Abstract
Aim
Recent studies revealed a correlation between skeletal muscle mass index and density with longevity; these studies largely evaluated appendicular skeletal muscles in older Caucasians. This retrospective cohort study assessed the association between axial skeletal muscles size and density with survival in African Americans with type 2 diabetes mellitus.
Methods
Psoas and paraspinous muscle mass index (cross sectional area/height2) and radiographic density (in Hounsfield Units) were measured using computed tomography in African American-Diabetes Heart Study participants, 314 women and 256 men, with median (25th, 75th quartile) age 55.0(48.0, 62.0) and 57.0(50.0, 64.0) years, respectively. Covariates in fully-adjusted model included age, sex, BMI, smoking, hormone replacement therapy (women), cardiovascular disease, hypertension, coronary artery calcified plaque mass, carotid artery calcified plaque mass, and African ancestry proportion.
Results
After median of 7.1(5.9, 8.2) years follow-up, 30(9.6%) of women and 49(19.1%) of men were deceased. In fully-adjusted models, psoas muscle mass index and paraspinous muscle mass index were inversely associated with mortality in men (psoas muscle mass index, hazard ratio [HR]=0.61, P=0.004; paraspinous muscle mass index, HR=0.64, P=0.004), but not in women. Psoas and paraspinous muscle densities did not associate with all-cause mortality. A penalized Cox regression that involved all covariates and predictors associated with mortality showed that only paraspinous muscle mass index remained a significant predictor of mortality (HR=0.65, P=0.02).
Conclusion
Independent from established risk factors for mortality, higher psoas and paraspinous muscle index associate with reduced all-cause mortality in middle-aged African American men with type 2 diabetes mellitus.
Keywords: African American, mortality, muscle, computed tomography, type 2 diabetes
Introduction
Epidemiological studies have shown that body weight and body mass index (BMI) alone are inadequate for accurately predicting all-cause and cardiovascular disease (CVD)-related mortality.1;2 Other parameters of body composition may better prognosticate subsequent mortality. Imaging methods for evaluating skeletal muscle size and adipose tissue distributions have helped refine the relationship between body composition and health.3;4 For example, visceral adiposity (e.g., accumulation of intra-abdominal fat) has emerged as a better indicator of cardiometabolic risk and life expectancy than body weight, BMI or total adipose mass.1;5 Redistribution of adipose cells from subcutaneous compartments to ectopic depots (e.g., in and around the liver, pancreas, heart, kidneys, skeletal muscle) associates with higher risk of insulin resistance, type 2 diabetes mellitus (T2D), CVD events and all-cause mortality and defines the risk phenotype described as “metabolically unhealthy adiposity.”6
Skeletal muscle is the major contributor to lean body mass. Computed tomography (CT) offers accurate assessments of muscle, including skeletal muscle mass and skeletal muscle adipose content.7 Cross-sectional and longitudinal studies revealed that myosteatosis (skeletal muscle fat infiltration) increases with age,8–10 contributes to the development of T2D,10–12 and correlates with impaired longevity in older Caucasians.13 In addition, reduced skeletal muscle mass is associated with unfavorable physical function, an unhealthy inflammatory status and predicts higher mortality in many medical scenarios (surgical oncology, solid organ transplantation, vascular surgery, intensive care, and trauma patients).14
Association between skeletal muscle mass and myosteatosis with mortality in middle-aged African Americans has not yet been evaluated. This study analyzed the relationship between CT-measured muscle mass and myosteatosis of the psoas and paraspinous muscles with mortality in African American-Diabetes Heart Study (AA-DHS) participants. Given the roles of muscle mass depletion and myosteatosis in cardiometabolic health and survival, we hypothesized that reduced muscle mass and increased myosteatosis in skeletal muscles visible on abdominal CT would associate with higher risk of mortality. The present studies tested whether relationships with muscle phenotypes were independent from BMI, age, lifestyle factors, subclinical atherosclerosis (coronary artery calcified atherosclerotic plaque), glycemic control, medications influencing skeletal muscle metabolism and African ancestry proportion.
Methods
Study Participants
The AA-DHS includes all African Americans with T2D recruited in two Wake Forest School of Medicine (WFSM) studies: the family-based Diabetes Heart Study (DHS) and unrelated individuals in AA-DHS. DHS is a cross-sectional study of European American and African American sibling pairs concordant for T2D. AA-DHS was initiated after DHS and enrolled unrelated African Americans using identical inclusion criteria. T2D was diagnosed in all participants developing diabetes after the age of 30 years in the absence of diabetic ketoacidosis. Diabetes was defined as fasting blood glucose (FBG) ≥126 mg/dL or a random glucose ≥200 mg/dL, history of physician diagnosis of diabetes, or use of insulin or an oral hypoglycemic agent. Baseline assessments consisted of interviews for medical history, anthropometric measurements, blood pressures, fasting blood measurements (glucose, serum creatinine, hemoglobin A1c [HbA1c], lipid panels), and spot urine albumin-to-creatinine ratio (UACR). Estimated glomerular filtration rate (eGFR) was computed using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.15 Treatment with insulin, oral hypoglycemic agents, aspirin, lipid lowering drug (statin and non-statin), angiotensin converting enzyme inhibitor (ACEi), and angiotensin receptor blocker (ARB) were recorded. Hypertension was defined based on physician diagnosis, study visit blood pressure >140/90 mmHg, and/or receipt of antihypertensive medications. The study was approved by the WFSM Institutional Review Board and all participants provided written informed consent.
