Abstract
The outgrowth of neurites is a critical step in neuronal maturation, and it is well established that the actin cytoskeleton is involved in this process. Investigators from our laboratory recently described a novel protein named palladin, which has been shown to play an essential role in organizing the actin cytoskeleton in cultured fibroblasts. We investigated the expression of palladin in the developing rat brain by Western blot and found that the E18 brain contained a unique variant of palladin that is significantly smaller (∼85 kDa) than the common form found in other developing tissues (90–92 kDa). Because the expression of a tissue-specific isoform suggests the possibility of a cell type-specific function, we investigated the localization and function of palladin in cultured cortical neurons. Palladin was found preferentially targeted to the developing axon but not the dendrites and was strongly localized to the axonal growth cone. When palladin expression was attenuated by transfection with antisense constructs in both the B35 neuroblastoma cell line and in primary cortical neurons, a reduction in the expression of palladin resulted in a failure of neurite outgrowth. These results implicate palladin as a critical component of the developing nervous system, with an important role in axonal extension.
INTRODUCTION
A key step in the development of the nervous system is neurite outgrowth, in which axons and dendrites are extended from the differentiating neuron. Growth cones of newly formed axons migrate by appropriate environmental cues to locate their target cells (Goodman and Shatz, 1993; Tessier-Lavigne and Goodman, 1996; Goodhill, 1998). Growth cone motility is an actin-dependent process (Forscher et al., 1992) and requires a complex coordination of signaling pathways that regulate the polymerization of actin and its assembly into organized arrays (Marsh and Letourneau, 1984; Tanaka and Sabry, 1995; Suter and Forscher, 1998). Although the details of cytoskeletal regulation in neurons are not known, growth cones appear to share similarities with related actin-based structures in nonneuronal cells.
Two types of cytoskeletal structures, filopodia and lamellipodia, play important roles in growth cone migration. Because in nonneuronal cells the assembly of distinct actin arrays is regulated by different members of the Rho family of GTPases (Hall, 1994; reviewed by Hall, 1998), the role of these pathways in axonal outgrowth has received much attention. Multiple Rho family members play a role in growth cone assembly and motility and are involved in axon and dendrite formation during neurogenesis (Kozma et al., 1995; Luo et al., 1996; Symons, 1996; Kozma et al., 1997; Luo et al., 1997; Threadgill et al., 1997; Zipkin et al., 1997; Hall, 1998; Renaudin et al., 1999; Bito et al., 2000). In particular, Brown et al. (2000) showed that cdc42, the GTPase that regulates the formation of filopodia in fibroblasts, stimulates neurite outgrowth in primary cultures of spinal cord neurons. Similarly, the scaffold protein N-WASP, a downstream target of cdc42 in fibroblasts, plays an important role in neurite extension in both PC12 cells and primary hippocampal neurons (Banzai et al., 2000). These results suggest that cytoskeletal proteins common to both neuronal and nonneuronal cells are involved in the assembly of actin arrays that serve to extend the axonal growth cone during neural development.
We characterized previously a novel, widely expressed protein named palladin (Parast and Otey, 2000). Palladin is a component of the actin cytoskeleton and localizes both in actin cables and in cell adhesions in a variety of nonneuronal cells. Most importantly, antisense experiments demonstrated that palladin was required to maintain the integrity of the actin cytoskeleton, and thus the well spread morphology, of cultured fibroblasts. Its sequence homology with known cytoskeletal proteins suggests that palladin possesses the features of a potent cytoskeletal scaffold. In the current study, we show that there is a specific size variant of palladin that is enriched in the developing brain and concentrated in the axons. In addition, we show that palladin is developmentally regulated during neuronal development and plays a critical role in neurite outgrowth in cultured embryonic neurons.
MATERIALS AND METHODS
Cell Culture
Rat B35 neuroblastoma cells (Schubert et al., 1974) were grown in DMEM (Life Technologies) containing 10% fetal bovine serum (FBS; Life Technologies, Rockville, MD). Cath.a-differentiated (CAD) cells were grown as previously described (Qi et al., 1997) at 37°C and in 5% CO2, in Ham's F12/EMEM medium (Life Technologies), supplemented with 8% FBS and 1% penicillin/streptomycin (Life Technologies). To induce morphological differentiation, CAD cells were switched to the same medium without serum supplementation for 12–24 h.
For the cultured cortical neurons, brains from E18 stage rat embryos were removed and transferred to a fresh culture dish containing calcium-magnesium free (CMF)-Hanks' balanced salt solution (HBSS). The cortex was dissected from each brain, minced, and placed in a 15-ml tube containing 5 ml of CMF-HBSS. Dispase was added to a final concentration of 2.5 U/ml, the tube was sealed, and the tissue was incubated for 15–20 min at 36°C. The tube was transferred to a sterile hood and the tissue pieces were gently triturated with a 10-ml pipette. The tissue pieces were allowed to settle for 2 min and the cells in suspension (3–4 ml) were transferred to a sterile culture tube containing 25 ml of complete culture medium (MEM plus 10% FBS plus 20 μg/ml gentamicin). An equivalent volume of CMF-HBSS was added back to the tube and the procedure was repeated until most of the tissue was dissociated (five to six cycles). Dissociated cells were diluted in 40–50 ml of complete medium at a concentration of 1–2 × 106 cells/ml. Cells were seeded into poly-d-lysine–treated 24-well plates at a final density of 50,000 cells/cm2. For immunocytochemical staining, the cells were grown on a 12-mm coverslip coated with poly-d-lysine. Cells were fed on the after day by a complete medium exchange to eliminate debris, followed by a 50% exchange every 2–3 d thereafter.
