Abstract
Background
Advanced-stage serous ovarian carcinoma results in the majority of deaths from ovarian carcinoma. The histone chaperone, Holliday junction-recognizing protein (HJURP), binds with centromere protein-A (CENP-A) and its expression has been shown to be a prognostic biomarker in some cancers. The aim of this study was to investigate the role of HJURP expression in advanced-stage serous ovarian carcinoma.
Material/Methods
Ninety-eight patients with advanced-stage serous ovarian carcinoma, who had tumor tissue samples available, were studied. Expression levels of HJURP were detected using immunohistochemistry (IHC) and were correlated with HJURP expression and patient clinicopathological factors. Fisher’s correlation coefficient, Kaplan-Meier survival curves, the log-rank test, and Cox’s regression proportional hazards model were performed to analyze the significance of factors affecting survival rate and independent prognostic factors.
Results
Increased expression levels of HJURP in advanced-stage serous ovarian carcinoma were found in 33.67% (33/98) of cases; low expression levels of HJURP were found in 66.33% (65/98) of cases. High expression levels of HJURP were significantly associated with lymph node metastases (P=0.018), and lower overall survival (P=0.002). HJURP expression was identified as an independent prognostic biomarker for patients with advanced serous ovarian cancer in this study group of 98 patients (P=0.013).
Conclusions
Increased expression of HJURP was identified as an independent negative prognostic biomarker for patients with advanced serous ovarian cancer in this study. Further studies are required to determine whether HJURP expression in serous ovarian carcinoma may have a role in guiding clinical management by stratifying patients according to risk.
MeSH Keywords: Biological Markers, Ovarian Neoplasms, Prognosis
Background
Worldwide, ovarian cancer is the fourth most common cause of cancer-related deaths in women and advanced-stage serous ovarian carcinoma results in the majority of deaths from ovarian carcinoma [1]. The high mortality rate for ovarian cancer is due to the lack of symptoms when the tumor is early-stage, and the high recurrence rate following surgical treatment [2]. Epithelial ovarian carcinoma accounts for approximately 90% of all ovarian cancers and can be further divided histologically into serous, endometrioid, clear cell, and mucinous adenocarcinoma. Annually, advanced-stage serous ovarian carcinoma leads to the majority of epithelial ovarian cancer deaths in the United States [3]. Although recent surgical and chemotherapy treatment regimens for serous ovarian carcinoma have been improved rapidly, the 5-year overall survival (OS) for ovarian carcinoma is still unsatisfactory, ranging from between 25–35% [4]. The validation of diagnostic and prognostic biomarkers for epithelial ovarian carcinoma is still emerging, and there remains a need for biomarkers that can predict prognosis or response to specific therapies, particularly in the era of targeted personalized treatment.
The centromere is a particular component of the eukaryotic chromosome, which is required for the assembly of the kinetochore during cell division. The maintenance of a normal centromere is critical for gene stability, and defects of the chromosome lead to aneuploidy and tumorigenesis [5]. The structure of the centromere has not been previously well investigated, but centromere protein-A (CENP-A) has been shown to be responsible for the assembly and the stability of centromeres [6]. CENP-A can be deposited on centromeric DNA by associating with chaperone proteins in the form of a CENP-A pre-nucleosomal complex [7].
The histone chaperone, Holliday junction-recognizing protein (HJURP), binds with centromere protein-A (CENP-A), and its expression has been shown to be a prognostic biomarker in some cancers [8]. HJURP has dual roles in DNA repair and chromosome segregation during mitosis, and the ectopic activation of HJURP has been shown to be associated with the immortality of cancer cells [7]. Overexpression of HJURP has now been reported in hepatocellular carcinoma and breast cancer [9,10]. However, there remains limited understanding on whether HJURP expression is a prognostic biomarker in ovarian cancer, despite continuing reports of HJURP in carcinogenesis.
