An optical series showing a transmigrated melanoma cell spreading on the Matrigel under the endothelium. An enrichment of αvβ3 persisted in the heterophilic contact regions in all the X/Y sections (arrowheads). In comparison, the endothelial junctions were only weakly stained (arrows). Bars, 10 μm.
Inhibition of Melanoma Cell Transendothelial Migration by an Anti-αvβ3 Antibody
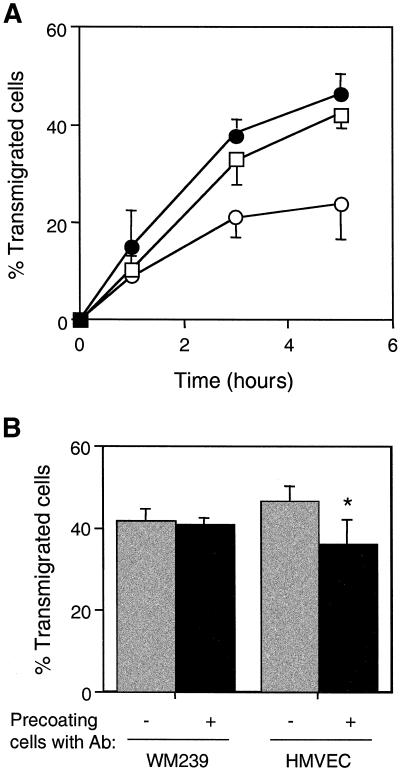
Given that αvβ3 was found in the heterotypic contacts during melanoma cell transmigration, we next examined the effects of the function-blocking mAb LM609 on melanoma cell transendothelial migration. When the antibody was added to the cocultures, melanoma transendothelial migration was reduced by 40–50% at 5 h (Figure 3A). The inclusion of a nonblocking control mAb in the assay did not result in any inhibition. The antibody did not affect the attachment of WM239 cells, because comparable numbers of melanoma cells were found associated with the HMVEC monolayer in cocultures incubated either in the presence or absence of LM609.
Figure 3.
Inhibition of melanoma transmigration through HMVECs by mAb LM609 directed against αvβ3. (A) DiI-labeled WM239 melanoma cells were seeded on HMVEC monolayers and transmigration was allowed to occur in the presence of LM609 IgG (40 μg/ml). Coverslips were fixed at 1, 3, and 5 h of coculture. The number of transmigrated cells was scored as described in MATERIALS AND METHODS. Cocultures were incubated in the absence of antibody (▵), or in the presence of mAb LM609 against αvβ3 (○) or mAb P2B1 against PECAM-1 (●). (B) Effects of preincubating cells with mAb LM609 on the transendothelial migration of melanoma cells. Either WM239 cells or HMVECs were preincubated with LM609 (40 μg/ml) for 30 min and the unbound antibody was removed. Melanoma cells were then added to the HMVEC monolayer and cocultured for 5 h. Assays were carried out with cells either precoated with mAb (solid bars) or without prior precoating (shaded bars). Data represent the mean ± SD (n = 9). The asterisk indicates a statistically significant reduction in the percentage of transmigrated cells compared with the control (Student's t test, p < 0.01).
Because both melanoma and endothelial cells express αvβ3, we next determined whether αvβ3 molecules expressed on both cell types were involved equally in the transmigration process of melanoma cells. To address this issue, either melanoma cells or endothelial cells were preincubated with mAb LM609 and then washed to remove unbound antibody before coculture. Preincubation of the WM239 cells resulted in a 40% reduction in the number of transmigrated cells at 5 h (Figure 3B). In contrast, no significant inhibition of WM239 cell transendothelial migration was observed when HMVECs were preincubated with the antibody. The results thus indicate that the integrin αvβ3 on melanoma cells, and not endothelial cells, is involved in the transmigration process.
αvβ3-Negative Melanoma Cells Are Impaired in Transendothelial Migration
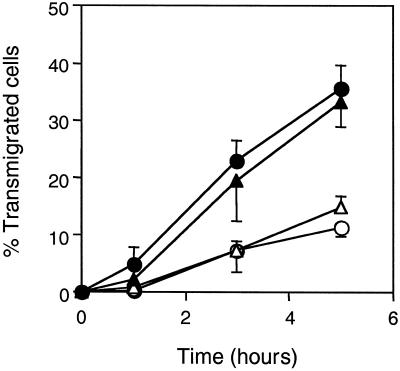
The key role of αvβ3 expressed by melanoma cells in the transendothelial migration process was further evaluated with the use of M21 melanoma cell variants either expressing or lacking the αv subunit (Cheresh and Spiro, 1987; Felding-Habermann et al., 1992). The cell line M21-L does not synthesize αv and lacks αvβ3. The M21-L4 cell line (derived from M21-L cells transfected with the αv cDNA) expresses αvβ3, whereas the M21-L12 line (derived from mock transfectants) does not. The ability of these cell lines to undergo transendothelial migration was examined (Figure 4). Whereas the αv-positive cell lines M21 and M21-L4 showed the normal kinetics of transmigration with levels comparable with that of WM239 cells, the αv-negative variants M21-L and M21-L12 were compromised in their ability to undergo transendothelial migration with <15% of cells transmigrated by 5 h. These results demonstrate that the transendothelial migration of melanoma cells is dependent on the its expression of αvβ3.
Figure 4.
