Abstract
Introduction
Open abdominal aortic aneurysm (AAA) repair effectively prevents rupture for patients with AAA, and is commonly studied as a metric reflecting hospital and surgeon expertise in cardiovascular care. However, given recent advances in endovascular abdominal aortic aneurysm repair (EVAR) such as branched/fenestrated EVAR, it is unknown how commonly open surgical repair is still used in everyday practice.
Methods
We analyzed trends in open AAA repair, EVAR, and branched/fenestrated EVAR for AAA in Medicare beneficiaries from 2003 to 2013. We used Medicare Part B claims to ascertain counts of these repair types and annually over the study period. We assessed regional and national trends in patient characteristics and procedure volume.
Results
Between 2003 and 2013, the total number of AAA repairs performed in fee-for-service Medicare patients declined by 26% from 31,582 to 23,421 (p<0.001), after a peak number of 32,540 was performed in 2005 (28% decline since 2005). The number of open AAA repairs steadily declined by a total of 76%, from 20,533 in 2003 to 4,916 in 2013 (p < 0.001). While the number of EVARs increased from 11,049 in 2003 to 19,247 in 2011 (p <0.001), it has since declined a total of 15% to only 16,362 repairs in 2013 (p <0.001). After its introduction in 2011, the number of branched/fenestrated EVAR cases continuously rose from 335 procedures in 2011 to 2,143 procedures in 2013 (p < 0.001). By 2013 virtually all hospital referral regions in the United States had rates of open AAA repair which would have been in the lowest quintile of volume in 2003.
Conclusions
The number of open AAA repairs fell by nearly 80% during the last decade, while traditional EVAR declined slightly and branched/fenestrated EVAR rapidly disseminated into national practice. These results suggest that open AAA repair is now performed too infrequently to be used as a metric in the assessment of hospital and surgeon quality in cardiovascular care. Further, surgical training paradigms will need to reflect the changing dynamics necessary to ensure surgeons and interventionists can safely perform these high-risk surgical procedures.
Introduction
Abdominal aortic aneurysm (AAA) is the 15th leading cause of death among people over the age of 65 in the United States.1 Because it is a technically complex, high-risk procedure with readily measurable complications such as mortality,2, 3 open AAA repair has been traditionally used to assess quality in vascular care by numerous quality assurance organizations, federal agencies, national insurers, and researchers.4, 5 Finally, the number of open AAA repairs performed by surgical trainees is an often-studied marker of the size and prestige of vascular surgery training programs.6–9
However, endovascular AAA repair (EVAR) began to steadily replace open AAA repair beginning in September, 1999 when the FDA approved the use of endovascular devices for patients with AAA.10 While initially limited to aneurysms with adequate normal infrarenal aorta, the last decade has seen considerable progress in techniques such as branched EVAR to allow endovascular repair for aneurysms involving the renal and visceral arteries.11, 12 The first generation of branched/fenestrated EVAR devices gained FDA approval in 2011, just over a decade after infrarenal EVAR became widely available.13
For several years now, a multitude of treatment options – open surgical repair, EVAR, and branched/fenestrated EVAR – exists for patients facing treatment for AAA.3, 10, 13, 14 While each of these modalities is used commonly for certain patients and treatment settings, a clear distribution of the current practice trends is not readily available. By understanding treatment trends and changes over time, we can better assess the applicability of open aortic aneurysm repairs a quality measure and as guideline for surgical training. Further, we can learn about the adaptation of complex endovascular aortic repair geographically over time. For these reasons, we used Medicare claims to examine the regional and national use of AAA repair types – open surgical repair, EVAR, and branched/fenestrated EVAR – between 2003 and 2013.
Methods
Overall analysis
Using national datasets from Medicare claims,15 we conducted a series of trend analyses. First, we used Part B Medicare claims to examine secular trends in the use of different types of AAA repair in fee-for-service Medicare patients. Second, we used the 307 hospital referral regions as defined in the Dartmouth Atlas of Healthcare16 to examine regional rates of the use of AAA repair overall, as well as the rates of each the individual procedures over time. Finally, we examined patient level demographics, comorbidities, and in-hospital outcomes, both overall and for each individual AAA repair type.
Secular trend analyses of AAA procedures
We used the International Classification of Disease procedure codes, version 9, (ICD-9) described in the supplementary appendix (Table Appendix) to identify fee for service Medicare patients treated for infrarenal and paravisceral abdominal aortic aneurysm between 2003 and 2013. We excluded ruptured aneurysm repairs as well as aneurysms with a thoracic component. The unit of analysis was the patient, and each patient was assigned to the first procedure type reported in Medicare claims. We used the total number of procedures per year divided by the mid-year population of Medicare beneficiaries to calculate procedure counts, as well as procedure rates per 100,000 Medicare beneficiaries.
