Abstract
Frontotemporal dementia is an umbrella clinical term that encompasses a group of neurodegenerative diseases characterised by progressive deficits in behaviour, executive function, or language. Frontotemporal dementia is a common type of dementia, particularly in patients younger than 65 years. The disease can mimic many psychiatric disorders because of the prominent behavioural features. Various underlying neuropathological entities lead to the frontotemporal dementia clinical phenotype, all of which are characterised by the selective degeneration of the frontal and temporal cortices. Genetics is an important risk factor for frontotemporal dementia. Advances in clinical, imaging, and molecular characterisation have increased the accuracy of frontotemporal dementia diagnosis, thus allowing for the accurate differentiation of these syndromes from psychiatric disorders. As the understanding of the molecular basis for frontotemporal dementia improves, rational therapies are beginning to emerge.
Introduction
Frontotemporal dementia is an insidious neurodegenerative clinical syndrome characterised by progressive deficits in behaviour, executive function, and language. The disorder is the third most common form of dementia across all age groups, after Alzheimer’s disease and dementia with Lewy bodies, and is a leading type of early-onset dementia.1 The first description of a patient with frontotemporal dementia was made by Arnold Pick in 1892;2 the patient had aphasia, lobar atrophy, and presenile dementia. In 1911, Alois Alzheimer recognised the characteristic association with Pick bodies and named the clinicopathological entity Pick’s disease,3 which led to the use of Pick’s disease as a synonym for frontotemporal dementia. In 1982, Mesulam described a language subtype of the disorder, later defined as primary progressive aphasia.4 Revised diagnostic criteria have recently been issued.5,6
Because of the close similarity of behavioural changes in patients with frontotemporal dementia to those seen in patients with psychiatric disorders, diagnosis is challenging. Here we review the clinical and laboratory features, epidemiology, genetics, and neuropathology of frontotemporal dementia to provide a comprehensive insight into this disorder and help differentiate it from psychiatric disorders and other neurodegenerative diseases. We also discuss therapeutic strategies for symptom management, and the most promising areas of therapeutic development.
Epidemiology
WHO estimates that dementia rates will double every 20 years, reaching 115·4 million in 2050.7 In a meta-analysis of 73 articles of early-onset dementia (patient age <65 years), frontotemporal dementia was the second or third most prevalent dementia subtype in most studies, with a prevalence ranging from 3% to 26%.1 Table 1 summarises the epidemiology of frontotemporal dementia.8–10 Because the disorder is still missed and misdiagnosed, most numbers probably underestimate its true prevalence.
Table 1.
| Age (years) | Prevalence | |
|---|---|---|
| Patients with early-onset dementia | <65 | 3–26% |
|
| ||
| US general population | 45–64 | 15–22 per 100 000 |
|
| ||
| UK general population | 45–64 | 3–26 per 100 000 |
|
| ||
| Italy general population | 45–64 | 22 per 100 000 |
|
| ||
| Japan, UK, and Netherlands | 30–64 | 1–16 per 100 000 |
|
| ||
| Most common FTD variant | ||
| USA and Europe | ·· | BV-FTD (50–70%) |
| Asia | ·· | SV-PPA or BV-FTD |
|
| ||
| Age at onset | ||
| <45 years | ·· | 10% |
| 45–64 years | ·· | 60% |
| >64 years | ·· | 30% |
|
| ||
| Survival time | ||
| From symptom onset | ·· | 6–11 years |
| From clinical diagnosis | ||
| FTD-MND | ·· | 2 years |
| BV-FTD | ·· | 3–4 years |
| NFV-PPA | ·· | 5 years |
| SV-PPA | ·· | 5+ years |
FTD=frontotemporal dementia. BV=behavioural variant. MND=motor neuron disease. PPA=primary progressive aphasia. NFV=non-fluent variant. SV=semantic variant.
Clinical features
Frontotemporal dementia is classified into three clinical variants: behavioural-variant frontotemporal dementia, which is associated with early behavioural and executive deficits; non-fluent variant primary progressive aphasia, with progressive deficits in speech, grammar, and word output; and semantic-variant primary progressive aphasia, which is a progressive disorder of semantic knowledge and naming. The diagnostic criteria outline features, (ie, clinical, imaging-supported, and genetically confirmed diagnosis), that increase the likelihood that frontotemporal dementia-related neuropathology will be identified5,6 (figure 1). As frontotemporal dementia progresses, the symptoms of the three clinical variants can converge, as an initially focal degeneration becomes more diffuse and spreads to affect large regions in the frontal and temporal lobes. Over time, patients develop global cognitive impairment and motor deficits, including parkinsonism, and motor neuron disease in some patients. Patients with end-stage disease have difficulty eating, moving, and swallowing. Death usually happens about 8 years after symptom onset and is typically caused by pneumonia or other secondary infections.
Figure 1. Diagnostic criteria for clinical diagnosis of FTD.
These criteria focus mostly on symptoms that are present early in the illness, which tend to more clearly delineate the three variants of FTD. FTD=frontotemporal dementia. BV=behavioural variant. SV=semantic variant. NFV=non-fluent variant. PPA=primary progressive aphasia.
Behavioural-variant frontotemporal dementia
The most pronounced early symptoms of behavioural-variant frontotemporal dementia include personality changes, disinhibition, and apathy (table 2). Behavioural disinhibition can result in tactless and socially inappropriate behaviour (eg, approaching strangers without respect for physical and social boundaries); impulsive or careless actions (such as reckless spending); new criminal behaviours (eg, theft, urination in public, sexual advances, or hit-and-run accidents); and embarrassing personal remarks. Reduced inhibition often results in bad fiscal decisions that can lead to financial ruin. Although patients might make inappropriate sexual comments, they usually have decreased libido. Apathy manifests as reduced interest in work, hobbies, social interaction, and hygiene, and can be mistaken for depression. Patients show a loss of sympathy and empathy towards their families and friends, and a decrease in social interest and responsiveness to the emotions and needs of other people. One of our patients was more concerned about going to the grocery store than attending the funeral of his spouse’s parent. As a result, family members and friends can often resent the patient. Patients show stereotyped behaviours, including simple repetitive movements, compulsive ritualistic behaviours, and repetitive use of verbal phrases. Binge eating, increased consumption of sweets or alcohol, and weight gain are different aspects of the hyperorality in behavioural-variant frontotemporal dementia. Patients with behavioural variant frontotemporal dementia often show deficits in various executive tasks, although their visuospatial skills are fairly normal at first. Patients have little insight into their own behaviour and might not recognise many of the changes that are reported by a knowledgeable informant. Some patients have decreased sensitivity to pain.11
Table 2.