The aim of this study was to investigate the role of HJURP expression in advanced-stage serous ovarian carcinoma, using immunohistochemistry (IHC), and to evaluate its prognostic value in this important cancer.
Material and Methods
Patients and ethics
Between 2009–2015, 388 patients underwent surgery for ovarian carcinoma at the Affiliated Hospital of Shandong Medical College and the Linyi Peoples’ Hospital. Ninety-eight patients were diagnosed with advanced-stage serous ovarian carcinoma by routine histopathology. Patients were diagnosed with TNM Stage IIIB–IV ovarian carcinoma, according to the current of the American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC) staging system.
Of the total number of 388 patients treated for ovarian cancer, 98 patients with advanced-stage serous ovarian cancer were selected using the following study inclusion criteria: tumor tissue was available for histology and immunohistochemistry (IHC); patients were available for clinical follow-up following treatment; none of the patients had chemotherapy or radiotherapy before surgery; all patients were treated with systemic platinum-based chemotherapy following surgery; and, there were no post-operative complications, and no other types of tumor were identified. The optimal cytoreductive surgical status was defined as the presence of residual tumor of no more than 1.0 cm in diameter [11].
Tissue samples of serous ovarian carcinoma were all obtained from the Pathology Department of the Affiliated Hospital of Shandong Medical College and the Linyi Peoples’ Hospital, with the written informed consent of the patients or their families.
Immunohistochemistry (IHC) for Holliday junction-recognizing protein (HJURP)
The streptavidin-biotin-complex immunoperoxidase immunohistochemistry (IHC) method was used to detect the expression of Holliday junction-recognizing protein (HJURP) in tumor tissues, according to the method previously reported [12–14]. Briefly, the tumor tissue samples were deparaffinized in xylene and rehydrated in graded alcohols, and then washed in citrate buffer to improve antigen retrieval. Endogenous peroxidase was blocked by incubation in 3% hydrogen peroxide for 10 minutes, and the non-specific antibody binding was blocked using phosphate buffered saline (PBS) containing 5% bovine serum albumin (BSA). The mouse monoclonal primary antibody to Holliday junction-recognizing protein (HJURP) (1: 200) (Catalog No. ab175577) (Abcam Ltd., Cambridge, UK) was incubated on the tissue sections overnight at 4°C. After washing three times with PBS, the secondary antibody, labeled with streptavidin-biotin peroxidase was added and incubates followed by washing with PBS, followed by incubation with 3,3′-diaminobenzidine (DAB) solution for visual localization of the immunostaining (brown), and the sections were counterstained with hematoxylin, and mounted with glass coverslips.
Visual scoring for the IHC localization of HJURP was evaluated by two senior pathologists, who were unaware of the clinical data of the patients. The IHC score was defined, as previously described, as the product of staining intensity multiplied by the percentage of positive cells [15,16]. The IHC staining intensity was defined as: negative (0), weak (1), moderate (2) and strong (3); the positive cell percentage was scored as: 1 (<25% positive cells); 2 (25–50% positive cells); 3 (50–75% positive cells); 4 (75–100% positive cells). Tumor tissue samples from the 98 patients were divided into the group with high HJURP expression, and the group with a low HJURP expression The cut-off IHC expression level was set as the point with the greatest combined of sensitivity plus specificity in the receiver operating characteristic (ROC) curve.
Statistical analysis
All the data were analyzed using SPSS 22.0 software (IBM, Chicago, IL, USA). Fisher’s correlation coefficient was used to evaluate the relationship between the expression of HJURP expression and the clinicopathological factors. Kaplan-Meier survival curves were constructed, the log-rank test was used to analyze the statistical differences. The Cox regression proportional hazards model were performed to analyze the significance of factors affecting survival rate and independent prognostic factors. A P-value <0.05 was statistically significant.
