Abstract
Background
The prevalence of severe obesity, often considered a contraindication to peritoneal dialysis (PD), has increased over time. However, mortality has decreased more rapidly in the PD population than the hemodialysis (HD) population in the United States. The association between obesity and clinical outcomes among patients with end-stage kidney disease remains unclear in the current era.
Study Design
Historical cohort study.
Setting & Participants
15,573 incident PD patients from a large US dialysis organization (2007–2011).
Predictor
Body mass index (BMI).
Outcomes
Modality longevity, residual renal creatinine clearance, peritonitis, and survival.
Results
Higher BMI was significantly associated with shorter time to transfer to HD (P for trend <0.001), longer time to kidney transplantation (P for trend <0.001), and, with borderline significance, with more frequent peritonitis-related hospitalization (P for trend =0.05). Compared to lean patients, obese patients had faster declines in residual kidney function (P for trend <0.001), and consistently achieved lower total Kt/V over time (P for trend <0.001) despite greater increases in dialysis Kt/V (P for trend <0.001). There was a U-shaped association between BMI and mortality, with the greatest survival associated with the BMI range of 30–<35 kg/m2 in the case-mix adjusted model. Compared to matched HD patients, PD patients had lower mortality for BMI <35 kg/m2 (P for interaction =0.1 for BMI 25–<35 [versus <25] kg/m2) and had equivalent survival in the BMI category ≥35 kg/m2 (P for interaction =0.001). This attenuation in survival difference among patients with severe obesity was only observed in patients with diabetes, but not in those without diabetes.
Limitations
Inability to evaluate causal associations. Potential indication bias.
Conclusions
Whereas obese PD patients had a higher risk of complications than non-obese PD patients, their survival was no worse than matched HD patients.
Keywords: peritoneal dialysis (PD), hemodialysis (HD), obesity, diabetes, mortality, survival advantage, technical failure, technical survival, peritonitis, residual kidney function (RKF), creatinine clearance (CLcr), kidney transplantation, obesity paradox, dialysis modality, end-stage renal disease (ESRD), renal replacement therapy (RRT)
The prevalence and severity of obesity among patients with end-stage renal disease (ESRD) has increased over time since the mid-1980s in developed countries.1 This epidemic has occurred in parallel with trends in the general population, but has been more dramatic among patients with ESRD in the United States.2–4 This difference in the observed secular trends of obesity can be explained by the heightened risk of chronic kidney disease among obese patients and those with diabetes,5,6 and also by the “obesity paradox” of ESRD patients in whom obesity is unexpectedly associated with greater survival.7–10
Interestingly, whereas the obesity paradox has consistently been observed among ESRD patients on hemodialysis (HD), there are inconsistent data among those receiving peritoneal dialysis (PD).11–17 It is generally thought that dialysis clearance may be less adequate in obese PD versus HD patients due to less efficient solute and fluid removal, although a small observational study has shown feasibility of achieving adequate solute clearance in obese PD patients.18 Other studies have shown that obesity is associated with higher risk of peritonitis and more rapid decline in residual kidney function,19,20 both important risk factors for death and transfer to HD.21–23 Indeed, a previous study of US Renal Data System (USRDS) data demonstrated that PD patients with obesity, defined as those with BMI ≥30 kg/m2, showed faster transfer to HD yet similar survival compared to PD patients without obesity.14 Furthermore, among ESRD patients who are obese, receipt of PD has been associated with equivalent or higher mortality as compared with receipt of HD.24,25 These data have discouraged nephrologists from recommending PD as a treatment option for obese ESRD patients,26,27 and some facilities have listed severe obesity as a contraindication to PD.28–32
However, most of these data were derived from non-contemporary cohorts who may not be generalizable to present-day dialysis populations. Mortality has decreased more rapidly in the PD versus HD population in the United States,4,33 likely due to advances in PD delivery, efficacy, and safety over the past two decades. Indeed, there is a progressive attenuation in mortality risk associated with PD in more recent cohorts.34 Nevertheless, it remains unclear if these advances have influenced the association between obesity and clinical outcomes in the current era. Thus, we hypothesized that the severity of obesity, expressed as body mass index (BMI), is incrementally associated with adverse clinical outcomes, and that the survival advantage of PD is attenuated among obese ESRD patients.
METHODS
Patients
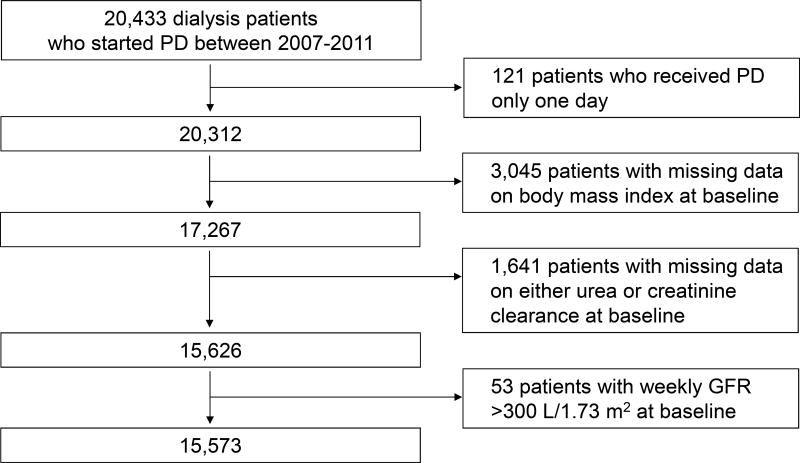
We retrospectively extracted, refined, and examined data from all incident ESRD patients who were aged 18 years or older in facilities operated by a large dialysis organization in the United States from January 1, 2007 through December 31, 2011.35 Data used for analyses were de-identified. We selected patients who underwent PD more than 1 day during the follow-up period. We then excluded patients without data on BMI or residual renal clearances of urea and creatinine during the first 91 days of PD (Figure 1). We further excluded patients with weekly renal creatinine clearance >300 L/1.73 m2. Differences in characteristics at PD initiation between included versus excluded patients were compared by standardized differences due to the relatively large sample size of this study (Table S1, available as online supplementary material).36,37
Figure 1.
Flow diagram summarizing the criteria used to constitute the analytic cohort. Abbreviations: PD, peritoneal dialysis; GFR, glomerular filtration rate.
This study was approved by the Institutional Review Committees of the Los Angeles Biomedical Research Institute at Harbor-UCLA, University of California Irvine Medical Center, and the University of Washington with the exemption of obtaining written consent given the large sample size, anonymity of the patients studied, and nonintrusive nature of the research.
Demographic, Clinical, and Laboratory Measures
All information were obtained from the electronic database of the dialysis provider. Blood samples were drawn using uniform techniques in all dialysis clinics and were transported to a central laboratory in Deland, Florida, typically within 24 hours. To minimize measurement variability, all repeated measures for each patient during the first quarter (or 91 days) of PD were averaged and then used as baseline data in all analyses. We calculated residual renal creatinine clearance (ClCr) as the average of renal urea and creatinine clearances, indexing to body surface area.38 Actual body weight, not ideal body weight, was used to calculate Kt/V.