Clinical symptoms characteristic of FTD
| BV-FTD | SV-PPA | NFV-PPA | |
|---|---|---|---|
| Social symptoms | |||
|
| |||
| Disinhibition, inappropriate or offensive behaviour, excessive jocularity, exaggerated emotional display, impulsivity, inappropriate sexual remarks, lack of embarrassment | 73–98% | 59% | 18% |
| Loss of empathy, lack of emotional insight, social coldness | 49–78% | 28–49% | 18% |
| Selfishness, disregard for others’ feelings | 83–89% | 91% | No |
| Aggression | 25–61% | 28–64% | 12% |
| Personal neglect, neglect of hygiene | 83–92% | 64% | No |
| Strange manner of dressing | Yes | Right-sided cases | No |
| Personality changes | Sometimes | Sometimes | Rarely |
|
| |||
| Emotional symptoms | |||
|
| |||
| Apathy, low motivation, aspontaneity, decreased initiation of behaviour | 54–96% | 18–47% | 41% |
| Depression, emotional detachment | Common | 44% | Sometimes depression |
| Irritability | 33% | 50% | 47% |
| Anxiety, social avoidance | No | Not usually | Yes |
| Exaggerated emotional display | 33–39% | 55% | Rarely |
|
| |||
| Eating and oral behaviours | |||
|
| |||
| Overeating, gluttony | 61–83% | 36% | Rarely |
| Reduced selectivity, indiscriminate eating | 41–55% | 9% | Rarely |
| Increased selectivity, food fads | 8–22% | 55% | Rarely |
| Preference for sweet foods | 25–56% | 36% | Rarely |
| Preference for savoury foods | 0% | 9% | Rarely |
| Hyperorality | 0–22% | 18% | Rarely |
|
| |||
| Repetitive or compulsive behaviours | |||
|
| |||
| Behavioural stereotypies | 95% | 72% | 24% |
| Compulsive behaviours | 5–15% | 60–80% | Rarely |
| Word obsessions, repetitious use of verbal expressions | Sometimes | Often | Rarely |
|
| |||
| Sensory symptoms | |||
|
| |||
| Reduced pain response | 39–45% | 27% | Rarely |
| Increased pain response, increased heat/cold response | 0–33% | 45–55% | Rarely |
| Incontinence | Sometimes | Sometimes | Rarely |
|
| |||
| Motor symptoms | |||
|
| |||
| Motor neuron disease | 15% | Rarely | Rarely |
| Parkinsonism | 18–20% | 3% | 6% |
| Progressive supranuclear palsy syndrome | Rarely | Not known | Sometimes |
| Corticobasal syndrome | Rarely | Not known | Sometimes |
|
| |||
| Executive symptoms | |||
|
| |||
| Mental rigidity, inflexibility | 41% | 38% | 12% |
| Inattention, disorganisation | Sometimes | Sometimes | Rarely |
| Loss of sensitivity to negative consequences, bad financial judgment | Sometimes | Sometimes | Rarely |
|
| |||
| Language symptoms | |||
|
| |||
| Impaired word finding | 45% | 84% | 71% |
| Apraxia of speech | 5% | 6% | 94% |
| Agrammatism | 0% | 0% | 53% |
| Impaired confrontation naming | 30% | 97% | 71% |
| Impaired single-word comprehension | 5% | 84% | 18% |
| Object agnosia | 2% | 34% | 0% |
| Phonological errors | 2% | 9% | 71% |
| Word repetition errors | 0% | 0% | 53% |
| Sentence repetition errors | 2% | 6% | 35% |
| Sentence comprehension errors | 23% | 72% | 88% |
| Surface dyslexia | 11% | 81% | 71% |
|
| |||
| Neuropsychiatric symptoms | |||
|
| |||
| Delusions | 14% | 9% | 6% |
| Hallucinations | 20% | 6% | 6% |
|
| |||
| Other symptoms | |||
|
| |||
| Sleep disorders | Sometimes | Rarely | Rarely |
| Restlessness | Sometimes | Sometimes | Rarely |
| Prosopagnosia | 3% | 47% | 0% |
| Decreased libido | Sometimes | Sometimes | Rarely |
| Episodic memory deficits | Rarely | Rarely | Rarely |
BV-FTD=behavioural-variant frontotemporal dementia. SV=semanticvariant. NFV= non-fluentvariant. PPA=primary progressive aphasia.
Some individuals who meet diagnostic criteria for behavioural-variant frontotemporal dementia have a very slow disease course (over decades) with slow progression of cognitive impairment and often normal MRI and PET studies. Their disease is classified as frontotemporal dementia phenocopy. Some of these individuals have a primary psychiatric disturbance such as bipolar disorder, Asperger’s syndrome, or factitious disease,12,13 whereas others might have a slow sporadic or genetic form of frontotemporal dementia.14
Primary progressive aphasia
Patients with primary progressive aphasia have a progressive, insidious decline in linguistic skills during the initial phase of the disease. Language dysfunction is the main symptom for the first 2 years of the illness. Deficits include language production, object naming, syntax, or word comprehension, and are apparent during conversation or through speech and language assessment. Language deficit is the main cause of impaired activities of daily living. Although the underlying cause is more often frontotemporal dementia, primary progressive aphasia can be associated with Alzheimer’s disease. If prominent visuospatial impairment or episodic or visual memory impairments are present, Alzheimer’s disease should be considered. The patient should not show behavioural disturbances during the initial disease phase; such changes are indicative of behavioural-variant frontotemporal dementia.4
Semantic-variant primary progressive aphasia
The term semantic dementia was used to describe a syndrome characterised by semantic aphasia and associative agnosia.15 Symptoms result from early asymmetrical degeneration of anterior temporal lobes and amygdala. Semantic loss causes anomia for people, places, and objects; word-finding difficulties; and impaired word comprehension. Left temporal lobe variant presents with mainly linguistic semantic loss (semantic-variant primary progressive aphasia), whereas the right temporal lobe variant presents with behaviour changes.16 Left temporal lobe variant is roughly three times more common than right temporal lobe variant.17 Comprehension of individual words is impaired, especially for words that are not routinely used by the patient. Anomia tends to be more pronounced for nouns than for verbs or pronouns. Patients have surface dyslexia and dysgraphia, impairments in which words with atypical spelling or pronunciation are regularised (eg, bouquet is read as bo-ket). Other language domains are spared, especially during the initial disease phase, and patients retain correct grammar and fluent speech. The deficits in recognition of objects and people go beyond the visual domain and tactile, olfactory, or gustatory clues do not help. As the disease spreads from the temporal lobes into the orbitofrontal cortex, behavioural changes occur, such as irritability, emotional withdrawal, insomnia, and strict or selective eating, often focused around one particular type of food; sometimes depression emerges.16 Although semantics are lost in left temporal lobe variant, functions pertaining to the right side, such as visual attention, are sometimes heightened. Therefore, patients with left temporal lobe variant tend to develop visual compulsions such as repetitive playing of puzzles, jewellery beading, collecting coins, gardening, painting, and collecting brightly coloured objects. By contrast, patients with right temporal lobe variant develop verbal compulsions involving words and symbols, such as writing notes, letters, and telephone numbers; making puns; or playing Solitaire.
Non-fluent variant primary progressive aphasia
Non-fluent variant primary progressive aphasia is characterised by slow, laboured, and halting speech production, and by omission or misuse of grammar (agrammatism). Patients often make inconsistent speech sound errors, including insertions, deletions, substitutions, transpositions, and distortions. Patients might have trouble understanding sentences with complex syntactic constructions (eg, “the girl that the boy likes stood up”), but retain the ability to understand simpler sentences with the same semantic content (eg, “the boy likes a girl; that girl stood up”). Early in the disease, written language production and syntactic comprehension tests reveal mild grammatical errors. Some patients maintain intact writing despite the presence of marked deficits in spoken language. Single-word comprehension and object knowledge are not affected, although patients can have a mild anomia that is usually more pronounced for verbs than for nouns.6
Motor symptoms
About 12·5% of patients with behavioural-variant frontotemporal dementia develop motor neuron disease, typically including upper motor neuron signs (hyper-reflexia, extensor plantar response, spasticity), lower motor neuron signs (weakness, muscle atrophy, fasciculations), dysarthria, dysphagia, and pseudobulbar affect. Mild features of motor neuron disease can occur in up to 40% of patients with frontotemporal dementia.18 Among the frontotemporal dementia variants, motor neuron disease arises frequently in patients with behavioural-variant frontotemporal dementia and less often in patients with semantic-variant primary progressive aphasia or non-fluent variant primary progressive aphasia.
