Abstract
Objective
To test the hypothesis that echocardiographic markers of pulmonary vascular disease (PVD) exist in asymptomatic preterm infants at one year corrected age (CA).
Study design
We conducted a prospective cohort study of 80 preterm (<29 weeks gestation) and 100 age- and weight-matched term infants, and compared broad-based conventional and quantitative echocardiographic measures of pulmonary hemodynamics at one year CA. Pulmonary artery acceleration time (PAAT), a validated index of pulmonary vascular resistance, arterial pressure and compliance, was utilized to assess pulmonary hemodynamics. Lower PAAT is indicative of PVD. Sub-analyses were performed in infants with bronchopulmonary dysplasia (BPD, n=48, 59%) and/or late onset pulmonary hypertension (PH, n=12, 15%)
Results
At one year, there were no differences between conventional measures of PH in the term-and preterm-born infants. All preterm infants had significantly lower values of PAAT than term-born infants (73 ± 8 vs. 98 ± 5 msec, P < .001). Preterm-born infants with BPD had even lower PAAT than those without BPD (69 ± 5 vs. 79 ± 4 msec, p<0.01). The degree of PVD at one year CA was inversely related to gestation in all preterm infants. Data analysis included adjustment for ventricular function and other confounding factors.
Conclusions
In comparison with term-born infants, preterm-born infants exhibit abnormal PAAT at one year CA irrespective of neonatal lung disease status, suggesting the existence of PVD beyond infancy. PAAT measurements offer a reliable non-invasive tool for screening and longitudinal monitoring of pulmonary hemodynamics in infants.
Keywords: Pulmonary vascular disease, Pulmonary Hypertension, Prematurity, Echocardiography
Pulmonary vascular disease (PVD) and its most severe form, pulmonary hypertension (PH), are common associations of neonatal respiratory diseases of prematurity, including bronchopulmonary dysplasia (BPD).1 Although the reported incidence of PH is 14–44% in the infants with established BPD, depending on the definition of each disease2–6, recent evidence indicates that up to 20% of extremely low gestational age neonates without BPD will develop some degree of PVD during the neonatal period.3–6 Although BPD and PH share similar risk factors and overlapping symptoms7, PVD may be a distinct entity in premature infants.3–10 The diagnosis of PVD and the true prevalence of PH in preterm infants have been dif cult to discern due to a paucity of reliable non-invasive measures to evaluate pulmonary hemodynamics,1,11,12 and inconsistent screening for PVD and PH in extremely low gestational age neonates.13 Use of different clinical and echocardiographic screening algorithms for detection of PH in premature infants have been proposed, but primarily focus on infants with BPD.11,13
The non-invasive echocardiographic assessment of pulmonary hemodynamics in children relies on a combination of qualitative evaluations (ventricular septal wall position and right ventricle morphological changes) and quantitative estimates based on the Doppler velocity of tricuspid regurgitation (DVTR); measures that often imprecisely correlate with cardiac catheterization indices.14 Although right heart catheterization (RHC) remains the gold standard for assessment of pulmonary hemodynamics, its invasive nature and risk of radiation exposure limits its use as screening and monitoring tool in this population.15 Recent studies have established Doppler derived pulmonary artery acceleration time (PAAT) as a comprehensive quantitative index of pulmonary artery pressure, vascular resistance and compliance against simultaneous RHC derived pulmonary hemodynamic in children.15 PAAT assesses the blood flow velocity characteristics in the RV outflow tract (RVOT) in response to changes in RV mechanical performance and pulmonary vascular load, and has superior diagnostic sensitivity for assessing PVD and following progression.15,16 Normative values and maturational patterns of PAAT have recently been established in healthy children.16
We hypothesize that developmental and maturational disruption to the pulmonary vasculature from preterm birth results in PVD as a distinct pathophysiology from BPD that exists at one year corrected age (CA). Our aim was to prospectively identify PVD using Doppler echocardiography derived PAAT in a cohort of preterm-born infants (with and without BPD) and compare it to term-born healthy infants at one year of age.
METHODS
In a prospective observational study, we recruited a cohort of preterm infants at birth and longitudinally followed them to one year CA. The infants were enrolled through the Prematurity and Respiratory Outcomes Program (PROP) at Washington University School of Medicine/Saint Louis Children’s Hospital neonatal intensive care unit between August 2011 and November 2013. The detailed design of the PROP Study (ClinicalTrials.gov: NCT01435187) has been described.17,18 Reports on reference values and maturational patterns of ventricular function, namely RV fractional area of change (FAC) and left ventricle (LV) and RV strain from these cohorts have been recently published,4,19 but PAAT and pulmonary hemodynamic data have not been reported. Subjects were eligible for inclusion if they were born between 23 0/7 weeks and 28 6/7 weeks gestational age and available for follow-up at one year CA. Subjects were excluded if they had any suspected congenital anomalies of the airways, congenital heart disease, intrauterine growth restriction or small for gestational age (birth weight < 10th centile for gestation). A cohort of healthy, age, and sex matched term-born infants were recruited for the control group. These infants were referred to the Washington University Outpatient Pediatric Cardiology Clinic at St. Louis Children’s Hospital from August 2011 to November 2013 for heart murmur evaluation and found to have normal cardiac structure and function (Figure 1). All subjects underwent a comprehensive clinical evaluation as part of the standard of care. The preterm infants’ perinatal clinical information and demographic characteristic were obtained. The Institutional Review Board for human studies at Washington University approved the study. Written informed consents were obtained from the parents/guardians of the study subjects.
Figure I.
Study subject flow diagram.
GA, gestational age; PMA, postmenstrual age; CA, corrected age
BPD, bronchopulmonary dysplasia. Late PH, pulmonary hypertension diagnosed at 36 weeks PMA by conventional echocardiography.3
Echocardiography was performed using commercially available ultrasound imaging systems (Vivid 7 and 9; GE Medical Systems, Milwaukee, WI) by one designated certified pediatric cardiac sonographer using a phased-array transducer (7.5–12 MHz). The echocardiographic images were acquired using a standardized image acquisition protocol based on the guidelines of the American Society of Echocardiography.20 The image data were acquired digitally at 36 weeks postmenstrual age (PMA) and one year CA in preterm infants and at one year of age in term infants and stored in raw Digital Imaging and Communications in Medicine cine-loop format for offline analysis. Vital signs, including heart rate and blood pressure readings, were recorded at the time of each echocardiogram.
