Abstract
Recent large scale genomic studies from the Clinical Lung Cancer Genome Project have identified different driver gene mutations in the subtypes of non-small cell lung carcinoma (NSCLC). These findings not only lead to remarkable progress in targeted therapies for lung cancer patients, but also provide fundamental knowledge for the subclassification of NSCLC. More recently, the advancement and clinical application of immunotherapy have reinforced the need for the accurate subclassification of NSCLC. In 2015, the World Health Organization (WHO) and the International Association for the Study of Lung Cancer (IASLC) updated their guidelines for the subclassification of lung cancers. These guidelines emphasize: (1) the subclassification of NSCLC, (2) the critical role of molecular characterization of tumors for targeted therapy, (3) the unique terminology for subclassifying NSCLC using small biopsy specimens, and (4) the utility of IHC biomarkers in the accurate diagnosis and subclassification of lung cancer. The guidelines have significant prognostic impact on oncologic practice and patient care. In this review, we summarize the current WHO guidelines for the classification of lung cancer, discuss advancements of targeted therapy and immunotherapy, and address the utility and limitation of immunomarkers in the subclassification of NSCLC, as well as the prospective future of the field.
Keywords: non-small cell lung carcinoma (NSCLC), EGFR mutations, fine needle aspiration (FNA) biopsy, targeted therapy, PD-L1 immunotherapy
Background
Lung cancer is a heterogeneous group of tumors, consisting of more than 50 histomorphological subtypes [1–3]. Over the past few decades, non-small cell lung carcinoma (NSCLC) and small cell lung carcinoma (SCLC) were the most frequently used diagnostic terms for lung cancer, mainly due to limited treatment options, which usually did not require further morphological subclassification. NSCLC comprises approximately 80–85% of all lung cancers [1, 2] with adenocarcinoma (ADC, approximately 40–50% of cases) and squamous cell carcinoma (SqCC, approximately 20–30% of cases) comprising the predominant histological subtypes of NSCLC [1–3]. Clinically, only a small portion of NSCLC patients are diagnosed at an early stage (stage I or II), when the tumor can be treated by surgical resection [2]. Over 60% of lung cancer patients present with locally advanced or metastatic disease (stage III or IV) at the time of diagnosis, at which point surgical resection may not be an option. Until recently, conventional chemotherapy and radiation therapy were the main stays of treatment for lung cancer patients.
Within the last decade, the discovery of EGFR (epidermal growth factor receptor) gene alterations and EML4-ALK (echinoderm microtubule-associated protein-like 4 and anaplastic lymphoma kinase) gene rearrangement in lung ADCs has led to the development of targeted therapy using TKIs (tyrosine kinase inhibitors) and crizotinib, respectively [4–7]. These targeted therapies have become the standard of care, and have led to improved clinical outcomes in a subset of lung cancer patients, whose tumors harbor EGFR or EML4-ALK alterations. The development of targeted therapies has advanced the therapeutic strategy from conventional chemo- and radiation-based therapy to genetic alteration-guided targeted therapy [8, 9]. Recently, large scale genomic studies from the Clinical Lung Cancer Genome Project have identified different driver gene alterations in certain subtypes of NSCLC. In ADC, EGFR activating mutations and EML4-ALK rearrangements are detected in approximately 25% of tumors. In addition, loss-of-function mutations in LKB1/STK11, NF1, CDKN2A, SMARCA4 and KEAP1, mutations in KRAS, amplification of MET, and rearrangements of ROS1 and RET have also been identified [10]. In contrast, SqCCs rarely harbor EGFR mutations or EML4-ALK rearrangements. Instead, SqCCs have alterations inRTKs , DDR2 and FGGRs, as well as inactivating mutations inCDKN2A, PTEN, KEAP1, MLL2, HLA-A, NFE2L2, NOTCH1 and RB1 [11]. Based on genomic studies and analyses of molecular pathways in different subtypes of lung cancer, the development of targeted therapies and clinical trials have increased rapidly (Table 1).
Table 1.