Early parkinsonism is present in up to 20% of patients with frontotemporal dementia and is most often seen in patients with behavioural-variant frontotemporal dementia, followed by those with non-fluent variant primary progressive aphasia.19 Patients with frontotemporal dementia have features of corticobasal syndrome or progressive supranuclear palsy syndrome. Corticobasal syndrome is characterised by asymmetrical parkinsonism, sensory–motor cortical dysfunction, alien-limb syndrome, and dystonia. Progressive supranuclear palsy syndrome is characterised by vertical supranuclear palsy, decreased saccade velocity, and early postural instability with falls. Behavioural changes, including executive dysfunction, apathy, and impulsivity, are common.20
The panel shows a clinical vignette describing a typical patient with frontotemporal dementia.
Differential diagnosis
Differential diagnosis of frontotemporal dementia needs a careful history that examines the progression of behavioural changes, family history, behaviour in face-to-face interviews, performance on neuropsychological testing, laboratory studies, and neuroimaging. Blood work should include a comprehensive metabolic panel including liver and kidney function tests, complete blood count, vitamin B12 concentration, and thyroid studies. Cerebrospinal fluid assessment should be done in atypical cases. Toxic (heavy metals, illicit drugs), inflammatory (autoimmune, paraneoplastic), or infectious (syphilis, HIV) causes should be considered. Patients should be screened for obstructive sleep apnoea. Normal pressure hydrocephalus and low intracranial pressure syndromes might cause dementia that mimics behavioural-variant frontotemporal dementia. Low cerebrospinal fluid β-amyloid concentrations suggest Alzheimer’s disease, whereas very high cerebrospinal tau concentrations could suggest rapidly progressive dementia. β-amyloid imaging is helpful, particularly in young patients, to rule out Alzheimer’s disease, although Alzheimer’s disease and frontotemporal lobar degeneration neuropathology can co-occur. Family history of dementia, movement disorders, or psychosis might warrant genetic counselling and search for genes that cause frontotemporal dementia.
Adult-onset psychiatric disorders
Psychiatric disorders can mimic frontotemporal dementia. The repetitive and compulsive behaviours noted in patients with behavioural-variant frontotemporal dementia might lead to a misdiagnosis of obsessive-compulsive disorder. Similarly, apathy and emotional withdrawal might lead to a misdiagnosis of depression, although patients with frontotemporal dementia do not usually have other symptoms typical of depression and often deny sadness. Frontotemporal dementia can cause delusions and euphoria, which are features of bipolar disorder and schizophrenia. Of the frontotemporal dementia variants, behavioural-variant is the most likely to be misdiagnosed as a psychiatric disorder (such as schizophrenia, bipolar disorder, or depression).21 Personality disorders can be the heralding sign of the behavioural-variant and borderline, antisocial, schizoid, schizotypal personality changes, and addictive disorders are common features of patients in the early stages of behavioural-variant frontotemporal dementia. A high rate of late-onset psychosis is a characteristic feature of inherited frontotemporal dementia associated with C9orf72 mutations, the most common genetic form of the disorder. Furthermore, C9orf72 mutations have been associated with schizophrenia and bipolar disorder.22–24
Alzheimer’s disease and other neurodegenerative diseases
There is a substantial overlap of symptoms between Alzheimer’s disease and frontotemporal dementia. Alzheimer’s pathology could be suggested by predominance of memory and visuospatial deficits, social appropriateness, normal neurological examination, and evidence of generalised brain atrophy on imaging. A form of progressive aphasia with prominent anomia, acalculia, and word-finding pauses (logopenic-variant primary progressive aphasia) is usually caused by Alzheimer’s neuropathology.6
The movement abnormalities in frontotemporal dementia, progressive supranuclear palsy, and corticobasal degeneration are typically less responsive to levodopa than those in classic Parkinson’s disease. Executive dysfunction, parkinsonism, and hallucinations can be seen in both dementia with Lewy bodies and frontotemporal dementia; however, patients with dementia with Lewy bodies have more pronounced parkinsonism, visuospatial deficits, and cognitive fluctuations compared with patients with frontotemporal dementia.25,26 A diagnosis of progressive supranuclear palsy is suggested with predominant postural imbalance, slowing of saccadic velocities, a history of early falls, dysphagia, and pseudobulbar affect. Progressive supranuclear palsy and corticobasal degeneration can initially present as either behavioural-variant frontotemporal dementia or non-fluent variant primary progressive aphasia syndrome.20
Imaging
With continuing advances in imaging techniques, several methods can be used to help in the diagnosis of frontotemporal dementia. Structural MRI and CT show patterns of atrophy: frontotemporal dementia is characterised by predominant frontal or temporal atrophy, and atrophy in the frontoinsular region is especially indicative of frontotemporal dementia.27 Fluorodeoxyglucose PET, functional MRI, and single-photon-emission CT likewise show disproportionate hypoperfusion and hypometabolism in these regions.19 Research in molecular PET imaging is very active, not only because of the specificity it allows for differentiation of frontotemporal dementia from Alzheimer’s disease, but also because of its potential for further differentiating among frontotemporal lobar degeneration syndromes. Amyloid tracer imaging can distinguish frontotemporal dementia from Alzheimer’s disease, because amyloid deposition is not a neuropathological feature of frontotemporal dementia; guidelines recommend amyloid imaging in patients younger than 65 years with persistent or progressive unexplained mild cognitive impairment or atypical or mixed dementia presentation. Diffusion tensor imaging can show widespread white-matter degeneration in frontotemporal dementia, exceeding that seen in Alzheimer’s disease.28
Functional connectivity network mapping, pioneered by Raichle and colleagues29 and Buckner and colleagues,30 is a technique to identify specific brain circuits with MRI. Seeley and colleagues31 have shown that the frontotemporal dementia variants, Alzheimer’s disease, and corticobasal syndrome each selectively affect a distinct functional connectivity network. Decreased intrinsic connectivity within the salience network is reported in behavioural-variant frontotemporal dementia in association with increased activity in the default mode network of brain regions. This pattern is reversed in Alzheimer’s disease. In both cases, increased activity of spared cortical areas might suggest a compensatory effect of unimpaired networks. By examining how functional networks are affected, connectivity maps can improve differential diagnosis and longitudinal tracking of disease progress. The next frontier in molecular PET imaging is tau imaging. Various forms of tau tracers are being tested in patients with dementia. Tau imaging can potentially differentiate between Alzheimer’s disease, non-Alzheimer’s tauopathies, and tau-negative dementias.32