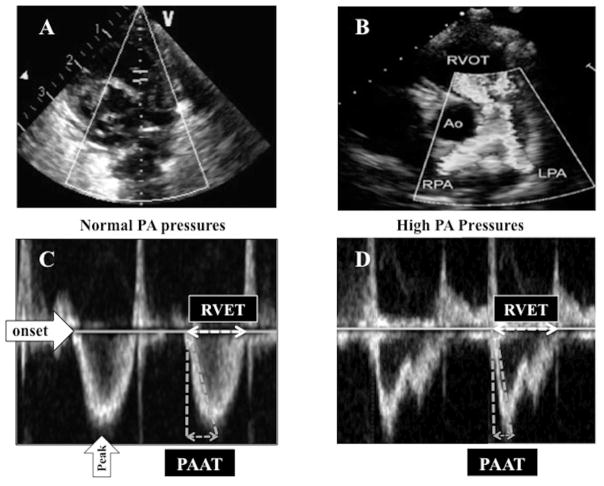
We assessed conventional echocardiographic measures of pulmonary hemodynamics, including the estimated RV systolic pressure (RVSP) using the Doppler velocity of tricuspid regurgitation (DVTR), the ratio of RVSP to systemic blood pressure (sBP), cardiac shunts and their directional flow, septal wall motion and position, and RV and right atrial morphology. We measured PAAT utilizing a published protocol for PAAT image acquisition and post-processing data analysis.15 A spectral Doppler image was obtained by placing a pulsed Doppler sample volume at the pulmonary valve annulus in the parasternal short-axis view (Figure II; available at www.jpeds.com). Maximal alignment of Doppler interrogation with blood flow direction was achieved with the placement of the sample volume at the annulus of the pulmonary valve and not more proximally in the RVOT. We calculated PAAT as the time interval between the onset of systolic pulmonary arterial flow (onset of ejection) and peak flow velocity. To account for the potential impact of heart rate variability, PAAT was adjusted for RV ejection time (RVET) with a ratio of PAAT:RVET (Figure 2).15 As we and others have previously demonstrated no correlation between PAAT or PAAT:RVET ratio and R-R interval in neonates,15,21 both PAAT and the ratio of PAAT:RVET were not corrected for R-R interval or its square root value in the analysis.
Figure II (online).
(A) A spectral Doppler image is obtained by placing a pulsed Doppler sample volume at the pulmonary valve annulus in the parasternal short-axis view. (B) Maximal alignment of Doppler interrogation with blood flow direction was achieved with the placement of the sample volume at the annulus of the pulmonary valve and not more proximally in the right ventricular outflow tract (RVOT). (C) Pulmonary artery (PA) Doppler velocity wave-form in a patient with normal PAP. PAAT is defined as the interval between the onset of systolic pulmonary arterial flow (onset of ejection) and peak flow velocity. RVET was measured from the interval between the onset of RV ejection to the point of systolic pulmonary arterial flow cessation. (D) PA Doppler velocity wave-form with shortened PAAT in a patient with high PAP. Ao, Aorta; LPA, left pulmonary artery; RPA, right pulmonary artery.
Because a lower PAAT reflects the triad of elevated PVR, decreased pulmonary arterial compliance, and RV mechanical performance,15 we assessed measures of RV function and morphology. We evaluated RV function using speckle-tracking echocardiography (STE)–derived free wall longitudinal systolic strain (FWLS) and FAC by methods previously reported.4,19 We measured RV areas in systole and diastole19, RV 2D chamber dimensions at the basal and mid ventricular levels, and RV length from the apex to the base.20 To test if acceleration time across the RVOT is dependent on its diameter22, we measured the proximal and distal RVOT diameters.20 We also examined LV function with STE-derived LV FWLS to assess the interventricular dependence.
We assessed for the contributions of BPD (using a modified definition of the 2001 National Institutes of Health BPD workshop18) and late onset PH (utilizing a broad echocardiogram-based definition described by Mourani et al 3) on PAAT measures at one year corrected age. Preterm infants were classified with late onset PH at 36 weeks PMA if they had 2 or more of the conventional echocardiographic measures of pulmonary hemodynamics listed above. We used an established pediatric cut-off value of PAAT < 90 msec and PAAT:RVET < 0.31 for detection of PVD (PVRi > 3 WU × m2, mPAP > 25 mm Hg, sPAP > 35 mm HG) at one year CA.15 The independent effect of the risk factors for BPD and PH, including gestational age, sex, total oxygen days, length of hospital stay, perinatal factors, common neonatal morbidities (necrotizing enterocolitis, intraventricular hemorrhage, and retinopathy of prematurity), and presence of a patent ductus arteriosus (PDA) at 36 weeks PMA23 on PAAT were also accounted for in the analysis.
Intra- and inter-observer variability for PAAT and PAAT:RVET were measured in 50% of the infants in each cohort by observers who were blinded to the clinical and demographic data. Each observer utilized the same measurement protocol15 and was blinded to the other’s results. Bland-Altman plot analysis (percentage bias, 95% limits of agreements), coefficient of variation (CV), and intraclass correlation coefficient were used to assess the reproducibility.
Statistical Analyses
All demographic data are expressed as median with interquartile ranges or as percentages, and all echocardiographic data are expressed as mean ± SD. Continuous variables of PAAT were tested for normality using the Kolmogorov-Smirnov test and a histogram illustration of the data. Analysis of variance tests were used to compare the changes in PAAT values amongst preterm infants with and without BPD and/or PH with term born infants. Student t-tests were separately utilized to compare the patterns between uncomplicated preterm infants and those with BPD and/or PH. All outcome variables with non-normal distributions were analyzed in simple comparisons using Wilcoxon rank sum tests or Kruskal-Wallis one-way analysis of variance for tests with more than two independent groups. Chi-square tests (or Fisher Exact test as appropriate) were used to assess the association between categorical variable. Univariate analysis was used to determine the best predictors to enter in the model and then backward step-wise regression was performed to assess the independent effect of gestational age, sex, total oxygen days, length of stay, and common neonatal morbidities (necrotizing enterocolitis, intraventricular hemorrhage, and retinopathy of prematurity), while adjusting for weight and body surface area at examination. We performed stepwise regression to analyze the influence of PH and BPD on PAAT patterns at one year corrected age.1,3 The statistical analysis was performed using SPSS version 14.0 (SPSS, Inc, Chicago, IL).
RESULTS
A total of 180 infants (80 preterm-born and 100 term-born) were included in this study (Figure 1). Of the 137 preterm-born infants recruited at birth, 13 infants died prior to hospital discharge, 5 transferred, and 2 withdrew from the study, leaving 117 infants with data for analysis at 36 weeks PMA. At one year CA, 80 preterm-born infants (69%) returned for follow-up and an echocardiogram was performed. The median gestational age at birth of the 80 preterm-born infants was 27.0 weeks (IQR 26.0–28.0) and the median birthweight was 960 g (IQR 800–1,138). BPD was diagnosed at 36 weeks PMA in 48 of the 80 preterm-born infants (59%) that received echocardiograms at one year CA. The median gestational age at birth of the 100 term-born infants was 39.0 weeks (IQR 38.0 – 40.0) and the median birthweight was 3165 g (IQR 2900–3760).
We compared the maternal and infant characteristics between preterm-born infants with and without BPD in Table I. Among infant demographic variables, only gestational age at birth was statistically significantly different between preterm-born infant with BPD and those without BPD (26 ± 1 weeks vs. 27 ± 1 weeks PMA, P<0.001). None of the infants received inotropes, inhaled nitric oxide or other pulmonary vasodilators in the first year of age after discharge from the NICU. Among the maternal variables that may affect pulmonary vascular growth and development, including chorioamnionitis, pre-eclampsia, placental abruption, and smoking, there were no significant differences between the preterm-born infants with and without BPD, Table I.
Table I.