Major Somatic Alterations and Targeted therapies in Lung Cancer (adopted from reference #20)
| Gene | PATHWAY | ABERRATIONS | %ADC | %SqCC | %SCLC | Drugs, approved and investigational |
|---|---|---|---|---|---|---|
| EGFR | RTK | Mutation Amplification |
20–30% >20% |
rare 7% |
Erlotinib, Gefitinib, Afatinib (approved), Dacomitinib, Cetuximab, Necitumumab, Neratinib | |
| ALK | Fusion with EML4 and other rare partners | 3–13% | Crizotinib (approved), X-396, Ceritinib (LDK378); Ganetispib, AUY922, AT13387 | |||
| MET | Mutation, amplification post-treatment with EGFR inhibitor | 5% 20% |
1. TKI: Tivantinib, Cabozantinib, Crizotinib 2. Monoclonal Ab: Onartuzumab, AMG102, Ficlatuzumab |
|||
| ERBB2 | Mutation, Amplification | 2–4% 5–10% |
Trastuzumab, Afatinib, Neratinib, MGHA22 | |||
| ERBB3 | Mutation | 2% | MM-121 | |||
| ROS | Mutation | 1.5 % | Crizotinib, AT13387 (HSP90) | |||
| RET | Translocation with KIF5B and other genes | 1–2% | Vandetanib, Cabozantinib ? | |||
| FGFR1 | Amplification | 1–3% | 22% | 6% | AZD4547, BGJ 398, BIBF 1120/nintedanib, dovitinib, HGS1036 | |
| DDR2 | Mutation | 3.8% | Dasatinib | |||
| IGF1R | Overexpression | ND | ND | 95% | AXL1717, OSI-906 | |
| KRAS | RAS | Mutation | 30% | 5% | Selumetinib, Trametinib, MEK162, and BKM120, everolimus,sirolimus AUY922, BYL719,Reolysin MEK162 |
|
| NF1 | 8–10% | 11% | ||||
| HRAS | 3% | |||||
| NRAS | <1% | <1% | ||||
| RASA1 | 4% | |||||
| BRAF | RAF | Mutations | 6% | 4% | Vemurafenib (only for V600E) | |
| PIK3CA | PI3K | Mutation | rare | 16% | BKM120, PX-866, GDC-0941 | |
| PTEN | Deletion | rare | 8% | BKM120, PX-866, GDC-0941 (PI3K), MK-2206 (AKT) | ||
| AKT1,2,3 | rare | 16%(AKT3) 20% all |
MK-2206 | |||
| TSC1,2 | 6% | Everolimus, sirolimus, temsirolimus | ||||
| LKB1 | LKB1/AMPK | Mutation | 15–30% | 2% | Biguanide compounds | |
| MDM2 | TP53 | Amplification | 20% | Inhibitors of TP53 – MDM2 interaction | ||
| CDKNA2/p16INK4 | RB1/ CDK | Deletions, silencing, mutation | >20% | 70% | CDK inhibitors PD0332991, BAY1000394 | |
| MYC MYCN MYCL |
Transcriptional regulators | Amplification | 31% | rare | 16% | Aurora kinase inhibitors, BH3 mimetics |
Although targeted therapy has improved clinical outcomes in certain subsets of lung cancer patients, the 5-year survival rate is still less than 20% [1–3]. More recently, immunotherapy has been developed and increasingly used in lung cancer patients. For example, PD-L1 (programmed cell death ligand 1) is a key immunoregulatory molecule which, upon interacting with its receptor, PD-1, inhibits the CD8 cytotoxic immune response and the resultant antitumor immune response [12–14]. In a variety of tumors, including lung cancer, PD-L1 is over expressed on tumor cells and plays an important role in the modulation of the immune response to both the tumor and host cells. Currently, there are several clinical trials involving FDA-approved immune checkpoint inhibitors, which attack PD-L1 expressing tumor cells by blocking the PD-L1/PD-1 signaling pathway. The use of these agents not only requires accurate subclassification of tumor types, but also requires specific testing to assess the level of PD-L1 expression on tumor cells by IHC (immunohistochemical) stains [15, 16]. Different anti-PD-L1/PD-1 agents require different detection methods and assay conditions (Table 2). For example, pembrolizumab requires the detection of PD-L1 expression on tumor cells by using the PD-L1 IHC 22C3 assay kit, whereas, atezolizumab requires the detection of PD-L1 expression on tumor cells by using the PD-L1 IHC 28–8 assay kit. The interpretation of results and scoring system are also different for each antibody and assay. For example, the reporting for the assay with the 28–8 antibody uses a 3 tiered scoring system for positive staining (1%, 5%, and 10%), while the assay with the 22C3 antibody uses a different 3 tiered scoring system (<1%, 1%–49%, and ≥50%)[17]. Therefore, it is necessary to develop a novel and universal biomarker to assess PD-L1 expression, rather than using individual markers and/or individual scoring system s.
Table 2.
PD-L1 immunotherapies in lung cancers and PD-L1 assay conditions.
| Checkpoint inhibitors | Target | Antibody Clone | Assay conditions | Detection System |
|---|---|---|---|---|
| Atezolizumab1 [Tecentriq, Genentech/Roche, South San Francisco, CA] | PD-L1 | SP142 [Ventana] Rabbit monoclonal | Ventana SP142 assay | Ventana Benchmark ULTRA platform |
| Durvalumab2 [AstraZeneca, Wilmington, DE] | PD-L1 | SP263 [Ventana] Rabbit monoclonal | Ventana SP263 assay | Ventana Benchmark ULTRA platform |
| Nivolumab1 [Opdivo, Bristol- Muers Squibb, Lawrenceville, NJ] | PD-1 | 28–8 [Dako] Rabbit monoclonal | Dako/Agilent 28–8 assay | Dako Link 48 platform |
| Pembrolizumab1 [Keytruda, Merck&Co Inc, Kenilworth, NJ] | PD-1 | 22C3 [Dako] Mouse monoclonal | Dako/Agilent 22c3 assay | Dako Link 48 platform |
| N/A | N/A | E1L3N3 [Cell Signaling] Rabbit monoclonal | PD- 1(E1L3N)XP from Cell Signaling | Leica Bond platform |
FDA approved therapy
in clinical trials
in investigation
The genetic characteristics of tumors provide the foundation for the development of personalized medicine in lung cancer patients. The current advances in targeted therapy and immunotherapy require accurate morphological subclassification of tumors. However, lung cancer, particularly NSCLC, consists of a heterogeneous group of tumors. This morphological heterogeneity may present diagnostic challenges, particularly when using small biopsy specimens. Clinically, most NSCLCs can be subclassified by evaluating histomorphological features using H&E (hematoxylin and eosin) stained sections. However, IHC markers are necessary for the accurate subclassification of lung cancers in daily practice. In this review, we will discuss the updated WHO (World Health Organization) guidelines for the classification of lung cancer, the utility and limitations of current IHC markers, and the prospective future of the discovery of potential IHC markers in lung cancer.