Transendothelial migration of the M21 melanoma cells through HMVEC monolayers. The percentages of transmigrated cells were scored at different times of coculture for M21 cells (αv+) (●), M21-L cells (αv−) (○), M21-L4 cells (αv+, M21-L cells transfected with αv cDNA (▴) and M21-L12 cells (αv−, M21-L mock transfectant) (▵). Data represent the mean ± SD (n = 9).
L1 Expression in Melanoma Cells and Endothelial Cells
To which adhesion receptor on endothelial cells does the melanoma αvβ3 bind? In addition to vitronectin and other ECM components, αvβ3 is known to undergo heterophilic binding with the cell adhesion molecules PECAM-1 and L1 (Buckley et al., 1996; Montgomery et al., 1996). Although both PECAM-1 and L1 are expressed by HMVECs, we have found that PECAM-1 redistributes away from the endothelial junction and is not required for melanoma cell transmigration (Voura et al., 2001). Therefore, our studies were focused on the potential involvement of L1. We first examined the expression of L1 in HMVECs and WM239 cells. Protein blot analysis showed that L1 was synthesized in both WM239 cells and HMVECs (Figure 5a). Whereas L1 was secreted by WM239 cells, L1 was not detected in the HMVEC-conditioned medium. Immunolocalization studies revealed several interesting features of L1 in WM239 cells (Figure 5, b and c). Whereas the plasma membrane of HMVECs showed clear staining of L1, L1 staining was barely detectable on the membrane of WM239 cells. However, perinuclear staining of L1 was evident in both types of cells, indicating that they were actively synthesizing L1. Membrane protrusions loaded with L1 were occasionally observed at the cell periphery, suggesting that L1 might be released into the medium by evagination of the plasma membrane.
Figure 5.
Expression of L1 in HMVEC and WM239 cells. (a) Protein blots stained with anti-L1 antibody (1:500 dilution): i) total cell protein of WM239 cells, ii) total cell protein of HMVECs, iii) WM239 conditioned medium, and iv) HMVEC conditioned medium. Cell were also fixed with paraformaldehyde and stained with anti-L1 antibody: HMVEC (b), WM239 cells (c). Although endothelial junctions were clearly stained with L1, the plasma membrane of melanoma cells was only weakly stained. Granules filled with L1 were occasionally observed at the cell membrane (arrowhead). Double immunostaining was carried out to examine the localization of L1 (d and f) and αvβ3 (e and g) during transendothelial migration of melanoma cells. Melanoma cells were preloaded with Hoechst dye for identification. A round WM239 cell attached on the endothelium is shown in d and e. Arrows indicate a higher concentration of L1 and αvβ3 associated with the lamellipodial structure. The staining of L1 and αvβ3 was more intense at the heterotypic contacts (arrow) than the homotypic endothelial contacts (arrowheads). A melanoma cell intercalated among endothelium cells is shown in f and g. Bars, 10 μm.
Interactions between L1 and αvβ3 during transendothelial migration of melanoma cells would predict colocalization of these molecules during the transmigration process. Double immunolocalization experiments were carried out and cells at different stages of transmigration were examined. Both L1 and αvβ3 showed punctate staining along the periphery of cells. In the initial stages of cell-cell interaction, high concentrations of L1 and αvβ3 were observed in regions where lamellipodia and pseudopodia of melanoma cells were in contact with the endothelium (Figure 5, d and e), suggesting the involvement of both cell adhesion molecules in the early stage of penetration. When melanoma cells became intercalated among endothelial cells, both L1 and αvβ3 were enriched along the heterotypic contacts although their staining patterns did not show complete overlap (Figure 5, f and g).
Inhibition of Transendothelial Migration of Melanoma Cells by RGD Peptides
Peptide inhibition studies were undertaken to evaluate the role of L1-αvβ3 interaction in melanoma cell transendothelial migration. The integrin αvβ3 mediates adhesion via an RGD sequence (Rouslahti and Obrink, 1996), and human L1 contains an RGD motif in 6th Ig-like domain (Ig6) (Hlavin and Lemmon, 1991). Therefore, a synthetic peptide containing the RGD motif and its flanking sequences in L1 (PSITWRGDGRDLQEL) and its control RAD peptide (PSITWRADGRDLQEL) were tested for their effects on melanoma transendothelial migration. The linear RGD peptide inhibited melanoma cell transmigration by 40%, whereas the inactive RAD peptide had no effect (Figure 6A). The cyclic RGD peptide (cyclo-RGDfV) has been reported to bind αvβ3 at high affinity and block its function effectively at low concentrations (Pfaff et al., 1994; Brooks et al., 1996; Kerr et al., 1999). Dose experiments showed that the cyclic RGD peptide was able to inhibit transendothelial migration of melanoma cells by 70%. Fifty percent inhibition was achieved at ∼5 μM peptide. In contrast, the cyclic RAD peptide did not have significant effects on the transmigration of melanoma cells. Time course studies with the use of the cyclic RGD peptide (Figure 6B) indicated that, although inhibitory at all time points, the peptide had its greatest effect at 5 h of coculture. To rule out negative effects of the cyclic peptide on cell attachment, the number of melanoma cells associated with the HMVEC monolayer was estimated for all time points. Comparable numbers of cells were obtained in all cases, indicating that the RGD peptide did not affect cell attachment.
Figure 6.