Maternal and infant characteristics and associations with BPD
| Characteristics | Whole Preterm cohort (n=80) | No BPD (n=32) | BPD (n=48) | P value |
|---|---|---|---|---|
| Birthweight (g) | 890 [765,1010] | 960 [825,1125] | 863[717,980] | 0.002 |
| Birthweight strata (g) | ||||
| 500 – 749 (n=16) | 660 [590,690] | 690 [655,705] | 640 [578.685] | 0.23 |
| 750 – 999 (n=41) | 890 [825,955] | 853 [800,945] | 895 [835,940] | 0.37 |
| 1000 – 1250 (n=23) | 1125 [905,1265] | 1140 [1050,1260] | 1100 [1040,1220] | 0.61 |
| Gestational age | 27 [26,28] | 27 [26,28] | 26 [25,27] | < 0.01 |
| Male sex | 39 (49%) | 18 (56%) | 21 (41%) | 0.22 |
| Multiple gestation | 14 (18%) | 3 (9%) | 11 (23%) | 0.17 |
| Infant Race | 0.66 | |||
| White | 38 (48%) | 12 (38%) | 26 (54%) | |
| Black | 42 (52%) | 20 (62%) | 22 (46%) | |
| Asian | 0 | 0 | 0 | |
| Other | 0 | 0 | 0 | |
| Ethnicity | 0.46 | |||
| Hispanic or Latino | 1 (13%) | 0 | 1 (2%) | |
| Not Hispanic or Latino | 79 (87%) | 32 (100%) | 47 (98%) | |
| Maternal Smoking | 16 (20%) | 5 (16%) | 11 (23%) | 0.48 |
| Antenatal steroids | 77 (96%) | 32 (100%_ | 45 (94%) | 0.16 |
| Surfactant Replacement Therapy | 80 (100%) | 32 (100%) | 48 (100%) | 0.84 |
| Cesarean section | 55 (69%) | 21 (66%) | 34 (71%) | 0.86 |
| Maternal complications | ||||
| Gestational DM | 4 (5%) | 1 (3%) | 3 (6%) | 0.56 |
| Gestational HTN | 22 (28%) | 12 (38%) | 10 (21%) | 0.28 |
| Prolonged rupture of membranes | 38 (17%) | 15 (15%) | 23 (19%) | 0.12 |
| Chorioamnionitis | 8 (10%) | 4 (13%) | 4 (8%) | 0.51 |
| Preeclampsia | 22 (28%) | 10 (31%) | 12 (25%) | 0.51 |
| Placental Abruption | 14 (18%) | 8 (25%) | 6 (13%) | 0.13 |
| Late onset PH | 12 (15%) | 4 (12.5%) | 8 (17%) | 0.45 |
| Presence of PDA at 36 weeks PMA* | 9 (11%) | 3 (9%) | 6 (13%) | 0.36 |
| Necrotizing enterocolitis | 7 (9%) | 2 (6%) | 5 (10%) | 0.56 |
| ROP threshold (Stage 2 or higher) | 30 (38%) | 6 (19%) | 24 (50%) | 0.02 |
| IVH (Grade 3 or 4) | 9 (11%) | 2 (6%) | 7 (15%) | 0.54 |
| Total oxygen days (NICU) | 87 [46,109] | 38 [18,58] | 100 [88,115] | <0.001 |
| Length of stay (NICU) | 93 [79,114] | 80 [69,100] | 106 [91,120] | <0.001 |
Data are presented as median [interquartile range], or number (Percentage). Data are presented as column percentages
BPD, bronchopulmonary dysplasia; PH, pulmonary hypertension; PDA, patent ductus arteriosus
ROP, retinopathy of prematurity; IVH, intraventricular hemorrhage
Presence of PDA, patent ductus arteriosus, at 36 weeks PMA, post-menstrual age.
All preterm-born infants had significantly lower values of PAAT and PAAT:RVET ratio than those of term-born infants (73 ± 8 msec vs. 98 ± 5 msec and 0.31 ± 0.04 vs. 0.40 ± 0.02 respectively, p< 0.001 for both), that persisted after adjusting for sex (β = 2.3, p=0.001 and β = 2.4, p=0.001, respectively) at one year CA. PAAT and PAAT:RVET significantly correlated with chronological age corrected for prematurity in both infants with BPD (r = 0.67 and 0.71) and infants without BPD (r = 0.55 and 0.62), (Figure IV; available at www.jpeds.com). In the preterm-born infants without BPD, step-wise regression analysis of the effects of sex, birth weight, total oxygen days, total ventilator days, length of stay, common neonatal morbidities (necrotizing enterocolitis, intraventricular hemorrhage, and retinopathy of prematurity), and length of exposure to a PDA also revealed that the relation of decreased PAAT and PAAT:RVET at one year CA persisted with decreasing gestational age at birth.
Figure IV (online).
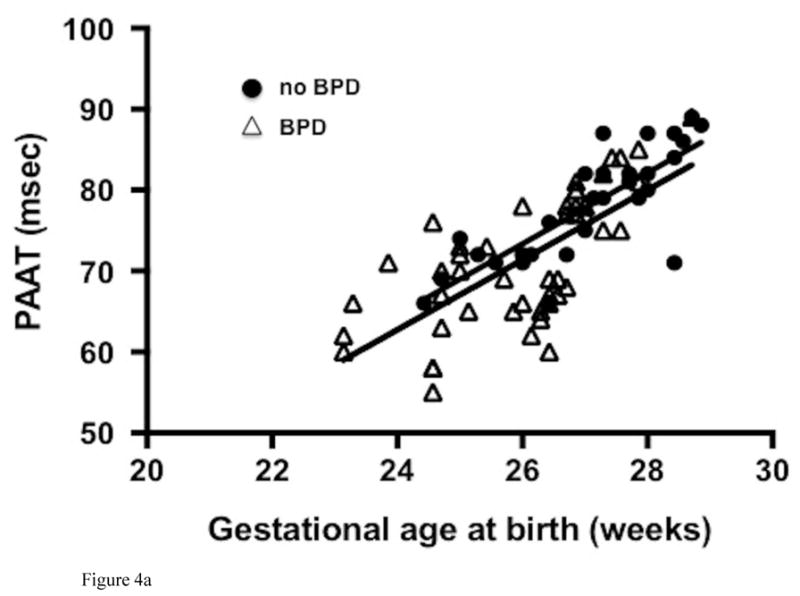
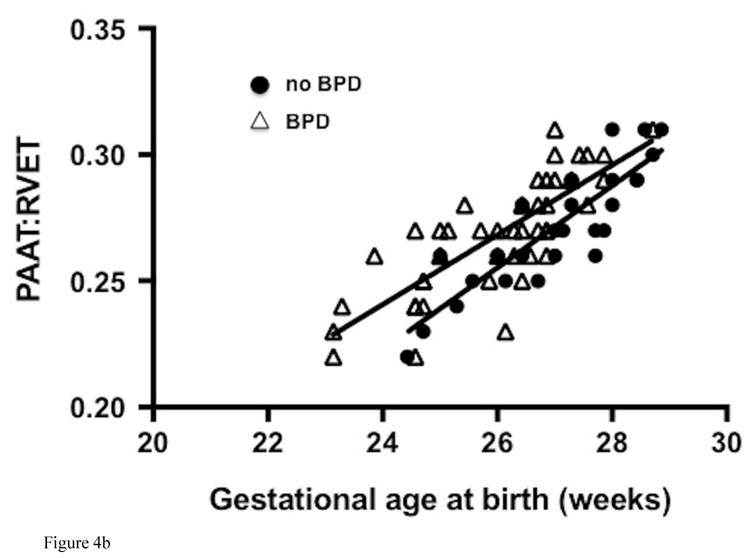
Correlation of (A) PAAT and (B) PAAT:RVET measured at one year corrected age with gestational age at birth in preterm-born infants with bronchopulmonary dysplasia, BPD (white triangles) and without BPD (black circles).