Muscle imaging
Skeletal muscle index and radiographic density (attenuation) was assessed by CT. All scanners were from one institution (WFSM). The muscles were measured on a single CT slice (image), at the level of L4 pedicle. The proximal and distal “borders” were determined by the single CT slice thickness of 2.5mm. Using PACS software, the free-hand region of interest tool was used to define the periphery of psoas and paraspinous muscles without Hounsfield Units (HU) thresholding for measurement of mean muscle attenuation in HU and muscle cross sectional area (CSA). Lower CT-measured skeletal muscle densities by this approach reflect greater intermuscular and intramuscular fat content. CT scans with left-right asymmetry between muscles (due to scoliosis, degenerative diseases, or prior surgery) as well as scans with internal or external artifacts were excluded (N=11). The skeletal muscle index (SMI) was calculated by dividing muscle CSA by patient height squared. This index is commonly used instead of cross sectional area in CT studies of sarcopenia to adjust for differences in body size.
CT measurements of muscle were made by a fellow in musculoskeletal radiology. Images with measurements were archived and validated for measurement accuracy by a single musculoskeletal radiology faculty member (LL). The muscle segmentation process was standardized by following the Standard Operating Procedures manual.16
Vascular imaging
A standardized scanning protocol based on the National Heart, Lung, and Blood Institute Multi-Ethnic Study of Atherosclerosis was used to measure coronary artery calcified atherosclerotic plaque (CAC), as well as calcified plaque in the carotid arteries and infra-renal aorta.17 Scoring parameters included a 90 HU threshold and two adjacent pixels to define the maximum calcified lesion size. Because traditional Agatston (calcium) scores add noise to the CT measurement of calcified plaque compared with volume-based measures, we used the calcium mass score (milligrams of calcium) derived from the volume score and accounting for density of calcified plaque on a pixel by pixel basis.18 We used Log10(CAC+1) as the covariate to make it possible to include individuals without evidence of subclinical vascular calcification in a given vascular bed. This smoothed the distribution of CAC scores and reduced the impact of extreme values. Participants who had previously undergone coronary artery bypass grafting, stenting, or angioplasty were empirically assigned a CAC mass scores of 500.
Vital Status
Vital status was assessed through Dec 31, 2015 using the National Death Index. Cause of death was classified based on the primary factor reported on death certificates. There were 79 deaths; 26 (32.9%) were attributed to CVD, 24.1% to cancer, 7.6% to infection, 5.1% to type 2 diabetes and 30.4% to other causes. Since causes of death were not adjudicated, all-cause mortality was selected as the primary outcome.
Statistical Analyses
Demographic and laboratory characteristics of participants were contrasted by survival status using Wilcoxon two-sample tests for the continuous variables of skeletal muscle density and muscle mass index in the psoas and paraspinous muscle distributions. Analyses were performed in the full sample and stratified by sex. The primary outcome was time to death, determined by the interval between the date of study enrollment and death. Study participants who were known to be alive as of December 31, 2015 were censored. Cox proportional hazard models were subsequently fitted. Covariates were selected to limit confounding effects and ensure that reported effects were not due to other measured variables not accounted for in the model. Association results are presented for a minimally-adjusted model accounting for age, sex, BMI, and smoking status, as well as a fully-adjusted model with all covariates in the minimally-adjusted model plus hormone replacement therapy (women), self-reported history of clinical CVD (angina, myocardial infarction, coronary artery bypass surgery, coronary angioplasty, stroke or carotid endarterectomy), hypertension, CAC mass score, carotid artery calcified atherosclerotic plaque mass score, and African ancestry proportion. Kaplan Meier curves were constructed to compare survival between muscle index tertiles.
Spearman rank correlation was used to estimate the pairwise correlation between all the predictors and covariates considered in these analyses. The absolute values of the Spearman rank correlation between the predictors considered in these models were moderate, varying from a minimum of 0.01 (between age and sex) to >0.9 (between the muscle mass index and density). Given the correlation between these predictors, a penalized Cox regression, assuming the L1 penalty (LASSO) was then fitted with all of the predictors considered to identify the main contribution of these variables to mortality, including calcified atherosclerotic plaque mass score in the coronary and carotid arteries, age, diabetes duration, smoking status, BMI, African ancestry proportion (genome-wide estimates based on results from the Illumina 5 million single nucleotide polymorphism chip), psoas density and index and paraspinous density and index. The LASSO model was fitted using the Cox model implemented in glmnet Package in R.19 The shrinkage parameter was determined using cross-validation. All variables that had a non-zero parameter estimate in the model corresponding to the optimum lambda (shrinkage parameter) were selected and used to fit a Cox proportional hazard model. This model also included age, sex, BMI, African Ancestry proportion and smoking status as covariates to account for their potential confounding effects.
Results
The study included 570 unrelated African Americans with T2D, median (25th, 75th percentile) age 56.0 years (49.0, 63.0) and diabetes duration at enrollment 8.0 years (5.0, 14.0). Baseline demographic and clinical characteristics in the full sample and stratified by sex are displayed in Table 1. Compared to women, men had lower BMI (36.0 vs. 30.9, P=7.0×10−5), more current or past smokers (55.1% vs. 71.9%, P=4.0×10−5), more self-reported myocardial infarction (6.7% vs. 14.9%, P=0.002), more coronary artery bypass surgery (1.9% vs. 5.9%, P=0.01), and higher diastolic blood pressure (76.2 vs. 79.0 mmHg, P=0.03). Medications included ACEi/ARB in 48.4% of participants, insulin in 39.5%, oral hypoglycemic medications in 96.5%, aspirin in 48.5%, and statins in 47.5%. In addition, 22.8% of women were prescribed hormone replacement therapy.
Table 1.