Neuropathology
Frontotemporal lobar degeneration is characterised by neuronal loss, gliosis, and microvacuolar changes of frontal lobes, anterior temporal lobes, anterior cingulate cortex, and insular cortex. Subtypes are associated with characteristic patterns of abnormal protein deposition.33 Initial changes occur in the anterior cingulate cortex, fronto-insular cortex, orbitofrontal cortex, and cingulate-frontal transitional zones.34 These regions have von Economo neurons and fork cells in layer 5 of the cortex, which are thought to play a central part in the integration of cortical and subcortical networks, and degenerate very early in behavioural-variant frontotemporal dementia.35 Either the microtubule-associated protein tau (MAPT), the TAR DNA-binding protein with molecular weight 43 kDa (TDP-43), or the fused-in-sarcoma (FUS) protein account for nearly all cases of frontotemporal lobar degeneration. The corresponding pathological subtypes of frontotemporal lobar degeneration are frontotemporal lobar degeneration-tau, frontotemporal lobar degeneration-TDP, and frontotemporal lobar degeneration-FUS. A few cases of frontotemporal lobar degeneration have ubiquitin-only or p62-only positive inclusions, or no inclusions at all.33
Frontotemporal lobar degeneration-tau
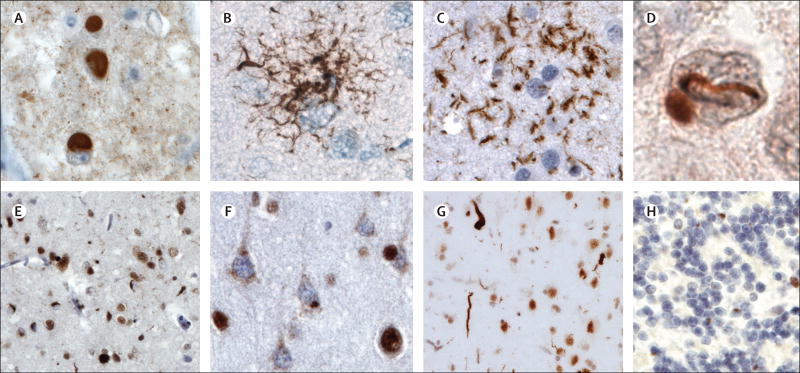
Frontotemporal lobar degeneration-tau accounts for 36–50% of all cases of frontotemporal lobar degeneration according to different pathological series.36–38 The most common subtypes of frontotemporal lobar degeneration-tau are Pick’s disease, corticobasal degeneration, and progressive supranuclear palsy. Pick’s disease constitutes 5% of all dementia cases and up to 30% of frontotemporal lobar degeneration-tau cases in various autopsy series.37,39 The disease is characterised by striking knife-edge atrophy of frontal, temporal, and cingulate gyri, whereas the parietal lobe is better preserved. Pick bodies are the pathological hallmark of Pick’s disease (figure 2A).40 Corticobasal degeneration accounts for about 35% of frontotemporal lobar degeneration-tau cases.37 Corticobasal degeneration shows predominant involvement of dorsal prefrontal cortex, supplemental motor area, peri-Rolandic cortex, and subcortical nuclei.40 Microscopically, corticobasal degeneration is characterised by pre-tangles, neuritic threads, ballooned neurons, astrocytic plaques (figure 2C), and oligodendroglial coiled bodies.40,41 Progressive supranuclear palsy accounts for about 31% of all cases of frontotemporal lobar degeneration-tau.37 Progressive supranuclear palsy is associated with atrophy of the frontal convexity, milder than in corticobasal degeneration.42 Subcortical atrophy is severe at the level of the globus pallidus, subthalamic nucleus, and brainstem nuclei. Microscopically, neuronal granular inclusions, tufted astrocytes, and globose tangles are seen (figure 2B) .40
Figure 2. Neuropathology in FTLD-tau and FTLD-TDP.
FTLD-tau (A) Pick bodies in Pick’s disease; (B) a tufted astrocyte in progressive supranuclear palsy; (C) an astrocytic plaque in corticobasal degeneration; FTLD-TDP (E) small compact or crescentic neuronal cytoplasmic inclusions and short, then neuropil threads in FTLD-TDP type A; (F) diffuse or granular neuronal cytoplasmic inclusions (with a relative paucity of neuropil threads) in FTLD-TDP type B; and (G) long, tortuous dystrophic neurites in FTLD-TDP type C.TDP can be seen within the nucleus in neurons lacking inclusions but mislocalises to the cytoplasm and forms inclusions in FTLD-TDP. The remaining FTLD cases are characterised by FUS-immunoreactive inclusions that stain negatively for tau and TDP-43; a vermiform neuronal nuclear inclusion in a dentate gyrus granule cell is shown (D); this neuron contains an ovoid cytoplasmic inclusion. In patients with hexanucleotide expansions in C9orf72, small juxtanuclear ubiquitin-positive, TDP-negative inclusions (H) are pathognomonic for the disorder. These inclusions contain dipeptide repeat proteins translated from the GGGGCC repeat in one of six reading frames. lmmunostains are 3-repeat tau (A), phospho-tau (B and C), FUS (D), TDP-43 (E–G) and ubiquitin (H). Sections are counterstained with haematoxylin. Scale bar applies to all panels and represents 50 µm in A, B, C, and H; 12 µm in D; and 100 µm in E and G. FTLD=frontotemporal lobar degeneration. TDP=TAR DNA-binding protein. FUS=fused-in-sarcoma.
Frontotemporal lobar degeneration-TDP
Frontotemporal lobar degeneration-TDP accounts for about 50% of all cases of frontotemporal lobar degeneration.37,38 Three major subtypes of frontotemporal lobar degeneration-TDP (types A, B, and C) are recognised on the basis of the patterns of cytoplasmic or intranuclear pathology, and cortical association (figure 2E–G and figure 3).43,44 In our centre, frontotemporal lobar degeneration-TDP type A accounts for about 50% of all cases of non-fluent variant primary progressive aphasia, 25% of suspected corticobasal degeneration cases, and a small proportion of behavioural-variant frontotemporal dementia cases with or without motor neuron disease, whereas frontotemporal lobar degeneration-TDP type B accounts for about two-thirds of frontotemporal dementia–motor neuron disease cases and 25% of behavioural-variant frontotemporal dementia cases. Frontotemporal lobar degeneration-TDP type C accounts for about 90% of all cases of semantic-variant primary progressive aphasia (left) or temporal-variant behavioural-variant frontotemporal dementia (right).
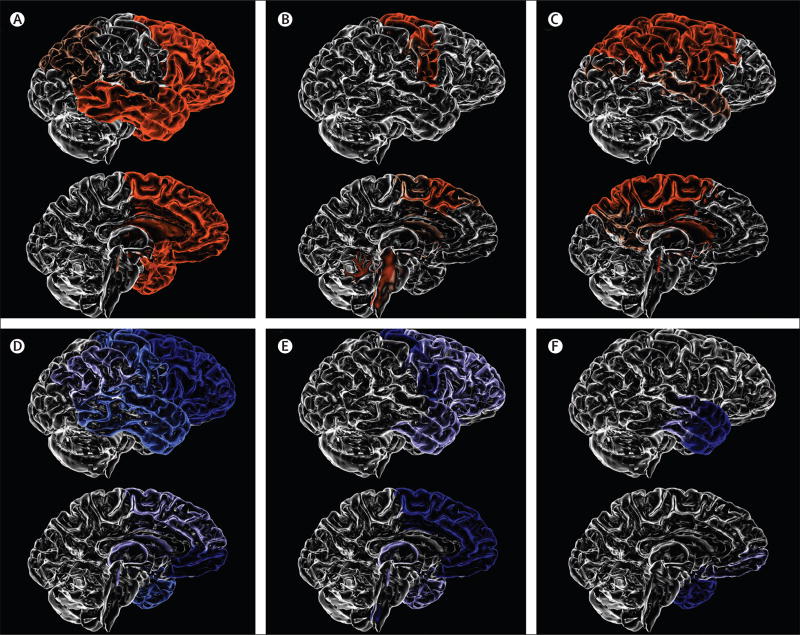
Figure 3. Patterns of brain atrophy in FTLD pathologies.
(A) Pick’s disease; (B) progressive supranuclear palsy; (C) corticobasal degeneration; (D) FTLD-TDP type A; (E) FTLD-TDP type B; (F) FTLD-TDP type C. FTLD-TDP type A is associated with an asymmetrical dorsal pattern of atrophy that includes the frontal lobe and temporal lobe (anterior, medial, and posterior regions), orbitofrontal cortex, anterior cingulate gyrus, inferior parietal lobe, striatum, and thalamus. FTLD-TDP type B is associated with a more medial pattern of atrophy mainly involving the medial and polar temporal lobe, anterior insular, cingulate and medial prefrontal cortices, and the orbitofrontal cortex. The frontal lobe is more severely affected in the posterior areas. FTLD-TDP type C is associated with either right-predominant or left-predominant anterior temporal lobe atrophy, with additional involvement of the amygdala, hippocampus, orbitofrontal cortex, and insular cortex. FTLD=frontotemporal lobar degeneration. TDP=TAR DN A-binding protein 43.