Inhibition of melanoma transmigration through HMVECs by RGD peptides. (A) Cocultures of WM239 cells and HMVECs were carried out in the presence or absence of different concentrations of synthetic peptides: linear RGD (▪), linear RAD (□), cyclic RGD (●), and cyclic RAD (○). The percentage of transmigrated cells was determined at 5 h and then normalized to the minus-peptide control. (B) Time course experiment for the transmigration of WM239 melanoma cells in the presence of 90 μM cyclic RGD peptide (solid bars). Control assays (stippled bars) were carried out in the absence of the cyclic peptide. Data represent the mean ± SD (n = 9). The asterisks indicate a statistically significant reduction in the percentage of transmigrated cells (Student's t test, p < 0.01).
Transendothelial Migration of Melanoma Cells Involves L1 on Endothelial Cells
To determine whether L1 was directly involved in melanoma cell transmigration, we made use of a rabbit antibody raised against L1 Ig4–6, which was previously found to inhibit L1-αvβ3 interactions (Yip et al., 1998). Coculture assays were carried out in the presence of these antibodies and the percentage of transmigrated cells was quantified at various time points (Figure 7A). The number of transmigrated melanoma cells was reduced by ∼50% at 5 h.
Figure 7.
Effects of anti-L1 antibodies on the transendothelial migration of melanoma cells. (A) WM239 melanoma cells were added to HMVEC monolayers for transmigration in the absence of antibody (●) or in the presence of anti-L1-Ig1–3 antibody (□) or anti-L1-Ig4–6 antibody (○). (B) Effects of preincubation of cells with antibodies on WM239 cell transmigration. WM239 melanoma cells or HMVECs were preincubated with the anti-L1-Ig4–6 antibody (1:10 dilution) for 30 min at 37°C. Unbound antibodies were removed by washing before the coculture assay. The percentages of transmigrated cells were scored at 5 h. Data represent the mean ± SD (n = 9; *p < 0.05).
Because L1 is expressed in both melanoma and endothelial cells, it is possible that L1-L1 homophilic interactions at the heterotypic contacts might play a role in the transmigration of melanoma cells. Therefore, we tested the effects of a rabbit antibody raised against the first three Ig-like domains of L1. Although this antibody is known to block L1 homophilic binding centered at the Ig2 domain (Zhao and Siu, 1995, 1996), it did not exert significant effects on the transendothelial migration of melanoma cells (Figure 7A). The data thus indicate that transendothelial migration does not involve L1-L1 homophilic binding.
These results led us to speculate that L1 on endothelial cells, and not melanoma cells, was involved in tumor cell extravasation. To address this issue, either endothelial cells or melanoma cells were preincubated with the anti-L1-Ig4–6 antibody. Cells were washed to remove unbound antibody before seeding the melanoma cells on the HMVEC monolayer. Preincubation of the WM239 melanoma cells with the antibody did not inhibit transendothelial migration (Figure 7B). However, preincubating HMVECs with the antibody did produce a small, but reproducible, level of inhibition. The number of transmigrated cells was reduced by ∼20%. The data are consistent with the idea that L1 on the endothelial cells, and not the melanoma cells, has a role during transendothelial migration of melanoma cells.
The above-mentioned results suggested that L1 and αvβ3 might function as an adhesive pair during melanoma extravasation. To address this issue, we tested whether the addition of both antibodies against L1 and αvβ3 would elicit additive inhibitory effects on the transmigration process. The results showed that the inhibitory effects of these two antibodies were not additive (Figure 8). An ∼40% inhibition was achieved whether the antibodies were added singly or together to the coculture. These results thus support the notion that L1 and αvβ3 are components of the same adhesive system.
Figure 8.
Inhibition of transmigration by a combination of antibodies against αvβ3 and L1. The transmigration assay was carried out in the presence of mAb LM609 (40 μg/ml) and anti-L1-Ig4–6 antibody (1:10 dilution), either singly or combined. Controls were carried out in either mAb P2B1 or rabbit preimmune serum. All values were normalized to that of the minus-antibody control. Data represent the mean ± SD (n = 9; *p < 0.01).
DISCUSSION
In this article, we have demonstrated the involvement of integrin αvβ3 in melanoma transendothelial migration. Whereas interactions between integrins and ECM are known to play an important role in the malignant behavior of melanoma cells, this is the first report to our knowledge that demonstrates a role for αvβ3 during tumor cell transendothelial migration. The expression of the integrin αvβ3 has been shown to associate with the invasiveness of a subset of tumors that eventually leave the primary tumor and cause secondary growth. Our studies have focused on the transendothelial migration process and we have found that αvβ3 on melanoma cells interacts with L1 on endothelial cells and that their interactions play a crucial role in the transmigration process.
Immunofluorescence labeling of cells in the coculture assay has revealed that αvβ3 becomes enriched in the heterotypic contacts from the initial stages of melanoma cell invasion, when membrane protrusions extending from the basolateral surfaces begin to contact the surface of the endothelium. In addition to adhesive interactions, the membrane protrusions may facilitate transmigration by releasing signaling molecules and/or proteases into the microenvironment (Basbaum and Werb, 1996; Ginestra et al., 1997). A high concentration of αvβ3 persists on the contact surfaces between melanoma cells and endothelial cells throughout the transmigration process, even when the transmigrated cells are spreading on the Matrigel. αvβ3 has been found to cluster in focal contacts and to promote both cell adhesion and cell motility (Leavesley et al., 1992; Seftor et al., 1992; Danen et al., 1994). Therefore, the ligation of αvβ3 with adhesion receptors may facilitate the passage of melanoma cells through the endothelial junction and subsequent migration on the Matrigel.