Immunocytochemistry
Cells grown on glass coverslips were fixed for 15 min with 4% paraformaldehyde in 0.01 M phosphate-buffered saline (PBS), then permeabilized in 0.2% Triton X-100 in PBS for 4 min, and rinsed three times in blocking/wash buffer (2% BSA in PBS). The cells were preincubated for 30 min in blocking/wash buffer and incubated with the primary antibody diluted in that buffer for 1 h at room temperature. The cells were rinsed four times with wash buffer and then incubated with the secondary antibodies diluted in wash buffer for 1 h. Cells were rinsed three times in wash buffer and given a final rinse in PBS. Coverslips were examined in a TCS-NT laser scanning confocal microscope (Leica, Deerfield, IL) and images were processed with the use of Adobe Photoshop 5.0 (Adobe Systems, Mountain View, CA). The following antibodies were used: anti-palladin monoclonals (clone 1E6, 7C6), characterized previously by Parast and Otey (2000), polyclonal anti-synaptophysin (Dako, Carpinteria, CA), polyclonal anti-MAP2 (a generous gift from Dr. Shelley Halpain, Scripps Research Institute, La Jolla, CA), polyclonal anti-growth–associated protein-43 (GAP-43; Chemicon International, Temecula, CA), fluorescein isothiocyanate-conjugated phalloidin (Sigma, St. Louis, Mo), Alexa fluorescein isothiocyanate 488 (Molecular Probes, Eugene, OR), Texas Red-conjugated anti-mouse (Jackson ImmunoResearch, West Grove, PA), and anti-rabbit (Jackson ImmunoResearch).
PAGE and Immunoblotting
Cultures were rinsed with PBS and then scraped at 4°C in RIPA buffer (150 mM NaCl, 50 mM Tris (pH 8.0), 1% Nonidet P40, 0.5% deoxycholate, and 0.1% SDS) containing 10 μg/ml leupeptin (Boehringer Mannheim, Indianapolis, IN, 1 mM phenylmethylsulfonyl fluoride (Sigma), 1 μg/ml aprotinin (Boehringer Mannheim) 1 μg/ml pepstatin, 1 mM Pefabloc (Boehringer Mannheim), 2 mM EDTA, and 1 mM orthovanadate (Sigma). Brains were Dounce homogenized in the same buffer. After centrifugation at 14,000 rpm for 10 min in a microcentrifuge at 4°C, the supernatant was mixed diluted 1:1 in Laemmli sample buffer and then boiled for 5 min at 95°C. Protein concentration was determined with the use of the bicinchoninic acid assay (Pierce, Rockford, IL). Samples were resolved by SDS-PAGE and then transferred to a nitrocellulose membrane. The membrane was blocked overnight at 4°C in 5% dry nonfat milk in TBST (0.05% Tween 20 in Tris-buffered saline) and then incubated for 1 h with 1 μg/ml antibodies directed against palladin (clone 7C6 and 1E6) or actin (Chemicon), diluted in TBST. Membranes were rinsed four times for 10 min each with TBST, then incubated with a goat anti-mouse horseradish peroxidase-conjugated antibody (Jackson ImmunoResearch, West Grove, PA), and again washed four times for 10 min each with TBST. Finally, the blots were developed on x-ray film (Kodak, Rochester, NY) with the use of ECL Western blotting detection reagent (Amersham, Arlington Heights, IL).
Transfections
The palladin antisense construct was characterized and described previously (Parast and Otey, 2000). Briefly, a partial mouse cDNA coding for palladin (GenBank accession number AF205078) was cloned in the antisense orientation into the adenovirus shuttle vectors pAdlox or pAdtrack and also in the sense orientation for use as a control. These constructs were transiently transfected into cultured cells. B35 neuroblastoma cells (50% of confluency) or CAD cells were transferred to serum-free Opti-MEM (Life Technologies) and transfected with indicated plasmid DNA (1 μg) for 8 h at 37°C with the use of Lipofectamine PLUS reagent (Life Technologies). DMEM containing (10%) FBS was added, and cells were incubated for an additional 16 h. Cells were then detached by trituration and plated on poly-d-lysine–coated glass coverslips. Cells were grown for 1 d in DMEM/10% FBS, and then (for the B35 cells) the medium was replaced with DMEM containing 0.5% FBS and 1 mM dibutyryl cAMP (Sigma) to induce neuronal differentiation. Cells were incubated for a further 24–72 h.
Primary cortical neurons in culture were transfected 3 h after they were plated with the use of Lipofectamine PLUS reagent as described below or by electroporation with a stimulator pulse (model S48, Grass Instruments, Quincy, MA), in 25 μl of a solution containing 157.7 mM NaCl, 3.4 mM KCl, 1.0 mM Mg2Cl, 10.0 mM glucose, and 5.0 mM HEPES, pH 7.4, with the indicated plasmid (0.5 μg/μl). Three voltage pulses of 140 V/cm, lasting 60 ms and opposite polarity were given at 20-s intervals. After electroporation, this buffer was removed, and cells were placed in culture in complete medium and then kept in culture for 4 d. Finally, cells were fixed with 4% paraformaldehyde in PBS for 15 min, washed three times with PBS, and visualized in a microscope (Zeiss, Oberkochen, Germany). The following plasmids were used: green fluorescence protein (GFP) plasmid alone (Clontech, Palo Alto, CA), and GFP along with the empty vector, the sense construct or the antisense construct.