Current WHO guidelines for the subclassification of lung cancer
The WHO Classification of Lung Cancer provides diagnostic terminology and classification criteria for lung tumors [2, 3]. It also serves as a foundation for appropriate clinical management and the development of clinical trials. Based on molecular advances and the clinical demand for the accurate subclassification of lung cancer, the WHO and the IASLC (International Association for the Study of Lung Cancer) have updated their guidelines in 2015 [2–3]. These new guidelines have had significant impact on daily practice and patient care.
Major updates in the current guidelines include: (1) the molecular and immunohistochemical characterization of tumors and the accurate subclassification of lung cancer for personalized medicine, (2) the reclassification of “bronchioloalveolar carcinoma” into several different subtypes, including adenocarcinoma in situ, minimally invasive ADC, and ADC with lepidic growth pattern, (3) the subclassification of SqCC into basaloid, keratinizing, and non-keratinizing types, and the requirement of immunohistochemical evidence of squamous differentiation in non-keratinizing SqCC, (4) the change in diagnostic terminology for several tumors, including “sclerosing hemangioma” to “sclerosing pneumocytoma”, and “hamartoma” to “pulmonary hamartoma”, (5) the addition of diagnostic entities based on molecular studies, including “NUT carcinoma”, “pulmonary myxoid sarcoma with an EWSR1–CREB1 translocation”, “myoepithelioma and myoepithelial carcinomas with EWSR1 gene rearrangements”, “epithelioid hemangioendotheliomas with WWTR1–CAMTA1 fusions”, and “PEComatous tumors” to include “benign and malignant PEComa” and “lymphangioleiomyomatosis”.
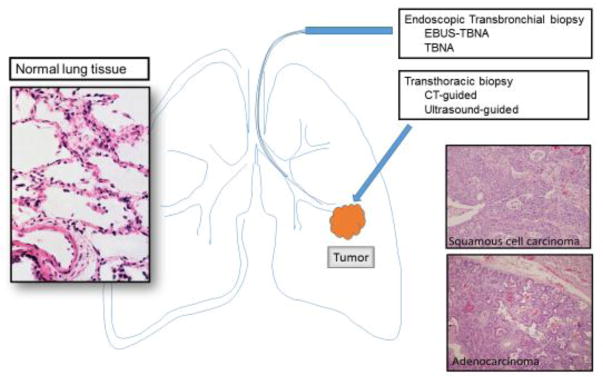
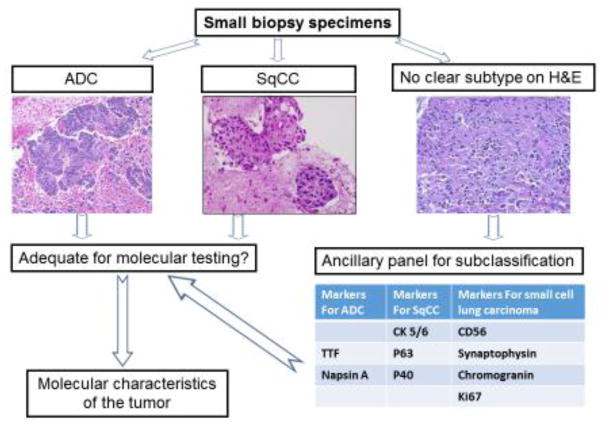
Clinically, the majority of lung cancer patients present with locally advanced disease or with distant metastasis at the time of diagnosis, and surgical resection of the tumor for morphological diagnosis may not be an option. Several minimally invasive approaches can be used to obtain tumor tissue, such as endobronchial ultrasound-guided transbronchial fine needle aspiration (EBUS-TBNA), and computerized tomography (CT) or ultrasound guided transthoracic fine needle aspiration (Figure 1) [18, 19]. However, these minimally invasive approaches often yield only small biopsy specimens, which can make accurate diagnosis challenging and often requires additional ancillary studies [2–3]. Recognizing the difficulty of evaluating small biopsy specimens in comparison to resected tumor tissue, the WHO guidelines have also recommended the following: (1) a separate set of criteria for the subclassification of small biopsy specimens from those for resected tumor tissue, (2) the requirement for IHC markers for the subclassification of lung cancer, and (3) the use of the diagnostic term NSCLC in certain biopsy cases. Certain tumors can only be classified using resected tumor tissue, rather than using small biopsy specimens, such as “large cell carcinoma”, which lacks any distinct morphologic or immunohistochemical differentiation, and can only be assessed when the tumor is resected. Another example is lung ADC with solid growth pattern, which can be confused with non-keratinizing SqCC on small biopsy specimens. In certain small biopsy specimens, the subclassification of the tumor, in addition to the morphologic evaluation and immunohistochemical characteristics of the tumor, may be still difficult. For these reasons, the guidelines emphasize the critical role of IHC markers in the accurate subclassification of lung tumors in addition to morphological evaluation, particularly in small biopsy specimens (Figure 2).
