Abstract
MicroRNA expression has not been studied during placentation in pregnancies that develop preeclampsia, where it likely manifests. In this pilot study, miRNA expression in late first trimester placenta from four pregnancies that developed severe preeclampsia matched to controls using the Affymetrix GeneChip® miRNA 3.0 Array identified 9 miRNAs differentially expressed, with MiR-202-3p the most significantly overexpressed in severe preeclampsia. Real-time reverse transcription polymerase chain reaction (qRT-PCR) confirmed overexpression of MiR-202-3p in a validation cohort, with a 7-fold increase in pregnancies that developed severe preeclampsia (p≤ 0.05). Differential miRNA expression, specifically miR-202-3p, is seen in first trimester placenta in severe preeclampsia.
Keywords: Chorionic villus sampling (CVS), first trimester placenta, microRNA (miRNA), preeclampsia
Introduction
Preeclampsia, affecting 2–8% of pregnancies, is associated with risk of maternal and fetal morbidity and mortality. (1–3) Pathogenesis is linked to aberrant trophoblast invasion leading to chronic placental malperfusion, maternal endothelial dysfunction and hypertension with adverse outcomes. (2) Gene expression changes in first trimester placenta occur (4) however, most studies are derived from placental tissue at delivery. (5) Since differences exist between the first and third trimester placenta, (6) detection of early changes are also critical for identification of potential mechanisms.
MiRNAs are small noncoding RNA that control target genes by blocking translation. Single miRNAs can impact hundreds of genes leading to cascading effects. Aberrant miRNA expression is linked to development of disease states (7, 8) which led to the use of miRNA profiling for prognostics and therapeutic targeting.(9–13)
Genome-wide studies of miRNAs in normal and pathological human placentas exist but none in first trimester. (5, 7, 14–17) Thus, the goal of this pilot study was to identify differential miRNA expression in first trimester placenta of pregnancies that developed severe preeclampsia to understand early pathogenesis.
Methods
Subjects were identified from the Cedars-Sinai Medical Center (CSMC) Prenatal Biorepository, through an IRB protocol. Chorionic villi from 11–13 week gestations were placed in RNAlater (Qiagen, Valencia CA) and stored as described. (18)
Severe preeclampsia was defined per American College of Obstetricians and Gynecologists Guidelines (19). Patients with medical complications (pregestational/gestational diabetes, chronic hypertension, tobacco dependence), or multiple gestation were excluded.
Four samples were identified from subjects that developed severe preeclampsia and matched with controls for gestational age at CVS (+/− 6 days), fetal sex, parity, which delivered at term without complications.
The validation cohort of 4 CVS samples from subjects that developed severe preeclampsia were matched with 4 controls for fetal sex and maternal parity.
Specimens were transferred to RNA-Bee reagent (Fisher Scientific) and RNA extracted using the RNA-Bee manufacturer’s method. Quantity and quality was assessed with NanoDrop 8000 Spectrophotometer and Agilent 2100 Bioanalyzer.
Total RNA was labeled with FlashTag™ Biotin HSR RNA Labeling Kit. Hybridization was carried out on an Affymetrix GeneChip® miRNA 3.0 Array containing 5,639 human targets. Arrays were scanned using the Affymetrix® GeneChip® Scanner 3000. MiRNA expression was analyzed using Affymetrix Expression Console.
Comparative real-time reverse transcription polymerase chain reaction (qRT-PCR) was used in the validation cohort. RNA was reverse-transcribed using microRNA-specific primers (Qiagen Inc). cDNA was amplified using Qiagen miScript PCR System on the Viia7 Real Time PCR machine (Applied BioSystems).
Descriptive statistics were performed utilizing StataIC 13. T-tests, Mann Whitney U and Chi-square was used as appropriate.
Exploratory Principal Component Analysis (PCA) was generated to evaluate the samples using Rgl package (Version 0.93) in R and Bioconductor. ANOVA was used for batch effects and compare data across groups. qRT-PCR was analyzed using ΔCT values quantitated from comparison to the reference miRNA, RNU6-2 using the Mann-Whitney U test.