RESULTS
Palladin Is Expressed in the Embryonic Brain
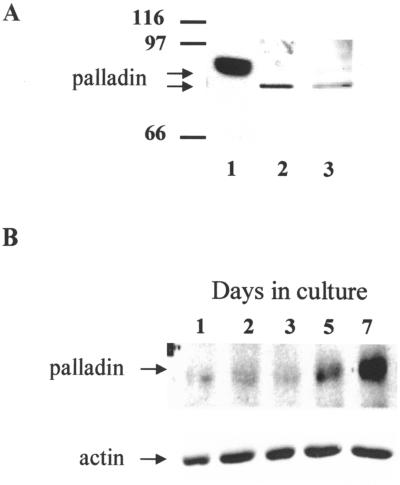
Previous results have shown that the cytoskeleton-associated protein palladin exists as multiple isoforms. The most widely expressed form migrates with an apparent molecular mass of 90–92 kDa by SDS-PAGE, and larger forms of ∼140 and ∼200 kDa have been detected in certain tissue and cell types. In addition to this tissue-specific expression pattern, palladin expression also appears to be under developmental control. Although the 90–92 kDa isoform of palladin has been shown to be ubiquitous in embryonic mouse tissues, it is dramatically down-regulated in many adult tissues (Parast and Otey, 2000). To determine whether palladin is expressed in the developing rat brain, we performed Western blot analysis on brains extracted from day E18 rat embryos. Because cultured fibroblasts have been shown to express predominantly the most ubiquitous form of palladin, lysates of these cells were run on the same blot for size comparison (lane 1). The immunoblot results are shown in Figure 1A and demonstrate that anti-palladin antibodies detect a major band of ≈85 kDa in rat embryo brains (lane 2), suggesting that palladin exists as a tissue-specific size variant in the developing brain.
Figure 1.
Palladin is expressed in brain, and its expression is regulated during neuronal development. (A) Western blot of palladin in lysate from chick embryo fibroblasts (lane 1), embryonic rat brain homogenized in RIPA buffer (lane 2), and cell lysate from 7-DIV neurons scraped in RIPA buffer (lane 3). Note that palladin in both brain and cultured neurons migrates at a lower position by SDS-PAGE than fibroblast palladin. (B) Western blot of palladin in primary cortical neurons maintained in culture for 1, 2, 3, 5, and 7 d and then scraped in RIPA lysis buffer. Total protein (5 μg) was loaded from each time point.
Intact brain contains a complex mixture of cell types, including neurons and many different types of glia. To determine whether palladin is expressed in neurons, we made use of primary cultures enriched in brain cortical neurons. These cultures were analyzed by Western blot, as shown in Figure 1A. The 85-kDa form of palladin was detected in cultured neurons (lane 3), and its expression increased at later times in culture (Figure 1B). This result suggests that palladin may play a role in the maturation of neurons and their morphological differentiation.
Localization of Palladin in Cultured Cortical Neurons
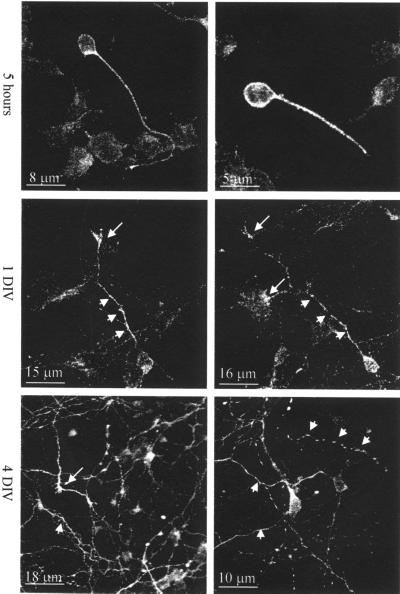
When neurons are grown in culture, they undergo a sequence of morphological changes that have been well documented (Dotti et al., 1988). To investigate palladin localization during neuronal differentiation in culture, immunofluorescence staining and confocal imaging were performed. After dissociation and within a few hours after plating, cortical neurons are round and exhibit lamellipodia. After 5 h in culture, the neurons start to extend minor processes. At this stage, palladin was highly concentrated in the cell body and along the longest process, the nascent axon (Figure 2, top). After 1 d in culture, the axons elongate and the dendrites start to develop, and both acquire large growth cones at their tips. In neurons at this stage, palladin was detected along the axons and in the growth cones and appeared to be preferentially localized to the axonal growth cone (Figure 2, middle). After >4 d in vitro (DIV), cortical neurons reach morphological maturation, with numerous dendrites, synaptic contacts, and axonal branches. At this point, palladin staining was concentrated in the growth cones and as bright dots along the processes (Figure 2, bottom).
Figure 2.
Immunofluorescence analysis of palladin during development of primary cortical neurons. Neurons cultured for 5 h, 1 d, and 4 d were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and then stained for palladin. After 5 h in culture, palladin is localized in the cell body and the first process. In 1-DIV neurons, palladin localized to the cell body and the nascent axon (short arrows) and axonal growth cone (long arrows). In 4-DIV neurons, palladin is concentrated in the processes (short arrows) and axonal growth cones (long arrows).