Results
Expression of Holliday junction-recognizing protein (HJURP) in serous ovarian cancer tissues
The findings of this study showed that Holliday junction-recognizing protein (HJURP) was mainly expressed in the nucleus of serous ovarian cancer cells (Figure 1A, 1B). This pattern of immunolocalization was consistent with previously reported findings in malignant human cells and was consistent with the known binding of centromere protein-A (CENP-A) in centromeres. Of the 98 cases of advanced-stage serous ovarian cancer and the tissue samples studied, increased expression levels of HJURP in advanced-stage serous ovarian carcinoma were found in 33.67% (33/98) of cases; low expression levels of HJURP were found in 66.33% (65/98) of cases. High expression levels of HJURP were significantly associated with lymph node metastases (P=0.018), and lower overall survival (P=0.002).
Figure 1.
Representative photomicrographs of the immunostaining for Holliday junction-recognizing protein (HJURP) shows low expression and high expression in serous ovarian carcinoma tissue samples. (A) Immunohistochemical staining shows low expression levels of Holliday junction-recognizing protein (HJURP). Scale bar: 50 μM. (B) Immunohistochemical staining shows high expression levels of Holliday junction-recognizing protein (HJURP). Scale bar: 50 μM.
The correlation between HJURP expression and patient clinicopathological factors
Table 1 summarizes the clinicopathological features in the 98 patients studied, including the age of patients, the histological grade of the serous ovarian carcinomas, tumor size, the presence of lymph node metastasis, the TNM stage, response to chemotherapy, and the surgical cytoreduction status. The correlation between HJURP expression and the patient clinicopathologic factors was analyzed with Fisher’s correlation coefficient (Table 1).
Table 1.
Correlation between HJURP and clinicopatholgic factors.
| Characters | Number | HJURP | P | |
|---|---|---|---|---|
| Low | High | |||
| Age | ||||
| <50 | 19 | 14 | 5 | 0.591 |
| ≥50 | 79 | 51 | 28 | |
| Histological grade | ||||
| I | 14 | 11 | 3 | 0.277 |
| II | 38 | 27 | 11 | |
| III | 46 | 27 | 19 | |
| Tumor size | ||||
| ≥5 cm | 37 | 23 | 14 | 0.497 |
| <5 cm | 61 | 42 | 19 | |
| Lymph node metastasis | ||||
| No | 49 | 38 | 11 | 0.018 |
| Yes | 49 | 27 | 22 | |
| TNM stage | ||||
| III | 49 | 37 | 12 | 0.053 |
| IV | 49 | 28 | 21 | |
| Response to chemotherapy | ||||
| No | 21 | 12 | 9 | 0.435 |
| Yes | 77 | 53 | 24 | |
| Cytoreduction status | ||||
| Optimal | 53 | 35 | 18 | 0.948 |
| Suboptimal | 45 | 30 | 15 | |
Means calculated by Fisher test;
HJURP – holliday junction-recognizing protein.
High expression levels of HJURP in tissue samples of serous ovarian carcinoma was shown to be significantly associated with lymph node metastasis. High expression levels of HJURP were significantly associated with positive lymph node metastases (P=0.018), indicating that HJURP may promote the lymphatic invasion in serous ovarian carcinoma. High HJURP expression was correlated with advanced TNM tumor stage (P=0.053), but this association did not reach statistical significance; this finding may reflect the association between high HJURP expression that was associated with lymph node metastasis, which is a factor in TNM tumor staging.