Frontotemporal lobar degeneration-FUS
Behavioural-variant frontotemporal dementia associated with frontotemporal lobar degeneration-FUS accounts for about 10% of all frontotemporal lobar degeneration cases.45 This entity is characterised by sporadic, early-onset frontotemporal dementia with severe disinhibition, sometimes psychosis, and other psychiatric and behavioural abnormalities in the absence of motor or linguistic deficits. Patients show distinctive FUS-immunoreactive inclusions that are abundant in the dentate gyrus (figure 2D), and severe striatal atrophy.46
Genetics
A family history of dementia is reported in up to 40% of cases of frontotemporal lobar degeneration, although a clear autosomal dominant history accounts for only 10% of cases.47 Mutations in C9orf72, MAPT, and GRN genes account for about 60% of all cases of inherited frontotemporal lobar degeneration.48 Genetic testing should be considered in patients with frontotemporal dementia with a strong family history of autosomal dominant neurological disorders including frontotemporal dementia, Alzheimer’s disease, parkinsonism, motor neuron disease, inclusion body myopathy, or late-onset psychosis.48,49
C9orf72 gene mutations account for about 25% of familial cases of frontotemporal lobar degeneration and are the most common genetic cause of frontotemporal dementia and amyotrophic lateral sclerosis.50 MAPT and GRN mutations each account for 5–20% of familial frontotemporal lobar degeneration cases.51 The prevalence of mutation types varies from region to region and might reflect founder effects. The expansion of a non-coding GGGGCC hexanucleotide repeat in the C9orf72 gene is the most common cause of inherited frontotemporal dementia and amyotrophic lateral sclerosis worldwide, and accounts for a smaller proportion of sporadic cases.50,52 C9orf72 mutations are most frequent in Scandinavia and northern Europe, and account for more than 50% of all frontotemporal lobar degeneration mutations at our centre in San Francisco, but are rare in Asia. MAPT mutations were the first identified monogenic cause of inherited frontotemporal dementia and lead to neurodegeneration through changed microtubule stabilisation and increased propensity of tau self-aggregation.53 GRN mutations are associated with haploinsufficiency with loss of two-thirds of functional progranulin concentrations in the serum and cerebrospinal fluid.54,55 Progranulin is a secreted protein involved in cell-cycle regulation, wound repair, axonal growth, and modulation of inflammation.56 Progranulin binds to the tumour necrosis factor (TNF) receptor, therefore potentially exercising a competing role against TNFα-induced inflammation.57 A higher prevalence of non-thyroid-related autoimmune disorder has been reported in carriers of GRN mutations.58 These mutations are most common in southern Europe, particularly Spain and Italy (table 3). TDP-43 and FUS are RNA-binding ribonucleoproteins associated with regulation of transcription and translation, alternative splicing, and RNA transport and stabilisation. Mutations in either TARDBP, the gene encoding TDP-43, or FUS are associated with familial amyotrophic lateral sclerosis with or without frontotemporal dementia.59 Table 3 summarises patterns of brain atrophy and proposed mechanisms of neurodegeneration associated with C9orf72, MAPT, GRN, TARDBP, FUS, VCP, or CHMP2B gene mutations.46,59–71
Table 3.
Genetic and pathological correlation in FTLD
| Prevalence among familial FTD cases |
Geographic prevalence of mutation carriers |
Atrophy patterns | Common clinical presentations |
FTLD proteinopathy | Mechanisms of neurodegeneration |
|
|---|---|---|---|---|---|---|
| C9orf72 (9p21.2) | 13–50% | Scandinavia, west Europe, USA, Australia, rare in Asia | Symmetrical, orbitofrontal, medial and dorsolateral frontal, followed by temporal lobes, parietal and occipital lobes, cerebellum, posterior thalamus | BV-FTD FTD-MND ALS Parkinsonism Late-onset psychosis | TDP type B (less commonly type A) dipeptide repeat proteins in neocortex, thalamus, cerebellum, and hippocampus | Endosomal trafficking dysregulation; RNA-foci formation and impaired transcription processing; dipeptide repeat protein toxicity |
| MAPT (17q21.1) | 5–20% | Northwest Europe, USA | Symmetric frontal, anterior cingulate cortex, insular, anterior, and medial temporal lobe | BV-FTD Parkinsonism | Tau (often unclassifiable, occasionally resembling Pick’s disease), corticobasal degeneration or progressive supranuclear palsy | Altered microtubule stabilisation and/or increased propensity of tau self-aggregation |
| GRN (17q21.32) | 5–20% | England, central and southern Europe, USA | Asymmetrical, anterior temporal, temporo-parietal, frontal (left > PPA; right > BV-FTD), anterior cingulate cortex, insular | BV-FTD NFV-PPA Parkinsonism CBS | TDP type A | Impaired neurotrophic function, promotion of inflammation, impaired lysosomal-mediated protein degradation |
| TARDBP (1p36.22) | Rare | Italy, France, North America, Japan, China, Australia | Symmetrical frontal (type A, DLPFC; type B, VMPFC), OFC, temporal atrophy | ALS FTD-MND | TDP type A or B | Impaired RNA-binding, transcription, translation, alternative splicing, RNA transport and stabilisation |
| FUS (16p11.2) | Rare | Worldwide | Frontal and temporal atrophy with striking striatal atrophy | ALS FTD-MND | FUS | Impaired RNA-binding, transcription, translation, alternative splicing, RNA transport and stabilisation |
| VCP (9p13.3) | Rare | West Europe, USA, Brazil, Korea, Australia | Frontal, temporal, and parietal lobes, especially prefrontal cortex and superior temporal gyrus; hippocampus, caudate nucleus, amygdala | BV-FTD FTD-MND Inclusion body myopathy Paget’s disease of the bone | TDP type D | Impaired ubiquitin-proteasome mediated protein degradation and autophagy |
| CHMP2B (3p11.2) | Rare | Denmark | Generalised cortical atrophy, mostly severe in frontal and temporal cortices | BV-FTD FTD-MND | Ubiquitin proteasome system proteins | Impaired endosomal-lysosomal and autophagic protein degradation pathway |
Pathology of frontotemporal lobar degeneration in patients with mutations in named genes, with chromosomal locations indicated in parentheses. FTD=frontotemporal dementia. FTLD=frontotemporal lobar degeneration. BV=behavioural variant. SV=semantic variant. NFV=non-fluent variant. PPA=primary progressive aphasia. MND=motor neuron disease. ALS=amyotrophic lateral sclerosis. TDP=TAR DNA-binding protein 43. FUS=fused-in-sarcoma. CBS=corticobasal syndrome. DLPFC=dorsolateral prefrontal cortex. VMPFC=ventromedial prefrontal cortex. OFC=orbitofrontal cortex.