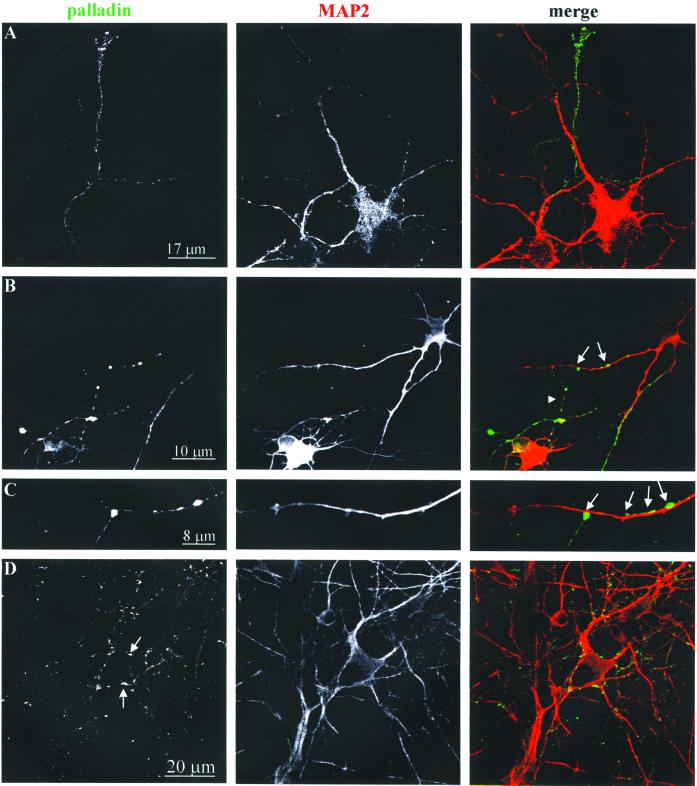
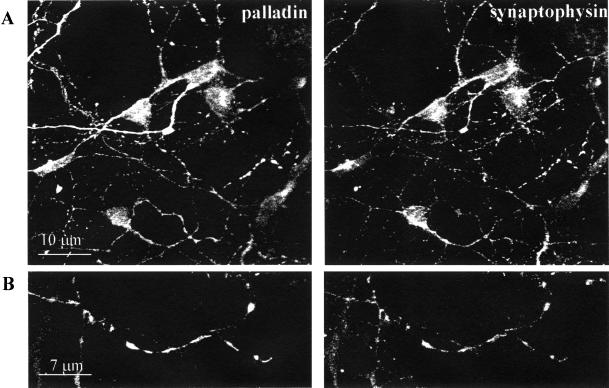
To further explore the subcellular distribution of palladin, 4-DIV and 7-DIV neurons were double-stained with antibodies to palladin and to MAP2, as a marker for the dendritic compartment. As shown in Figure 3, palladin largely failed to colocalize with MAP2 in both 4-DIV neurons (Figure 3, A–C) and 7-DIV neurons (Figure 3D). These results suggest that palladin is largely excluded from the dendrites. In fact, in the more mature 7-DIV neurons, palladin was strongly concentrated in puncta that resembled nascent synapses (arrows in Figure 3D). To determine whether palladin is concentrated in the axons and presynaptic compartment, neurons were double-labeled for palladin and synaptophysin. In 4-DIV neurons, the overall localization patterns of palladin and synaptophysin were very similar, although there were clear differences in the intensity of staining (Figure 4). Taken together, these double-label immunofluorescence data indicate that palladin is concentrated in the axons, and not the dendrites, of developing neurons.
Figure 3.
Palladin is localized in the axonal compartment. Primary cortical neurons at 4 DIV (A– C) or 7 DIV (D) were fixed, permeabilized, and then double-stained for palladin and MAP2 (a dendrite marker). The cells were imaged with a confocal microscope. Note the overall lack of overlap in the palladin- and MAP2-staining patterns. Palladin localized to the axon and in the presynaptic compartment of the synapses (arrow).
Figure 4.
Palladin and synaptophysin largely colocalize. Cultured cortical neurons (4 DIV) were fixed, permeabilized, and then double-stained for palladin and synaptophysin. Cells were analyzed by confocal imaging. Overall, the staining patterns were similar. Palladin was found in the axons, where it colocalized with synaptophysin in nascent synapses and varicosities.
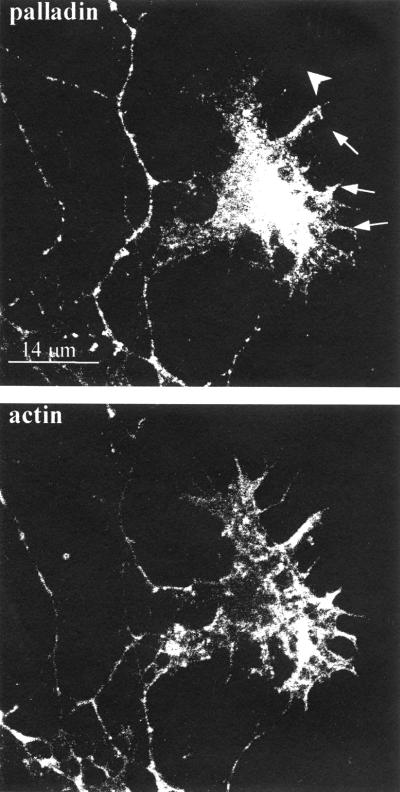
We also examined the distribution of palladin in axons at higher magnification. By 4 d in culture, palladin was detected strongly in the axonal growth cone (Boukhelifa and Otey, our unpublished results), as shown by double-labeling with a polyclonal antibody to GAP-43, an axonal growth cone marker (Goslin et al., 1988). Because palladin has been shown previously to be concentrated in actin-rich structures in nonneuronal cells, we asked whether palladin colocalizes with filamentous actin in the growth cone by counterstaining with phalloidin. As shown in Figure 5, there was a partial overlap in the staining patterns of palladin and F-actin. Palladin was concentrated in the central core of the growth cone and was detected in some, but not all, of the filopodia and fine actin-rich processes in the distal region of the growth cone.