BMI was categorized in six groups (<20, 20–<25, 25–<30, 30–<35, 35–<40, and ≥40 kg/m2). Given the established cardiovascular risk profiles among HD patients, severe obesity was defined as a BMI of ≥35 kg/m2.39
Statistics for Clinical Outcomes Among Patients Receiving PD
Patients were followed up from their first day of PD until their death or 60 days after transfer to HD or kidney transplantation (KTx). The outcomes of interest were all-cause death, transfer to HD, KTx, peritonitis-related and non-peritonitis-related hospitalization, and trajectories of solute clearance indices (i.e., renal ClCr and renal, peritoneal, and total weekly Kt/V). Transfer to HD was defined as not undergoing PD for ≥60 days.
The associations of the BMI categories with the competing outcomes of death, transfer to HD, and KTx were examined in the entire PD cohort using cause-specific hazards models by treating competing events as censoring. Proportional hazards assumptions were tested using log-log plots and Schoenfeld residuals. The associations with peritonitis-related and non-peritonitis-related hospitalization were also examined by the negative binominal regression model.
Models were examined with three-level sequential adjustments as follows: 1) Unadjusted model that included the BMI categories only; 2) Case-mix adjusted model that included age, sex, race/ethnicity, cause of ESRD, six comorbidities in Table 1, log-transformed dialysis vintage, and log-transformed 5-year cumulative number of incident PD patients treated per facility (as a proxy for "PD facility experience"); and 3) Fully adjusted model which included all of the covariates in the case-mix model plus log-transformed weekly renal ClCr, weekly peritoneal Kt/V, normalized protein nitrogen appearance (nPNA), and six laboratory variables in Table 1. The case-mix adjusted model was a priori defined as the primary model given the potential over-adjustment in the fully adjusted model.
Table 1.
Characteristics of 15,573 incident PD patients (2007–2011) who have data on both BMI and renal clearances of urea and creatinine at baseline across six BMI categories.
| <20 kg/m2 | 20 – <25 kg/m2 | 25 – <30 kg/m2 | 30 – <35 kg/m2 | 35 – <40 kg/m2 | ≥40 kg/m2 |
P for trend |
|
|---|---|---|---|---|---|---|---|
| Variable | (n=861) | (n=3,971) | (n=4,869) | (n=3,121) | (n=1,487) | (n=820) | |
| BMI (kg/m2) | 19 (18 to 20) | 23 (22 to 24) | 27 (26 to 29) | 32 (31 to 33) | 37 (36 to 38) | 43 (41 to 46) | <0.001 |
| Age (years) | 54±19 | 57±17 | 58±15 | 56±14 | 54±13 | 51±12 | <0.001 |
| Male sex | 37% | 59% | 64% | 60% | 49% | 39% | <0.001 |
| Race | |||||||
| Non-Hispanic white | 56% | 59% | 59% | 58% | 57% | 55% | 0.1 |
| Non-Hispanic black | 19% | 18% | 21% | 24% | 27% | 31% | <0.001 |
| Hispanic | 10% | 12% | 14% | 13% | 11% | 12% | 0.7 |
| Other races | 15% | 11% | 7% | 5% | 4% | 3% | <0.001 |
| Cause of ESRD | |||||||
| Diabetes | 18% | 29% | 41% | 49% | 53% | 55% | <0.001 |
| Hypertension | 30% | 29% | 28% | 25% | 23% | 21% | <0.001 |
| Other causes | 51% | 42% | 30% | 26% | 25% | 24% | <0.001 |
| Dialysis vintage (months) | 2 (1 to 5) | 1 (1 to 5) | 1 (0 to 5) | 1 (1 to 5) | 1 (1 to 6) | 2 (1 to 7) | 0.08 |
| Comorbid conditions | |||||||
| Diabetes | 38% | 51% | 64% | 72% | 73% | 76% | <0.001 |
| Congestive heart failure | 8% | 9% | 8% | 8% | 8% | 9% | 0.9 |
| Myocardial infarction | 11% | 14% | 13% | 13% | 12% | 12% | 0.2 |
| Other cardiac diseases | 15% | 14% | 14% | 13% | 12% | 13% | 0.03 |
| Hypertension | 53% | 54% | 53% | 51% | 51% | 48% | 0.001 |
| COPD | 3% | 3% | 2% | 3% | 3% | 2% | 0.9 |
| No. of 5-year cumulative incident PD patients | 31 (17 to 49) | 32 (19 to 54) | 33 (18 to 54) | 32 (18 to 54) | 32 (18 to 53) | 32 (17 to 57) | 0.5 |
| Weekly CLcr (L/1.73m2) | |||||||
| Renal | 52 (28 to 87) | 55 (30 to 91) | 61 (34 to 96) | 64 (36 to 100) | 63 (34 to 101) | 62 (35 to 101) | <0.001 |
| Peritoneal | 37±13 | 37±12 | 36±11 | 34±11 | 34±11 | 35±10 | <0.001 |
| Total | 90 (67 to 122) | 94 (69 to 125) | 97 (72 to 130) | 99 (73 to 133) | 97 (71 to 132) | 95 (71 to 134) | <0.001 |
| Weekly Kt/V | |||||||
| Renal | 0.9 (0.5 to 1.5) | 0.9 (0.5 to 1.5) | 1.0 (0.5 to 1.5) | 1.0 (0.5 to 1.5) | 0.9 (0.5 to 1.5) | 0.9 (0.5 to 1.4) | 0.5 |
| Peritoneal | 1.7±0.5 | 1.6±0.4 | 1.5±0.4 | 1.4±0.4 | 1.3±0.4 | 1.3±0.4 | <0.001 |
| Total | 2.6 (2.1 to 3.2) | 2.5 (2.1 to 3.0) | 2.4 (2.0 to 3.0) | 2.4 (2.0 to 2.9) | 2.3 (1.9 to 2.8) | 2.2 (1.8 to 2.7) | <0.001 |
| Normalized PNA (g/kg/d) | 1.05±0.30 | 1.01±0.26 | 0.98±0.26 | 0.94±0.25 | 0.92±0.24 | 0.88±0.23 | <0.001 |
| Laboratory variables | |||||||
| Hemoglobin (g/dL) | 11.9±1.5 | 11.8±1.3 | 11.8±1.3 | 11.7±1.3 | 11.6±1.2 | 11.4±1.2 | <0.001 |
| Albumin (g/dL) | 3.5±0.5 | 3.6±0.5 | 3.6±0.5 | 3.7±0.4 | 3.7±0.4 | 3.6±0.4 | <0.001 |
| Creatinine (mg/dL) | 5.6 (4.2 to 8.1) | 6.0 (4.5 to 8.4) | 6.1 (4.5 to 8.4) | 6.1 (4.6 to 8.3) | 6.2 (4.6 to 8.5) | 6.2 (4.5 to 8.5) | <0.001 |
| Corrected calcium (mg/dL) | 9.2±0.6 | 9.1±0.6 | 9.1±0.6 | 9.1±0.6 | 9.1±0.6 | 9.1±0.6 | <0.001 |
| Phosphorus (mg/dL) | 4.9±1.3 | 4.8±1.3 | 4.9±1.2 | 4.9±1.2 | 5.0±1.3 | 5.0±1.3 | 0.01 |
| Intact PTH (pg/mL) | 258 (143 to 440) | 268 (165 to 433) | 284 (180 to 439) | 315 (195 to 488) | 334 (218 to 510) | 331 (209 to 567) | <0.001 |
Note: Values for categorical variables are given as percentages; values for continuous variables, as mean ± standard deviation or median [interquartile range]. Baseline refers to first 91 days of PD. Trends across BMI categories were evaluated by non-parametric trend tests. Conversion factors for units: calcium in mg/dL to mmol/L, ×0.2495; creatinine in mg/dL to µmol/L, ×88.4; phosphorus in mg/dL to mmol/L, ×0.3229.