Treatment
No approved disease-modifying drugs are available for the treatment of frontotemporal dementia. Treatment is focused on management of behavioural symptoms. Severity of compulsion, agitation, aggressiveness, impulsivity, and aberrant eating behaviour can improve with the use of selective serotonin reuptake inhibitors.72 Behavioural abnormalities can be managed with low doses of atypical antipsychotics.73 Caution should be used when treating elderly patients with dementia with atypical antipsychotics because of the heightened risk of mortality secondary to cardiac events, falls, and infections.74 Cholinesterase inhibitors are not beneficial and can worsen behavioural abnormalities seen in patients with frontotemporal dementia.75 Memantine does not improve or delay progression of frontotemporal dementia symptoms.76
Advances in the understanding of the pathobiology of frontotemporal dementia have identified new potential therapeutic targets for the development of effective disease-modifying drugs. The discovery that pathological tau protein spreads trans-synaptically in a prion-like manner to consecutive brain regions along anatomical pathways of intrinsic connectivity has increased interest in the possibility that antibodies could be used to prevent trans-neuronal spreading of pathological abnormalities, therefore hopefully preventing the disease from further spreading.31,77,78 Other therapeutic approaches to tauopathies under investigation are based on testing the efficacy of inhibitors of tau aggregation with methylene blue derivatives and microtubule stabilising drugs (eg, TPI-287), and by development of tau vaccines.79
The discovery of the pathogenic expansion in C9orf72 as the most common cause of inherited frontotemporal dementia has increased interest in the development of antisense oligonucleotides to reduce concentrations of potentially toxic mRNAs.80 This approach is under investigation with the aim of reducing the total amount of pathological tau species.81
Because neurodegeneration in PGRN mutation carriers is associated with progranulin haploinsufficiency and diminished progranulin serum concentrations, studies are attempting to curtail the progression of neurodegeneration in PGRN mutation carriers with molecules such as the histone deacetylase inhibitor suberoylanilide hydroxamic acid, which enhances progranulin transcription; alkalising compounds (such as chloroquine, bepridil, and amiodarone) that stimulate progranulin production; and inhibitors of the vacuolar ATPase, which all increase intracellular and secreted progranulin concentrations.82,83 A clinical trial testing the efficacy of nimodipine is in progress at our institution (NCT01835665).
Conclusion and future directions
Frontotemporal dementia is a common dementia, particularly in individuals younger than 65 years. The clinical presentation of behavioural-variant frontotemporal dementia is characterised by the predominance of behavioural symptoms. Differentiation of the initial changes of this devastating neurodegenerative disease from common psychiatric disorders, particularly schizophrenia, depression, bipolar disorder, delusional disorders, obsessive-compulsive behaviour, and borderline, schizoidal, and antisocial personality disorders can be difficult. Many novel biological mechanisms leading to frontotemporal lobar degeneration have been identified. Nevertheless, the cause remains elusive and many questions remain unanswered.
What is the reason for the selective vulnerability of the frontal and temporal lobes of the brain to frontotemporal dementia? Insights into this selective susceptibility have come from the identification of von Economo neurons and fork cells as the initial target of degeneration in frontotemporal dementia.35 However, little is known about the biology of these cell types.
Who is at risk of developing frontotemporal dementia? Can preclinical disease detection of at-risk individuals lead to disease prevention? Development of neuroimaging and molecular biomarkers will lead to the early identification of disease changes and potentially prevent, halt, or even reverse the pathological process. Genetic efforts will allow the discovery of new pathogenic mutations and risk-associated polymorphisms in familial frontotemporal lobar degeneration. Frontotemporal dementia preclinical phenomenology needs to be better characterised. Current routine behavioural and emotional assessments are not sufficiently sensitive in prediction of disease onset.
International efforts are necessary to characterise patients affected with the inherited forms of frontotemporal dementia. The advantage of this approach relies on the theoretical possibility of predicting the natural history of disease in carriers of pathogenic mutations. Such studies will be fundamental in designing effective clinical trials of disease-modifying drugs, when potential molecular candidates become available. Molecular PET imaging promises to improve effectiveness and robustness of clinical trials through a better differentiation of frontotemporal lobar degeneration subtypes in vivo.
How does frontotemporal lobar degeneration spread in the brain? What is the role of selective neuronal vulnerability in view of the findings regarding prion-like spreading of proteinopathies? Various and often co-occurring mechanisms of neurodegeneration are emerging, suggesting that many targets of therapeutic intervention will be identified in the future. As a result, future therapeutic strategies will probably involve simultaneous use of various drugs, similar to the treatment of hypertension, cancer, or HIV/AIDS. On the other hand, improvement of biomarker characterisation will lead to the development of proteinopathy-specific therapies.
Finally, what can we learn from the study of frontotemporal lobar degeneration syndrome that can be applied to the management of psychiatric disorders? Reports of the high frequency of long-standing early psychiatric symptoms and late-onset psychosis in carriers of C9orf72 pathological expansion have renewed attention towards the clinical overlap of so-called pure psychiatric disorders and traditional forms of neurodegeneration. Increased knowledge of the pathophysiology of behavioural dysfunction in frontotemporal lobar degeneration will probably provide further insights into the neurobiology of psychiatric diseases. After several decades of segregation between behavioural neurology and psychiatry, we might be witnessing the rebirth of neuropsychiatry, as we enter one of the most exciting times for the understanding and hopefully the treatment of frontotemporal dementia.
Panel: Clinical vignette.
A 69-year-old right-handed man with a past medical history of complex partial seizures, hypertension, and hyperlipidaemia was referred because of a 5-year history of progressive behavioural changes. His initial symptoms consisted of apathy and diminished interest in household chores. He reported increasing frustration at work because of difficulties dealing with job duties and related paperwork. His ability to do manual work at home declined. He developed difficulties operating a microwave and began losing weight because he could not follow directions on how to process precooked meals. He became confused about credit card payments. 1 year later, he began making potentially hurtful remarks publicly towards strangers in a way that the random target would be able to hear (“Do you think she really wanted this hair colour?”; “Why does he need to have so many tattoos?”). He began engaging strangers in conversations and, on one occasion, addressed a security guard screaming “I need security” and proceeded to hug him. He started using sexually inappropriate language with his wife, although his interest in sex declined. He began repeating sentences in a stereotypical way, often out of context, and later started making faces or talking with a Donald Duck-like voice in public, irrespective of the circumstances. He shoplifted from a grocery store on one occasion. He became distant and less empathic towards his family members. At the same time, he became hyperemotional with regard to life events of strangers, such as when reminded of the assassination of John Lennon. His eating habits changed and he began eating large amounts of ice cream and smoothies, yoghurt, and milk. He also began reporting bizarre visual hallucinations, such as seeing a dinosaur in his neighbour’s backyard, and began talking to stuffed animals in the house. Later in the disease course, he developed hand clumsiness and began losing muscle bulk in his chest, shoulders, and legs. He developed motor stereotypies such as tapping and hitting his chest as a sign of strength, and started to have difficulties with swallowing. His family history was remarkable for epilepsy in his father and younger brother. His maternal grandmother had been unable to raise her children as a result of what was described as a nervous breakdown. One of his paternal uncles was affected with parkinsonism. The patient’s neurological examination was remarkable for poor orientation and inappropriate behaviour, mildly diminished muscle bulk in the left arm, and mild kinetic tremors. His neuropsychological assessment revealed predominant and severe executive impairment. His brain MRI showed severe volume loss at the level of the orbitofrontal cortex, insular cortex, dorsolateral and mesial frontal gyri, and anterior temporal lobes, which was more severe on the right cerebral hemisphere. There was atrophy of the caudate nuclei, hippocampi, and cerebellar hemispheres. He was diagnosed with probable behavioural-variant frontotemporal dementia, probably secondary to frontotemporal lobar degeneration, associated withTDP-43type-B pathology.5
Acknowledgments
University of California, San Francisco; Alzheimer Disease Research Center; Program Project Grant; the Tau Consortium; and the Consortium for Frontotemporal Dementia Research provided funding for this report. We thank Caroline Latham and John Fesenko for their help with the rendition and editing of the illustrations. We thank William W Seeley for providing the neuropathological illustrations.
Footnotes
Contributors
JB and SS contributed to the review of the literature, writing of the initial draft, and manuscript editing and revision. BLM contributed to the editing and critical revision of the manuscript.
Declaration of interests
We declare no competing interests.