Figure 5.
Palladin and F-actin localization in the axonal growth cone. Cultured cortical neurons (4 DIV) were fixed, permeabilized, and then double-stained for palladin and filamentous actin. Palladin is concentrated in the central region of the growth cone and partially colocalized with F-actin in some, but not all, of the filopodia. Arrows point to filopodia that stain positively for palladin; single arrowhead points to a filopodium that failed to stain for palladin.
Suppression of Palladin Expression in Neurons Inhibits Neurite Outgrowth
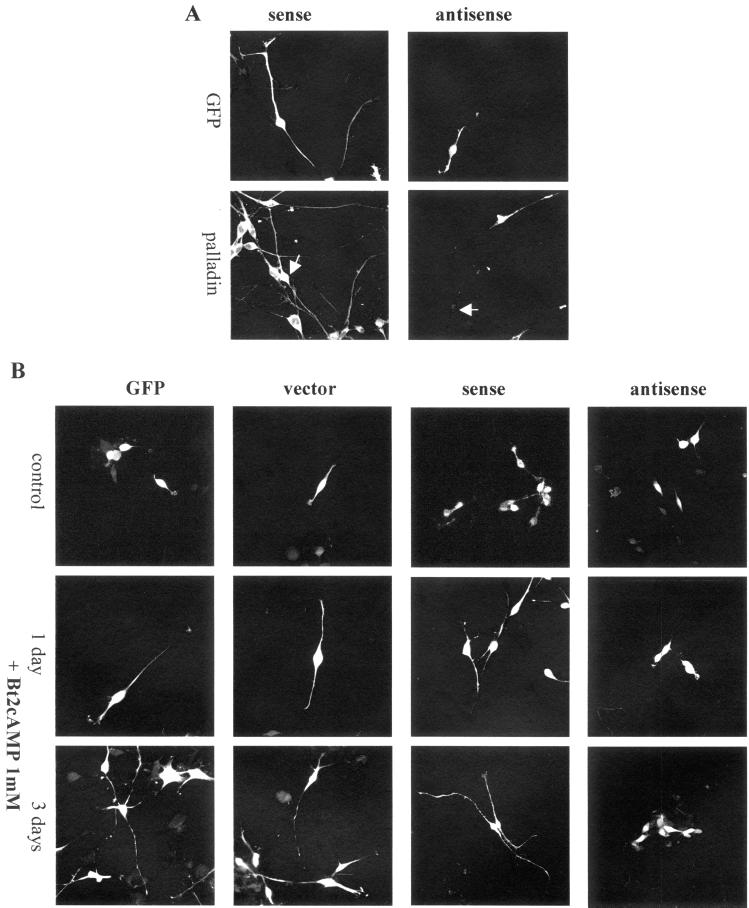
Palladin is present in neurons from the earliest stages of development and continues to be strongly expressed in the axon, growth cone and nascent synapses of the growing and mature neuron. This specific localization, taken together with previous results showing that palladin plays a role in cell morphology (Parast and Otey, 2000), suggests that palladin could be involved in axon elongation and neurite outgrowth. Therefore, we used an antisense approach to determine whether palladin is essential for neuronal morphogenesis. For these experiments, we first made use of the B35 rat neuroblastoma cell system. Western blot analysis confirmed that these cells express the same size variant of palladin that had been detected in cultured primary neurons and embryonic rat brain (Boukhelifa and Otey, our unpublished results). B35 cells were transfected with palladin antisense construct (cotransfected with GFP) or with the control plasmids: GFP alone, GFP cotransfected with empty vector, or GFP cotransfected with palladin sense construct. Immunofluorescence staining was performed to confirm a decrease in palladin immunoreactivity in the antisense-transfected, but not the control-transfected, B35 cells (Figure 6A). To monitor the effects of this loss of expression on neuronal maturation, a time-course experiment was performed. B35 cells are a useful experimental model for the study of neurite outgrowth because they undergo differentiation to a neuron-like phenotype in response to dibutyryl cAMP (Bt2cAMP). Accordingly, cultured B35 cells were transfected with palladin antisense or control plasmids and then treated with Bt2cAMP to stimulate neurite outgrowth. After 1 d in culture in the presence of Bt2cAMP, cells transfected with control plasmids began to extend neurites, whereas cells transfected with palladin antisense remained morphologically immature (Figure 6B). After 3 d with Bt2cAMP, control cells had formed numerous long processes, and the antisense transfected cells were essentially unchanged (Figure 6B, bottom row). In parallel experiments, an identical lack of neurite outgrowth was observed in a second neuron-like cell line, mouse CAD cells (Boukhelifa and Otey, our unpublished results). These results suggest that palladin plays an essential role in neuronal maturation and morphogenesis.
Figure 6.