Figure 1.
Collection of Tumor tissue by FNA (fine needle aspiration) biopsies, and histomorphology of normal lung parenchyma, ADC and SqCC. Tumor tissue may be collected by endoscopic techniques, such as transbronchial FNA with or without ultrasound guidance, or transthoracic techniques with CT or ultrasound guidance.
Figure 2.
Subclassification of small biopsy specimens. The diagnosis of ADC and SqCC can be made by finding evidence of glandular differentiation and cytokeration formation, respectively. However, the subclassification of a poorly differentiated carcinoma without obvious glandular or cytokeratin formation may require the use of a panel of IHC markers. The molecular characteristics of the tumor play a critical role for targeted therapy.
The Utility and Limitations of current IHC markers in the accurate subclassification of NSCLC
Although the majority of NSCLC cases can be subclassified based on histomorphological examination using hematoxylin and eosin (H&E) stained slides, sometimes the accurate subclassification of a tumor may be difficult due to a variety of reasons. For example, resected tumor tissue may have extensive necrosis and lack viable tumor cells, or the biopsy specimen may have scant tumor cells and lack the characteristic architecture of the tumor. Furthermore, artifacts during specimen preparation may also pose challenges. The degree of tumor cell differentiation is another factor which may potentially affect accurate diagnosis [20, 21]. For instance, poorly differentiated carcinomas lack the specific phenotypes and morphological features of either glandular or squamous differentiation (Figure 2). In these cases, IHC study of the tumor is necessary for diagnosis and determination of tumor subtype.
In daily clinical practice, the most commonly used IHC markers for the classification of NSLCLs are TTF-1 (thyroid transcription factor-1), Napsin A, CK5/6, P63 and P40 [22–24]. These markers have different sensitivities and specificities. Our studies and those of others have demonstrated that these markers have overall sensitivities and specificities ranging from 70% to 100% for the identification of ADCs, and from 60% to 100% for the identification of SqCCs [22, 23]. However, there are still limitations and drawbacks when using these markers.
TTF-1 and Napsin A are expressed by pneumocytes and are most useful for the identification of glandular differentiation in ADC [20–24]. For many years, TTF-1 has been the predominant marker employed for the identification of a neoplasm of lung origin, demonstrating a sensitivity ranging from 75% to 80% for lung ADC [20–23]. TTF-1 is a member of the Nkx2 family of transcription factors, and is also expressed by other tumors and normal tissues, such as thyroid tissue and thyroid tumors, as well as neuroendocrine tumors (SCLC and carcinoid tumors) [20–23]. The expression of TTF-1 is also inversely correlated with the degree of tumor differentiation, i.e. poorly differentiated ADCs are less likely to express TTF-1 compared to well differentiated tumors [22]. Additionally, Napsin A has also been identified as a marker for the detection of lung ADC [24]. Napsin A is an aspartic proteinase involved in the maturation of the surfactant protein B in lung tissue [24]. It is abundantly expressed in the cytoplasm of normal type II pneumocytes and Clara cells, in addition to lung ADCs, however, it is also expressed by kidney proximal and convoluted tubule cells, and renal cell carcinomas [20, 21, 24]. Additionally, the expression of Napsin A has been shown to be potentially regulated by TTF-1 [24]. Studies using resected tumor tissue have shown that Napsin A has better specificity than TTF-1 for the determination of lung origin among well to moderately differentiated lung ADCs [21]. However, its expression is decreased among poorly differentiated lung ADCs [20, 21].
Both P40 and P63 are products of the P63 gene on chromosome 3q27–29, and are expressed by the basal or progenitor cell layer of bronchial epithelium[2–5]. The full-length protein TAp63 (containing the N-terminal transactivation domain) can be identified using the antibody 4A4 (P63), and the N-terminal-truncated protein isoform of TAp63 can be identified using the antibody P40 [26 ].Both P63 and P40 are used for identification of squamous differentiation. The sensitivity and specificity of P40 have been reported to be100% and 98–100%, respectively, in identifying SqCCs in surgically resected specimens [ 25, 26]. By using tumor tissue microarrays (TMAs), we have demonstrated that P40 and P63 have sensitivities and specificities of 80.95% and 90.0% (P40) and 93.5% and 80.0% (P63) in identifying lung SqCC, respectively[ 27].Our previous study was also consistent with these findings in that P40 had a higher specificity and lower sensitivity than that of P63[23, 27, 32] .