Results
For the microarray study, maternal age, BMI, race, parity, and gestational age at CVS were similar. All fetuses were females. Gestational age at delivery (250 ± 32.9 vs 275 ± 9.1 days) and birth weight (2351 ± 1110.15 vs 3617 ± 564.2 g) were also similar. However, the median birth weight percentiles were lower in subjects that developed preeclampsia (10th vs 71st, p=0.0421).
For the validation cohort for qRT-PCR studies, maternal age, BMI, race, parity and gestational age at CVS were similar. Gestational age at delivery (233 ± 38 vs 279 ± 6 days, p=0.0548), birth weight (1670 ± 957 vs 3045 ± 143 g, p=0.0295) and birth weight percentiles (3rd vs 19.5th, p=0.0209) were lower in the pregnancies that developed preeclampsia.
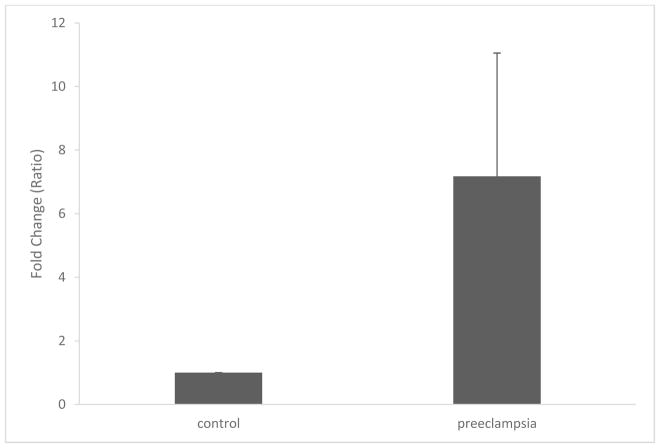
Nine miRNAs were differentially expressed in severe preeclampsia compared to controls (Table 1). MiR-202-3p was the most differentially expressed miRNA and therefore qRT-PCR of miR-202-3p was performed in a validation cohort. It was increased 7-fold in preeclampsia compared to control pregnancies (Figure 1).
Table 1.
Differentially Expression MicroRNAs in Microarray Scan
| MicroRNA | P value * | Fold change |
|---|---|---|
| has-miR-202-3p_st | 0.000115112 | 2.21 |
| hp_hsa-mir-320b-1_x_st | 0.000215714 | −1.5 |
| has-miR-432-star_st | 0.00749553 | −1.7 |
| ENSGOOOOO221611_st | 0.0129797 | 1.53 |
| hsa-miR-25-star_st | 0.0261407 | 1.66 |
| hsa-miR-4701-3p_st | 0.0306885 | −1.83 |
| hsa-miR-451_st | 0.0314716 | 2.15 |
| hp_hsa-miR-933_st | 0.0383562 | −1.53 |
| has-miR548aj_st | 0.0384967 | 2.03 |
ANOVA
Figure 1. qRT-PCR with miR-202-3p.
qRT-PCR of miR-202-3p is increased 7-fold in severe preeclampsia (fold change ± SD). P< 0.05.
Discussion
Nine miRNAs were differentially expressed in severe preeclampsia compared to controls in late first trimester placenta. MiR-202-3p, the most significantly overexpressed miRNA in microarray studies was also overexpressed in a validation cohort using qRT-PCR.