Baseline demographic and clinical characteristics of the African American-Diabetes Heart Study (AA-DHS) cohort*
| Variables |
P- value |
||||||
|---|---|---|---|---|---|---|---|
| N | Full sample | N | Women | N | Men | ||
| Age, years | 570 | 56.0 (49.0, 63.0) | 314 | 55.0 (48.0, 62.0) | 256 | 57.0 (50.0, 64.0) | 0.2 |
| Body mass index, kg/m2 | 569 | 33.5 (29.0, 38.8) | 314 | 36.0 (30.7, 41.6) | 255 | 30.9 (27.9, 35.7) | <0.001 |
| African ancestry proportion, % | 570 | 82.7 (75.8, 88.6) | 314 | 83.8 (77.4, 89.3) | 256 | 81.8 (74.8, 87.8) | 0.02 |
| Smoking, current or former, % | 570 | 62.6% | 314 | 55.1% | 256 | 71.9% | <0.001 |
| Diabetes duration at enrollment, years | 570 | 8.0 (5.0, 13.0) | 314 | 8.0 (5.0, 13.0) | 256 | 8.5 (4.0, 13.0) | 0.9 |
| Angina, % | 534 | 15.4% | 302 | 16.9% | 232 | 13.4% | 0.26 |
| Heart attack, % | 560 | 10.4% | 312 | 6.7% | 248 | 14.9% | 0.002 |
| Coronary artery bypass surgery, % | 570 | 3.7% | 314 | 1.9% | 256 | 5.9% | 0.01 |
| Coronary angioplasty, % | 567 | 12.2% | 314 | 11.5% | 253 | 13.0% | 0.57 |
| Stroke, % | 563 | 7.6% | 313 | 7.7% | 250 | 7.6% | 0.98 |
| Carotid endarterectomy, % | 564 | 0.2% | 311 | 0.3% | 253 | 0.0% | 0.37 |
| Hypertension, % | 570 | 81.8% | 314 | 83.4% | 256 | 79.7% | 0.25 |
| Systolic blood pressure, mmHg | 556 | 132.0 (121.0, 145.6) | 308 | 132.0 (121.0, 146.1) | 248 | 132.0 (121.0, 145.0) | 0.8 |
| Diastolic blood pressure, mmHg | 556 | 78.0 (70.0, 85.0) | 308 | 76.2 (69.0, 83.2) | 248 | 79.0 (70.0, 86.0) | 0.03 |
| ACEi/ARB use, % | 570 | 48.4% | 314 | 46.5% | 256 | 50.8% | 0.31 |
| Insulin use, % | 567 | 39.5% | 312 | 37.5% | 255 | 42.0% | 0.28 |
| Oral antidiabetic medication, % | 567 | 96.5% | 312 | 97.1% | 255 | 95.7% | 0.36 |
| Aspirin use, % | 520 | 48.5% | 283 | 49.8% | 237 | 46.8% | 0.52 |
| Statin use, % | 568 | 47.5% | 313 | 47.9% | 255 | 47.1% | 0.84 |
| Follow-up time, years | 570 | 6.9 (5.9, 7.8) | 314 | 6.9 (6.1, 7.8) | 256 | 6.8 (5.7, 7.8) | 0.08 |
Data presented as median (25th percentile, 75th percentile) for continuous variables or % for categorical variables.
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Table 2 displays biochemical profiles and CT measures in the full sample, and stratified by sex. Participants had median HbA1c 7.7% (6.7, 9.2), eGFR 85.0 ml/min/1.73m2 (68.0, 103.8), and UACR 13.9 mg/g (5.0, 53.3). Sex differences were present across several biochemical and vascular parameters. Compared to women, men had higher HbA1c (7.6% vs. 7.8%, P=0.03), more albuminuria (UACR 10.9 vs. 18.8 mg/g, P=0.03), and lower high density lipoprotein cholesterol concentrations (48 vs. 43 mg/dl, P=3.0×10−7). Men had greater burdens of CAC (CAC mass score, 68.5 vs. 244.8 mg Ca+, P=0.005; CAC presence [defined as >10mg Ca+], 63.0% vs. 73.0%, P=0.01), a higher prevalence of aorta calcified plaque [aorta Ca+ >10mg] (76.1% vs. 84.0%, P=0.02), and more vascular beds with detectable calcified plaque (P=0.004).
Table 2.