Correlation between HJURP expression and patient overall survival
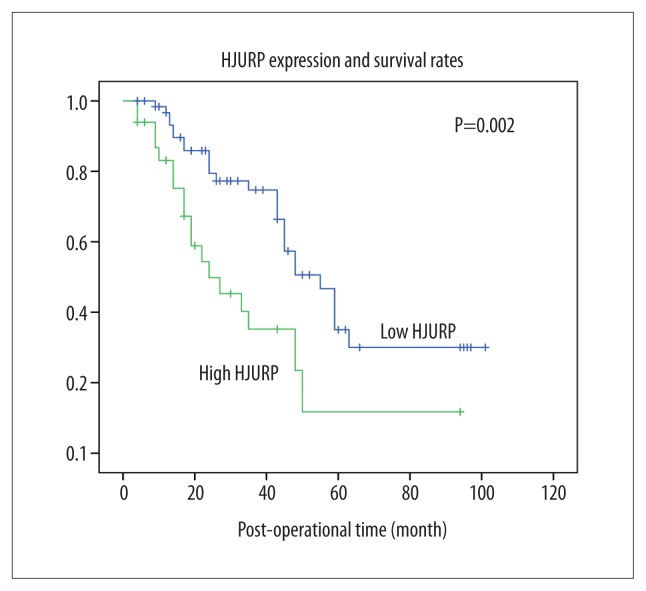
Univariate analysis using Kaplan-Meier survival curves that compared patients with high HJURP expression and low HJURP expression, and the log-rank test showed that high HJURP expression was significantly correlated with a lower patient survival rate (P=0.002) (Figure 2). Also, positive lymph node metastasis (P=0.018), advanced TNM stage (P=0.014), and poor response to chemotherapy (P=0.020) were all correlated with poor prognosis for patients with advanced serous ovarian cancer (Table 2).
Figure 2.
Kaplan-Meier survival curves showing the correlation between the expression of Holliday junction-recognizing protein (HJURP) and survival rates in patients with advanced-stage serous ovarian carcinoma. Kaplan-Meier curves show the survival rates of the groups of patients with advanced-stage serous ovarian carcinoma with high expression and low expression of Holliday junction-recognizing protein (HJURP), analyzed using the log-rank test. Patients with high expression of HJURP had lower survival rates when compared with patients with low expression of HJURP (P=0.002).
Table 2.
Correlation between HJURP and survival rates.
| Characters | Univariate analysis | |
|---|---|---|
| 5-year survival rate % | P | |
| Age | ||
| <50 | 41.7 | 0.661 |
| ≥50 | 24.5 | |
| Histological grade | ||
| I | 30.3 | 0.339 |
| II | 36.9 | |
| III | 17.4 | |
| Tumor size | ||
| ≥5 cm | 27.8 | 0.863 |
| <5 cm | 26.6 | |
| Lymph node metastasis | ||
| No | 44.4 | 0.018 |
| Yes | 5.6 | |
| TNM stage | ||
| III | 34.5 | 0.014 |
| IV | 19.0 | |
| Response to chemotherapy | ||
| Yes | 37.8 | 0.02 |
| No | 28.2 | |
| Cytoreduction status | ||
| Optimal | 34.8 | 0.838 |
| Suboptimal | 7.5 | |
| HJURP | ||
| Low | 35.0 | 0.002 |
| High | 11.7 | |
Means calculated by log-rank test;
HJURP – holliday junction-recognizing protein.
Independent prognostic factors in patients with advanced-stage serous ovarian carcinoma
Independent prognostic factors were identified and verified using the Cox regression proportional hazards model for patients with advanced-stage serous ovarian carcinoma. The identified prognostic factors that underwent multivariate analysis, including lymph node status, response to chemotherapy, and tumor tissue HJURP expression (Table 3). The TNM stage was excluded because of its direct association with the lymph node status, which was one parameter of the TNM staging system.
Using the Cox regression hazard model, a high HJURP expression level was shown to be an independent prognostic factor for patients with advanced-stage serous ovarian carcinoma (P=0.013) (HR=2.18, 95% CI=1.18–4.04), indicating that HJURP expression was a negative prognostic biomarker of advanced-stage serous ovarian carcinoma. Also, a reduced patient response to chemotherapy was an independent prognostic factor for patients with advanced-stage serous ovarian carcinoma (P=0.011) (HR=2.75, 95% CI=1.27–5.97). The presence of lymph node metastases resulted in a poor patient prognosis (P=0.059), but this association did not reach statistical significance.