References
- 1.Vieira RT, Caixeta L, Machado S, et al. Epidemiology of early-onset dementia: a review of the literature. Clin Pract Epidemiol Ment Health. 2013;9:88–95. doi: 10.2174/1745017901309010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pick A. Über die Beziehungen der senilen Hirnatrophie zur Aphasie. Prager Med Wochenschr. 1892;17:165–67. [Google Scholar]
- 3.Alzheimer A. Über eigenartige Krankheitsfälle der späteren Alters. Z Gesamte Neurol Psychiatr. 1911;4:356–85. [Google Scholar]
- 4.Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49:425–32. [PubMed] [Google Scholar]
- 5.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. [(accessed Nov 10, 2014)];Dementia fact sheet. 2015 Mar; http://www.who.int/mediacentre/factsheets/fs362/en/
- 8.Knopman DS, Roberts RO. Estimating the number of persons with frontotemporal lobar degeneration in the US population. J Mol Neurosci. 2011;45:330–35. doi: 10.1007/s12031-011-9538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert MA, Bickel H, Prince M, et al. Estimating the burden of early onset dementia; systematic review of disease prevalence. Eur J Neurol. 2014;21:563–69. doi: 10.1111/ene.12325. [DOI] [PubMed] [Google Scholar]
- 10.Hodges JR, Davies R, Xuereb J, Kril J, Halliday G. Survival in frontotemporal dementia. Neurology. 2003;61:349–54. doi: 10.1212/01.wnl.0000078928.20107.52. [DOI] [PubMed] [Google Scholar]
- 11.Miller BL, Boeve BF. The behavioral neurology of dementia. Cambridge, UK and New York, NY, USA: Cambridge University Press; 2009. [Google Scholar]
- 12.Davies RR, Kipps CM, Mitchell J, Kril JJ, Halliday GM, Hodges JR. Progression in frontotemporal dementia: identifying a benign behavioral variant by magnetic resonance imaging. Arch Neurol. 2006;63:1627–31. doi: 10.1001/archneur.63.11.1627. [DOI] [PubMed] [Google Scholar]
- 13.Kipps CM, Hodges JR, Hornberger M. Nonprogressive behavioural frontotemporal dementia: recent developments and clinical implications of the ‘bvFTD phenocopy syndrome’. Curr Opin Neurol. 2010;23:628–32. doi: 10.1097/WCO.0b013e3283404309. [DOI] [PubMed] [Google Scholar]
- 14.Khan BK, Yokoyama JS, Takada LT, et al. Atypical, slowly progressive behavioural variant frontotemporal dementia associated with C9orf72 hexanucleotide expansion. J Neurol Neurosurg Psychiatry. 2012;83:358–64. doi: 10.1136/jnnp-2011-301883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 16.Seeley WW, Bauer AM, Miller BL, et al. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64:1384–90. doi: 10.1212/01.WNL.0000158425.46019.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003;61:1196–203. doi: 10.1212/01.wnl.0000091868.28557.b8. [DOI] [PubMed] [Google Scholar]
- 18.Burrell JR, Kiernan MC, Vucic S, Hodges JR. Motor neuron dysfunction in frontotemporal dementia. Brain. 2011;134:2582–94. doi: 10.1093/brain/awr195. [DOI] [PubMed] [Google Scholar]
- 19.Le Ber I, Guedj E, Gabelle A, et al. and the French research network on FTD/FTD-MND. Demographic, neurological and behavioural characteristics and brain perfusion SPECT in frontal variant of frontotemporal dementia. Brain. 2006;129:3051–65. doi: 10.1093/brain/awl288. [DOI] [PubMed] [Google Scholar]
- 20.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Woolley JD, Khan BK, Murthy NK, Miller BL, Rankin KP. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry. 2011;72:126–33. doi: 10.4088/JCP.10m06382oli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galimberti D, Reif A, Dell’osso B, et al. C9orf72 hexanucleotide repeat expansion is a rare cause of schizophrenia. Neurobiol Aging. 2014;35:1214. e7–1214. e10. doi: 10.1016/j.neurobiolaging.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Galimberti D, Reif A, Dell’Osso B, et al. C9orf72 hexanucleotide repeat expansion as a rare cause of bipolar disorder. Bipolar Disord. 2014;16:448–49. doi: 10.1111/bdi.12169. [DOI] [PubMed] [Google Scholar]
- 24.Galimberti D, Fenoglio C, Serpente M, et al. Autosomal dominant frontotemporal lobar degeneration due to the C9orf72 hexanucleotide repeat expansion: late-onset psychotic clinical presentation. Biol Psychiatry. 2013;74:384–91. doi: 10.1016/j.biopsych.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 25.Claassen DO, Parisi JE, Giannini C, Boeve BF, Dickson DW, Josephs KA. Frontotemporal dementia mimicking dementia with Lewy bodies. Cogn Behav Neurol. 2008;21:157–63. doi: 10.1097/WNN.0b013e3181864a09. [DOI] [PubMed] [Google Scholar]
- 26.Perri R, Monaco M, Fadda L, Caltagirone C, Carlesimo GA. Neuropsychological correlates of behavioral symptoms in Alzheimer’s disease, frontal variant of frontotemporal, subcortical vascular, and lewy body dementias: a comparative study. J Alzheimers Dis. 2014;39:669–77. doi: 10.3233/JAD-131337. [DOI] [PubMed] [Google Scholar]
- 27.Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Schuff N, Du AT, et al. White matter damage in frontotemporal dementia and Alzheimer’s disease measured by diffusion MRI. Brain. 2009;132:2579–92. doi: 10.1093/brain/awp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–17. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maruyama M, Shimada H, Suhara T, et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 2013;79:1094–108. doi: 10.1016/j.neuron.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seeley WW, Crawford R, Rascovsky K, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65:249–55. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim EJ, Sidhu M, Gaus SE, et al. Selective frontoinsular von Economo neuron and fork cell loss in early behavioral variant frontotemporal dementia. Cereb Cortex. 2012;22:251–59. doi: 10.1093/cercor/bhr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baborie A, Griffiths TD, Jaros E, et al. Pathological correlates of frontotemporal lobar degeneration in the elderly. Acta Neuropathol. 2011;121:365–71. doi: 10.1007/s00401-010-0765-z. [DOI] [PubMed] [Google Scholar]
- 37.Josephs KA, Hodges JR, Snowden JS, et al. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol. 2011;122:137–53. doi: 10.1007/s00401-011-0839-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sieben A, Van Langenhove T, Engelborghs S, et al. The genetics and neuropathology of frontotemporal lobar degeneration. Acta Neuropathol. 2012;124:353–72. doi: 10.1007/s00401-012-1029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16:203–12. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Dickson DW, Hauw JJ, Agid Y, Litvan I. Progressive Supranuclear Palsy and Corticobasal Degeneration. Hoboken, NJ: Wiley-Blackwell; 2011. [Google Scholar]
- 41.Rebeiz JJ, Kolodny EH, Richardson EP., Jr Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol. 1968;18:20–33. doi: 10.1001/archneur.1968.00470310034003. [DOI] [PubMed] [Google Scholar]
- 42.Boxer AL, Geschwind MD, Belfor N, et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol. 2006;63:81–86. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- 43.Mackenzie IR, Neumann M, Baborie A, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–13. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rohrer JD, Geser F, Zhou J, et al. TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia. Neurology. 2010;75:2204–11. doi: 10.1212/WNL.0b013e318202038c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackenzie IR, Munoz DG, Kusaka H, et al. Distinct pathological subtypes of FTLD-FUS. Acta Neuropathol. 2011;121:207–18. doi: 10.1007/s00401-010-0764-0. [DOI] [PubMed] [Google Scholar]
- 46.Mackenzie IR, Foti D, Woulfe J, Hurwitz TA. Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain. 2008;131:1282–93. doi: 10.1093/brain/awn061. [DOI] [PubMed] [Google Scholar]