Baseline biochemical and radiological characteristics of the African American-Diabetes Heart Study (AA-DHS) cohort*
| Variables | P-value | ||||||
|---|---|---|---|---|---|---|---|
| N | Full sample | N | Women | N | Men | ||
| Hemoglobin A1c, % | 554 | 7.7 (6.7, 9.1) | 305 | 7.6 (6.6, 8.7) | 249 | 7.8 (6.8, 9.6) | 0.03 |
| Fasting glucose, mg/dl | 564 | 134.0 (107.8, 176.0) | 311 | 131 (106.5, 162.0) | 253 | 139 (111.0, 197.0) | 0.02 |
| Serum creatinine, mg/dl | 564 | 1.0 (0.8, 1.1) | 311 | 0.8 (0.7, 1.0) | 253 | 1.1 (0.9, 1.2) | <0.001 |
| Blood urea nitrogen, mg/dl | 562 | 14.0 (11.0, 17.0) | 309 | 14 (11.0, 17.0) | 253 | 14 (11.0, 17.0) | 0.26 |
| CKD-EPI eGFR, ml/min/1.73m2 | 564 | 88.3 (72.4, 108.4) | 311 | 90.1 (71.3, 110.5) | 253 | 87.5 (74.5, 104.6) | 0.74 |
| Urine albumin to creatinine ratio, mg/g | 555 | 13.0 (5.0, 51.1) | 305 | 10.9 (4.3, 47.0) | 250 | 18.8 (5.0, 58.8) | 0.03 |
| High density lipoprotein cholesterol, mg/dl | 561 | 45.0 (39.0, 53.0) | 311 | 48.0 (40.0, 57.0) | 250 | 43.0 (37.0, 50.0) | <0.001 |
| Low density lipoprotein cholesterol, mg/dl | 551 | 103.0 (83.0, 131.0) | 306 | 105.0 (86.0, 134.0) | 245 | 103.0 (77.0, 125.0) | 0.10 |
| Triglycerides, mg/dl | 561 | 104.0 (77.0, 145.0) | 311 | 101.0 (76.5, 145.0) | 250 | 107.0 (79.0, 146.0) | 0.32 |
| Coronary artery CP mass, mg Ca+ | 563 | 119 (2.5, 500) | 311 | 68.5 (1.8, 500) | 252 | 244.8 (4.9, 500) | 0.005 |
| Coronary artery CP >10 mg Ca+, % | 563 | 67.5% | 311 | 63.0% | 252 | 73.0% | 0.01 |
| Carotid artery CP mass, mg Ca+ | 563 | 4.0 (0.0, 107.0) | 311 | 2.5 (0.0, 89.8) | 252 | 8.5 (0.0, 155.9) | 0.11 |
| Carotid artery CP >10 mg Ca+, % | 563 | 44.9% | 311 | 41.8% | 252 | 48.8% | 0.10 |
| Aorta CP mass, mg Ca+ | 559 | 1192.0 (48.5, 6843.5) | 309 | 929.0 (19.0, 6837.0) | 250 | 1246.0 (94.5, 6876.2) | 0.23 |
| Aorta CP >10 mg Ca+, % | 559 | 79.6% | 309 | 76.1% | 250 | 84.0% | 0.02 |
| Vascular beds with CP, n | 557 | 2.0 (1.0, 3.0) | 307 | 2.0 (1.0, 3.0) | 250 | 2.0 (2.0, 3.0) | 0.004 |
| Psoas muscle index, cm2/m2 | 570 | 4.0 (3.3, 4.8) | 314 | 3.5 (3.0, 4.0) | 256 | 4.8 (4.0, 5.5) | <0.001 |
| Psoas muscle density, HU | 570 | 54.0 (50.2, 57.7) | 314 | 53.3 (49.5, 57.6) | 256 | 55.1 (51.5, 58.3) | 0.001 |
| Paraspinous muscle index, cm2/m2 | 570 | 8.1 (7.1, 9.0) | 314 | 8.0 (7.1, 9.0) | 256 | 8.1 (7.1, 9.0) | 0.87 |
| Paraspinous muscle density, HU | 570 | 40.7 (29.4, 49.0) | 314 | 34.7 (22.1, 42.7) | 256 | 47.2 (39.8, 53.2) | <0.001 |
CP, calcified plaque; HU, Hounsfield Units.
Median (25th percentile, 75th percentile) for continuous variables or % for categorical variables.
The median values of psoas muscle mass index, psoas muscle density, paraspinous muscle mass index, and paraspinous muscle density are provided in the full sample and stratified by sex (Table 2). Men had higher psoas muscle index (4.8 vs. 3.5 cm2/m2, P=2.0×10−40), higher psoas muscle densities (53.3 vs. 55.1 HU, P=0.001), and higher paraspinous muscle densities (34.7 vs. 47.2 HU, P=5.0×10−29) than women; while paraspinous muscle index was similar (8.0 vs. 8.1 cm2/m2, P=0.87).
After median follow-up of 7.1 years, (5.9, 8.2), 79 deaths were recorded (49 [19.1%] in men and 30 [9.6%] in women). Table 3 presents the results of multivariate association analyses for mortality, based on baseline psoas and paraspinous muscle mass index and density. In the full sample, psoas muscle mass index was significantly associated with all-cause mortality. For each 1 SD increase in psoas muscle mass index, the 7-year hazard ratio (HR) for death was 37% lower in the minimally-adjusted analysis (model 1, adjusted for age, sex, BMI, and smoking status; 95% confidence interval [CI], 0.47–0.83; P=0.001), and 32% lower in the fully-adjusted analysis (model 2, model 1 plus adjustment for hormone replacement therapy [women], history of CVD, hypertension, CAC mass score, and African ancestry proportion; 95% CI, 0.50–0.91; P=0.008). Paraspinous muscle mass index was significantly associated with death in the minimally-adjusted analysis (model 1); for each 1 SD increase in baseline paraspinous muscle mass index, the 7-year HR for death was 27% lower (HR 0.73, 95% CI, 0.55–0.98; P=0.03). A trend toward association was also present in the fully-adjusted analysis (model 2; HR, 0.77, 95%CI, 0.58–1.03; P=0.07). In contrast, muscle density was not associated with all-cause mortality in the full sample.
Table 3.