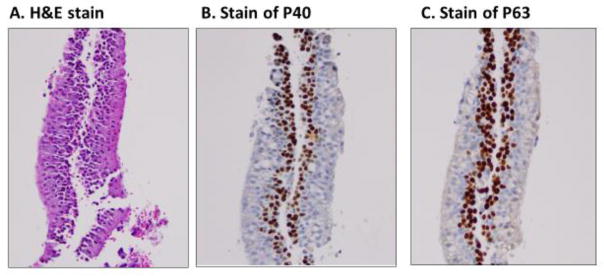
Cross-reaction with other tissue types and tumors is a limitation of current IHC markers [20–25]. For example, TTF-1 is immunoreactive with normal pulmonary alveolar macrophages, whereas, both P40 and P63 are immunoreactive with normal bronchial basal epithelial cells (Figure 3) [27]. This non-specific immunoreactivity of IHC markers with normal cells should not be confused with tumor cells. In addition, individual IHC markers require multiple sections of tumor tissue for the test to be performed. It is not uncommon for tumor tissue to be exhausted by IHC studies leaving little tissue for molecular analyses, particularly when using small biopsy specimens. Other limitations of IHC markers include: (1) the time-consuming nature of IHC testing, which can result in prolonged turnaround times, significantly affecting the patient’s clinical management, (2) the influence on the interpretation of IHC results by non-specific staining of non-neoplastic cells, such as Napsin A positivity in pulmonary alveolar macrophages, and P40 positivity in normal bronchial basal cells, and (3) the different staining patterns of different clones of the same antibody.
Figure 3.
Normal basal layer of bronchial cells is immunoreactive with P40 and P63. They should not be confused with squamous cell carcinoma. A, H&E stain of normal bronchial cells; B, immunostain of P40; and C, immunostain of P63.
To improve the efficiency and accuracy of subclassifying NSCLC, several recent studies have investigated the potential utility of combining several IHC markers into a single marker [28–31]. A dual marker combining TTF-1 and Napsin A demonstrated 74% sensitivity and 87% specificity [28], and 74% sensitivity and 88–96% specificity [29] in the identification of lung ADC using fine needle aspiration (FNA) material. A dual marker of P63 and CK5 demonstrated 100% sensitivity and 100% specificity in the identification of lung SqCC using FNA material [30]. Lung tumor tissue microarray (TMA) data using a dual marker of TTF-1 and P40 demonstrated 93% sensitivity and 92% specificity in diagnosing SqCC [31]. A marker combining desmoglein 3 and CK5 demonstrated 100% sensitivity and 100% specificity in diagnosing SqCC [31]. All of these studies demonstrate that dual or triple markers have similar or better sensitivities and specificities when compared to individual markers. Additionally, combined IHC markers also have the advantage of using minimal tumor tissue for immunohistochemical subclassification, thereby leaving more tissue available for molecular studies.
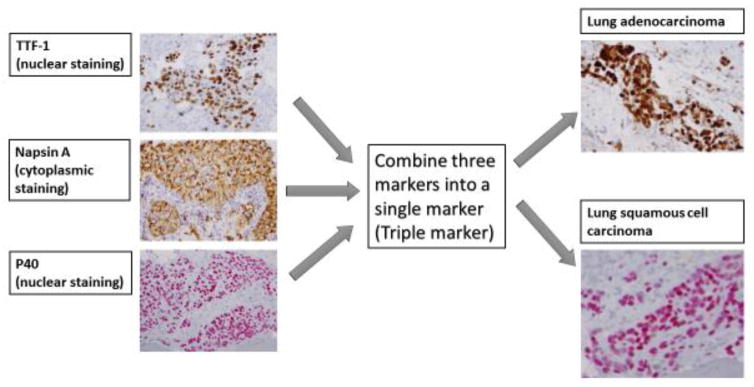
Recently, we tested a novel triple marker in our practice to subclassify NSCLC [32, 33] The triple marker consists of TTF-1, Napsin A and P40 (Figure 4), and has been developed on an automated staining instrument (BenchMark Ultra, Roche and Ventana Medical Systems, Tucson, AZ). In our study, the triple marker demonstrated a similar sensitivity and specificity when compared to individual IHC markers. The triple marker has a sensitivity of 86.0% and a specificity of 100% in identifying lung ADCs, and a sensitivity of 100% and a specificity of 97.1% in identifying lung SqCCs [32, 33]. Our approach has demonstrated that use of a combined IHC marker is a cost-effective method for subclassifying lung cancer in daily practice. Furthermore, the triple marker improves turnaround time and has the advantage of using minimal tumor tissue. It is now used routinely in our daily operation. The utility of the triple marker is summarized in Figure 5. However, the non-specific staining of normal pulmonary cells, such as alveolar macrophages and bronchial basal cells, remains a limitation of the triple marker. Therefore, the discovery of new markers is necessary to fulfill clinical demand for the accurate subclassification of NSCLC for the purposes of targeted therapy and personalized medicine.