Abbreviations: BMI, body mass index; CLcr, creatinine clearance; ESRD, end-stage renal disease; COPD, chronic obstructive pulmonary disease; PD, peritoneal dialysis; PNA, protein nitrogen appearance; PTH, parathyroid hormone
In order to examine the association of BMI with change in solute clearance indices over time, patient follow-up time was divided into quarters (or 91-day periods) from date of PD initiation up to two years, and we used the linear mixed-effects models with random intercept and slope using unstructured covariance matrices. Each of quarterly-averaged values in solute clearance indices served as the outcome, and we included the BMI categories, case-mix variables, and the interaction terms between quarters and the BMI categories. The interaction terms represent differences in the slopes from baseline.
Statistics for Mortality Comparison Between HD and PD
In order to evaluate whether the severity of obesity affects the between-modality difference in KTx-free mortality, we matched PD patients to HD patients in a 1:2 ratio using nested matching with replacement based on age (within ±2.5 years), sex, race/ethnicity, ESRD reason, dialysis vintage, the six aforementioned BMI categories, and Deyo-Charlson comorbidity index categories (2, 3–4, 5, 6, ≥7).40 In short, except for those who were ever treated with either home HD, infrequent HD, less frequent HD, or nocturnal HD, we identified the number of days from dialysis initiation to PD initiation (vintage day) for each PD patient. Each PD patient was then matched to two patients who were treated with HD at the same dialysis vintage regardless of whether or not matched patients initiated PD later on (as per the intention-to-treat principle). All patients were followed up from this matched vintage day. Matching with replacement generally decreases bias.41 The average of pre- and post-HD weight was used to calculate BMI among HD patients in order to minimize the influence of body fluid dynamics. Among 7,412 patients who were treated with HD and PD in the same quarter of dialysis and who had data on BMI during both modality periods, the within-individual difference in BMI was 0.0±1.3 kg/m2. Laboratory variables were not included for this analysis because they were considered intermediating factors.
The matched cohort of 14,007 PD patients and 28,014 matched HD patients totaled 42,021 patients, and comprised 40,289 individual patients. Patients were followed up even after switching dialysis modality but censored at loss-to-follow-up, or December 31, 2011. KTx was a competing event and treated as censoring in cause-specific hazards models. For calculating the difference in cumulative mortality between HD and PD over time, BMI was categorized into just 3 groups (<25, 25–<35, and ≥35 kg/m2) in order to ensure adequate sample sizes. Given violation of the proportional hazard assumption by treatment modality (i.e., PD vs. HD), the statistical significance of effect modification by BMI category was evaluated by their interaction terms with PD in the cause-specific hazards models including the interaction term between PD and log-transformed time. In subgroup analyses, the cumulative mortality and its between-group differences were provided with their 95% bootstrap confidence intervals (CIs) based on 1,000 bootstrap sampling. Given that the prevalence of diabetes, an established effect modifier of the association between dialysis modality and mortality,42–44 was incrementally higher across increasing BMI categories, we further stratified patients according to diabetes.
Linear assumptions among covariates were examined using restricted cubic spline functions and a likelihood ratio test for goodness of fit.45 Missing baseline data were 4% for dialysis facility and intact PTH and <1.2% for the remaining laboratory variables (Table S2), and were imputed by respective median values. Missing longitudinal data on solute clearance indices (~30% after the second quarter) were not imputed in mixed-effects models. Analyses were conducted using STATA MP version 13.1 (StataCorp, College Station, TX) and SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Baseline Demographic, Clinical, and Laboratory Characteristics
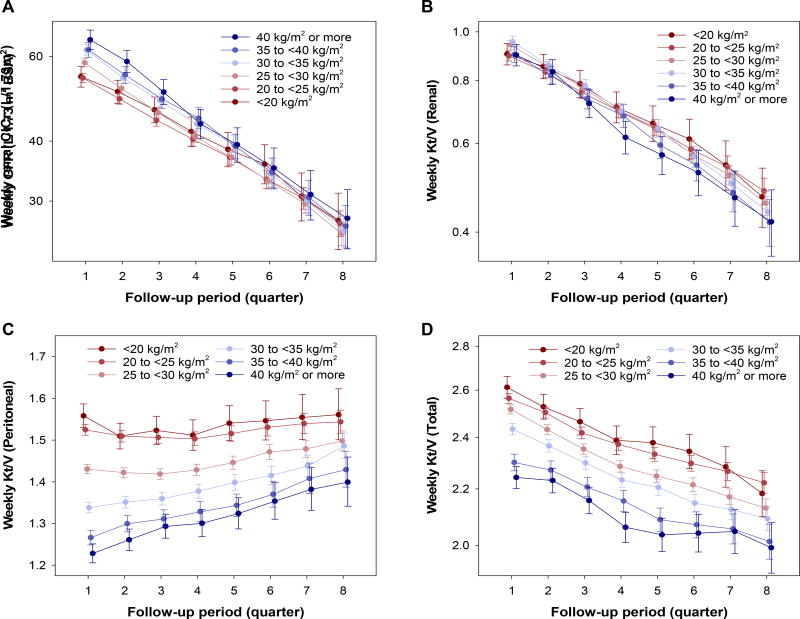
The mean age in the entire PD cohort was 56±15 (standard deviation) years, among whom 58% were male, 58% were non-Hispanic white, 22% were non-Hispanic black, and 62% had diabetes (Table 1). Their median BMI was 28 (interquartile range [IQR], 24–32) kg/m2, and the prevalence of BMI categories <20, 20–<25, 25–<30, 30–<35, 35–<40, and ≥40 kg/m2 was 5.5%, 26%, 32%, 21%, 10%, and 5.5%, respectively. Patients with higher BMI had greater renal ClCr and lower peritoneal ClCr, and there was a significant trend toward greater total ClCr across higher BMI. Meanwhile, renal Kt/V was not different across these BMI categories but patients with higher BMI showed lower peritoneal Kt/V, resulting in lower total Kt/V.
Association of BMI With Clinical Outcomes and Change in Renal ClCr
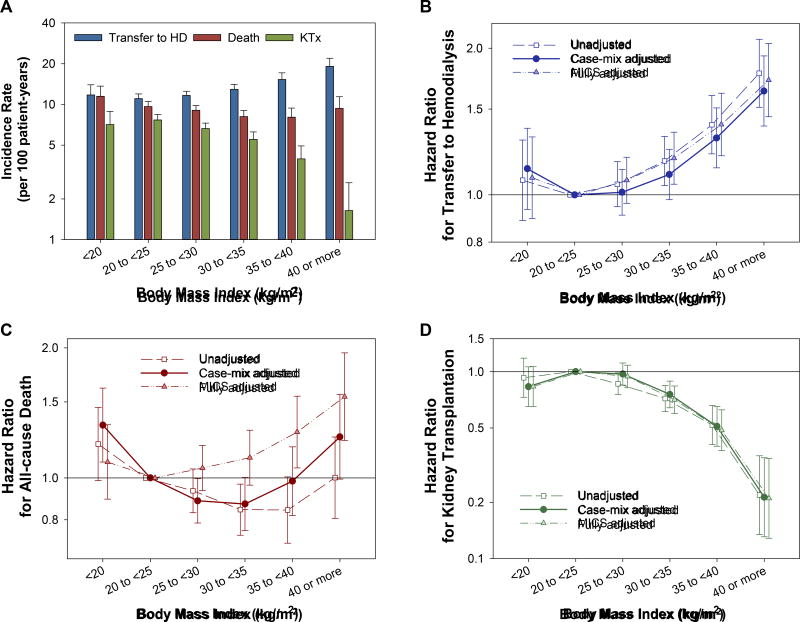
During the follow-up period, 2,568 patients transferred to HD. A total of 1,863 patients died while 1,271 underwent KTx during the follow-up period including 90 days after transferring to HD. The incidence rates of these outcomes across the six BMI categories are shown in Figure 2A. Patient characteristics during the last quarter of PD are summarized in Table 2, according to the outcomes. Specifically, among patients transferred to HD, the median total ClCr and total Kt/V during the last quarter of PD were 68 (IQR, 51–93) and 2.0 (IQR, 1.7–2.5), respectively, and 22% and 23% did not reach the target ClCr and Kt/V, respectively.