Preterm-born infants with BPD had decreased PAAT and PAAT:RVET at one year CA when compared with those without BPD (69 ± 5 msec vs. 79 ± 4 msec and 0.29 ± 0.02 vs. 0.33 ± 0.06 respectively, p<0.01 for both), Table II. Preterm-born infants without BPD had significantly lower values of PAAT and PAAT:RVET when compared with term-born healthy infants (79 ± 4 msec vs. 98 ± 5 msec and 0.33 ± 0.06 vs. 0.40 ± 0.02, both p < 0.001).
Table II.
Echocardiographic assessment of term and preterm-born infants at one year
| Measures | Term cohort | Whole Preterm cohort | No BPD | BPD | No PH | PH | P value | P value | P value |
|---|---|---|---|---|---|---|---|---|---|
| n=100 | n=80 | n=32 | n=48 | n=68 | n=12 | Term vs. Preterm | No BPD vs. BPD | No PH vs. PH | |
| CONVENTIONAL | |||||||||
| PDA* | 0 | 3 (4%) | 1 (3%) | 2 (4%) | 3 (4%) | 0 | 0.25 | 0.82 | 0.76 |
| PFO/ASD* | 7 (7%) | 10 (13%) | 4 (13%) | 6 (13%) | 7 (10%) | 3 (25%) | 0.67 | 0.97 | 0.93 |
| VSD* | 0 | 1 (1%) | 0 | 1 (2%) | 1 (1%) | 0 | 0.16 | 0.32 | 0.38 |
| DVTR present | 0 | 9 (11%) | 3 (9%) | 6 (13%) | 7 (10%) | 3 (25%) | 0.34 | 0.35 | 0.39 |
| RVSP > 40 mm Hg | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA | NA |
| RVSP/sBP > 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA | NA |
| Septal wall flattening | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA | NA |
| QUANTITATIVE | |||||||||
| Ventricular function | |||||||||
| RV FAC (%) | 35 ± 3 | 33 ± 2 | 34 ± 3 | 30 ± 4 | 34 ± 3 | 30 ± 4 | 0.05 | < 0.01 | < 0.01 |
| RV FWLS (%) | − 29 ± 4 | − 24 ± 2 | − 25 ± 1 | − 23 ± 3 | − 26 ± 1 | − 22 ± 1 | 0.01 | < 0.01 | < 0.01 |
| LV FLWS (%) | − 20 ± 2 | − 20 ± 3 | − 19 ± 5 | − 20 ± 2 | − 20 ± 3 | − 20 ± 1 | 0.34 | 0.23 | 0.27 |
| RV Morphology | |||||||||
| RV basal length (cm) | 2.0 ± 0.3 | 2.4 ± 0.3 | 2.3 ± 0.3 | 2.5 ± 0.3 | 2.4 ± 0.12 | 2.6 ± 0.2 | < 0.01 | < 0.01 | < 0001 |
| RV mid-cavity length (cm) | 1.7 ± 0.3 | 2.0 ± 0.3 | 1.8 ± 0.3 | 2.1 ± 0.2 | 1.9 ± 0.5 | 2.2 ± 0.3 | < 0.01 | < 0.01 | < 0.01 |
| RV major length (cm) | 3.9 ± 0.2 | 3.9 ± 0.3 | 3.9 ± 0.4 | 4.0 ± 0.3 | 3.9 ± 0.2 | 3.9 ± 0.3 | 0.56 | 0.31 | 0.37 |
| RV proximal diameter (cm) | 1.6 ± 0.2 | 1.6 ± 0.3 | 1.5 ± 0.3 | 1.5 ± 0.4 | 1.6 ± 0.1 | 1.5 ± 0.5 | 0.66 | 0.13 | 0.31 |
| RV distal diameter (cm) | 1.3 ± 0.2 | 1.3 ± 0.3 | 1.4 ± 0.3 | 1.3 ± 0.2 | 1.4 ± 0.4 | 1.3 ± 0.3 | 0.26 | 0.34 | 0.32 |
| RV systolic area (cm2) | 3.8 ± 0.2 | 4.4 ± 0.4 | 4.1 ± 0.3 | 4.7 ± 0.3 | 4.0 ± 0.4 | 4.8 ± 0.3 | < 0.01 | < 0.01 | < 0.01 |
| RV diastolic area (cm2) | 6.2 ± 0.4 | 6.9 ± 0.3 | 6.7 ± 0.3 | 6.9 ± 0.3 | 6.6 ± 0.2 | 7.0 ± 0.4 | < 0.01 | < 0.01 | < 0.01 |
| RV afterload (pulmonary hemodynamics) | |||||||||
| PAAT (msec) | 98 ± 5 | 73 ± 8 | 79 ± 4 | 69 ± 5 | 74 ± 4 | 70 ± 5 | < 0.01 | < 0.01 | < 0.01 |
| PAAT:RVET | 0.40 ± 0.02 | 0.30 ± 0.04 | 0.31 ± 0.04 | 0.28 ± 0.06 | 0.32 ± 0.03 | 0.29 ± 0.03 | < 0.01 | < 0.01 | < 0.01 |
BPD, bronchopulmonary dysplasia; PH, pulmonary hypertension
PDA, patent ductus arteriosus; PFO, patent foramen ovale; ASD, atrial septal defect; DVTR, Doppler velocity tricuspid regurgitation
RVSP, right ventricle systolic pressure; sBP, systolic blood pressure
RV FAC, right ventricle fractional area of change;
RV FWLS, free wall longitudinal strain;
PAAT, pulmonary artery acceleration time; RVET, right ventricle ejection time; NA, not applicable
All left-to-right shunt patterns
Postnatal steroids were administered in 26 infants (33%) at least one time during their hospital course; of these 23 infants received postnatal steroids to treat lung disease beyond the first month of age and were also diagnosed with BPD. The three remaining infants received steroids in the first month of age to facilitate a trial of extubation. All preterm-born infants received caffeine from birth through 34 weeks PMA, and 15/80 (18%) of infants that returned at one year CA received at least one dose of diuretics during their neonatal course, no infants were on either medication at 36 weeks PMA. There was no statistical difference in the PAAT and PAAT:RVET between those infants that did and did not receive postnatal steroids (p=0.45), and those that did and did not received diuretics (p=0.34), although the study was not adequately powered to answer these questions.