Hazard ratios for 7-year mortality per 1SD increase in baseline psoas and paraspinous muscle measures
| Predictor | SD | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Full sample | |||||||
| Psoas muscle index, cm2/m2 | 1.2 | 0.63 | 0.47, 0.83 | 0.001 | 0.68 | 0.50, 0.91 | 0.008 |
| Psoas muscle density, HU | 6.8 | 0.87 | 0.72, 1.05 | 0.15 | 0.91 | 0.75, 1.11 | 0.35 |
| Paraspinous muscle index, cm2/m2 | 1.5 | 0.73 | 0.55, 0.98 | 0.03 | 0.77 | 0.58, 1.03 | 0.07 |
| Paraspinous muscle density, HU | 15.1 | 0.89 | 0.67, 1.18 | 0.40 | 0.91 | 0.69, 1.22 | 0.53 |
| Men | |||||||
| Psoas muscle index, cm2/m2 | 1.1 | 0.59 | 0.42, 0.83 | 0.002 | 0.61 | 0.44, 0.86 | 0.004 |
| Psoas muscle density, HU | 7.4 | 0.89 | 0.69, 1.16 | 0.39 | 0.91 | 0.71, 1.17 | 0.47 |
| Paraspinous muscle index, cm2/m2 | 1.4 | 0.62 | 0.45, 0.85 | 0.003 | 0.64 | 0.46, 0.87 | 0.004 |
| Paraspinous muscle density, HU | 11.4 | 0.89 | 0.67, 1.19 | 0.44 | 0.98 | 0.72, 1.32 | 0.88 |
| Women | |||||||
| Psoas muscle index, cm2/m2 | 0.8 | 0.97 | 0.69, 1.36 | 0.87 | 1.09 | 0.69, 1.73 | 0.71 |
| Psoas muscle density, HU | 6.2 | 0.87 | 0.60, 1.24 | 0.44 | 0.88 | 0.55, 1.40 | 0.59 |
| Paraspinous muscle index, cm2/m2 | 1.5 | 0.95 | 0.56, 1.61 | 0.86 | 1.03 | 0.63, 1.70 | 0.89 |
| Paraspinous muscle density, HU | 15.2 | 0.89 | 0.55, 1.44 | 0.64 | 0.74 | 0.46, 1.20 | 0.22 |
Hazard ratios (HR), 95% confidence intervals (CI) and P-values were obtained with Cox proportional hazard models, assuming 1 SD increase in the predictor. Model 1 (minimally-adjusted) included age, BMI and smoking (plus sex in the full sample). Model 2 (fully-adjusted) included all variables in Model 1, plus hormone replacement therapy (women), history of cardiovascular disease (angina, heart attack, coronary artery bypass surgery, coronary angioplasty, stroke, carotid endarterectomy), hypertension, coronary artery calcified atherosclerotic plaque mass score, carotid artery calcified atherosclerotic plaque mass score, and African ancestry proportion. SD, Standard deviation. HU, Hounsfield Units.
Cox proportional hazard models were performed stratified by sex. In men, both psoas muscle index and paraspinous muscle index significantly associated with lower mortality. For each 1 SD increase in psoas muscle mass index, the 7-year hazard ratio for death in men was 41% lower in model 1 (95% CI, 0.42–0.83; P=0.002) and 39% lower in model 2 (95% CI, 0.44–0.86; P=0.004). For each 1 SD increase in paraspinous muscle mass index, the hazard for death in men was 38% lower in model 1 (95% CI, 0.45–0.85; P=0.003) and 36% lower in model 2 (95% CI, 0.46–0.87; P=0.004). In contrast, significant associations between psoas and paraspinous muscle mass index with all-cause mortality were not observed in women. Neither psoas nor paraspinous muscle density was significantly associated with all-cause mortality in sex-stratified analyses.
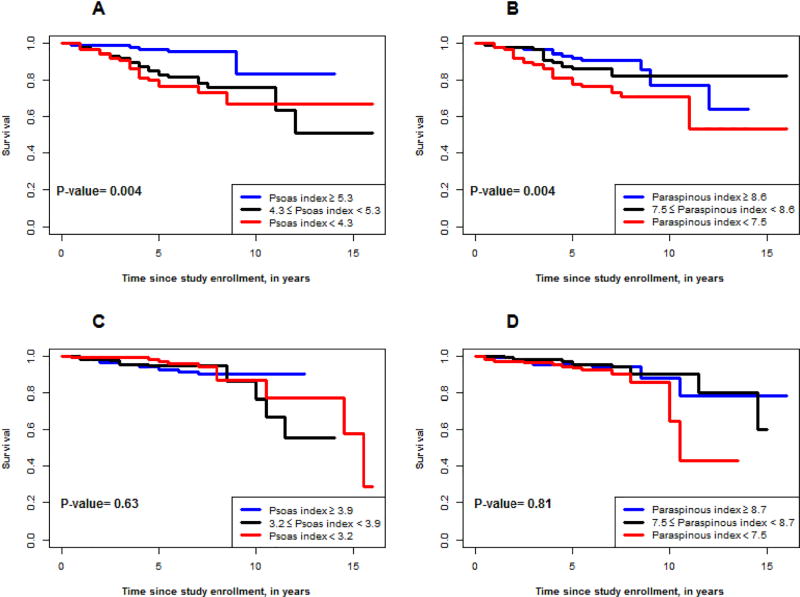
Kaplan–Meier survival estimates were generated by sex-stratified tertiles of psoas and paraspinous muscle mass index (Figure 1). In men, the estimated 7-year survival by psoas muscle index tertile was 90.6% in the highest, 84.1% in the middle, and 70.6% in the lowest tertile. For paraspinous index, the estimated 7-year survivals were 90.6%, 82.0% and 73.0% in the three tertiles, respectively. There were no associations with survival by tertiles of psoas muscle index or paraspinous muscle index in women.
Figure 1.
Kaplan Meier survival curves based on tertiles of psoas (1A and 1C) and paraspinous (1B and 1D) muscle mass index in males (1A and 1B) and females (1C and 1D).
Results of the LASSO multivariate model are presented in Table 4. LASSO selected predictors of mortality including carotid artery calcified plaque mass greater than 10 mg Ca+ (with an even stronger effect for carotid artery calcified plaque mass greater than 100 mg Ca+), psoas muscle mass index, psoas muscle density and paraspinous muscle mass index. LASSO parameter estimates are known to be biased because of the constraint imposed on them. Unconstrained estimates were obtained for these variables by fitting a model Cox proportional hazard model with age, sex, BMI and smoking status included as covariates to control for their possible cofounding effect. Across all variables included, only paraspinous muscle mass index was significant in this model with HR and 95% CI 0.65 (0.45, 0.93), P=0.02. Smoking status had the strongest effect, 2.07 (0.90, 4.77); however, this effect did not reach statistical significance p=0.09.