Figure 4.
The utility of a triple marker. We have combined three individual markers (TTF-1,Napsin A and P40) into a single marker to subclassify NSCLC.
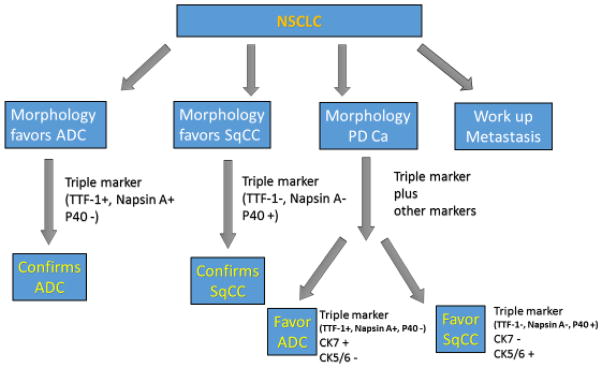
Figure 5.
The work flow for the differential diagnosis of NSCLC using the triple marker. Based on the histomorphological assessment of the specimen, different immunomarkers are performed.
Prospective future directions
Lung cancer development and progression are multistep processes [34]. They are characterized by aberrant genetic changes and protein expression, which subsequently lead to phenotypic transformation of cells and progression of the tumor [34–36]. This process involves complex intracellular signaling pathways and various cellular proteins [34]. In addition to the genetic characterization of lung cancer, it is also necessary to address the need for protein biomarkers for the accurate subclassification of lung cancer, as well as for monitoring disease progression and treatment response [34–36].
Over the past decade, numerous studies have been published related to discovery of candidate biomarkers in lung cancer. For example, a keyword search query in PubMed using “protein biomarkers in lung cancer” , yields more than twenty-thousand articles. Of this large number, more than thirteen-thousand articles are related to diagnostic biomarkers, and more than three-thousand articles are related to prognostic biomarkers. These studies represent tremendous effort in the discovery of potential biomarkers for the detection, classification and progression monitoring of lung cancer. Using advanced molecular technologies and proteomics, numerous studies have reported candidate biomarkers for lung cancer. These state-of-the-art technologies provide new platforms not only for the systematic study of intracellular proteins, but also for the characterization of the complex microenvironment of lung tissue. However, none of these biomarkers have been widely used in daily practice. Recently, several markers have been studied for the identification of ADC and SqCC. For example, it has been reported that SPATS2 has a sensitivity and specificity of 67% and 100% for the identification of SqCC, and ST6GALNAC1 has a sensitivity and specificity of 67% and 100% for the identification of ADC when using resected tumor tissue [37]. The utility of these candidate biomarkers still needs to be validated in well-designed, large scale studies.
In biomarker discovery and potential clinical application, it is important to use carefully selected clinical specimens in both the discovery process and subsequent validation phase. The potential success of biomarker discovery and application largely depends on the quality and availability of patient specimens. This requires a large number of carefully selected patient cohorts to determine the potential utility of the biomarker. Clinical validation of potential biomarkers must be conducted in a way to maximally avoid false positive and/or false negative results. For each candidate biomarker, robust and reproducible assays need to be developed and used in the validation phase.
When considering PD-L1 immunotherapy, three separate drug-specific assays are required for the clinical application of checkpoint inhibitors (Table 2). These FDA approved tests are either called companion (for pembrolizumab) or complementary (for atezolizumab and nivolumab) tests [15–17]. These tests use 3 different antibodies and 3 sets of assay conditions . The companion test for pembrolizumab uses the Dako/Agilent 22c3 assay, whereas, the two complementary tests for atezolizumab and nivolumab use the Ventana SP142 assay and the Dako/Agilent 28–8 assay, respectively. This practice has potential drawbacks. For example, the oncologist has to be aware of which test to order for a specific inhibitor. If no clinical information is provided, pathology personnel must spend time determining which test to perform. Furthermore, different assays require different scoring systems [15–17] which may cause problems in the interpretation of results. Therefore, a single test using a novel marker is needed to unify the detection of PD-L1 expression in tumor cells in order to improve patient care.
In summary, targeted therapies and immunotherapy have progressed rapidly, however, the overall progression-free survival rate of lung cancer patients is still suboptimal. The current WHO guidelines emphasize the importance of the accurate subclassification of NSCLC for personalized medicine. In order to improve clinical outcomes for lung cancer patients, biomarkers for the accurate subclassification of lung cancer and for monitoring response to targeted therapy, are urgently needed. Recent advances in molecular techniques have significantly improved the identification and validation of potential biomarkers in experimental settings. Although select candidate biomarkers have been studied and evaluated using clinical specimens, further improvement of the workflow and validation process in large scale cohorts remains necessary. Finally, the lack of validation of prognostic markers is also a concern in clinical practice, therefore, clinical specimens and patient cohorts should be selected carefully in both the discovery process and subsequent validation phase.