Antibody inhibition studies have demonstrated that the participation of αvβ3 is vital to the transmigration of melanoma cells. Although retardation of transmigration was observed 1 h after coculture, the inhibitory effects were most prominent at 5 h. Most melanoma cells were arrested at the stage when they became intercalated between endothelial cells. Similar data were obtained with the use of the cyclic RGD peptide to inhibit the function of αvβ3, suggesting that αvβ3 may play a more important role during the later stages of transmigration. αvβ3 is known to bind the RGD motif of a number of ECM components (Cheresh, 1987; Pfaff et al. 1994; Horton, 1997). Thus, antibody blocking of αvβ3 can impair the cell spreading process and delay the transmigration process.
Studies on the M21 cell line and its variants further support the role of αvβ3 during transendothelial migration of melanoma cells. Whereas the transmigration efficiency of M21 cells is comparable with that of WM239 cells, the αv-deficient variants transmigrated poorly, correlating very well with their decreased tumorigenicity in nude mice (Felding-Habermann et al., 1992). Both transmigration efficiency and tumorigenicity are rescued when αv expression is restored by cDNA transfection. These findings suggest that the reduction in transmigration efficiency may contribute to the decreased tumorigenicity of the αv-deficient cells. Our previous findings (Voura et al., 1998a) on the poorly metastatic WM35 melanoma cell line (Bani et al., 1996) are reminiscent of the M21 variants. The migratory ability of WM35 cells, like the αv-negative M21 cells, was depressed compared with their more aggressive counterparts. Tumor necrosis factor-α can stimulate the ability of the WM35 cells to transmigrate. However, this effect is inhibited by the anti-αvβ3 mAb LM609 (Voura and Siu, unpublished data).
Although both melanoma cells and endothelial cells express αvβ3, blocking the αvβ3 molecules on melanoma cells, and not endothelial cells, with mAb LM609 inhibits transmigration. Thus, transmigration of melanoma cells likely requires the participation of only those αvβ3 molecules expressed on melanoma cells. Although the expression of αvβ3 on endothelial cells has been implicated in endothelial cell motility and angiogenesis (Brooks et al., 1994, Kim et al. 2000; Kumar et al., 2000), endothelial αvβ3 may not be directly involved in the transmigration process of melanoma cells.
We have identified L1 as the major adhesion receptor on endothelial cells that binds αvβ3, although both L1 and PECAM-1 on endothelial cells can serve as ligands for αvβ3 (Piali et al., 1995; Buckley et al., 1996; Montgomery et al., 1996; Felding-Habermann et al., 1997). Whereas PECAM-1 has been found to play an important role in leukocyte migration (Muller, 1995), we have found that PECAM-1 is not required for the transendothelial migration of melanoma cells. In fact, PECAM-1 is redistributed away from endothelial junctions associated with transmigrating melanoma cells and is absent in the heterotypic contacts (Voura et al., 2001). This leaves L1 as the major target for ligation with αvβ3 on melanoma cells. Consistent with other reports that tumor cell αvβ3 can adhere to L1-coated substrates via the RGD sequence in the L1 Ig6 domain (Ebeling et al., 1996; Montgomery et al., 1996; Duczmal et al., 1997), RGD peptides inhibit the transmigration of melanoma cells. Our studies with the use of cells precoated with antibodies further demonstrate that it is the L1 present on endothelial cells, and not melanoma cells, that is involved in transendothelial migration. These results might explain why we did not observe complete colocalization between L1 and αvβ3 in heterotypic contacts during the transmigration process.
The expression of L1 has been found in a metastatic variant of the melanoma cell line K1735, whereas nonmetastasizing cells are L1-negative, suggesting a role for L1 in tumor progression (Linnemann et al., 1989). In addition to melanoma, L1 has been found in malignant cells of diverse origin, including neuroblastoma, osteogenic sarcoma, squamous lung carcinoma, and skin carcinoma cell lines (Mujoo et al., 1986; Linnemann et al., 1989; Reid and Hemperly, 1992). Our morphological studies show that only a low level of L1 is associated with the plasma membrane of melanoma cells. The homophilic binding site of L1 has been mapped to a sequence in the Ig2 domain (Zhao et al., 1998). Antibody blocking experiments with the use of anti-L1-Ig1–3 antibody indicate that L1-L1 pairing between melanoma cells and endothelial cells does not contribute significantly to the adhesion and transmigration of melanoma cells. Because a substantial amount of L1 is released into the medium by melanoma cells, we speculate that L1 molecules released by melanoma cells may adhere to the surface of endothelial cells and augment the binding interactions between melanoma αvβ3 and L1 on endothelial cells.
Metalloproteinases, as well as other ECM-degrading enzymes, have been shown to be important for cancer progression, and the localization of these enzymes to the surface of invasive cells is important for their function (de Vries et al., 1996; Chapman, 1997; Werb, 1997; Brunner et al., 1998; Deryugina et al., 1998). In addition to cell adhesion and migration, αvβ3 has been found to localize matrix metalloproteinase-2 (MMP-2) to the surface of invasive cells and this interaction can be inhibited by the αvβ3 function-blocking antibody LM609 (Brooks et al., 1996). The interaction of MMP-2 with αvβ3 also plays a role in tumor angiogenesis (Brooks et al., 1998; Silletti et al., 2001). Membrane type-1 MMP can also localize MMP-2 on the cell surface of invasive cells via tissue inhibitor of metalloproteinase-2. Therefore, inhibiting MMP-2 interaction with the αvβ3 integrin may not preclude the membrane association of the enzyme during melanoma cell extravasation (Strongin et al., 1995; Werb, 1997). However, recent evidence suggests that the cooperation of both αvβ3 and membrane-type-1 MMP is required for the localization of active MMP-2 to the cell surface (Deryugina et al., 2000, 2001; Hofmann et al., 2000). Therefore, it is possible that a further component of our observed αvβ3 inhibition studies is provided by a reduced localized activation of MMP-2 on the surface of the melanoma cells during transmigration. Such blocking would most likely result in reduced melanoma cell spreading on the Matrigel matrix.