Discussion
Currently, the standard first-line therapy for ovarian cancer is the cytoreductive surgery followed by the platinum-based chemotherapy combined with paclitaxel [17]. Recent developments and modifications in chemotherapy are some of the reasons for improved patient survival in ovarian cancer. However, the development of patient treatments, including chemotherapy, is dependent on the discovery of new prognostic biomarkers. The findings of this study, in 98 patients, showed that increased expression of Holliday junction-recognizing protein (HJURP) was an independent negative prognostic biomarker of advanced-stage serous ovarian carcinoma. Further studies are required to determine whether HJURP expression in serous ovarian carcinoma may have a role in guiding clinical management by stratifying patients according to risk.
The findings of this study are supported by previous in vitro and in vivo studies on the function and significance of HJURP expression in the progression of malignancy [7,18]. The biological function of HJURP involves its interaction with centromere protein-A (CENP-A) and with the deposition of CENP-A in the centromere [19,20]. However, activation of HJURP is required for chromosomal stability and is also involved in immortalizing cancer cells [7]. The aberrant upregulation of HJURP has been reported in several human cancers, including breast cancer [9,10]. These findings support the role of HJURP as both a prognostic biomarker and also as a potential novel therapeutic target for the development of anticancer drugs.
The findings of this study showed that high expression of HJURP was significantly associated with positive lymph node status (lymph node metastasis) in patients with advanced-stage serous ovarian carcinoma, indicating that HJURP expression may promote lymphatic invasion in ovarian cancer. However, the role of HJURP expression in lymphatic invasion and lymph node metastasis requires support from future studies.
Also, the findings of this study showed that the correlation between HJURP expression and lymphatic invasion could contribute to the poorer prognosis of patients with high HJURP expression. Previous studies have shown that increased levels of HJURP mRNA was a prognostic factor for disease-free survival and overall survival in patients with breast cancer and that HJURP mRNA expression was a predictive biomarker for sensitivity and response to radiotherapy [10]. However, the role of HJURP expression as a predictive biomarker for sensitivity to chemotherapy and radiotherapy require support from future studies.
This study has shown that the detection of HJURP expression levels in tumor tissue samples from patients with advanced-stage serous ovarian carcinoma may not only be used to stratify high-risk patients but also select suitable patients for radiotherapy. Until now, little has been known about the role of HJURP expression in human cancer, and there is still no specific HJURP inhibitor or treatment that may block HJURP function in cancer cells. However, inhibitors of HJURP, or its downstream proteins, may be developed in future, but require further studies on the mechanisms of HJURP expression in human cancer progression.
Conclusions
The findings of this study have shown that increased expression of Holliday junction-recognizing protein (HJURP) was an independent negative prognostic biomarker in 98 patients diagnosed with advanced serous ovarian for patients with advanced serous ovarian carcinoma. Further studies are required to determine whether HJURP expression in serous ovarian carcinoma may have a role in guiding clinical management by stratifying patients according to risk.