Regional rates over time
Next, we examined the regional rates of each type of aortic aneurysm repair between 2003 and 2013, using the hospital referral regions (HRR) defined in the Dartmouth Atlas of Healthcare.16 We calculated both individual procedure counts as well as procedure rates per 1,000 Medicare patients per year, using the Medicare patient population from the Denominator file for each individual year.15 Regions take into account the location of the patient’s recorded address, rather than that of the institution where the procedure is performed.
Analysis of demographics, comorbidities and outcome
We recorded the average age, race, and gender of each of the patients studied in our cohort. We also examined the Charlson comorbidity index for each patient at the time of surgical treatment,17 and then measured in-hospital mortality rates. Mortality rates were similarly assessed for patients who underwent AAA repair for non-ruptured aneurysms without a thoracic component. We used non-parametric tests of trend to assess differences in repair rates over time.
Our study was approved by the Center for Protection of Human Subjects at Dartmouth’s Geisel School of Medicine. Informed consent was not necessary given the de-identified nature of all data. All analyses were performed using STATA software, version 13 (College Station, TX).
Results
Overall trends in AAA repair
We found that between 2003 and 2013, the number of AAA repairs performed in fee-for-service Medicare beneficiaries declined from 31,582 procedures in 2003 to 23,421 in 2013. The absolute highest number of procedures was performed in 2004, and comprised over 32,000 procedures per year in fee-for-service Medicare patients. Patients were an average of 76 years old for those underdoing EVAR, while those undergoing open AAA repair were approximately 74 years old (Table I). These ages did not change significantly over time. Finally, age ranges for branched/fenestrated EVAR were more similar to those for EVAR than for open repair (Table I).
Table I.
Cohort Characteristics Over Time
Age, gender, and Charlson Comorbidity score in 2003, 2007, and 2013 for open AAA repair, EVAR, and branched EVAR. AAA, abdominal aortic aneurysm; EVAR, endovascular aneurysm repair.
| 2003 | 2007 | 2013 | |
|---|---|---|---|
| All Repairs | |||
| n | 31,582 | 30,213 | 23,421 |
| Age, mean (CI) | 74.6 (74.6, 74.7) | 75.2 (75.1, 75.3) | 75.64 (75.6, 75.7) |
| Female, % (CI) | 27.0 (26.5, 27.5) | 25.5 (25.0, 25.9) | 26.0 (25.4, 26.6) |
| Charlson Index | 2.90 (2.88, 2.92) | 3.11 (3.09, 3.14) | 3.39 (3.36, 3.42) |
| Open Repair | |||
| n | 20,533 | 11,919 | 4,916 |
| Age, mean (CI) | 74.1 (74.0, 74.2) | 74.0 (73.9, 74.1) | 73.5 (73.3, 73.7) |
| Female, % (CI) | 31.6 (31.0, 32.3) | 30.9 (29.9, 32.0) | 39.8 (38.4, 41.1) |
| Charlson Index | 2.94 (2.91, 2.96) | 3.15 (3.12, 3.19) | 3.65 (3.58, 3.71) |
| EVAR | |||
| n | 11,049 | 18,294 | 16,362 |
| Age, mean (CI) | 75.7 (75.6, 75.8) | 76.0 (75.9, 76.1) | 76.2 (76.1, 76.3) |
| Female, % (CI) | 18.4 (17.7, 19.1) | 19.5 (18.9, 20.0) | 22.4 (21.7, 23.0) |
| Charlson Index | 2.83 (2.79, 2.87) | 3.09 (3.05, 3.12) | 3.30 (3.27, 3.34) |
| Branched-EVAR | |||
| n | N/A | N/A | 2,143 |
| Age, mean (CI) | 76.2 (75.9, 76.5) | ||
| Female, % (CI) | 22.3 (20.5, 24.0) | ||
| Charlson Index | 3.44 (3.34, 3.54) | ||
CI, 95% confidence interval; CCI, Charlson Comorbidity Index; EVAR, endovascular aneurysm repair.