Our studies have established a role for αvβ3-L1 interactions during transendothelial migration of melanoma cells. However, although antibodies directed against L1 and αvβ3, as well as cyclic RGD peptides, are potent inhibitors of this process, we are unable to achieve complete inhibition with the use of these reagents. It is, therefore, likely that other adhesion receptors are involved. Our previous work has suggested the involvement of classic cadherins (Sandig et al., 1997). Other junctional structures, such as gap junctions, may also be involved (El-Sabban and Pauli, 1991). Future studies will focus on the identification and characterization of additional adhesion receptors involved in this important step of cancer metastasis.
ACKNOWLEDGMENTS
We thank Drs. Avrum Gotlieb and Fred Keeley of the University of Toronto and Dr. Martin Sandig of the University of Western Ontario for valuable advice and discussion. This work was supported by Operating Grant MT-11443 from the Canadian Institutes of Health Research (to C.-H.S.), and by National Cancer Institute Grant CA-69112 and National Heart, Lung, and Blood Institute Grant HL-62477 (to A.M.P.M.). E.B.V. was supported by a Studentship from the Canadian Institutes of Health Research and R.A.R. was supported in part by a scholarship from the Frank Fletcher Memorial Fund, University of Toronto.
Abbreviations used:
- ECM
extracellular matrix
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine
- HMVEC
human microvascular endothelial cell
- Ig
immunoglobulin
- mAb
monoclonal antibody
- MMP
matrix metalloproteinase
- PECAM-1
platelet endothelial cell adhesion molecule-1
REFERENCES
- Albelda SM, Mette SA, Elder DE, Stewart R, Damjanovich L, Herlyn M, Buck CA. Integrin distribution in malignant melanoma: association of the β3 subunit with tumor progression. Cancer Res. 1990;50:6757–6764. [PubMed] [Google Scholar]
- Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991;114:1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman LK, Aylett GW, Cambareri AC, Cole SR. Different epitopes of the CD31 antigen identified by monoclonal antibodies: cell type-specific patterns of expression. Tissue Antigens. 1991;38:199–207. doi: 10.1111/j.1399-0039.1991.tb01898.x. [DOI] [PubMed] [Google Scholar]
- Bani MR, Rak J, Adachi D, Wiltshire R, Trent JM, Kerbel RS, Ben-David Y. Multiple features of advanced melanoma recapitulated in tumorigenic variants of early stage (radial growth phase) human melanoma cell lines: evidence for a dominant phenotype. Cancer Res. 1996;56:3075–3086. [PubMed] [Google Scholar]
- Basbaum C, Werb Z. Focalized proteolysis: spatial and temporal regulation of extracellular matrix degradation at the cell surface. Curr Opin Cell Biol. 1996;8:731–738. doi: 10.1016/s0955-0674(96)80116-5. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Sillitti S, von Schalacha TL, Friedlander M, Cheresh DA. Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell. 1998;92:391–400. doi: 10.1016/s0092-8674(00)80931-9. [DOI] [PubMed] [Google Scholar]
- Brooks P, Stromblad S, Sanders L, von Schalscha T, Aimes R, Stetler-Stevenson W, Quigley J, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Brunner G, Reimbold K, Meissauer A, Schirrmacher V, Erkell L. Sulfated glycosaminoglycans enhance tumor cell invasion in vitro by stimulating plasminogen activation. Exp Cell Res. 1998;239:301–310. doi: 10.1006/excr.1997.3877. [DOI] [PubMed] [Google Scholar]
- Buckley CD, Doyonnas R, Newton JP, Blystone SD, Brown EJ, Watt SM, Simmons DL. Identification of αvβ3 as a heterotypic ligand for CD31/PECAM-1. J Cell Sci. 1996;109:437–445. doi: 10.1242/jcs.109.2.437. [DOI] [PubMed] [Google Scholar]
- Chapman H. Plasminogen activators, integrins and the coordinated regulation of cell adhesion and migration. Curr Opin Cell Biol. 1997;9:714–724. doi: 10.1016/s0955-0674(97)80126-3. [DOI] [PubMed] [Google Scholar]
- Cheresh D. Human endothelial cells synthesize and express and Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci USA. 1987;84:6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D, Spiro R. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen and von Willebrand factor. J Biol Chem. 1987;262:17703–17711. [PubMed] [Google Scholar]
- Danen EH, Ten Berge PJ, Van Muigen GN, Van't Hof-Grootenboer AE, Brocker EB, Ruiter DJ. Emergence of Symbol“ § 125Symbol” § 121 fibronectin- and αvβ3 vitronectin-receptor expression in melanocytic tumor progression. Histopathology. 1994;24:249–256. doi: 10.1111/j.1365-2559.1994.tb00517.x. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Bourdon MA, Jungwirth K, Smith JW, Strongin AY. Functional activation of integrin αvβ3 in tumor cells expressing membrane-type 1 matrix metalloproteinase. Int J Cancer. 2000;86:15–23. doi: 10.1002/(sici)1097-0215(20000401)86:1<15::aid-ijc3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Bourdon MA, Reisfeld RA, Strongin AY. Remodeling of collagen matrix by human tumor cells requires activation and cell surface association of matrix metalloproteinase-2. Cancer Res. 1998;58:3743–3750. [PubMed] [Google Scholar]
- Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, Smith JW, Strongin AY. MT1-MMP initiates activation of pro-MMP-2 and integrin αvβ3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res. 2001;15:209–223. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- de Vries T, de Wit P, Clemmensen I, Verspaget H, Weidle U, Brocker E, Ruiter D, van Muijen G. Tetranectin and plasmin/plasminogen are similarly distributed at the invasive front of cutaneous melanoma lesions. J Pathol. 1996;179:260–265. doi: 10.1002/(SICI)1096-9896(199607)179:3<260::AID-PATH586>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Duczmal A, Schollhammer S, Katich S, Ebeling O, Schwartz-Albiez R, Altevogt P. The L1 adhesion molecule supports αvβ3-mediated migration of human tumor cells and activated T lymphocytes. Biochem Biophys Res Commun. 1997;232:236–239. doi: 10.1006/bbrc.1997.6265. [DOI] [PubMed] [Google Scholar]
- Ebeling O, Duczmal A, Aigner S, Geiger C, Schollhammer S, Kemshead JT, Moller P, Schwartz-Albeiz R, Altevogt P. L1 adhesion molecule on human lymphocytes and monocytes: expression and involvement in binding to αvβ3 integrin. Eur J Immunol. 1996;26:2508–2516. doi: 10.1002/eji.1830261035. [DOI] [PubMed] [Google Scholar]
- El-Sabban ME, Pauli BU. Cytoplasmic dye transfer between metastatic tumor cells and vascular endothelium. J Cell Biol. 1991;115:1375–1382. doi: 10.1083/jcb.115.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felding-Habermann B, Mueller B, Romerdahl C, Cheresh D. Involvement of integrin αv gene expression in human melanoma tumorigenicity. J Clin Invest. 1992;89:2018–2022. doi: 10.1172/JCI115811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felding-Habermann B, Silletti S, Mei F, Siu C-H, Yip PM, Brooks P, Cheresh D, O'Toole T, Ginsberg M, Montgomery AMP. A single immunoglobulin-like domain of the human neural cell adhesion molecule L1 supports adhesion by multiple vascular and platelet integrins. J Cell Biol. 1997;139:1567–1581. doi: 10.1083/jcb.139.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestra A, Monea S, Seghezzi G, Dolo V, Nagase H, Mignatti P, Vittorelli M. Urokinase plasminogen activator and gelatinases are associated with membrane vesicles shed by human HT1080 fibrosarcoma cells. J Biol Chem. 1997;272:17216–17222. doi: 10.1074/jbc.272.27.17216. [DOI] [PubMed] [Google Scholar]
- Hall H, Carbonetto S, Schachner M. L1/HNK-1 carbohydrate- and β1 integrin-dependent neural cell adhesion to laminin-1. J Neurochem. 1997;68:544–553. doi: 10.1046/j.1471-4159.1997.68020544.x. [DOI] [PubMed] [Google Scholar]
- Hlavin M, Lemmon V. Molecular structure and functional testing of human L1-CAM: an interspecies comparison. Genomics. 1991;11:416–423. doi: 10.1016/0888-7543(91)90150-d. [DOI] [PubMed] [Google Scholar]
- Hofmann UB, Wesphal JR, van Kraats AA, Ruiter DJ, van Muijen GN. Expression of integrin αvβ3 correlates with activation of membrane-type matrix metalloproteinase-1 (MT1-MMP) and matrix metalloproteinase-2 (MMP-2) in human melanoma cells in vitro and in vivo. Int J Cancer. 2000;87:12–19. doi: 10.1002/1097-0215(20000701)87:1<12::aid-ijc3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Horstkorte R, Schachner M, Hagyar J, Verherr T, Schmitz B. The fourth immunoglobulin-like domain of NCAM contains a carbohydrate recognition domain for oligomannosidic glycans implicated in association with L1 and neurite outgrowth. J Cell Biol. 1993;121:1409–1421. doi: 10.1083/jcb.121.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton MA. The αvβ3 integrin “vitronectin receptor”. Int J Biochem Cell Biol. 1997;29:721–725. doi: 10.1016/s1357-2725(96)00155-0. [DOI] [PubMed] [Google Scholar]
- Hortsch M. The L1 family of neural cell adhesion molecules: old proteins performing new tricks. Neuron. 1996;17:587–593. doi: 10.1016/s0896-6273(00)80192-0. [DOI] [PubMed] [Google Scholar]
- Izumoto S, Ohnishi T, Arita N, Hiraga S, Taki T, Hayakawa T. Gene expression of neural cell adhesion molecule L1 in malignant gliomas and biological significance of L1 in glioma invasion. Cancer Res. 1996;56:1440–1444. [PubMed] [Google Scholar]
- Johnson JP. Cell adhesion molecules in the development and progression of malignant melanoma. Cancer Metastasis Rev. 1999;18:345–357. doi: 10.1023/a:1006304806799. [DOI] [PubMed] [Google Scholar]