A PDA was present on echocardiogram at 36 weeks PMA in 13% (n=6) of preterm-born infants with BPD compared with 9% (n=3) without BPD that returned at one year CA. There were no differences in PAAT or PAAT:RVET at one year CA between preterm-born infants who still had PDA at 36 weeks PMA (n=9, 11%, adjusted for gestational age, sex, and PDA size) and those who did not have a PDA (p = 0.36, p= 0.32, respectively).
On the basis of conventional echocardiographic evidence, we found an late PH in 15% (n = 17 of 117 preterm-born infants) at 36 weeks PMA and none at one-year corrected age. Of the 17 infants with late PH at 36 weeks, 13 met BPD criteria and four did not. Twelve of the 17 infants with late PH returned for follow-up at one-year CA, of which eight (75%) were classified with BPD and four (25%) without BPD at 36 weeks PMA (Figure I and Table III [available at www.jpeds.com]). None of these 12 infants required oxygen or respiratory support at one year corrected age. There were no significant differences among the maternal variables that may affect pulmonary vasculature development between the preterm infants with and without PH (Table I).
Table III (online).
Criteria for pulmonary hypertension diagnosis at 36 weeks post-menstrual age.3
| Patient | GA | BPD | Late-onset PH | Primary PH Criteria* | Alternative PH criteria 1* | Alternative PH criteria 2* | Designated criteria for Diagnosis of PH | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bidrectional or R > L PDA shunt | Bidrectional or R > L PFO/ASD shunt | Bidrectional or R > L VSD shunt | RVSP ** >40 mmHg | RVSP/s BP > 0.5 | Septal Wall flattening | |||||||
| 1 | 28 | No | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Moderate |
| 2 | 27 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | None |
| 3 | 25 | No | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Moderate |
| 4 | 25 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | None |
| 5 | 27 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | None |
| 6 | 24 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | None |
| 7 | 24 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | None |
| 8 | 27 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Moderate |
| 9 | 27 | No | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | None |
| 10 | 25 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Moderate |
| 11 | 25 | No | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | None |
| 12 | 26 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Moderate |
PH, pulmonary hypertension; Patients were classified with late onset PH at 36 weeks PMA if they had two or more of the designated criteria
BPD, bronchopulmonary dysplasia (based on a modified definition of the 2001 National Institutes of Health BPD workshop)18
RVSP, right ventricle systolic pressure; sBP, systolic blood pressure; PDA, patent ductus arteriosus,
PFO, patent foramen ovale; ASD, atrial septal defect;
Criteria adapted from Mourani et al.3
Primary PH criteria: A single positive finding in any individual component [bidirectional or right to left shunting through a PDA, PFO, ASD, or VSD, RVSP > 40 mmHg, RVSP/sBP > 0.5, or any degree (mild, moderate, or sever) of septal wall flattening] of the designated criteria is sufficient for the diagnosis of PH.
Alternative PH criteria 1: Excludes the presence of mild septal wall flattening
Alternative PH criteria 2: Excludes the presence of any degree of septal wall flattening
RVSP was based on the presence of the Doppler velocity tricuspid regurgitate jet (modified Bernoulli equation). An interpretable DVTR was present in 14.5% (17/117) patients at 36 weeks post-menstrual age. Only 5/117 (4%) had any degree of septal wall flattening at 36 weeks PMA. Only patient #2 had a PDA at 36 weeks PMA. All 12 patients had either a PFO or ASD. None of the 12 infants had a VSD at 36 weeks PMA.
Preterm-born Infants with late PH at 36 weeks PMA had decreased PAAT and PAAT:RVET at one year corrected age when compared with infants without late PH (70 ± 4 msec vs. 74 ± 4 msec and 0.29 ± 0.03 vs. 0.32 ± 0.03 respectively, p<0.01 for both), even after adjusting for the presence of BPD and presence of PDA at 36 weeks PMA on regression analysis (β = 2.4, p=0.01, β = 2.5, p<0.01 and β = 2.3, p<0.01, β = 2.6, p<0.01, respectively). Preterm-born infants without late PH also had significantly lower values of PAAT and PAAT:RVET when compared with term-born health infants (74 ± 4 msec vs. 98 ± 5 msec and 0.33 ± 0.06 vs. 0.40 ± 0.02, both p < 0.001). All 80 (100%) preterm-born infants had PAAT < 90 msec and 51 preterm-born infants (64%) had PAAT:RVET < 0.31). All term-born infants had PAAT > 90 msec and PAAT:RVET > 34.
In the preterm cohort, RV free wall longitudinal strain (FWLS) ranged from 21% to 26% and there were strong linear correlations between PAAT and PAAT:RVET with RV FWLS (r = 0.86 and r = 0.84, respectively, P < 0.001) at one year corrected age. Because of the linear interdependence of PAAT measures with RV FWLS in the same direction, RV FWLS was not taken into account in analysis of PAAT and PAAT:RVET with BPD and PH. We have previously demonstrated in this cohort that infants with BPD and/or PH have decreased RV FWLS when compared with preterm-born infants without BPD and/or PH.4 The inverse relationships between RV fractional area of change (FAC) with PAAT and PAAT:RVET were significant, but not as strong (r < 0.75 for all measures). PAAT < 90 msec and PAAT/RVET < 0.31 detected an RV FWLS cutoff value of 24% with sensitivity of 87% and specificity of 89%, with an AUC of 0.88 (95% CI, 0.84–0.97). The basal and mid-cavity diameters, and the RV areas at end-systole and end-diastole were larger in all the preterm-born infants, and even more pronounced in infants with BPD and/or PH. The longitudinal diameter from the apex to the base and the proximal and distal RVOT diameters were similar in all preterm-born and term-born infants, regardless of the presence of BPD and/or PH. We demonstrated that LV FWLS was similar between term-born and all preterm-born infants at one year CA, irrespective of a diagnosis of BPD or late PH at 36 weeks PMA (p=0.45).4 All values for comparisons can be found in Table II.
The measurements of PAAT were feasible in 100% of term-born and preterm-born infants using the methods previously described by our group.15 (A DVTR was present in 11% (9/80) of preterm infants at one CA, Table II). There was high degree of intra-observer agreement (bias 4.2%, 95% LOA −12.0 to +12.0; CV 5.6%) and inter-observer agreement (bias 7.2%, 95% LOA −8.8 to +8.6; CV 5.0%) for PAAT measurements. PAAT:RVET had similar results.