Footnotes
Source of support: Departmental sources
References
- 1.Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: Targeted therapies in ovarian cancer. Nat Rev Cancer. 2009;9:167–81. doi: 10.1038/nrc2583. [DOI] [PubMed] [Google Scholar]
- 2.Basu M, Mukhopadhyay S, Chatterjee U, Roy SS. FGF16 promotes invasive behavior of SKOV-3 ovarian cancer cells through activation of mitogen-activated protein kinase (MAPK) signaling pathway. J Biol Chem. 2014;289:1415–28. doi: 10.1074/jbc.M113.535427. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 4.Colombo PE, Fabbro M, Theillet C, et al. Sensitivity and resistance to treatment in the primary management of epithelial ovarian cancer. Crit Rev Oncol Hematol. 2014;89:207–16. doi: 10.1016/j.critrevonc.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Shuaib M, Ouararhni K, Dimitrov S, Hamiche A. HJURP binds CENP-A via a highly conserved N-terminal domain and mediates its deposition at centromeres. Proc Natl Acad Sci USA. 2010;107:1349–54. doi: 10.1073/pnas.0913709107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad K, Henikoff S. Centromeres are specialized replication domains in heterochromatin. J Cell Biol. 2001;153:101–10. doi: 10.1083/jcb.153.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato T, Sato N, Hayama S, et al. Activation of Holliday junction-recognizing protein involved in the chromosomal stability and immortality of cancer cells. Cancer Res. 2007;67:8544–53. doi: 10.1158/0008-5472.CAN-07-1307. [DOI] [PubMed] [Google Scholar]
- 8.Foltz DR, Jansen LE, Black BE, et al. The human CENP-A centromeric nucleosome-associated complex. Nat Cell Biol. 2006;8:458–69. doi: 10.1038/ncb1397. [DOI] [PubMed] [Google Scholar]
- 9.Hu B, Wang Q, Wang Y, et al. Holliday junction-recognizing protein promotes cell proliferation and correlates with unfavorable clinical outcome of hepatocellular carcinoma. Onco Targets Ther. 2017;10:2601–7. doi: 10.2147/OTT.S127738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Z, Huang G, Sadanandam A, et al. The expression level of HJURP has an independent prognostic impact and predicts the sensitivity to radiotherapy in breast cancer. Breast Cancer Res. 2010;12:R18. doi: 10.1186/bcr2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaid TM, Yeung TL, Thompson MS, et al. Identification of FGFR4 as a potential therapeutic target for advanced-stage, high-grade serous ovarian cancer. Clin Cancer Res. 2013;19:809–20. doi: 10.1158/1078-0432.CCR-12-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H, Xu Y, Zhang Q, et al. Prognostic significance of TBL1XR1 in predicting liver metastasis for early stage colorectal cancer. Surg Oncol. 2017;26:13–20. doi: 10.1016/j.suronc.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Xu YF, Yang XQ, Lu XF, et al. Fibroblast growth factor receptor 4 promotes progression and correlates to poor prognosis in cholangiocarcinoma. Biochem Biophys Res Commun. 2014;446:54–60. doi: 10.1016/j.bbrc.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 14.Yang XQ, Xu YF, Guo S, et al. Clinical significance of nerve growth factor and tropomyosin-receptor-kinase signaling pathway in intrahepatic cholangiocarcinoma. World J Gastroenterol. 2014;20:4076–84. doi: 10.3748/wjg.v20.i14.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Xu Y, Zhang Q, et al. Correlations between TBL1XR1 and recurrence of colorectal cancer. Sci Rep. 2017;7:44275. doi: 10.1038/srep44275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Yang X, Li Z, et al. Sprouty2 correlates with favorable prognosis of gastric adenocarcinoma via suppressing FGFR2-induced ERK phosphorylation and cancer progression. Oncotarget. 2017;8:4888–900. doi: 10.18632/oncotarget.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.du Bois A, Luck HJ, Meier W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–29. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- 18.de Tayrac M, Aubry M, Saikali S, et al. A 4-gene signature associated with clinical outcome in high-grade gliomas. Clin Cancer Res. 2011;17:317–27. doi: 10.1158/1078-0432.CCR-10-1126. [DOI] [PubMed] [Google Scholar]
- 19.Lacoste N, Woolfe A, Tachiwana H, et al. Mislocalization of the centromeric histone variant CenH3/CENP-A in human cells depends on the chaperone DAXX. Mol Cell. 2014;53:631–44. doi: 10.1016/j.molcel.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Tachiwana H, Muller S, Blumer J, et al. HJURP involvement in de novo CenH3(CENP-A) and CENP-C recruitment. Cell Rep. 2015;11:22–32. doi: 10.1016/j.celrep.2015.03.013. [DOI] [PubMed] [Google Scholar]