- 47.Rohrer JD, Guerreiro R, Vandrovcova J, et al. The heritability and genetics of frontotemporal lobar degeneration. Neurology. 2009;73:1451–56. doi: 10.1212/WNL.0b013e3181bf997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Ber I. Genetics of frontotemporal lobar degeneration: an up-date and diagnosis algorithm. Rev Neurol. 2013;169:811–19. doi: 10.1016/j.neurol.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 49.Goldman JS, Rademakers R, Huey ED, et al. An algorithm for genetic testing of frontotemporal lobar degeneration. Neurology. 2011;76:475–83. doi: 10.1212/WNL.0b013e31820a0d13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9orf72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–56. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rademakers R, Neumann M, Mackenzie IR. Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol. 2012;8:423–34. doi: 10.1038/nrneurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Renton AE, Majounie E, Waite A, et al. and the ITALSGEN Consortium. A hexanucleotide repeat expansion in C9orf72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–68. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–05. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 54.Gijselinck I, Van Broeckhoven C, Cruts M. Granulin mutations associated with frontotemporal lobar degeneration and related disorders: an update. Hum Mutat. 2008;29:1373–86. doi: 10.1002/humu.20785. [DOI] [PubMed] [Google Scholar]
- 55.Shankaran SS, Capell A, Hruscha AT, et al. Missense mutations in the progranulin gene linked to frontotemporal lobar degeneration with ubiquitin-immunoreactive inclusions reduce progranulin production and secretion. J Biol Chem. 2008;283:1744–53. doi: 10.1074/jbc.M705115200. [DOI] [PubMed] [Google Scholar]
- 56.Toh H, Chitramuthu BP, Bennett HP, Bateman A. Structure, function, and mechanism of progranulin; the brain and beyond. J Mol Neurosci. 2011;45:538–48. doi: 10.1007/s12031-011-9569-4. [DOI] [PubMed] [Google Scholar]
- 57.Tang W, Lu Y, Tian QY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478–84. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller ZA, Rankin KP, Graff -Radford NR, et al. TDP-43 frontotemporal lobar degeneration and autoimmune disease. J Neurol Neurosurg Psychiatry. 2013;84:956–62. doi: 10.1136/jnnp-2012-304644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lattante S, Rouleau GA, Kabashi E. TARDBP and FUS mutations associated with amyotrophic lateral sclerosis: summary and update. Hum Mutat. 2013;34:812–26. doi: 10.1002/humu.22319. [DOI] [PubMed] [Google Scholar]
- 60.Gendron TF, Bieniek KF, Zhang YJ, et al. Antisense transcripts of the expanded C9orf72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS. Acta Neuropathol. 2013;126:829–44. doi: 10.1007/s00401-013-1192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mizielinska S, Lashley T, Norona FE, et al. C9orf72 frontotemporal lobar degeneration is characterised by frequent neuronal sense and antisense RNA foci. Acta Neuropathol. 2013;126:845–57. doi: 10.1007/s00401-013-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mori K, Weng SM, Arzberger T, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ ALS. Science. 2013;339:1335–38. doi: 10.1126/science.1232927. [DOI] [PubMed] [Google Scholar]
- 63.Farg MA, Sundaramoorthy V, Sultana JM, et al. C9orf72, implicated in amytrophic lateral sclerosis and frontotemporal dementia, regulates endosomal trafficking. Hum Mol Genet. 2014;23:3579–95. doi: 10.1093/hmg/ddu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mackenzie IR, Frick P, Neumann M. The neuropathology associated with repeat expansions in the C9orf72 gene. Acta Neuropathol. 2014;127:347–57. doi: 10.1007/s00401-013-1232-4. [DOI] [PubMed] [Google Scholar]
- 65.Whitwell JL, Weigand SD, Boeve BF, et al. Neuroimaging signatures of frontotemporal dementia genetics: C9orf72, tau, progranulin and sporadics. Brain. 2012;135:794–806. doi: 10.1093/brain/aws001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rohrer JD, Ridgway GR, Modat M, et al. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. Neuroimage. 2010;53:1070–76. doi: 10.1016/j.neuroimage.2009.12.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cruts M, Theuns J, Van Broeckhoven C. Locus-specific mutation databases for neurodegenerative brain diseases. Hum Mutat. 2012;33:1340–44. doi: 10.1002/humu.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y, Yu JT, Zong Y, Zhou J, Tan L. C9orf72 mutations in neurodegenerative diseases. Mol Neurobiol. 2014;49:386–98. doi: 10.1007/s12035-013-8528-1. [DOI] [PubMed] [Google Scholar]
- 69.Nalbandian A, Donkervoort S, Dec E, et al. The multiple faces of valosin-containing protein-associated diseases: inclusion body myopathy with Paget’s disease of bone, frontotemporal dementia, and amyotrophic lateral sclerosis. J Mol Neurosci. 2011;45:522–31. doi: 10.1007/s12031-011-9627-y. [DOI] [PubMed] [Google Scholar]
- 70.Holm IE, Englund E, Mackenzie IR, Johannsen P, Isaacs AM. A reassessment of the neuropathology of frontotemporal dementia linked to chromosome 3. J Neuropathol Exp Neurol. 2007;66:884–91. doi: 10.1097/nen.0b013e3181567f02. [DOI] [PubMed] [Google Scholar]
- 71.Boxer A. Establishing therapeutic efficacy in familial frontotemporal degeneration. San Francisco, CA, USA: Nov 14–15, 2013. [Google Scholar]
- 72.Lebert F, Stekke W, Hasenbroekx C, Pasquier F. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17:355–59. doi: 10.1159/000077171. [DOI] [PubMed] [Google Scholar]
- 73.Asmal L, Flegar SJ, Wang J, Rummel-Kluge C, Komossa K, Leucht S. Quetiapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2013;11:CD006625. doi: 10.1002/14651858.CD006625.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.US Food and Drug Administration. [(accessed Nov 10, 2014)];Information for healthcare professionals: conventional antipsychotics. 2008 Jun 16; http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124830.htm.
- 75.Mendez MF, Shapira JS, McMurtray A, Licht E. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry. 2007;15:84–87. doi: 10.1097/01.JGP.0000231744.69631.33. [DOI] [PubMed] [Google Scholar]
- 76.Boxer AL, Knopman DS, Kaufer DI, et al. Memantine in patients with frontotemporal lobar degeneration: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2013;12:149–56. doi: 10.1016/S1474-4422(12)70320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanders DW, Kaufman SK, DeVos SL, et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron. 2014;82:1271–88. doi: 10.1016/j.neuron.2014.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yanamandra K, Kfoury N, Jiang H, et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron. 2013;80:402–14. doi: 10.1016/j.neuron.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wischik CM, Harrington CR, Storey JM. Tau-aggregation inhibitor therapy for Alzheimer’s disease. Biochem Pharmacol. 2014;88:529–39. doi: 10.1016/j.bcp.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 80.Lagier-Tourenne C, Baughn M, Rigo F, et al. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc Natl Acad Sci USA. 2013;110:E4530–39. doi: 10.1073/pnas.1318835110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeVos SL, Goncharoff DK, Chen G, et al. Antisense reduction of tau in adult mice protects against seizures. J Neurosci. 2013;33:12887–97. doi: 10.1523/JNEUROSCI.2107-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cenik B, Sephton CF, Dewey CM, et al. Suberoylanilide hydroxamic acid (vorinostat) up-regulates progranulin transcription: rational therapeutic approach to frontotemporal dementia. J Biol Chem. 2011;286:16101–08. doi: 10.1074/jbc.M110.193433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Capell A, Liebscher S, Fellerer K, et al. Rescue of progranulin deficiency associated with frontotemporal lobar degeneration by alkalizing reagents and inhibition of vacuolar ATPase. J Neurosci. 2011;31:1885–94. doi: 10.1523/JNEUROSCI.5757-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]