DISCUSSION
This prospective longitudinal study demonstrated that extreme preterm-born infants exhibit characteristics of PVD that include increased resistance, elevated pressure, and decreased compliance of the pulmonary vasculature at one year corrected age. The PVD, as assessed using pulmonary artery Doppler derived PAAT and PAAT:RVET, appears to be a distinct pathology of prematurity that is present independent of clinically apparent lung disease. We found that the degree of PVD at one year CA was inversely related to the gestational age in both healthy preterm infants and preterm infants with established BPD and/or late PH. These findings add to the growing list of complications of being born premature that may increase susceptibility or be a marker for greater risk of late cardiovascular disease beyond the neonatal period and into early childhood, adolescence and adulthood.24,25
A major barrier in detection of PVD and PH to discern their true prevalence and allow for larger scale studies of promising therapies has been the lack of accurate screening tools or universal screening guidelines for identification and stratification of patients with PH.11,13,26 PVR and pulmonary arterial pressure (PAP) are conventionally assessed non-invasively in infants using two Doppler echocardiography methods: (1) measurement of the maximal velocity of tricuspid regurgitation with the application of the modified Bernoulli equation3,27; and (2) measurement of pulmonary flow velocity indices.21,28 Recent studies in children and infants comparing the DVTR and right heart catheterization methods have revealed clinically relevant discrepancies between Doppler-estimated and catheter-measured PAP.29 In a significant number of infants, the DVTR is either absent or insufficient to measure.3,27 PAAT is reemerging as a quantitative method to assess pulmonary blood flow velocity characteristics in the RVOT in infants and children.15,16 There now exists data showing reliable correlation and agreement between PAAT and measured PAP, vascular resistance, and compliance by cardiac catheterization in children and infants that validate this non-invasive method as sensitive measure for assessing pulmonary hemodynamics.15,16
A shortened PAAT reflects elevated PVR and low pulmonary arterial compliance with a larger afterload to RV ejection, altering RV mechanical performance. When PAAT is modified to take into account heart rate (PAAT:RVET), the correlation is further enhanced.15 A PAAT of < 90 msec and PAAT:RVET < 0.31 detects infants with PVD and RV dysfunction with high a degree of sensitivity and specificity.15 In this study, all preterm infants had a PAAT < 90 msec, PAAT:RVET < 0.31, and RV strain values < 26% (normal RV strain at one year of age is > −29%)30, further highlighting that shorter PAAT is associated with differing degrees of RV dysfunction in patients with PVD. This implies that RV afterload is a major contributor to the observed differences in RV function.15 In the setting of depressed RV function, elevated PVR, and decreased compliance there is an increased time needed by the RV to develop the wall tension necessary to overcome the elevated afterload.
Impaired ventricular contraction alone might not cause an early peaking of deceleration of pulmonary systolic flow if the pulmonary vascular bed is normal,31 however the combined consequences of depressed RV function, altered pulmonary hemodynamics, and decreased vascular compliance lead to a shorter PAAT.15 PAAT also integrates the “impedance” to the forward pulsatile blood flow from backward pressure wave reflections from multiple bifurcations of the pulmonary circulation.15 In PVD, the reduced compliant nature of the distal vascular bed causes impedance to increase and forward blood flow to decelerate early in systole as the reflected pressure wave front reaches the pulmonary trunk prematurely, causing an early systolic deceleration and early peaking of flow velocity in the pulmonary artery.32 Thus, PAAT shortening may indicate the presence of underlying pulmonary arterial disease in children as the time to peak velocity in the pulmonary artery decreases and PAAT shortens.
Although numerous cohort and epidemiologic reports indicate that PVD is associated with the presence and degree of BPD5,6,8,33,34, it is important to recognize that the pulmonary vascular hemodynamics, similar to lung function, of most extremely low birth weight infants without BPD may be abnormal, and that those infants also are at increased risk of adverse outcomes.7,33,35 In this study all preterm infants had decreased PAAT and PAAT:RVET, even in the absence of clinical lung disease, when compared with healthy term born infants at one year of age. Subhedar et al prospectively followed a cohort of preterm infants to 52 weeks CA and also reported lower ratios of PAAT:RVET in preterm infants with established BPD, as compared with infants without BPD. 28 Several cross sectional studies have described raised PA pressures, as measured by decreased PAAT or PAAT:RVET, in child survivors of BPD in comparison with healthy preterm 9,21,27,36 and term born-children.37,38 Benetar et al27 and Kwon et al9 even reported lower values of PAAT in children diagnosed with BPD and late PH during the neonatal period. On the other hand, Joshi et al.39 and Korhonen et al found that preterm survivors of BPD had no signs of elevated pulmonary pressures at 7–12 years of age, even after a trial of hypoxic exposure.39,40 Even if PVR and PAP “normalize” by school age in some preterm infants, pulmonary vascular reactivity to changes in oxygen tension and inhaled nitric oxide may still persist into adolescence.41 These studies consisted of small patient cohorts of heterogeneous prematurity and did not assess reproducibility. In comparison, our study used established and validated PAAT values of consistent reliability in relatively larger cohort of prospectively and longitudinally followed extremely low gestational preterm infants.
The high incidence of persistent PVD21,28,37 in extremely premature infants parallels the association of BPD with decreasing gestational age.7 Clinical risk factors associated with persistent PVD include lower gestational age5 and low-birth-weight z score.3 In our study, multivariate analysis showed that PAAT and PAAT:RVET at one year CA were directly related to gestational age at birth in both preterm infants with and without BPD and/or late PH. Subhedar et al.28 and Fitzgerald et al.21 also reported that lower PAAT:RVET in all preterm-born children corresponded to lower gestational age at birth. Although the mechanistic inter-relation of both pathologies in more prematurely born infants is confounded by the tandem development of pulmonary airspace and vasculature42, and the variability in the definitions of each disease,3,18,43 some preterm infants with severe chronic lung injury have normal pulmonary vascular pressures and right heart function,8–10 and other preterm infants without apparent lung disease or BPD demonstrate echocardiographic features of PVD and right ventricular (RV) failure.3–6 Could PVD be a distinct pathology with low PAAT as a feature of prematurity, rather than a manifestation of BPD?12,44 Although premature birth earlier in gestation disrupts the programing of alveolar and vascular development, the response of the pulmonary circulatory system following preterm birth may subsequently induce pathologic remodeling that is characteristic of PVD with lower values of PAAT, and lead to the development of PH.45
The confounding variables for interpretation of the PAAT and PAAT:RVET ratio are heart rate, RV dysfunction, and the presence of large left to right shunts.15 As the PAAT:RVET ratio may fall with increasing heart rate, we plotted the R-R interval against the ratio and saw no statistically significant association between these variables. Consequently, we did not assess PAAT:RVET corrected for heart rate.15 We characterized RV function with RV FLWS and FAC and saw a significant correlation between PAAT and measures of RV function, further confirming the interdependence of PAAT with RV systolic function. Because of the dependence on these variables, PAAT should be interpreted in conjunction with measures of right heart function. A large ASD with increased left to right flow will increase volume to the right heart and falsely elevate PAAT. In this study only 10 patients (13%) of the extreme preterm infants had an ASD or PFO, and none had an effect on PAAT, even after adjusting for BPD, PH, and gestational age. However, this study was not powered to account for the impact of an ASD on PAAT, and the true influence of shunts is an area for further investigation. Altered LV dynamics may also contribute to abnormal pulmonary hemodynamics through its interventricular interactions with the RV and/or left heart failure that can lead to pulmonary venous congestion and PH. In this study, we demonstrated that LV FWLS, as a measure of LV systolic function, was similar between term-born and all preterm-born infants at one year CA, irrespective of a diagnosis of BPD or late PH at 36 weeks PMA.4 Although peak velocity across the RVOT depends on the diameter, based on the notion that velocity is inversely proportional to the cross sectional area, the time to peak velocity (PAAT) had no relationship with diameter across the RVOT. There were no patients with valve narrowing or stenosis in this study.22
In conclusion, preterm-born infants exhibit PVD characteristics at one year CA, regardless of neonatal lung disease status, suggesting the existence of PVD beyond infancy. Although this PVD is commonly associated with BPD, it also appears to be a distinct pathology of prematurity. PAAT measurements may offer a reliable non-invasive tool for screening and longitudinal monitoring of pulmonary hemodynamics in infants.