Figure 2.
(A) Incidence rate for transfer to HD, all-cause death, and KTx, and the hazard ratios for (B) transfer HD, (C) all-cause death, and (D) KTx across six BMI categories among 15,573 incident PD patients (2007–2011). The association with each outcome was estimated with three-level sequential adjustments. Bars represent 95% confidence intervals. Abbreviations: BMI, body mass index; HD, hemodialysis; PD, peritoneal dialysis; KTx, kidney transplantation.
Table 2.
Patient characteristics during last quarter of PD, stratified by outcomes
| Variable | Transfer to HD | Death | Kidney Transplant | Censoring events |
|---|---|---|---|---|
| (n=2,568) | (n=1,863) | (n=1,271) | (n=9,871) | |
| BMI (kg/m2) | 28 (25 to 33) | 27 (24 to 32) | 26 (23 to 30) | 28 (24 to 32) |
| Age (years) | 57±15 | 66±13 | 48±13 | 57±15 |
| Male sex | 58% | 61% | 59% | 57% |
| Race | ||||
| Non-Hispanic white | 54% | 74% | 64% | 56% |
| Non-Hispanic black | 27% | 13% | 18% | 23% |
| Hispanic | 14% | 8% | 10% | 13% |
| Other races | 6% | 6% | 9% | 8% |
| Cause of ESRD | ||||
| Diabetes | 45% | 51% | 27% | 39% |
| Hypertension | 28% | 26% | 22% | 28% |
| Other causes | 27% | 23% | 51% | 33% |
| Dialysis vintage (months) | 17 (11 to 26) | 18 (10 to 28) | 16 (9 to 26) | 18 (9 to 30) |
| Comorbid conditions | ||||
| Diabetes | 71% | 71% | 44% | 61% |
| Congestive heart failure | 15% | 13% | 3% | 6% |
| Myocardial infarction | 17% | 21% | 8% | 11% |
| Other cardiac diseases | 20% | 20% | 10% | 12% |
| Hypertension | 60% | 48% | 47% | 52% |
| COPD | 4% | 4% | 1% | 2% |
| No. of Five-year cumulative incident PD patients | 31 (18 to 53) | 34 (19 to 54) | 36 (21 to 55) | 32 (17 to 53) |
| Weekly CLcr | ||||
| Renal (L/week) | 25 (7 to 55) | 15 (0 to 42) | 42 (14 to 83) | 41 (13 to 79) |
| Peritoneal (L/week) | 39±13 | 40±12 | 35±13 | 36±13 |
| Total (L/week) | 68 (51 to 93) | 60 (48 to 81) | 79 (56 to 111) | 77 (56 to 111) |
| Total <50 L/week (%) | 22% | 31% | 17% | 16% |
| Weekly Kt/V | ||||
| Renal | 0.5 (0.3 to 1.0) | 0.4 (0.2 to 0.9) | 0.9 (0.4 to 1.5) | 0.8 (0.4 to 1.4) |
| Peritoneal | 1.5±0.5 | 1.6±0.4 | 1.5±0.4 | 1.5±0.4 |
| Total | 2.0 (1.7 to 2.5) | 1.9 (1.7 to 2.3) | 2.2 (1.9 to 2.7) | 2.2 (1.9 to 2.6) |
| Total <1.7 (%) | 23% | 24% | 8% | 10% |
| Laboratory variables | ||||
| Hemoglobin (g/dL) | 11.2±1.4 | 11.2±1.4 | 11.6±1.3 | 11.0±1.3 |
| Albumin (g/dL) | 3.4±0.5 | 3.1±0.5 | 3.8±0.4 | 3.7±0.5 |
| Creatinine (mg/dL) | 8.3 (5.9 to 11.3) | 7.3 (5.3 to 9.5) | 8.6 (5.9 to 12.4) | 7.7 (5.3 to 10.8) |
| Corrected calcium (mg/dL) | 9.1±0.7 | 9.2±0.6 | 9.2±0.5 | 9.1±0.6 |
| Phosphorus (mg/dL) | 5.5±1.8 | 5.3±1.6 | 5.3±1.3 | 5.1±1.4 |
| Intact PTH (pg/mL) | 314 (203 to 486) | 257 (159 to 417) | 303 (213 to 457) | 332 (213 to 509) |
Note: Note: Values for categorical variables are given as percentages; values for continuous variables, as mean ± standard deviation or median [interquartile range]. Conversion factors for units: calcium in mg/dL to mmol/L, ×0.2495; creatinine in mg/dL to µmol/L, ×88.4; phosphorus in mg/dL to mmol/L, ×0.3229.
Abbreviations: CLcr, creatinine clearance; ESRD, end-stage renal disease; COPD, chronic obstructive pulmonary disease; HD. Hemodialysis; PD, peritoneal dialysis; PTH, parathyroid hormone
There was a trend towards higher risk of transfer to HD across higher BMI categories (Ptrend <0.001 for all adjustment models; Figure 2B). In the primary model adjusted for case-mix covariates using the “optimal” BMI (i.e., 20 to <25 kg/m2)1 as the reference group, the risk for transfer to HD was significantly higher in BMI categories of 35–<40 and ≥40 kg/m2. The risk associated with BMI categories of <20 and 25–<30 kg/m2 were not significant in all models, while the BMI category of 30–<35 kg/m2 was associated with shorter time to transfer to HD in the unadjusted and fully-adjusted models but not in the case-mix adjusted mode.
We observed a U-shaped association between BMI and all-cause mortality, with the greatest survival in the BMI range of 30–<35 kg/m2 in the case-mix adjusted model (Figure 2C). The mortality risk of the lowest of the six BMI categories (i.e., <20 kg/m2) was incrementally attenuated with sequential adjustments. Lower mortality risk associated with the higher BMI categories (i.e., ≥25 kg/m2) was also attenuated by these adjustments and even reversed in the BMI categories ≥35 kg/m2 after adjustment for laboratory variables. Higher BMI showed consistent associations with lower likelihood of undergoing KTx across all adjustment models, particularly in BMI categories >30 kg/m2 (Ptrend <0.001; Figure 2D).