Mi-202, encoded on chromosome 10, is the precursor of two different mature miRNA sequences from the same stem-loop, miR-202-3p and miR-202-5p, each having different targets. (http://www.ncbi.nlm.nih.gov/nuccore/NR_030170; http://mirbase.org/cgi-bin/mirna_summary.pl?fam=MIPF0000121) MiR202-3p was upregulated in first trimester placenta from pregnancies that developed severe preeclampsia. MiR-202-3p promotes apoptosis and inhibits proliferation in cancer cells by post-transcriptionally targeting expression of various proteins. (9, 20, 21) Among the other differentially expressed miRNA’s, miR-451 and miR-548 also inhibit proliferation, miR-451 with tumor-suppressor properties in colon cancer cell lines and miR-548 with tumor-suppressor properties and pro-apoptotic effects in breast cancer cell lines. (22, 23) The overexpression of miR-202-3p, as well as miR-451 and miR-548, in the late first trimester placenta might have a similar role in apoptosis and inadequate trophoblast proliferation and invasion, however, this remains to be determined.
Differential expression of miRNAs in the third trimester placenta that develop preeclampsia have been described.(14) However, miR202-3p was not described. Oxidative stress may alter the expression of miRNAs in placenta (24) and chronic hypoxia in preeclampsia may lead to changes in placental miRNA expression throughout gestation. Our studies utilized samples early in gestation, identifying potential events during placentation that can lead to severe preeclampsia, before clinical onset, which may permit assessment of cause rather than effects of disease.
Limitations include the small sample size. However, significant differences in miRNA expression by microarray in the initial cohort was confirmed in a validation cohort. The majority of samples were from Caucasian women of advanced reproductive age, thus results may not be applicable to all groups. Larger studies are necessary.
Our findings support existing evidence that fundamental biological processes leading to severe preeclampsia occur in the first trimester (2, 25) and miRNA expression is altered in first trimester placenta of women who develop severe preeclampsia, which differs from the third trimester. The biologic function of the identified novel candidate miRNA miR-202-3p in first trimester placenta warrants further investigation and may ultimately be used as a biomarker for development of preeclampsia.
Highlights.
MiR-202-3p is expressed early in gestations that develop severe preeclampsia.
MiR-202-3p is 7-fold higher in first trimester placenta developing preeclampsia.
Differences in miRNA expression exist among first and third trimester placenta.
Acknowledgments
Funding Source: The research was supported by the NICHD of the National Institutes of Health under award number R01HD074368 and also by the Helping Hand of Los Angeles, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Seminars in perinatology. 2012;36(1):56–9. doi: 10.1053/j.semperi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Choudhury M, Friedman JE. Epigenetics and microRNAs in preeclampsia. Clinical and experimental hypertension. 2012;34(5):334–41. doi: 10.3109/10641963.2011.649931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennstrom M, et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. The New England journal of medicine. 2016;374(1):13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 4.Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009;30(1):15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleinrouweler CE, van Uitert M, Moerland PD, Ris-Stalpers C, van der Post JA, Afink GB. Differentially expressed genes in the pre-eclamptic placenta: a systematic review and meta-analysis. PloS one. 2013;8(7):e68991. doi: 10.1371/journal.pone.0068991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Song W, Na Q. The emerging roles of placenta-specific microRNAs in regulating trophoblast proliferation during the first trimester. The Australian & New Zealand journal of obstetrics & gynaecology. 2012;52(6):565–70. doi: 10.1111/j.1479-828X.2012.01481.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen DB, Wang W. Human placental microRNAs and preeclampsia. Biology of reproduction. 2013;88(5):130. doi: 10.1095/biolreprod.113.107805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuang JC, Jones PA. Epigenetics and microRNAs. Pediatric research. 2007;61(5 Pt 2):24R–9R. doi: 10.1203/pdr.0b013e3180457684. [DOI] [PubMed] [Google Scholar]