Figure III.
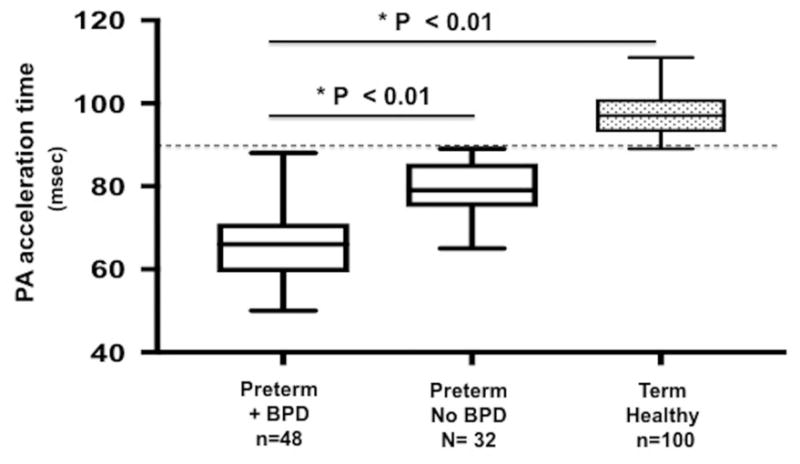
Comparison of PAAT between preterm born- and term born-infants at one year of age. Dash line shows established cut-off value of PAAT <90 msec for detection of pulmonary vascular disease (PVRi > 3 WU × m2, mPAP > 25 mm Hg, sPAP > 35 mm HG) in children.15
Acknowledgments
Supported by the Premature and Respiratory Outcomes Program (NIH 1U01 HL1014650, U01 HL101794), NIH R21 HL106417, Pediatric Physician Scientist Training Grant (NIH 5 T32 HD043010-09), and the Postdoctoral Mentored Training Program in Clinical Investigation (NIH UL1 TR000448).
Abbreviations
- 2DSTE
Two-dimensional speckle tracking echocardiography
- BPD
Bronchopulmonary dysplasia
- CA
Corrected age
- DVTR
Doppler velocity of tricuspid regurgitation
- FAC
Fractional area of change
- FWLS
Free wall longitudinal systolic strain
- PAAT
Pulmonary artery acceleration time
- PDA
Patent ductus arteriosus
- PH
Pulmonary hypertension
- PMA
Postmenstrual age
- PVD
Pulmonary vascular disease
- RVET
Right ventricular ejection time
- RVOT
Right ventricular outflow tract
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mourani PM, Abman SH. Pulmonary Hypertension and Vascular Abnormalities in Bronchopulmonary Dysplasia. Clin Perinatol. 2015;42:839–55. doi: 10.1016/j.clp.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkelhamer SK, Mestan KK, Steinhorn RH. Pulmonary hypertension in bronchopulmonary dysplasia. Semin Perinatol. 2013;37:124–31. doi: 10.1053/j.semperi.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mourani PM, Sontag MK, Younoszai A, Miller JI, Kinsella JP, Baker CD, et al. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;191:87–95. doi: 10.1164/rccm.201409-1594OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy PT, El-khuffash A, Patel MD, Breatnach CR, James AT, Sanchze AA, et al. Maturational patterns of systolic ventricular deformation mechanics by two-dimensional speckle tracking echocardiography in preterm infants over the first year of age. J Am Soc Echocardiogr. 2017;30:685–98. doi: 10.1016/j.echo.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weismann CG, Asnes JD, Bazzy-Asaad A, Tolomeo C, Ehrenkranz RA, Bizzarro MJ. Pulmonary hypertension in preterm infants: results of a prospective screening program. J Perinatol. 2017;37:572–77. doi: 10.1038/jp.2016.255. [DOI] [PubMed] [Google Scholar]
- 6.Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics. 2012;129:e682–89. doi: 10.1542/peds.2011-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagiub M, Kanaan U, Simon D, Guglani L. Risk Factors for Development of Pulmonary Hypertension in Infants with Bronchopulmonary Dysplasia: Systematic Review and Meta-Analysis. Paediatr Respir Rev. 2017;23:27–32. doi: 10.1016/j.prrv.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Farquhar M, Fitzgerald DA. Pulmonary hypertension in chronic neonatal lung disease. Paediatr Respir Rev. 2010;11:149–53. doi: 10.1016/j.prrv.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Kwon HW, Kim H-S, An HS, Kwon BS, Kim GB, Shin SH, et al. Long-Term Outcomes of Pulmonary Hypertension in Preterm Infants with Bronchopulmonary Dysplasia. Neonatology. 2016;110:181–9. doi: 10.1159/000445476. [DOI] [PubMed] [Google Scholar]
- 10.Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120:1260–9.1. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 11.Nagiub M, Lee S, Guglani L. Echocardiographic Assessment of Pulmonary Hypertension in Infants with Bronchopulmonary Dysplasia: Systematic Review of Literature and a Proposed Algorithm for Assessment. Echocardiography. 2015;32:819–33. doi: 10.1111/echo.12738. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor MG, Cornfield DN, Austin ED. Pulmonary hypertension in the premature infant: a challenging comorbidity in a vulnerable population. Curr Opin Pediatr. 2016;28:324–30. doi: 10.1097/MOP.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnan U, Feinstein JA, Adatia I, Austin ED, Mullen MP, Hopper RK, et al. Evaluation and Management of Pulmonary Hypertension in Children with Bronchopulmonary Dysplasia. J Pediatr. 2017:1–12. doi: 10.1016/j.jpeds.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 14.Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical Utility of Echocardiography for the Diagnosis and Management of Pulmonary Vascular Disease in Young Children With Chronic Lung Disease. Pediarics. 2008;12:317–325. doi: 10.1542/peds.2007-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy PT, Patel MD, Groh G, Choudhry S, Murphy J, Holland MR, et al. Pulmonary Artery Acceleration Time Provides a Reliable Estimate of Invasive Pulmonary Hemodynamics in Children. J Am Soc Echocardiogr. 2016 Nov;29:1056–65. doi: 10.1016/j.echo.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koestenberger M, Grangl G, Avian A, Gamillscheg A, Grillitsch M, Cvirn G, et al. Normal Reference Values and z Scores of the Pulmonary Artery Acceleration Time in Children and Its Importance for the Assessment of Pulmonary Hypertension. Circ Cardiovasc Imaging. 2017;10:e00533617. doi: 10.1161/CIRCIMAGING.116.005336. [DOI] [PubMed] [Google Scholar]