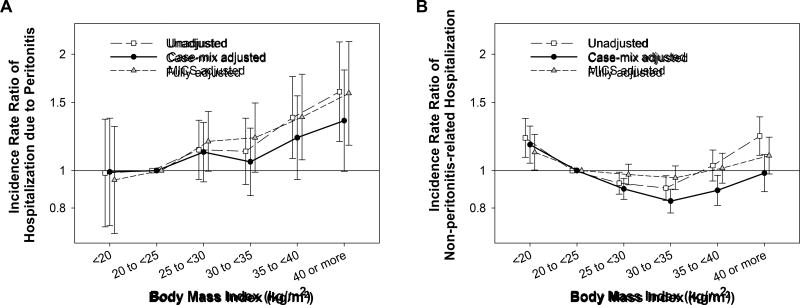
We then examined the associations of BMI with peritonitis-related and non-peritonitis-related hospitalizations. There were 2,331 hospitalizations due to peritonitis among 1,315 patients and 32,723 non-peritonitis-related hospitalizations among 9,442 patients. There was a trend towards higher incidence of peritonitis-related hospitalization across higher BMI categories in all adjustment models (Ptrend = <0.001, 0.05, and <0.001 in the unadjusted, case-mix, and fully adjusted models, respectively; Figure 3A). Conversely, higher BMI was associated with lower incidence of non-peritonitis-related hospitalization in the case-mix adjusted model (Ptrend <0.001) although this association was not significant in the unadjusted and fully adjusted models (Ptrend = 0.8 and 0.9, respectively; Figure 3B).
Figure 3.
The association of BMI with incidence rate of (A) peritonitis-related and (B) non-peritonitis-related hospitalization among 15,573 incident PD patients (2007–2011). Incidence rate ratios were estimated with three-level sequential adjustments. Bars represent 95% confidence intervals. Abbreviations: BMI, body mass index; PD, peritoneal dialysis.
We also compared changes in solute clearance indices across the six BMI categories. Obese patients started PD at higher renal ClCr levels but had faster decline in renal ClCr (Ptrend <0.001; Figure 4A). Renal Kt/V was comparable at baseline across BMI categories, but it also declined faster among obese patients (Ptrend <0.001; Figure 4B). Obese patients, when compared with lean patients, showed lower peritoneal Kt/V at baseline and had greater increase in peritoneal Kt/V (Ptrend <0.001; Figure 4C). Consequently, the rate of decline in total Kt/V was not different across BMI categories (Ptrend =0.6), and lower total Kt/V among obese versus lean patients persisted during two years of PD (Ptrend <0.001; Figure 4D).
Figure 4.
Two-year trajectories of weekly (A) renal ClCr and (B) renal, (C) peritoneal, and (D) total Kt/V across BMI categories among 15,573 incident PD patients (2007–2011) estimated by linear mixed-effects model with adjustment for case-mix variables. Points and bars represent estimates and 95% CIs, respectively. Abbreviations: BMI, body mass index; BSA, body surface area; ClCr, creatinine clearance.
Mortality Among Patients on PD Versus HD by BMI Category
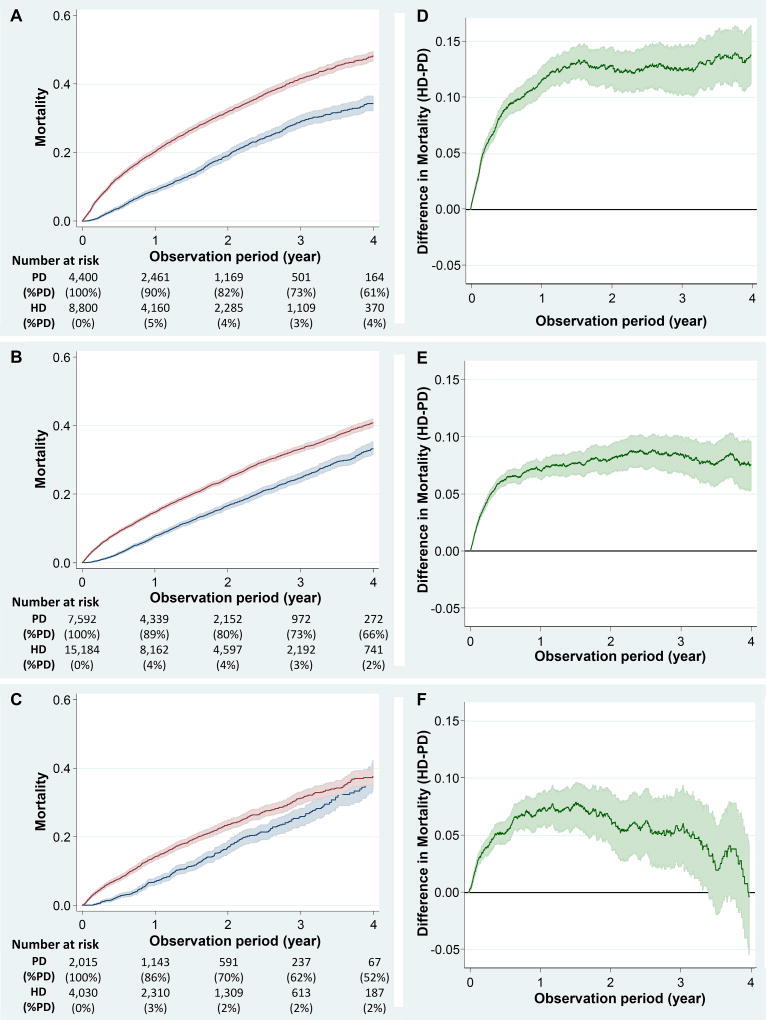
A total of 14,007 PD patients (90%) were matched to 28,014 HD patients using 1:2 matching based on BMI, age, sex, race/ethnicity, ESRD reason, dialysis vintage, the three BMI categories (ie, <25, 25–<35, and ≥35 kg/m2) used for this analysis, and Charlson comorbidity index. Patient characteristics are summarized in Table 3. Obese patients tended to be younger, female, and non-Hispanic black; were more likely to have diabetes as the cause of ESRD; and had higher Charlson comorbidity index scores (Ptrend <0.001 for all). In this matched cohort, patients were followed even after changing their treatment modality. Among incident PD patients, the proportion of patients still on PD at Year 4 was lowest (52%) among patients with BMI ≥35 kg/m2 when compared to those with BMI <25 kg/m2 versus 25–<35 kg/m2 (i.e., 61% vs. 66%, respectively) (Figure 5A–C). BMI significantly modified the association between dialysis modality and mortality (P for interaction =0.01). The between-group difference in mortality (HD – PD) increased especially during the first year across the three BMI categories, and was 11% (95% CI, 10%–13%), 7% (95% CI, 6%–8%), and 7% (95% CI, 6%–9%) at Year 1 in the lowest, middle, and highest BMI stratum, respectively (Figure 5D–F). While PD patients with BMI <35 kg/m2 consistently showed lower mortality for up to four years (Pinteraction =0.1), the survival advantage of PD over HD was attenuated over time among patients with BMI ≥35 kg/m2 (versus <25 kg/m2; Pinteraction =0.001), and the significant difference was limited to up to 1,250 days.
Table 3.