- Kerr JS, Wexler RS, Mousa SA, Robinson CS, Wexler EJ, Mohamed S, Voss ME, Devenny JJ, Czerniak PM, Gudzelak A, Slee AM. Novel small molecule alpha v integrin antagoniasts: comparative anti-cancer efficacy with known angiogenesis inhibitors. Anticancer Res. 1999;19:959–968. [PubMed] [Google Scholar]
- Kim S, Harris M, Varner JA. Regulation of integrin αvβ3-mediated endothelial cell migration and angiogenesis by integrin α5β1 and protein kinase A. J Biol Chem. 2000;275:33920–33928. doi: 10.1074/jbc.M003668200. [DOI] [PubMed] [Google Scholar]
- Kowitz A, Kadmon G, Eckert M, Schirrmacher V, Schachner M, Altevogt P. Expression and function of the neural cell adhesion molecule L1 in mouse leukocytes. Eur J Immunol. 1992;22:1199–1205. doi: 10.1002/eji.1830220514. [DOI] [PubMed] [Google Scholar]
- Kuhn T, Stoeckli E, Condrau M, Rathjen F, Sonderegger P. Neurite outgrowth on immobilized axonin-1 is mediated by a heterophilic interaction with L1 (G4) J Cell Biol. 1991;115:1113–1126. doi: 10.1083/jcb.115.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujat R, Miragall F, Krause D, Dermietzel R, Wrobel K-H. Immunolocalization of the neural cell adhesion molecule L1 in non-proliferating epithelial cells of the male urogenital tract. Histochemistry. 1995;103:311–321. doi: 10.1007/BF01457416. [DOI] [PubMed] [Google Scholar]
- Kumar CC, Armstrong L, Yin Z, Malkowski M, Maxwell E, Ling H, Yaremko B, Liu M, Varner J, Smith EM, Neustadt B, Nechuta T. Targeting integrins αvβ3and αvβ5 for blocking tumor-induced angiogenesis. Adv Exp Med Biol. 2000;476:169–180. [PubMed] [Google Scholar]
- Lafrenie RM, Gallo S, Podor TJ, Buchanan MR, Orr FW. The relative roles of vitronectin receptor, E-selectin and α4β1 in cancer cell adhesion to interleukin-1-treated endothelial cells. Eur J Cancer. 1994;30A:2151–2158. doi: 10.1016/0959-8049(94)00354-8. [DOI] [PubMed] [Google Scholar]
- Leavesley DI, Ferguson GD, Wayner EA, Cheresh DA. Requirement of the integrin beta 3 subunit for carcinoma cell spreading or migration on vitronectin and fibrinogen. J Cell Biol. 1992;117:1101–1107. doi: 10.1083/jcb.117.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemann D, Raz A, Bock E. Differential expression of cell adhesion molecules in variants of K1735 melanoma cells differing in metastatic capacity. Int J Cancer. 1989;43:709–712. doi: 10.1002/ijc.2910430428. [DOI] [PubMed] [Google Scholar]
- Miura M, Asou H, Kobayashi M, Uyemura K. Functional expression of a full-length cDNA coding for rat neural cell adhesion molecule L1 mediate homophilic intercellular adhesion and migration of cerebellar neurons. J Biol Chem. 1992;267:10752–10758. [PubMed] [Google Scholar]
- Montgomery AMP, Becker JC, Siu C-H, Lemmon VP, Cheresh DA, Pancook JD, Zhao X, Reisfeld RA. Human neural cell adhesion molecule L1 and Rat homologue NILE are ligands for αvβ3. J Cell Biol. 1996;132:475–485. doi: 10.1083/jcb.132.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujoo K, Spiro RC, Reisfeld RA. Characterization of a unique glycoprotein antigen expressed on the surface of human neuroblastoma cells. J Biol Chem. 1986;261:10299–10305. [PubMed] [Google Scholar]
- Muller WA. The role of PECAM-1 (CD31) in leukocyte emigration: studies in vitro and in vivo. J Leukoc Biol. 1995;57:523–528. doi: 10.1002/jlb.57.4.523. [DOI] [PubMed] [Google Scholar]
- Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natali PG, Hamby CV, Felding-Habermann B, Laing B, Nicotra MR, Di Filippo F, Giannarelli D, Temponi M, Ferrone S. Clinical significance of αvβ3 integrin and intercellular adhesion molecule-1 expression in cutaneous malignant melanoma lesions. Cancer Res. 1997;57:1554–1560. [PubMed] [Google Scholar]
- Nicolson G. Cancer Metastasis: tumor cell and host organ properties important in metastasis to specific secondary sites. Biochim Biophys Acta. 1988;948:175–244. doi: 10.1016/0304-419x(88)90010-8. [DOI] [PubMed] [Google Scholar]
- Orr FW, Wang HH, Lafrenie RM, Scherbarth S, Nance DM. Interactions between cancer cells and the endothelium in metastasis. J Pathol. 2000;190:310–329. doi: 10.1002/(SICI)1096-9896(200002)190:3<310::AID-PATH525>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Pancook J, Reisfeld R, Varki N, Vitiello A, Fox R, Montgomery A. Expression and regulation of the neural cell adhesion molecule L1 on human cells of myelomonocytic and lymphoid origin. J Immunol. 1997;158:4413–4421. [PubMed] [Google Scholar]
- Pfaff M, Tangemann K, Muller B, Gurrath M, Muller G, Kessler H, Timpl R, Engel J. Selective recognition of cyclic RGD peptides of NMR defined conformation by αIIbSymbol“ § 123,Symbol” § 12vSymbol“ § 123 and α5β1 integrins. J Biol Chem. 1994;269:20233–20238. [PubMed] [Google Scholar]
- Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA. CD31/PECAM-1 is a ligand for αvβ3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol. 1995;130:451–460. doi: 10.1083/jcb.130.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RA, Hemperly JJ. Variants of human L1 cell adhesion molecule arise through alternate splicing of RNA. J Mol Neurosci. 1992;3:127–135. doi: 10.1007/BF02919404. [DOI] [PubMed] [Google Scholar]
- Rouslahti E, Obrink B. Common principles in cell adhesion. Exp Cell Res. 1996;227:1–11. doi: 10.1006/excr.1996.0243. [DOI] [PubMed] [Google Scholar]
- Ruppert M, Aigner S, Hubbe M, Yagita H, Altevogt P. The L1 adhesion molecule is a cellular ligand for VLA-5. J Cell Biol. 1995;131:1881–1891. doi: 10.1083/jcb.131.6.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandig M, Voura EB, Kalnins VI, Siu C-H. Role of cadherins in the transendothelial migration of melanoma cells in culture. Cell Motil Cytoskeleton. 1997;38:351–364. doi: 10.1002/(SICI)1097-0169(1997)38:4<351::AID-CM5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Seftor RE, Seftor EA, Gehlsen KR, Stetler-Stevenson WG, Brown PD, Ruoslahti E, Hendrix MJ. Role of the αvβ3 integrin in human melanoma cell invasion. Proc Natl Acad Sci USA. 1992;89:1557–1561. doi: 10.1073/pnas.89.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silletti S, Kessler T, Goldberg J, Boger DL, Cheresh DA. Disruption of matrix metalloproteinase 2 binding to integrin αvβ3 by an organic molecule inhibits angiogenesis and tumor growth in vivo. Proc Natl Acad Sci USA. 2001;98:119–124. doi: 10.1073/pnas.011343298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Cheresh DA. Integrin (αvβ3)-ligand interaction. Identification of a heterodimeric RGD binding site on the vitronectin receptor. J Biol Chem. 1990;265:2168–2172. [PubMed] [Google Scholar]
- Stetler-Stevenson W, Aznavoorian S, Liotta L. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- Strongin A, Collier I, Bannikov G, Marmer B, Grant G, Goldberg G. Mechanism of cell surface activation of 72-kDa type IV collagenase. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- Voura, E.B., Chen, N., and Siu, C.-H. (2001). Platelet-endothelial cell adhesion molecule-1 (CD31) redistributes from the endothelial junction and is not required for the transendothelial migration of melanoma cells. Clin. Exp. Metastasis (in press). [DOI] [PubMed]
- Voura EB, Sandig M, Kalnins VI, Siu C-H. Cell shape changes and cytoskeleton reorganization during transendothelial migration of human melanoma cells. Cell Tissue Res. 1998a;293:375–387. doi: 10.1007/s004410051129. [DOI] [PubMed] [Google Scholar]
- Voura EB, Sandig M, Siu C-H. Cell-cell interactions during transendothelial migration of tumor cells. Microsc Res Tech. 1998b;43:265–273. doi: 10.1002/(SICI)1097-0029(19981101)43:3<265::AID-JEMT9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- Weterman M, van Muijen G, Bloemers H, Ruiter D. Biology of disease; molecular markers of melanocytic tumor progression. Lab Invest. 1994;70:593–608. [PubMed] [Google Scholar]
- Yip PM, Siu CH. PC12 cells utilize the homophilic binding site of L1 for cell-cell adhesion but L1-αvβ3 interaction for neurite outgrowth. J Neurochem. 2001;76:1552–1564. doi: 10.1046/j.1471-4159.2001.00152.x. [DOI] [PubMed] [Google Scholar]
- Yip PM, Zhao X, Montgomery A, Siu C-H. The Arg-Gly-Asp motif in the cell adhesion molecule L1 promotes neurite outgrowth via interaction with the αvβ3 integrin. Mol Biol Cell. 1998;9:277–290. doi: 10.1091/mbc.9.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun Z, Menter G, Nicolson G. Involvement of integrin αvβ3 in cell adhesion motility and liver metastasis of murine RAW117 large cell lymphoma. Cancer Res. 1996;56:3103–3111. [PubMed] [Google Scholar]
- Zhao X, Siu C-H. Colocalization of the homophilic binding site and the neuritogenic activity of the cell adhesion molecule L1 to its second Ig-like domain. J Biol Chem. 1995;270:29413–29421. doi: 10.1074/jbc.270.49.29413. [DOI] [PubMed] [Google Scholar]
- Zhao X, Siu C-H. Differential effects of two hydrocephalus/MASA syndrome-related mutations on the homophilic binding and neuritogenic activities of the cell adhesion molecule L1. J Biol Chem. 1996;271:6563–6566. doi: 10.1074/jbc.271.12.6563. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yip PM, Siu C-H. Identification of a homophilic binding site in Ig-like domain 2 of the cell adhesion molecule L1. J Neurochem. 1998;71:960–971. doi: 10.1046/j.1471-4159.1998.71030960.x. [DOI] [PubMed] [Google Scholar]