- 17.Pryhuber GS, Maitre NL, Ballard RA, Cifelli D, Davis SD, Ellenberg JH, et al. Prematurity and Respiratory Outcomes Program (PROP): study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States. BMC Pediatr. 2016;10:37. doi: 10.1186/s12887-015-0346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poindexter BB, Feng R, Schmidt B, Aschner JL, Ballard RA, Hamvas A, et al. Comparisons and limitations of current definitions of bronchopulmonary dysplasia for the prematurity and respiratory outcomes program. Ann Am Thorac Soc. 2016;12:1822–30. doi: 10.1513/AnnalsATS.201504-218OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy PT, Dioneda B, Holland MR, Sekarski TJ, Lee CK, Mathur A, et al. Right ventricular function in preterm and term neonates: reference values for right ventricle areas and fractional area of change. J Am Soc Echocardiogr. 2015;28:559–69. doi: 10.1016/j.echo.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for chamber quantification. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald D, Evans N, Van Asperen P, Henderson-Smart D. Subclinical persisting pulmonary hypertension in chronic neonatal lung disease. Arch Dis Child Fetal Neonatal Ed. 1994;70:F118–122. doi: 10.1136/fn.70.2.f118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamimura D, Hans S, Suzuki T, Fox RT, Hall ME, Musani SK, et al. Delayed Time to Peak Velocity Is Useful for Detecting Severe Aortic Stenosis. J Am Heart Assoc. 2016;5:e003907. doi: 10.1161/JAHA.116.003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schena F, Francescato G, Cappelleri A, et al. Association between Hemodynamically Significant Patent Ductus Arteriosus and Bronchopulmonary Dysplasia. J Pediatr. 2015;166:1488–1492. doi: 10.1016/j.jpeds.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Raju TNK, Buist AS, Blaisdell CJ, Moxey-Mims M, Saigal S. Adults born preterm: a review of general health and system-specific outcomes. Acta Paediatr. 2017;106:1409–1437. doi: 10.1111/apa.13880. [DOI] [PubMed] [Google Scholar]
- 25.Naumburg E, Söderström L, Huber D, Axelsson I. Risk factors for pulmonary arterial hypertension in children and young adults. Pediatr Pulmonol. 2016;52:636–641. doi: 10.1002/ppul.23633. [DOI] [PubMed] [Google Scholar]
- 26.Altit G, Lee HC, Hintz S, Tacy TA, Feinstein JA, Bhombal S. Practices surrounding pulmonary hypertension and bronchopulmonary dysplasia amongst neonatologists caring for premature infants. J Perinatol. 2017:1–6. doi: 10.1038/s41372-017-0025-3. [DOI] [PubMed] [Google Scholar]
- 27.Benatar A, Clarke J, Silverman M. Pulmonary hypertension in infants with chronic lung disease: non-invasive evaluation and short term effect of oxygen treatment. Arch Dis Child Fetal Neonatal Ed. 2008;72:F14–F19. doi: 10.1136/fn.72.1.f14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subhedar NV, shaw NJ. Changes in pulmonary arterial pressure in preterm infants with chronic lung disease. Arch Dis Child Fetal Neonatal Ed. 2000;82:F243–7. doi: 10.1136/fn.82.3.F243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groh GK, Levy PT, Holland MR, Murphy JJ, Sekarski TJ, Meyers CL, et al. Doppler echocardiography inaccurately estimates right ventricular pressure in children with elevated right heart pressure. J Am Soc Echocardiogr. 2014;27:163–71. doi: 10.1016/j.echo.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy PT, Sanchez A, Machefsky A, Fowler S, Holland MR, Singh GK. Normal ranges of right ventricular systolic and diastolic strain measures in children: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2014;27:549–60. doi: 10.1016/j.echo.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuda M, Sekiguchi T, Sugishita Y, Kuwako K, Iida K, Ito I. Reliability of non-invasive estimates of pulmonary hypertension by pulsed Doppler echocardiography. Br Heart J. 1986;56:158–64. doi: 10.1136/hrt.56.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Champion HC, Michelakis ED, Hassoun PM. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: state of the art and clinical and research implications. Circulation. 2009;120:992–1007. doi: 10.1161/CIRCULATIONAHA.106.674028. [DOI] [PubMed] [Google Scholar]
- 33.Jobe AH, Steinhorn R. Can We Define Bronchopulmonary Dysplasia? J Pediatr. 2017:1–5. doi: 10.1016/j.jpeds.2017.06.064. [DOI] [PubMed] [Google Scholar]
- 34.An HS, Bae EJ, Kim GB, et al. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J. 2010;40:131–136. doi: 10.4070/kcj.2010.40.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hjalmarson O, Sandberg K. Abnormal lung function in healthy preterm infants. Am J Respir Crit Care Med. 2002;165:83–7. doi: 10.1164/ajrccm.165.1.2107093. [DOI] [PubMed] [Google Scholar]
- 36.Farstad T, Brockmeir F, Bratlid D. Cardiopulmonary functionin premature infantswith bronchopulmonary dysplasia-a 2-year follow up. Eur J Pediatr. 1995;154:853–8. doi: 10.1007/BF01959797. [DOI] [PubMed] [Google Scholar]
- 37.Koroglu OA, Yalaz M, Levent E, Akisu M, Kültürsay N. Cardiovascular Consequences of Bronchopulmonary Dysplasia in Prematurely Born Preschool Children. Neonatology. 2014;104:283–9. doi: 10.1159/000354542. [DOI] [PubMed] [Google Scholar]
- 38.Kazanci E, Karagoz T, Tekinalp G, Ozkutlu S, Yurdakök M, Yiğit S, et al. Myocardial performance index by tissue Doppler in bronchopulmonary dysplasia survivors. Turk J Pediatr. 2011;53:388–96. [PubMed] [Google Scholar]
- 39.Joshi S, Wilson DG, Kotecha S, Pickerd N, Fraser AG, Kotecha S. Cardiovascular function in children who had chronic lung disease of prematurity. Arch Dis Child Fetal Neonatal E. 2014;99:F373–9. doi: 10.1136/archdischild-2013-305185. [DOI] [PubMed] [Google Scholar]
- 40.Korhonen P, Hyödynmaa E, Lautamatti V, Iivainen T, Tammela O. Cardiovascular findings in very low birthweight schoolchildren with and without bronchopulmonary dysplasia. Early Hum Dev. 2005;81:497–505. doi: 10.1016/j.earlhumdev.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 41.Mourani PM, Ivy DD, Gao D, Abman SH. Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2004;17:1006–13. doi: 10.1164/rccm.200310-1483OC. [DOI] [PubMed] [Google Scholar]
- 42.Hislop A. Developmental biology of the pulmonary circulation. Paediatr Respir Rev. 2005;6:35–43. doi: 10.1016/j.prrv.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE. Understanding the Short- and Long-Term Respiratory Outcomes of Prematurity and Bronchopulmonary Dysplasia. Am J Respir Crit Care Med. 2015;19:134–156. doi: 10.1164/rccm.201412-2142PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farrow KN, Steinhorn RH. Pulmonary Hypertension in Premature Infants Sharpening the Tools of Detection. Am J Respir Crit Care Med. 2015;191:12–4. doi: 10.1164/rccm.201411-2112ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol. 2005;67:623–61. doi: 10.1146/annurev.physiol.67.040403.102229. [DOI] [PubMed] [Google Scholar]