Characteristics of 14,007 PD patients versus 28,014 matched HD patients across three BMI categories.
| BMI <25 kg/m2 | BMI 25–<35 kg/m2 | BMI ≥35 kg/m2 | ||||
|---|---|---|---|---|---|---|
| HD | PD | HD | PD | HD | PD | |
| BMI (kg/m2) | 22 (21 to 24) | 23 (21 to 24) | 29 (27 to 31) | 29 (27 to 31) | 39 (37 to 42) | 38 (36 to 42) |
| Age (years) | 57±17 | 57±17 | 58±14 | 58±14 | 54±12 | 54±12 |
| Male sex | 57% | 57% | 63% | 63% | 45% | 45% |
| Race/Ethnicity | ||||||
| Non-Hispanic white | 62% | 62% | 60% | 60% | 59% | 59% |
| Non-Hispanic black | 18% | 18% | 22% | 22% | 29% | 29% |
| Hispanic | 11% | 11% | 13% | 13% | 10% | 10% |
| Other races | 9% | 9% | 4% | 4% | 2% | 2% |
| Cause of ESRD | ||||||
| Diabetes | 29% | 29% | 46% | 46% | 59% | 59% |
| Hypertension | 30% | 30% | 27% | 27% | 22% | 22% |
| Other causes | 41% | 41% | 27% | 27% | 19% | 19% |
| Dialysis vintage (months) | 1 (1 to 4) | 1 (1 to 4) | 1 (0 to 4) | 1 (0 to 4) | 1 (1 to 5) | 1 (1 to 5) |
| Charlson comorbidity index | 2 (1 to 3) | 2 (1 to 3) | 2 (1 to 3) | 2 (1 to 3) | 2 (2 to 3) | 2 (2 to 3) |
| 2 | 44% | 44% | 29% | 29% | 21% | 21% |
| 3–4 | 30% | 30% | 33% | 33% | 34% | 34% |
| 5 | 21% | 21% | 31% | 31% | 36% | 36% |
| 6 | 4% | 4% | 6% | 6% | 7% | 7% |
| ≥7 | 2% | 2% | 2% | 2% | 2% | 2% |
Note: Note: Values for categorical variables are given as percentages; values for continuous variables, as mean ± standard deviation or median [interquartile range].
Abbreviations: BMI, body mass index; ESRD, end-stage renal disease; HD, hemodialysis; PD, peritoneal dialysis
Figure 5.
The incidence (A–C) and between-group difference (D–F) in all-cause death across three BMI categories among 14,007 PD patients vs. 28,014 HD patients matched based on BMI, age, sex, race/ethnicity, ESRD reason, Charlson comorbidity index, and dialysis vintage. Patients were followed up even after changing modality to either PD or HD. The curves were truncated at 4 years given <5% patients remaining at risk. Abbreviations: BMI, body mass index; HD, hemodialysis; PD, peritoneal dialysis.
Additionally, the association between dialysis modality and mortality was further modified by diabetes (Pinteraction <0.001). While PD was consistently associated with lower mortality among non-diabetic ESRD patients irrespective of BMI strata (Ptrend for interaction =0.6), attenuation in the survival difference was observed among patients with diabetes when BMI exceeded 35 kg/m2 (Pinteraction = 0.007 [versus <25 kg/m2]; Figure S1).
DISCUSSION
In this large, nationally representative and contemporary cohort of 15,573 incident PD patients in the United Sates, we found that higher BMI was associated with shorter time to transfer to HD, longer time to KTx, and more frequent peritonitis-related hospitalization, and that there was a U-shaped association between BMI and mortality. Obese patients had faster decline in residual kidney function, and consistently showed lower total Kt/V over time despite greater increase in dialysis Kt/V. When compared to matched HD patients, PD patients consistently showed lower mortality if their BMI was <35 kg/m2. However, there were no significant differences in mortality between modalities among severely obese patients, especially among those who had diabetes.
In previous studies, obesity has similarly been associated with shorter time to transfer to HD,14,46 higher risk of peritonitis,19 and faster decline in residual kidney function.20 However, most of these prior studies used BMI as a continuous variable or categorized BMI into 2–3 groups, limiting their ability to detect a threshold of BMI where risk starts to increase. The large sample size of this study enabled us to examine more granularly-defined BMI categories, and we found that in BMI categories ≥30 kg/m2, there was an incremental risk of transfer to HD along with faster decline in residual kidney function.
An increased risk of peritonitis-associated hospitalization was also observed in higher BMI, but the lack of significant association in each category may be attributed to the limited number of events captured in our administrative dataset. Peritonitis-related hospitalization was observed in 8% of the study population, but the overall peritonitis event should be more frequent if we accounted for those treated in the outpatient setting. Potential mechanisms include protracted wound healing, greater difficulties in the daily care of the exit site, and increased susceptibility against skin and soft tissue infection.
The reason for the transfer from PD to HD is often multifactorial, and we were unable to identify definite reason(s) for each individual case in the administrative data. However, our findings suggested that among patients who transferred to HD, approximately one-fourth of them did not reach the minimum solute clearance (i.e., 1.7 of weekly Kt/V)47 during the last quarter of PD. Obese patients consistently showed lower Kt/V over time than lean patients largely due to the mathematical coupling between BMI and urea distribution volume “V” based on actual body weight, which might have led to faster transfer to HD among obese patients. However, the Watson formula overestimates V in obese patients, resulting in lower-than-actual Kt/V.48 KDOQI (Kidney Disease Outcomes Quality Initiative) guidelines suggest considering the use of the patient’s ideal (or standard weight)38 or the use of body surface area instead of V.47 Another unsolved issue is that we cannot separate the contribution of different body compositions (i.e., muscle versus fat) based on the anthropometric measures. Further studies are needed to develop a better metric to assess dialysis adequacy among PD patients across a wide range of body weights and different body compositions.
The association between treatment modality and mortality has been shown to be modified by obesity in some studies,24,25 and has also changed over time favoring PD.4,34 Using USRDS data between 1995 and 1997, Stack et al. showed a heightened risk of death among PD patients with BMI ≥23.5 kg/m2 (versus HD patients in the same BMI categories) while comparable survival was observed among those with lower BMI.24 In a previous report from our group,25 we found comparable mortality risk among PD and HD patients with BMI ≥35 kg/m2, and lower mortality risk associated with PD among less obese patients using administrative data between 2001 and 2006 from the same dialysis organization. In the present study, we examined a more contemporary incident dialysis cohort (2007–2011), and consistently observed lower mortality among PD versus HD patients across BMI categories in non-diabetic patients. Diabetic PD patients also showed lower mortality compared with their diabetic HD counterparts if their BMI was <35 kg/m2, and they had similar mortality even in the presence of severe obesity. Advances in PD during these two decades have likely contributed to improved survival in the PD population, and might have modified the modality-mortality association in obese patients.
We acknowledge several limitations in this study. First, due to the observational nature of our study, we can neither exclude the presence of residual confounding and unmeasured confounders (i.e., regions,49 socioeconomic status,50 elective outpatient initiation51) nor prove causality between obesity and outcomes. Second, there may be selection bias because residual renal clearance was not consistently measured in all patients. Specifically, included patients were likely to be treated in facilities with more PD experience and to have greater residual kidney function (Table S1), and, hence, our results may not be extrapolated to patients who are treated in less experienced facilities or among patients with little or no residual kidney function. Third, we did not have post-KTx information. Censoring patients at KTx might have resulted in underestimation of mortality risk associated with obesity because non-obese patients are more likely to undergo KTx, which substantially improves survival of ESRD patients. However, this censoring is unlikely to affect the results of survival comparisons between PD versus HD because the relative likelihood of undergoing KTx between dialysis modalities are similar across BMI categories.25
In conclusion, obesity is associated with various adverse outcomes among PD patients but should not be considered an absolute contraindication to PD given the equivalent mortality of obese PD versus HD patients observed in our study. These findings make it imperative to implement interventions and strategies that would safely prolong time on PD among obese patients, such as larger dwell volumes, frequent evaluation of residual kidney function with proper adjustment of PD prescription, as well as prevention of peritonitis and residual kidney function decline. Further studies are also needed to test whether successful weight reduction reduces PD-related adverse events and enhances the survival advantage of PD among obese patients.