Acknowledgments
This work is partially supported by Drs. Ji and Li Family Cancer Research Foundation (QKL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017 doi: 10.3322/caac.21387. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer; 2015. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Brambilla E, Nicholson AG, et al. WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10(9):1243–60. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 4.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 5.Tsao M-S, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, Lorimer I, Zhang T, Liu N, Daneshmand M, Marrano P, da Cunha Santos G, Lagarde A, Richardson F, Seymour L, Whitehead M, Ding K, Pater J, Shepherd FA. Erlotinib in lung cancer — molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 6.Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Science. 2007;98:1817–1824. doi: 10.1111/j.1349-7006.2007.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H. Identification of the transforming EML4-ALK fusion gene in non- small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 8.Stella GM, Luisetti M, Pozzi E, Comoglio PM. Oncogenes in non-small-cell lung cancer: emerging connections and novel therapeutic dynamics. Lancet Respir Med. 2013;1:251–61. doi: 10.1016/S2213-2600(13)70009-2. [DOI] [PubMed] [Google Scholar]
- 9.Shtivelman E, Hensing T, Simon GR, Dennis PA, Otterson GA, Bueno R, Salgia R. Molecular pathways and therapeutic targets in lung cancer. Oncotarget. 2014 Mar 30;5(6):1392–433. doi: 10.18632/oncotarget.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–50. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519–25. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Facchinetti F, Marabelle A, Rossi G, Soria JC, Besse B, Tiseo M. Moving Immune Checkpoint Blockade in Thoracic Tumors beyond NSCLC. J Thorac Oncol. 2016;11:1819–1836. doi: 10.1016/j.jtho.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 13.Lastwika KJ, Wilson W, 3rd, Li QK, Norris J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, Liu NL, Gills JJ, Dennis PA. Control of PD-L1 expression by oncogenic activation of the AKT/mTOR pathway in non-small cell lung cancer. Cancer Res. 2016;76:227–238. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 14.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gniadek T, Li Q, Tully E, Chatterjee S, Nimmagadda S, Gabrielson E. Heterogeneous expression of PD-L1 in pulmonary squamous cell carcinoma and adenocarcinoma: implications for assessment by small biopsy. Mod Pathol. 2017 doi: 10.1038/modpathol.2016.213. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Rimm DL, Han G, Taube JM, Yi ES, Bridge JA, Flieder DB, Homer R, West WW, Wu H, Roden AC, Fujimoto J, Yu H, Anders R, Kowalewski A, Rivard C, Rehman J, Batenchuk C, Burns V, Hirsch FR, Wistuba II. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol. 2017 Mar 9; doi: 10.1001/jamaoncol.2017.0013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, Richardson W, Towne P, Hanks D, Vennapusa B, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12:208–222. doi: 10.1016/j.jtho.2016.11.2228. [DOI] [PubMed] [Google Scholar]
- 18.Feller-Kopman D, Yung RCW, Burroughs F, Li QK. Cytology of endobronchial ultrasound-guided transbronchial needle aspiration: a retrospective study with histology correlation. Cancer Cytopathol. 2009;117(6):482–490. doi: 10.1002/cncy.20049. [DOI] [PubMed] [Google Scholar]
- 19.Munoz M, Lechtzin N, Li QK, Yarmus L, Lee H, Feller-Kopman D. Bronchoscopy with endobronchial ultrasound guided transbronchial needle aspiration vs. transthoracic needle aspiration in lung cancer diagnosis and staging. J Thora Dis. 2017 doi: 10.21037/jtd.2017.07.26. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoll LM, Johnson MW, Gabrielson E, Askin F, Clark DP, Li QK. The utility of Napsin A in the identification of primary and metastatic lung adenocarcinoma among cytologically “poorly differentiated carcinoma”. Cancer Cytopathol. 2010;118:441–449. doi: 10.1002/cncy.20108. [DOI] [PubMed] [Google Scholar]
- 21.Bishop J, Sharma R, Illei P. Napsin A and Thyroid Transcription Factor -1 Expression in Carcinomas of the Lung, Breast, Pancreas, Colon, Kidney, Thyroid, and Malignant Mesothelioma. Human Pathol. 2010;41:20–25. doi: 10.1016/j.humpath.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Rekhtman N, Ang DC, Sima CS, Travis WD, Moreira AL. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod Pathol. 2011;24:1348–1359. doi: 10.1038/modpathol.2011.92. [DOI] [PubMed] [Google Scholar]