Treatment of cultured B35 neuroblastoma cells with palladin antisense results in impaired neurite outgrowth. (A) B35 cells were transfected with GFP plus control (sense) construct or GFP plus palladin antisense construct and then fixed and stained for palladin. Arrows point to transfected cells. Although antisense transfection led to a reduction in palladin immunoreactivity (right), transfection with the control construct did not (left). (B) B35 cells were transfected with GFP alone, GFP plus empty vector, GFP plus sense construct, or GFP plus palladin antisense construct. The top row of images shows undifferentiated control cells, and the second and third rows show cells stimulated with 1 mM Bt2cAMP. Note that cells treated with palladin antisense failed to extend neurites in response to Bt2cAMP (far right).
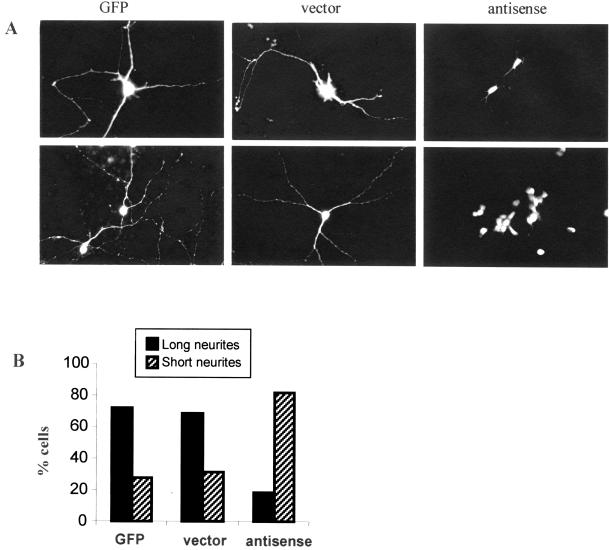
Although B35 cells are a valuable experimental model for studying neuronal development, they do not have all the properties of neurons. To examine the role of palladin in neurite extension in primary neurons, these experiments were repeated with the use of cultured cortical neurons that were transfected with palladin antisense or control constructs, with the use of two different methods: treatment with Lipofectamine PLUS (Figure 7A, top) or electroporation (Figure 7A, bottom). After transfection, the cells were maintained in culture for 4 d. The results with the use of both methods of transfection were essentially the same. In both cases, neurons treated with palladin antisense failed to achieve a normal morphology: the antisense-treated neurons exhibited dramatically shortened processes (Figure 7). In contrast, cultured neurons transfected with control plasmids established a normal morphology typical of the untreated neurons after 4 d in culture, with numerous long processes. To quantify these results, transfected cells were counted and divided into two groups: short neurites or long neurites. Cells with neurites greater than two times the length of perikaryon in the longitudinal plane were scored as “long.” The histogram in Figure 7B shows that 72% of the cells transfected with GFP alone and 69% of cells transfected with GFP plus empty vector were bearing long processes, whereas only 18% of the antisense-treated cells formed long neurites. These results suggest that the cytoskeletal protein palladin plays an essential role in neurite outgrowth and in the establishment of polarity during neuronal morphogenesis.
Figure 7.
Palladin antisense inhibits neurite extension from primary cortical neuron in culture. (A) GFP alone, GFP plus the empty vector, or GFP plus palladin antisense were transiently expressed in rat cortical primary culture cells with the use of two different methods: transfection with Lipofectamine PLUS (top) or electroporation (bottom). In both cases, cells were cultured for 4 d after transfection and then fixed and analyzed by confocal microscopy. Antisense-transfected cortical neurons exhibited a failure to extend neurites. (B) Quantification of the results shown above. Cells with neurites of greater than two body lengths were scored as long neurite-bearing cells; the others were scored as short-neurite bearing. At least 40–100 cells for each treatment were counted. Results are expressed as the mean percentages of short or long neurite-bearing cells. Similar results were obtained with both methods of antisense transfection.
DISCUSSION
Palladin was recently described as a novel protein with a critical role in maintaining the integrity of the organized cytoskeleton in cultured fibroblasts. It was demonstrated that suppression of palladin expression by antisense transfection results in a loss of stress fibers in fibroblast and trophoblast cell lines (Parast and Otey, 2000). In organized tissues, palladin is widely expressed as a 90- to 92-kDa protein. In nonneuronal cells such as fibroblasts and epithelial cells, palladin is distributed in stress fibers and cell adhesions throughout the cell (Parast and Otey, 2000). In these cells, all actin-rich structures appear to contain palladin. Surprisingly, this was not the case in cultured neurons. Whereas filamentous actin is concentrated in both dendrites and axons (Smart and Halpain, 2000), we detected palladin only in the axon and its growth cone. The observation that palladin is an actin-associated protein with an asymmetric distribution in neurons suggests that it may contribute to establishing the polarized morphology of the neuron. The molecular pathways that create and maintain the polarized phenotype are not well understood; however, it is known that another cytoskeletal element, the microtubule network, is polarized in neurons, because axons and dendrites differ in the orientation of their microtubules (Baas et al., 1989; Mandell and Banker, 1996). One microtubule-associated protein, MAP2, becomes restricted to the soma and dendrites early in the maturation of the neuron in culture (Matus et al., 1986). Our results show that palladin is targeted to the axon from the earliest stages of neuronal polarization and remains concentrated in the axon and axonal growth cone even in mature, highly branched neurons. Recently, a study of palladin localization in the adult rat brain, with the use of immuno-electron microscopy, demonstrated that palladin is concentrated in presynaptic nerve terminals and is not detected in mature dendrites (Hwang et al., 2001). Together with the current results, this suggests that palladin is targeted to the axonal compartment early in the development of the neurons and persists in this polarized distribution even in the adult brain.