Table 4.
Multivariate model with nonzero predictors of mortality in men in the LASSO model*
| Predictor | LASSO estimate |
Change/ status |
Hazard ratio |
95% Confidence interval |
P value |
|---|---|---|---|---|---|
| Age | - | 9.5 | 0.9 | (0.62, 1.31) | 0.59 |
| BMI | - | 6.1 | 0.97 | (0.66, 1.41) | 0.86 |
| Smoking status | - | 1 | 2.07 | (0.90, 4.77) | 0.09 |
| African ancestry proportion | - | 10.1 | 0.91 | (0.72, 1.16) | 0.46 |
| Carotid artery CP >10 mg Ca+, % | 0.09 | 1.2 | 1.14 | (0.65, 1.99) | 0.65 |
| Carotid artery CP >100 mg Ca+, % | 0.20 | 1 | 1.86 | (0.60, 5.77) | 0.28 |
| Psoas muscle index, cm2/m2 | −0.04 | 1.1 | 0.54 | (0.16, 1.75) | 0.30 |
| Psoas muscle density, HU | −0.02 | 7.4 | 1.25 | (0.42, 3.75) | 0.69 |
| Paraspinous muscle index, cm2/m2 | −0.12 | 1.4 | 0.65 | (0.45, 0.93) | 0.02 |
LASSO and final Cox regressions were fit based on a sample of 249 men with T2D and 49 total deaths.
Diabetes duration, hemoglobin A1c, cardiovascular disease, coronary artery calcified plaque, aorta calcified plaque, African ancestry proportion and paraspinous muscle density were included in the LASSO model, but their parameter estimates were shrunken to zero.
Discussion
The present study assessed associations between CT measures of psoas and paraspinous skeletal muscle index and density, with all-cause mortality in a large cohort of African Americans with T2D. The AA-DHS cohort has been intensively phenotyped for conventional CVD risk factors and has more than 7 years of follow-up. A significant inverse association was observed between psoas and paraspinous muscle index with all-cause mortality in these middle-aged African American men with T2D. Axial skeletal muscle indices were independent predictors of survival and the paraspinous muscle mass index was a better predictor of mortality than conventional CVD risk factors, which were also positively associated with mortality in this cohort.
Skeletal muscle mass and quality are becoming recognized as powerful predictors of adverse outcomes, including mortality. The pathophysiologic processes contributing to relationships between skeletal muscle mass and survival remain poorly understood. Mechanisms underlying muscle loss other than disuse include insulin resistance, chronic inflammation and mitochondrial dysfunction; conditions which are also associated with T2D. Insulin is an anabolic hormone that stimulates protein synthesis, including in skeletal muscle. In experimental models of insulin resistance, animals exhibited increases in markers of proteasomal protein degradation in skeletal muscles which led to decreased muscle size and function.20 As such, insulin resistance per se may impair muscle strength and performance in patients with T2D.21 In contrast, low skeletal mass may also contribute to the development of T2D due to its important role in glucose metabolism. Association between altered skeletal muscle fiber composition and subsequent development of insulin resistance was reported in previously healthy young men with low birth weights.22;23
A novel finding in this report was the inverse association between psoas and paraspinous muscle mass index with mortality in a cohort largely comprised of middle-aged individuals (median age 56.0 years). The magnitude of this association was clinically important in men and independent from established (age, BMI, smoking status, history of CVD, and hypertension) and novel predictors of mortality (CAC and African ancestry proportion). Several studies demonstrate that the incidence of skeletal muscle mass depletion increases with age and becomes clinically relevant in the elderly.24;25 This study may have been ‘powered’ to detect an association between muscle mass index and survival in middle-aged men perhaps because muscle mass depletion was more prevalent in AA-DHS participants affected by the presence of T2D which is a potent risk factor for muscle mass loss.26 Muscle fiber type proportions vary by individual, change with aging, and are relatively understudied in relation to psoas versus paraspinous muscle. We would expect denser muscle to be more metabolically efficient. The relative higher density (lower fat) of psoas muscle compared to paraspinous muscle could simply reflect greater compactness and reduced number of muscle groups limiting the amount of intermuscular fat between muscle groups. Physiologically, the denser muscle could metabolize glucose better than the less dense paraspinous muscle.
The present results complement prior studies demonstrating the prognostic utility of CT-measured skeletal muscle mass in other anatomical regions as clinically significant predictors of length of hospitalization and mortality after cardiac procedures, abdominal surgeries, patients admitted to critical care unit, and patients with malignancy or trauma. These studies had variable numbers of African American participants and few with T2D.27–31 Results support the use CT to measure skeletal muscle mass index to provide unique prognostic information not accounted for in standard risk models.
Sex-specific differences were observed in the association between skeletal muscle mass index and all-cause mortality in African Americans with T2D. Different pathophysiologic mechanisms appear to produce loss of muscle mass in clinical and experimental studies involving men and women.32–34 In men, skeletal muscle mass depletion appears to be driven by the catabolic influence of myostatin, while in women reductions in insulin-like growth factor 1 (IGF-1) may cause loss of muscle mass.32 It is possible that the processes underlying sex-specific skeletal muscle mass depletion contributed to the differential associations between skeletal muscle mass index and mortality in the AA-DHS cohort. Future studies and larger samples will be needed to verify whether sex-specific differences in the association between reduced skeletal muscle mass and mortality are maintained in other race groups and in other skeletal muscle regions.