Supplementary Material
Figure S1: Kaplan-Meier estimates in overall survival across three BMI categories among PD patients vs matched HD patients stratified by diabetes history.
Table S1: Characteristics of included vs excluded PD patients during first 91 days of PD.
Table S2: Missing frequencies and percentages among the covariates.
Acknowledgments
Support: The work in this study has been performed with the support of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institute of Health research grants R01-DK95668 (RM and KKZ), K24-DK091419 (KKZ), R01-DK078106 (KKZ) KKZ is supported by philanthropic grants from Mr. Harold Simmons, Mr. Louis Chang, Dr. Joseph Lee and Aveo. CPK is supported by NIDDK grants R01-DK096920 and U01-DK102163. CMR is supposed by NIDDK grant K23-DK102903. ES is supported by the VA Career Development Award IK2-CX 001266-01. YO is supported by the Uehara Memorial Foundation Research Fellowship. None of the funders had any role in study design; collection, analysis, or interpretation of data; or writing the report or the decision to submit the report for publication.
Dr Kalantar-Zadeh has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, American Society of Nephrology, Astra-Zeneca, Aveo, Chugai, DaVita, Fresenius, Genetech, Haymarket Media, Hospira, Kabi, Keryx, National Institutes of Health, National Kidney Foundation, Relypsa, Resverlogix, Sanofi, Shire, Vifor, and ZS-Pharma.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ Contributions: Research idea and study design: YO; data acquisition: RM, KK-Z; data analysis/interpretation: YO, ES, RM, MBR, CMR, MS, DLG, W-LL, CPK, KK-Z; statistical analysis: YO, ES, MS; supervision or mentorship: RM, CPK, KK-Z. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Financial Disclosure: The other authors declare that they have no other relevant financial interests.
Disclaimer: This study is based on data provided by DaVita Clinical Research. The manuscript was reviewed and approved by DaVita. The interpretation and conclusions are those of the authors and do not represent the views of DaVita.
References
- 1.Johnson DW. What is the optimal fat mass in peritoneal dialysis patients? Perit. Dial. Int. 2007;27(Suppl 2):S250–254. [PubMed] [Google Scholar]
- 2.Kramer HJ, Saranathan A, Luke A, et al. Increasing body mass index and obesity in the incident ESRD population. J. Am. Soc. Nephrol. 2006;17(5):1453–1459. doi: 10.1681/ASN.2005111241. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Renal Data System. USRDS 2016 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Vol. 2016 National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2016. [Google Scholar]
- 5.Stenvinkel P, Zoccali C, Ikizler TA. Obesity in CKD--what should nephrologists know? J. Am. Soc. Nephrol. 2013;24(11):1727–1736. doi: 10.1681/ASN.2013040330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu JL, Kalantar-Zadeh K, Ma JZ, Quarles LD, Kovesdy CP. Association of body mass index with outcomes in patients with CKD. J. Am. Soc. Nephrol. 2014;25(9):2088–2096. doi: 10.1681/ASN.2013070754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doshi M, Streja E, Rhee CM, et al. Examining the robustness of the obesity paradox in maintenance hemodialysis patients: a marginal structural model analysis. Nephrol. Dial. Transplant. 2016;31(8):1310–1319. doi: 10.1093/ndt/gfv379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vashistha T, Mehrotra R, Park J, et al. Effect of age and dialysis vintage on obesity paradox in long-term hemodialysis patients. Am. J. Kidney Dis. 2014;63(4):612–622. doi: 10.1053/j.ajkd.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhee CM, Ahmadi SF, Kalantar-Zadeh K. The dual roles of obesity in chronic kidney disease: a review of the current literature. Curr. Opin. Nephrol. Hypertens. 2016;25(3):208–216. doi: 10.1097/MNH.0000000000000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmadi SF, Zahmatkesh G, Streja E, et al. Association of Body Mass Index With Mortality in Peritoneal Dialysis Patients: A Systematic Review and Meta-Analysis. Perit. Dial. Int. 2016;36(3):315–325. doi: 10.3747/pdi.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson DW, Herzig KA, Purdie DM, et al. Is obesity a favorable prognostic factor in peritoneal dialysis patients? Perit. Dial. Int. 2000;20(6):715–721. [PubMed] [Google Scholar]
- 12.Aslam N, Bernardini J, Fried L, Piraino B. Large body mass index does not predict short-term survival in peritoneal dialysis patients. Perit. Dial. Int. 2002;22(2):191–196. [PubMed] [Google Scholar]
- 13.McDonald SP, Collins JF, Johnson DW. Obesity is associated with worse peritoneal dialysis outcomes in the Australia and New Zealand patient populations. J. Am. Soc. Nephrol. 2003;14(11):2894–2901. doi: 10.1097/01.asn.0000091587.55159.5f. [DOI] [PubMed] [Google Scholar]
- 14.Snyder JJ, Foley RN, Gilbertson DT, Vonesh EF, Collins AJ. Body size and outcomes on peritoneal dialysis in the United States. Kidney Int. 2003;64(5):1838–1844. doi: 10.1046/j.1523-1755.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- 15.Abbott KC, Glanton CW, Trespalacios FC, et al. Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int. 2004;65(2):597–605. doi: 10.1111/j.1523-1755.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 16.Johansen KL, Young B, Kaysen GA, Chertow GM. Association of body size with outcomes among patients beginning dialysis. Am. J. Clin. Nutr. 2004;80(2):324–332. doi: 10.1093/ajcn/80.2.324. [DOI] [PubMed] [Google Scholar]
- 17.de Mutsert R, Grootendorst DC, Boeschoten EW, Dekker FW, Krediet RT. Is obesity associated with a survival advantage in patients starting peritoneal dialysis? Contrib. Nephrol. 2009;163:124–131. doi: 10.1159/000223790. [DOI] [PubMed] [Google Scholar]
- 18.Shibagaki Y, Faber MD, Divine G, Shetty A. Feasibility of adequate solute clearance in obese patients on peritoneal dialysis: a cross-sectional study. Am. J. Kidney Dis. 2002;40(6):1295–1300. doi: 10.1053/ajkd.2002.36904. [DOI] [PubMed] [Google Scholar]
- 19.Piraino B, Bernardini J, Centa PK, Johnston JR, Sorkin MI. The effect of body weight on CAPD related infections and catheter loss. Perit. Dial. Int. 1991;11(1):64–68. [PubMed] [Google Scholar]
- 20.Johnson DW, Mudge DW, Sturtevant JM, et al. Predictors of decline of residual renal function in new peritoneal dialysis patients. Perit. Dial. Int. 2003;23(3):276–283. [PubMed] [Google Scholar]
- 21.Li PK, Szeto CC, Piraino B, et al. ISPD Peritonitis Recommendations: 2016 Update on Prevention and Treatment. Perit. Dial. Int. 2016;36(5):481–508. doi: 10.3747/pdi.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo WK, Bargman JM, Burkart J, et al. Guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis. Perit. Dial. Int. 2006;26(5):520–522. [PubMed] [Google Scholar]