When palladin expression was attenuated in cultured primary neurons by transfection with an antisense construct, the cells failed to extend neurites: even after 4 d, they retained the same morphology as when they were first plated. Identical results were obtained in two different neuronal cell lines. On the surface, it appears surprising that the cells failed to extend both dendrites and axons, even though palladin is not detected in dendritic growth cones. However, results from multiple laboratories suggest that an inhibition of axonal outgrowth disturbs the establishment of cell polarity so that dendrites also fail to form (reviewed by Bradke and Dotti, 2000). Similar observations have been reported when the axonal proteins synapsin II and GAP-43 are inhibited. Synapsin II is a neuron-specific phosphoprotein that has a role in the regulation of neurotransmitter release and in the formation of nerve terminals, and it also binds F-actin (Chilcote et al., 1994). GAP-43 is an actin-associated protein that is specifically targeted to the axonal growth cone (Hens et al., 1993). Neurons depleted of synapsin II by treatment with antisense oligonucleotides fail to extend any processes and remain round (Ferreira et al., 1994); similarly, neurons treated with GAP-43 antisense oligonucleotides do not exhibit growth cone spreading or neurite branching (Aigner and Caroni, 1995). Also, a targeted disruption of GAP-43 in P19 embryonal carcinoma cells inhibits neuronal differentiation as well as acquisition of the morphological phenotype (Mani et al., 2000). Taken together with our current results obtained with the use of palladin antisense constructs, these observations suggest that disruption of endogenous levels of the critical proteins involved in assembling the neuronal actin cytoskeleton results in a loss of the polarized phenotype.
The precise mechanism by which palladin contributes to neurite outgrowth will be the subject of future investigations and is likely to involve actin polymerization. The axonal growth cone was recently shown to contain unstable actin that is subjected to cycles of polymerization and depolymerization, and this cycling appears to be required for axon elongation and growth cone motility (Bradke and Dotti, 1999). Palladin partially colocalizes with F-actin in axonal growth cones, but no conserved binding site for F-actin has been found in palladin's sequence. Instead, palladin's sequence contains multiple conserved binding sites for proteins that regulate actin polymerization, including Mena and profilin (Parast and Otey, 2000). Both of these proteins have been shown to play important roles in axon outgrowth and neurite extension (Wills et al., 1999; Banzai et al., 2000; Goldberg et al., 2000). Our future efforts will focus on the possibility that palladin may contribute to cytoskeletal plasticity in the axonal growth cone by forming complexes with Mena/VASP proteins and/or profilin and regulating their interactions with actin monomers and polymers.
ACKNOWLEDGMENTS
The authors thank Kris Phend and Alda Fernandes for skilled technical assistance, Adam Hantman for helpful suggestions, Kathryn Akong for contributing to the early phases of the project, Drs. Shelley Halpain, Patricia Maness, and Gerry Oxford for gifts of antibodies and cell lines, Dr. Aldo Rustioni for critical reading of the manuscript and helpful discussions, Drs. William Snider and Annette Markus for assistance with electroporation, and Dr. Michael Chua for aid with confocal imaging. This work was supported by National Institutes of Health grants NS16264 and NS12440 to A. Rustioni and GM50974 to C. Otey.
REFERENCES
- Aigner L, Caroni P. Absence of persistent spreading, branching, and adhesion in GAP-43-depleted growth cones. J Cell Biol. 1995;128:647–660. doi: 10.1083/jcb.128.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Black MM, Banker GA. Changes in microtubule polarity orientation during the development of hippocampal neurons in culture. J Cell Biol. 1989;109:3085–3094. doi: 10.1083/jcb.109.6.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banzai Y, Miki H, Yamaguchi H, Takenawa T. Essential role of neural Wiskott-Aldrich syndrome protein in neurite extension in PC12 cells and rat hippocampal primary culture cells. J Biol Chem. 2000;275:11987–11992. doi: 10.1074/jbc.275.16.11987. [DOI] [PubMed] [Google Scholar]
- Bito H, Furuyashiki T, Ishihara H, Shibasaki Y, Ohashi K, Mizuno K, Maekawa M, Ishizaki T, Narumiya S. A critical role for a Rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron. 2000;26:431–441. doi: 10.1016/s0896-6273(00)81175-7. [DOI] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–1934. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr Opin Neurobiol. 2000;10:574–581. doi: 10.1016/s0959-4388(00)00124-0. [DOI] [PubMed] [Google Scholar]
- Brown MD, Cornejo BJ, Kuhn TB, Bamburg JR. Cdc42 stimulates neurite outgrowth and formation of growth cone filopodia and lamellipodia. J Neurobiol. 2000;43:352–364. doi: 10.1002/1097-4695(20000615)43:4<352::aid-neu4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Chilcote TJ, Siow YL, Schaeffer E, Greengard P, Thiel GJ. Synapsin IIa bundles actin filaments. Neurochemistry. 1994;63:1568–1571. doi: 10.1046/j.1471-4159.1994.63041568.x. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Kosik KS, Greengard P, Han HQ. Aberrant neurites and synaptic vesicle protein deficiency in synapsin II-depleted neurons. Science. 1994;264:977–979. doi: 10.1126/science.8178158. [DOI] [PubMed] [Google Scholar]