Psoas and paraspinous muscle density did not associate with mortality in this report. This is in contrast with other studies that found an association between myosteatosis and mortality in African Americans and Caucasians31;35–37 Different methodologies have been used to measure muscle metrics on CT examinations. These methodologic differences may explain some of the discrepancies between various studies reporting relationships between muscle size and muscle density and mortality. Differences in study designs and cohorts could also contribute to the lack of consistency in these relationships. First, although skeletal muscle fatty infiltration is greater in men of African descent compared to European descent, myosteatosis progresses with age.10;38–40 Therefore, inclusion of middle-aged African Americans (25% of whom were <49 years old) in AA-DHS might have reduced our power to detect an association between skeletal muscle fat infiltration and mortality. Second, the skeletal muscle territories assessed in AA-DHS differed from previous studies that analyzed appendicular skeletal muscles. Myosteatosis and mortality were analyzed in a large African ancestry population-based cohort including 1,652 men aged 40 years or older; 17% had T2D and mean follow-up was 5.9 years.37 That study identified independent associations between higher calf intramuscular and intermuscular fat depots with all-cause mortality limited to participants ≥65 years of age (HR 1.66 per SD lower muscle density, 95% CI 1.24–2.21 adjusted for BMI, smoking, alcohol intake, physical activity, television viewing time, health status, T2D, renal disease, stroke, cancer, myocardial infarction, and calf muscle area).37 Thus, additional studies in populations of African ancestry are needed to further describe and confirm the distribution of adipose tissue in various skeletal muscle territories and associations with mortality.
The present study has strengths and limitations. Participants were enrolled at one academic institution, as such it many not reflect patient populations or outcomes at other centers and should be externally validated in a prospective fashion. Although we standardized the muscle segmentation process, error measurements were not directly assessed. In our other studies using the same methods, the intra-class correlation coefficient was 0.9.16 Psoas and para-spinous muscle inter-muscular adipose tissue were not measured and we lacked muscle index and density in non-axial muscles because abdominal CT was used. Additional markers of physical function and clinical frailty were not obtained. Therefore, complementary (or incremental) effects of muscle mass index measures in combination with gait speed or performance variables could not be ascertained. Integration of parameters of physical performance along with muscle mass index might improve prognostication. In contrast, the AA-DHS cohort was extensively phenotyped for risk factors determining risk of mortality, including vascular calcification, medication use, and markers of glycemic control. Nevertheless, the association we observed between psoas muscle mass index and paraspinous muscle mass index with mortality in this retrospective study do not prove causality. It remains possible that underlying disease processes that caused changes in muscle mass index also contributed to the increase in mortality. Although the analyses adjusted for conventional risk factors for death, adjustments did not take into account disease severity, and residual confounding by disease severity or unmeasured confounders could not be excluded. Despite these limitations, the present results have important implications given that psoas and paraspinous muscle mass index were significant prognostic markers for long-term mortality in African American men with T2D. These muscle markers outperformed traditional clinical variables in terms of their strength of association and statistical significance. Multi-center studies in larger and more diverse populations are needed for confirmation.
In conclusion, CT-measured psoas and paraspinous muscle mass index were significant predictors of mortality in middle-aged African American men with T2D, but not African American women with T2D. Additional work is required to explore predictive effects of psoas and paraspinous mass index with other clinically relevant outcomes, including cause-specific hospitalizations and cause-specific mortality.
Acknowledgments
Funding: This work was financially supported by grants from the National Institute of Health R01 DK071891 (BIF) and HL67348 (DWB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no conflicts of interest.
References
- 1.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 2.Tomiyama AJ, Hunger JM, Nguyen-Cuu J, Wells C. Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005–2012. Int J Obes (Lond) 2016;40:883–886. doi: 10.1038/ijo.2016.17. [DOI] [PubMed] [Google Scholar]
- 3.De SA, Lavie CJ, Kachur S, Patel DA, Milani RV. Body composition and mortality in a large cohort with preserved ejection fraction: untangling the obesity paradox. Mayo Clin Proc. 2014;89:1072–1079. doi: 10.1016/j.mayocp.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Bosy-Westphal A, Muller MJ. Identification of skeletal muscle mass depletion across age and BMI groups in health and disease--there is need for a unified definition. Int J Obes (Lond) 2015;39:379–386. doi: 10.1038/ijo.2014.161. [DOI] [PubMed] [Google Scholar]
- 5.Tan CY, Vidal-Puig A. Adipose tissue expandability: the metabolic problems of obesity may arise from the inability to become more obese. Biochem Soc Trans. 2008;36:935–940. doi: 10.1042/BST0360935. [DOI] [PubMed] [Google Scholar]
- 6.Stefan N, Haring HU, Schulze MB. Metabolically healthy obesity: the low-hanging fruit in obesity treatment? Lancet Diabetes Endocrinol. 2017 doi: 10.1016/S2213-8587(17)30292-9. [DOI] [PubMed] [Google Scholar]
- 7.Goodpaster BH, Thaete FL, Kelley DE. Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci. 2000;904:18–24. doi: 10.1111/j.1749-6632.2000.tb06416.x. [DOI] [PubMed] [Google Scholar]