- 23.Mathew AT, Fishbane S, Obi Y, Kalantar-Zadeh K. Preservation of residual kidney function in hemodialysis patients: reviving an old concept. Kidney Int. 2016;90(2):262–271. doi: 10.1016/j.kint.2016.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stack AG, Murthy BV, Molony DA. Survival differences between peritoneal dialysis and hemodialysis among "large" ESRD patients in the United States. Kidney Int. 2004;65(6):2398–2408. doi: 10.1111/j.1523-1755.2004.00654.x. [DOI] [PubMed] [Google Scholar]
- 25.Lievense H, Kalantar-Zadeh K, Lukowsky LR, et al. Relationship of body size and initial dialysis modality on subsequent transplantation, mortality and weight gain of ESRD patients. Nephrol. Dial. Transplant. 2012;27(9):3631–3638. doi: 10.1093/ndt/gfs131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thamer M, Hwang W, Fink NE, et al. US nephrologists' recommendation of dialysis modality: results of a national survey. Am. J. Kidney Dis. 2000;36(6):1155–1165. doi: 10.1053/ajkd.2000.19829. [DOI] [PubMed] [Google Scholar]
- 27.Stack AG. Determinants of modality selection among incident US dialysis patients: results from a national study. J. Am. Soc. Nephrol. 2002;13(5):1279–1287. doi: 10.1681/ASN.V1351279. [DOI] [PubMed] [Google Scholar]
- 28.Little J, Irwin A, Marshall T, Rayner H, Smith S. Predicting a patient's choice of dialysis modality: experience in a United Kingdom renal department. Am. J. Kidney Dis. 2001;37(5):981–986. doi: 10.1016/s0272-6386(05)80014-9. [DOI] [PubMed] [Google Scholar]
- 29.Jager KJ, Korevaar JC, Dekker FW, Krediet RT, Boeschoten EW Netherlands Cooperative Study on the Adequacy of Dialysis Study G. The effect of contraindications and patient preference on dialysis modality selection in ESRD patients in The Netherlands. Am. J. Kidney Dis. 2004;43(5):891–899. doi: 10.1053/j.ajkd.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 30.Mehrotra R, Marsh D, Vonesh E, Peters V, Nissenson A. Patient education and access of ESRD patients to renal replacement therapies beyond in-center hemodialysis. Kidney Int. 2005;68(1):378–390. doi: 10.1111/j.1523-1755.2005.00453.x. [DOI] [PubMed] [Google Scholar]
- 31.Mendelssohn DC, Mujais SK, Soroka SD, et al. A prospective evaluation of renal replacement therapy modality eligibility. Nephrol. Dial. Transplant. 2009;24(2):555–561. doi: 10.1093/ndt/gfn484. [DOI] [PubMed] [Google Scholar]
- 32.Oliver MJ, Garg AX, Blake PG, et al. Impact of contraindications, barriers to self-care and support on incident peritoneal dialysis utilization. Nephrol. Dial. Transplant. 2010;25(8):2737–2744. doi: 10.1093/ndt/gfq085. [DOI] [PubMed] [Google Scholar]
- 33.Mehrotra R, Kermah D, Fried L, et al. Chronic peritoneal dialysis in the United States: declining utilization despite improving outcomes. J. Am. Soc. Nephrol. 2007;18(10):2781–2788. doi: 10.1681/ASN.2006101130. [DOI] [PubMed] [Google Scholar]
- 34.Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch. Intern. Med. 2011;171(2):110–118. doi: 10.1001/archinternmed.2010.352. [DOI] [PubMed] [Google Scholar]
- 35.Kuttykrishnan S, Kalantar-Zadeh K, Arah OA, et al. Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol. Dial. Transplant. 2015;30(7):1208–1217. doi: 10.1093/ndt/gfv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schacht A, Bogaerts K, Bluhmki E, Lesaffre E. A new nonparametric approach for baseline covariate adjustment for two-group comparative studies. Biometrics. 2008;64(4):1110–1116. doi: 10.1111/j.1541-0420.2008.00994.x. [DOI] [PubMed] [Google Scholar]
- 38.National Kidney Foundation. KDOQI Clinical Practice Guidelines for Peritoneal Dialysis Adequacy, Update 2006. Am. J. Kidney Dis. 2006;48(Suppl 1):S91–158. doi: 10.1053/j.ajkd.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 39.Gastrointestinal Surgery for Severe Obesity. [accessed January 9, 2017];NIH Consensus Statement Online. 1991 Mar 25–27;9(1):1–20. Vol 20171991. [PubMed] [Google Scholar]
- 40.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 41.Smith JA, Todd PE. Does matching overcome LaLonde's critique of nonexperimental estimators? Journal of econometrics. 2005;125(1):305–353. [Google Scholar]
- 42.Winkelmayer WC, Glynn RJ, Mittleman MA, Levin R, Pliskin JS, Avorn J. Comparing mortality of elderly patients on hemodialysis versus peritoneal dialysis: a propensity score approach. J. Am. Soc. Nephrol. 2002;13(9):2353–2362. doi: 10.1097/01.asn.0000025785.41314.76. [DOI] [PubMed] [Google Scholar]
- 43.Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J. Am. Soc. Nephrol. 2010;21(3):499–506. doi: 10.1681/ASN.2009060635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perl J, Wald R, McFarlane P, et al. Hemodialysis vascular access modifies the association between dialysis modality and survival. J. Am. Soc. Nephrol. 2011;22(6):1113–1121. doi: 10.1681/ASN.2010111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Royston P, Sauerbrei W, Freiburg G. Multivariable modeling with cubic regression splines: A principled approach. Stata Journal. 2007;7(1):45. [Google Scholar]
- 46.Pajek J, Hutchison AJ, Bhutani S, et al. Outcomes of peritoneal dialysis patients and switching to hemodialysis: a competing risks analysis. Perit. Dial. Int. 2014;34(3):289–298. doi: 10.3747/pdi.2012.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Kidney F. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am. J. Kidney Dis. 2015;66(5):884–930. doi: 10.1053/j.ajkd.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Johansson AC, Samuelsson O, Attman PO, Bosaeus I, Haraldsson B. Limitations in anthropometric calculations of total body water in patients on peritoneal dialysis. J. Am. Soc. Nephrol. 2001;12(3):568–573. doi: 10.1681/ASN.V123568. [DOI] [PubMed] [Google Scholar]
- 49.Mehrotra R, Story K, Guest S, Fedunyszyn M. Neighborhood location, rurality, geography, and outcomes of peritoneal dialysis patients in the United States. Perit. Dial. Int. 2012;32(3):322–331. doi: 10.3747/pdi.2011.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen JI, Mitani AA, Saxena AB, Goldstein BA, Winkelmayer WC. Determinants of peritoneal dialysis technique failure in incident US patients. Perit. Dial. Int. 2013;33(2):155–166. doi: 10.3747/pdi.2011.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quinn RR, Hux JE, Oliver MJ, Austin PC, Tonelli M, Laupacis A. Selection bias explains apparent differential mortality between dialysis modalities. J. Am. Soc. Nephrol. 2011;22(8):1534–1542. doi: 10.1681/ASN.2010121232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Kaplan-Meier estimates in overall survival across three BMI categories among PD patients vs matched HD patients stratified by diabetes history.
Table S1: Characteristics of included vs excluded PD patients during first 91 days of PD.
Table S2: Missing frequencies and percentages among the covariates.