- Forscher P, Lin CH, Thompson C. Novel form of growth cone motility involving site-directed actin filament assembly. Nature. 1992;357:515–518. doi: 10.1038/357515a0. [DOI] [PubMed] [Google Scholar]
- Goldberg DJ, Foley MS, Tang D, Grabham PW. Recruitment of the Arp2/3 complex and Mena for the stimulation of actin polymerization in growth cones by nerve growth factor. J Neurosci Res. 2000;60:458–467. doi: 10.1002/(SICI)1097-4547(20000515)60:4<458::AID-JNR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Goodhill GJ. Mathematical guidance for axons. Trends Neurosci. 1998;21:226–231. doi: 10.1016/s0166-2236(97)01203-4. [DOI] [PubMed] [Google Scholar]
- Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;72:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Goslin K, Schreyer DJ, Skene JH, Banker G. Development of neuronal polarity: GAP-43 distinguishes axonal from dendritic growth cones. Nature. 1988;336:672–674. doi: 10.1038/336672a0. [DOI] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hens JJ, Benfenati F, Nielander HB, Valtorta F, Gispen WH, De Graan PN. B-50/GAP-43 binds to actin filaments without affecting actin polymerization and filament organization. J Neurochem. 1993;61:1530–1533. doi: 10.1111/j.1471-4159.1993.tb13649.x. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Pagliardini S, Boukhelifa M, Parast MM, Otey CA, Rustioni A, Valtschanoff JG. Palladin is preferentially expressed in excitatory terminals in the rat central nervous system. J Comp Neurol. 2001;436:211–224. [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Hensch TK, Ackerman L, Barbel S, Jan LY, Jan YN. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature. 1996;379:837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- Luo L, Jan LY, Jan YN. Rho family GTP-binding proteins in growth cone signaling. Curr Opin Neurobiol. 1997;7:81–86. doi: 10.1016/s0959-4388(97)80124-9. [DOI] [PubMed] [Google Scholar]
- Mandell JW, Banker GA. A spatial gradient of tau protein phosphorylation in nascent axons. J Neurosci. 1996;16:5727–5740. doi: 10.1523/JNEUROSCI.16-18-05727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S, Schaefer J, Meiri KF. Targeted disruption of GAP-43 in P19 embryonal carcinoma cells inhibits neuronal differentiation, as well as acquisition of the morphological phenotype. Brain Res. 2000;853:384–395. doi: 10.1016/s0006-8993(99)02042-9. [DOI] [PubMed] [Google Scholar]
- Marsh L, Letourneau PC. Growth of neurites without filopodial or lamellipodial activity in the presence of cytochalasin B. J Cell Biol. 1984;99:2041–2047. doi: 10.1083/jcb.99.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A, Bernhardt R, Bodmer R, Alaimo D. Microtubule-associated protein 2 and tubulin are differently distributed in the dendrites of developing neurons. Neuroscience. 1986;17:371–389. doi: 10.1016/0306-4522(86)90253-8. [DOI] [PubMed] [Google Scholar]
- Parast MM, Otey CA. Characterization of palladin, a novel protein localized to stress fibers and cell adhesions. J Cell Biol. 2000;150:643–656. doi: 10.1083/jcb.150.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Wang JKT, McMillian M, Chikaraishi DM. Characterization of a CNS cell line, CAD, in which morphological differentiation is initiated by serum deprivation. J Neurosci. 1997;17:1217–1225. doi: 10.1523/JNEUROSCI.17-04-01217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudin A, Lehmann M, Girault J, McKerracher L. Organization of point contacts in neuronal growth cones. J Neurosci Res. 1999;55:458–471. doi: 10.1002/(SICI)1097-4547(19990215)55:4<458::AID-JNR6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Schubert D, Heinemann S, Carlisle W, Tarikas H, Kimes B, Patrick J, Steinback JH, Culp W, Brandt BL. Clonal cell lines from the rat central nervous system. Nature. 1974;249:224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- Smart FM, Halpain S. Regulation of dendritic spine stability. Hippocampus. 2000;10:542–554. doi: 10.1002/1098-1063(2000)10:5<542::AID-HIPO4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Suter DM, Forscher P. An emerging link between cytoskeletal dynamics and cell adhesion molecules in growth cone guidance. Curr Opin Neurobiol. 1998;8:106–116. doi: 10.1016/s0959-4388(98)80014-7. [DOI] [PubMed] [Google Scholar]
- Symons M. Rho family GTPases: the cytoskeleton and beyond. Trends Biochem Sci. 1996;21:178–181. [PubMed] [Google Scholar]
- Tanaka E, Sabry J. Making the connection: cytoskeletal rearrangements during growth cone guidance. Cell. 1995;83:171–176. doi: 10.1016/0092-8674(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Threadgill R, Bobb K, Ghosh A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron. 1997;19:625–634. doi: 10.1016/s0896-6273(00)80376-1. [DOI] [PubMed] [Google Scholar]
- Wills Z, Marr L, Zinn K, Goodman CS, Van Vector D. Profilin and the Abl tyrosine kinase are required for motor axon outgrowth in the Drosophila embryo. Neuron. 1999;22:291–299. doi: 10.1016/s0896-6273(00)81090-9. [DOI] [PubMed] [Google Scholar]
- Zipkin ID, Kindt RM, Kenyon CJ. Role of a new Rho family member in cell migration and axon guidance in C. elegans. Cell. 1997;90:883–894. doi: 10.1016/s0092-8674(00)80353-0. [DOI] [PubMed] [Google Scholar]