- 8.Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009;8:339–348. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miljkovic I, Kuipers AL, Cvejkus R, et al. Myosteatosis increases with aging and is associated with incident diabetes in African ancestry men. Obesity (Silver Spring) 2016;24:476–482. doi: 10.1002/oby.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 12.Brehm A, Krssak M, Schmid AI, Nowotny P, Waldhausl W, Roden M. Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes. 2006;55:136–140. [PubMed] [Google Scholar]
- 13.Wijsman CA, van Opstal AM, Kan HE, et al. Proton magnetic resonance spectroscopy shows lower intramyocellular lipid accumulation in middle-aged subjects predisposed to familial longevity. Am J Physiol Endocrinol Metab. 2012;302:E344–E348. doi: 10.1152/ajpendo.00455.2011. [DOI] [PubMed] [Google Scholar]
- 14.Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr. 2002;76:473–481. doi: 10.1093/ajcn/76.2.473. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boutin RD, Bamrungchart S, Bateni CP, et al. CT of Patients With Hip Fracture: Muscle Size and Attenuation Help Predict Mortality. AJR Am J Roentgenol. 2017;208:W208–W215. doi: 10.2214/AJR.16.17226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 18.Wagenknecht LE, Divers J, Register TC, et al. Bone Mineral Density and Progression of Subclinical Atherosclerosis in African-Americans With Type 2 Diabetes. J Clin Endocrinol Metab. 2016;101:4135–4141. doi: 10.1210/jc.2016-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon N, Friedman J, Hastie T, Tibshirani R. Regularization Paths for Cox's Proportional Hazards Model via Coordinate Descent. J Stat Softw. 2011;39:1–13. doi: 10.18637/jss.v039.i05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostler JE, Maurya SK, Dials J, et al. Effects of insulin resistance on skeletal muscle growth and exercise capacity in type 2 diabetic mouse models. Am J Physiol Endocrinol Metab. 2014;306:E592–E605. doi: 10.1152/ajpendo.00277.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolland Y, Czerwinski S, Abellan Van KG, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen CB, Storgaard H, Madsbad S, Richter EA, Vaag AA. Altered skeletal muscle fiber composition and size precede whole-body insulin resistance in young men with low birth weight. J Clin Endocrinol Metab. 2007;92:1530–1534. doi: 10.1210/jc.2006-2360. [DOI] [PubMed] [Google Scholar]
- 23.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One. 2010;5:e10805. doi: 10.1371/journal.pone.0010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aleman-Mateo H, Lopez Teros MT, Ramirez FA, Astiazaran-Garcia H. Association between insulin resistance and low relative appendicular skeletal muscle mass: evidence from a cohort study in community-dwelling older men and women participants. J Gerontol A Biol Sci Med Sci. 2014;69:871–877. doi: 10.1093/gerona/glt193. [DOI] [PubMed] [Google Scholar]
- 25.Jang HC. Sarcopenia, Frailty, and Diabetes in Older Adults. Diabetes Metab J. 2016;40:182–189. doi: 10.4093/dmj.2016.40.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umegaki H. Sarcopenia and diabetes: Hyperglycemia is a risk factor for age-associated muscle mass and functional reduction. J Diabetes Investig. 2015;6:623–624. doi: 10.1111/jdi.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weijs PJ, Looijaard WG, Dekker IM, et al. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care. 2014;18:R12. doi: 10.1186/cc13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heberton GA, Nassif M, Bierhals A, et al. Usefulness of Psoas Muscle Area Determined by Computed Tomography to Predict Mortality or Prolonged Length of Hospital Stay in Patients Undergoing Left Ventricular Assist Device Implantation. Am J Cardiol. 2016;118:1363–1367. doi: 10.1016/j.amjcard.2016.07.061. [DOI] [PubMed] [Google Scholar]
- 29.Looijaard WG, Dekker IM, Stapel SN, et al. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit Care. 2016;20:386. doi: 10.1186/s13054-016-1563-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoo T, Lo WD, Evans DC. Computed tomography measured psoas density predicts outcomes in trauma. Surgery. 2017;162:377–384. doi: 10.1016/j.surg.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teigen LM, John R, Kuchnia AJ, et al. Preoperative Pectoralis Muscle Quantity and Attenuation by Computed Tomography Are Novel and Powerful Predictors of Mortality After Left Ventricular Assist Device Implantation. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.117.004069. [DOI] [PubMed] [Google Scholar]
- 32.Tay L, Ding YY, Leung BP, et al. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. Age (Dordr) 2015;37:121. doi: 10.1007/s11357-015-9860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canon ME, Crimmins EM. Sex differences in the association between muscle quality, inflammatory markers, and cognitive decline. J Nutr Health Aging. 2011;15:695–698. doi: 10.1007/s12603-011-0340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kob R, Fellner C, Bertsch T, et al. Gender-specific differences in the development of sarcopenia in the rodent model of the ageing high-fat rat. J Cachexia Sarcopenia Muscle. 2015;6:181–191. doi: 10.1002/jcsm.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wijnhoven HA, Snijder MB, van Bokhorst-de van der Schueren MA, Deeg DJ, Visser M. Region-specific fat mass and muscle mass and mortality in community-dwelling older men and women. Gerontology. 2012;58:32–40. doi: 10.1159/000324027. [DOI] [PubMed] [Google Scholar]
- 36.Miljkovic I, Kuipers AL, Cauley JA, et al. Greater Skeletal Muscle Fat Infiltration Is Associated With Higher All-Cause and Cardiovascular Mortality in Older Men. J Gerontol A Biol Sci Med Sci. 2015;70:1133–1140. doi: 10.1093/gerona/glv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Q, Zmuda JM, Kuipers AL, et al. Greater skeletal muscle fat infiltration is associated with higher all-cause mortality among men of African ancestry. Age Ageing. 2016;45:529–534. doi: 10.1093/ageing/afw062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82:1210–1217. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torriani M, Grinspoon S. Racial differences in fat distribution: the importance of intermuscular fat. Am J Clin Nutr. 2005;81:731–732. doi: 10.1093/ajcn/81.4.731. [DOI] [PubMed] [Google Scholar]
- 40.Miljkovic I, Cauley JA, Petit MA, et al. Greater adipose tissue infiltration in skeletal muscle among older men of African ancestry. J Clin Endocrinol Metab. 2009;94:2735–2742. doi: 10.1210/jc.2008-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]