- 23.Gurda GT, Zhang L, Wang Y, et al. Utility of five commonly used immunohistochemical markers TTF-1, Napsin A, CK7, CK5/6 and P63 in primary and metastatic adenocarcinoma and squamous cell carcinoma of the lung: a retrospective study of 246 fine needle aspiration cases. Clin Transl Med. 2015;4:16. doi: 10.1186/s40169-015-0057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuman Y, Bergman A, Ueno T, et al. Napsin A, a Member of the Aspartic Protease Family, is Abundantly Expressed in Normal Lung and Kidney Tissue and is Expressed in Lung Adenocarcinomas. FEBS Letters. 1999;462:129–134. doi: 10.1016/s0014-5793(99)01493-3. [DOI] [PubMed] [Google Scholar]
- 25.Nobre AR, Albergaria A, Schmitt F. p40: A p63 Isoform Useful for Lung Cancer Diagnosis. A Review of the Physiological and Pathological Role of p63. Acta Cytol. 2012;57:1–8. doi: 10.1159/000345245. [DOI] [PubMed] [Google Scholar]
- 26.Pelosi G, Rossi G, Cavazza A, Righi L, Maisonneuve P, Barbareschi M, Graziano P, Pastorino U, Garassino M, de Braud F, Papotti M. Np63 (p40) Distribution Inside Lung Cancer: A Driver Biomarker Approach to Tumor Characterization. In J of Sur Pathol. 2013;21(3):229–239. doi: 10.1177/1066896913476750. [DOI] [PubMed] [Google Scholar]
- 27.Lilo MT, Allison D, Wang Y, Ao MH, Gabrielson E, Geddes S, Zhang H, Askin F, Li QK. Expression of P40 and P63 in lung cancers using fine needle aspiration (FNA) cases. Understanding clinical pitfalls and limitations. J Am Soc Cytopathol. 2016;5:123–132. doi: 10.1016/j.jasc.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fatima N, Cohen C, Lawson D, Siddiqui MT. TTF-1 and Napsin A double stain: a useful marker for diagnosing lung adenocarcinoma on fine-needle aspiration cell blocks. Cancer Cytopathol. 2011;119:127–133. doi: 10.1002/cncy.20135. [DOI] [PubMed] [Google Scholar]
- 29.Johnson H, Cohen C, Fatima N, Duncan D, Siddiqui MT. Thyroid transcription factor 1 and napsin a double stain: utilizing different vendor antibodies for diagnosing lung adenocarcinoma. Acta Cytol. 2012;56:596–602. doi: 10.1159/000339793. [DOI] [PubMed] [Google Scholar]
- 30.Sethi S, Geng L, Shidham VB, Archuletta P, Bandyophadhyay S, Feng J, Madan S, Shi D, Tranchida P, Giorgadze T. Dual color multiplex TTF-1 + Napsin A and p63 + CK5 immunostaining for subcategorizing of poorly differentiated pulmonary non-small carcinomas into adenocarcinoma and squamous cell carcinoma in fine needle aspiration specimens. Cytojournal. 2012;9:10. doi: 10.4103/1742-6413.94570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown AF, Sirohi D, Fukuoka J, Cagle P, Policarpio-Nicolas M, Tacha D, Jagirdar J. Tissue-Preserving Antibody Cocktails to Differentiate Primary Squamous Cell Carcinoma, Adenocarcinoma, and Small Cell Carcinoma of Lung. Arch Pathol Lab Med. 2013;137:1274–1281. doi: 10.5858/arpa.2012-0635-OA. [DOI] [PubMed] [Google Scholar]
- 32.Ao MH, Zhang H, Sakowski L, Sharma R, Illei PB, Gabrielson E, Askin F, Li QK. The utility of a novel triple marker (combination of TTF-1, Napsin A and p40) in the subclassification of non-small cell lung carcinoma (NSCLC) Human Pathol. 2014;45:926–934. doi: 10.1016/j.humpath.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma R, Wang Y, Gurda GT, Geddes S, Gabrielson E, Askin F, Li QK. Utility of the novel triple marker (combination of TTF-1, Napsin-A and p40) in the subclassification of non-small cell lung carcinomas (NSCLC) using fine needle aspiration (FNA) materials. Hum Pathol. 2016;54:8–16. doi: 10.1016/j.humpath.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 34.Zupa A, Improta G, Silvestri A, Pin E, Deng J, Aieta M, Musto P, Nitti D, Mammano E, Liotta L, Belluco C, Wulfkuhle J, Petricoin E., 3rd A pilot characterization of human lung NSCLC by protein pathway activation mapping. J Thorac Oncol. 2012;7(12):1755–66. doi: 10.1097/JTO.0b013e3182725fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buhrens RI, Amelung JT, Reymond MA, Beshay M. Protein expression in human non-small cell lung cancer: a systematic database. Pathobiology. 2009;76:277–285. doi: 10.1159/000245893. [DOI] [PubMed] [Google Scholar]
- 36.Yanagisawa K, Shyr Y, Xu BJ, Massion PP, Larsen PH, White BC, Roberts JR, Edgerton M, Gonzalez A, Nadaf S, Moore JH, Caprioli RM, Carbone DP. Proteomic patterns of tumour subsets in non-small-cell lung cancer. Lancet. 2003;362:433–439. doi: 10.1016/S0140-6736(03)14068-8. [DOI] [PubMed] [Google Scholar]
- 37.Takamochi K, Ohmiya H, Itoh M, Mogushi K, Saito T, Hara K, Mitani K, Kogo Y, Yamanaka Y, Kawai J, Hayashizaki Y, Oh S, Suzuki K, Kawaji H. Novel biomarkers that assist in accurate discrimination of squamous cell carcinoma from adenocarcinoma of the lung. BMC Cancer. 2016;16(1):760. doi: 10.1186/s12885-016-2792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]