- 9.Meng X, Chen X, Lu P, Ma W, Yue D, Song L, et al. MicroRNA-202 inhibits tumor progression by targeting LAMA1 in esophageal squamous cell carcinoma. Biochemical and biophysical research communications. 2016;473(4):821–7. doi: 10.1016/j.bbrc.2016.03.130. [DOI] [PubMed] [Google Scholar]
- 10.Orlicka-Plocka M, Gurda D, Fedoruk-Wyszomirska A, Smolarek I, Wyszko E. Circulating microRNAs in Cardiovascular Diseases. Acta biochimica Polonica. 2016 doi: 10.18388/abp.2016_1334. [DOI] [PubMed] [Google Scholar]
- 11.Alipoor SD, Adcock IM, Garssen J, Mortaz E, Varahram M, Mirsaeidi M, et al. The roles of miRNAs as potential biomarkers in lung diseases. European journal of pharmacology. 2016;791:395–404. doi: 10.1016/j.ejphar.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salarinia R, Sahebkar A, Peyvandi M, Mirzaei HR, Jaafari MR, Riahi MM, et al. Epi-Drugs and Epi-miRs: Moving Beyond Current Cancer Therapies. Current cancer drug targets. 2016;16(9):773–88. doi: 10.2174/1568009616666151207110143. [DOI] [PubMed] [Google Scholar]
- 13.Bordinhao AL, Evangelista AF, Oliveira RJ, Macedo T, Silveira HC, Reis RM, et al. MicroRNA profiling in human breast cancer cell lines exposed to the anti-neoplastic drug cediranib. Oncology reports. 2016 doi: 10.3892/or.2016.5153. [DOI] [PubMed] [Google Scholar]
- 14.Betoni JS, Derr K, Pahl MC, Rogers L, Muller CL, Packard RE, et al. MicroRNA analysis in placentas from patients with preeclampsia: comparison of new and published results. Hypertension in pregnancy. 2013;32(4):321–39. doi: 10.3109/10641955.2013.807819. [DOI] [PubMed] [Google Scholar]
- 15.Enquobahrie DA, Abetew DF, Sorensen TK, Willoughby D, Chidambaram K, Williams MA. Placental microRNA expression in pregnancies complicated by preeclampsia. American journal of obstetrics and gynecology. 2011;204(2):178e12–21. doi: 10.1016/j.ajog.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu XM, Han T, Sargent IL, Yin GW, Yao YQ. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. American journal of obstetrics and gynecology. 2009;200(6):661e1–7. doi: 10.1016/j.ajog.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 17.Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. American journal of obstetrics and gynecology. 2007;196(3):261e1–6. doi: 10.1016/j.ajog.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Pisarska MD, Akhlaghpour M, Lee B, Barlow GM, Xu N, Wang ET, et al. Optimization of techniques for multiple platform testing in small, precious samples such as Human Chorionic Villus Sampling. Prenatal diagnosis. 2016 doi: 10.1002/pd.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of O Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstetrics and gynecology. 2013;122(5):1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Li C, Wang M, Su L, Qu Y, Li J, et al. Decrease of miR-202-3p expression, a novel tumor suppressor, in gastric cancer. PloS one. 2013;8(7):e69756. doi: 10.1371/journal.pone.0069756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Huang Z, Guo W, Ni S, Xiao X, Wang L, et al. microRNA-202-3p inhibits cell proliferation by targeting ADP-ribosylation factor-like 5A in human colorectal carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20(5):1146–57. doi: 10.1158/1078-0432.CCR-13-1023. [DOI] [PubMed] [Google Scholar]
- 22.Mamoori A, Gopalan V, Lu CT, Chua TC, Morris DL, Smith RA, et al. Expression pattern of miR-451 and its target MIF (macrophage migration inhibitory factor) in colorectal cancer. J Clin Pathol. 2017;70(4):308–12. doi: 10.1136/jclinpath-2016-203972. [DOI] [PubMed] [Google Scholar]
- 23.Ke H, Zhao L, Feng X, Xu H, Zou L, Yang Q, et al. NEAT1 is Required for Survival of Breast Cancer Cells Through FUS and miR-548. Gene Regul Syst Bio. 2016;10(Suppl 1):11–7. doi: 10.4137/GRSB.S29414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muralimanoharan S, Maloyan A, Mele J, Guo C, Myatt LG, Myatt L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta. 2012;33(10):816–23. doi: 10.1016/j.placenta.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51(4):970–5. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]