Secular trends in open AAA, EVAR, and branched EVAR
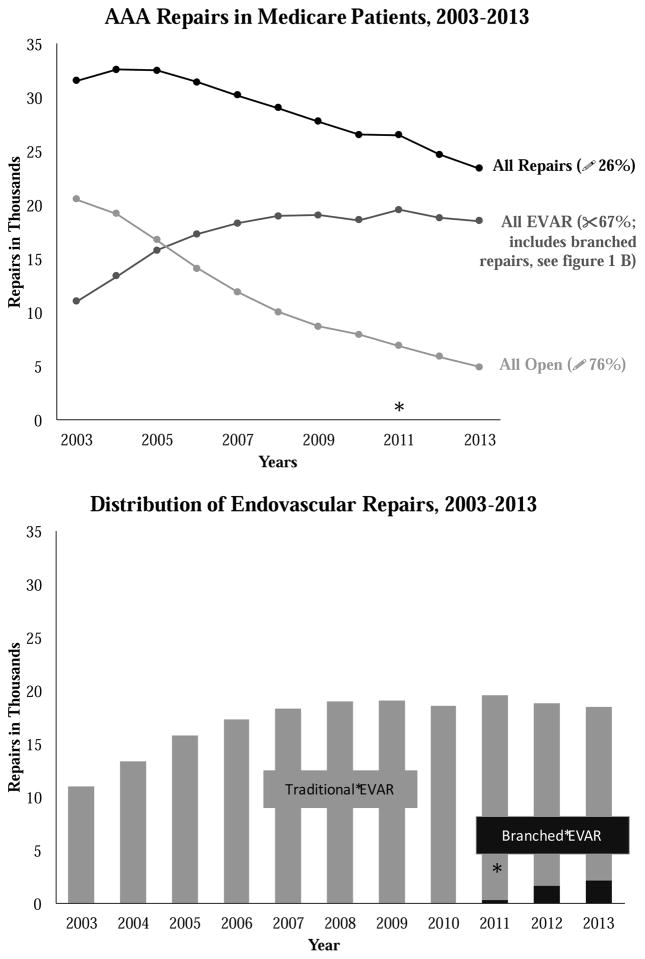
Between 2003 and 2013, the number of open AAA repairs in Medicare patients declined by 76%, from 20,533 procedures in 2003 to 4,916 procedures in 2013. EVAR procedures increased between 2003 and 2011, from 11,049 procedures in 2003 to a peak of 19,247 procedures in 2011, a 74% increase during that time period (p <0.001). Rates of EVAR then began to decline in 2012, falling 15% compared to the highest rates seen between 2011 and 2013 (p <0.001, Figure 1).
Figure I.
A&B: Trends in all repairs, open repair, and total EVAR from 2003 to 2013 in the adult Medicare population (A). Proportion of branched EVAR performed in comparison to total EVAR (B). The asterisk denotes the first year where a measureable number of branched repairs was performed. AAA, abdominal aortic aneurysm; EVAR, endovascular aneurysm repair.
The decline in traditional EVAR occurred at the same at which rates of branched/fenestrated EVAR began to increase. Specifically, while branched/fenestrated EVAR was fairly uncommon in 2011, with fewer than 400 procedures performed in fee-for-service Medicare patients, the procedure has been rapidly adopted, with more than six times as many branched/fenestrated EVARs performed in 2013 as compared to 2011 (2,143 versus 335, p<0.001).
Regional variation in the use of AAA procedure types
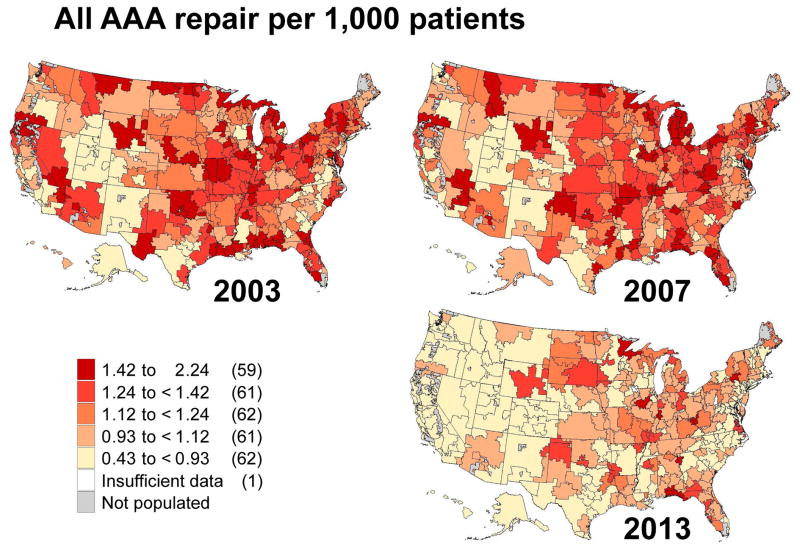
Next, we characterized the regional use of each of the procedure types using maps at three points in time during our study period: 2003, 2007, and 2013 (Figures 2, 3, 4 and 5). We found that overall rates of AAA repair declined over time, as shown by the lighter colors in the maps depicting overall aneurysm repair rates in 2013 as compared to 2003. For example, in 2003 there were 59 regions in the United States that performed more than 1.4 AAA repairs per 1,000 beneficiaries: places such as New England, New York, and northern California (Figure 2). However, by 2013, only 9 of the 307 hospital referral regions still performed AAA repairs of any kind at this frequency.
Figure II.
Regional rates of overall abdominal aortic aneurysm repair in 2003, 2007 and 2013 by hospital referral region.
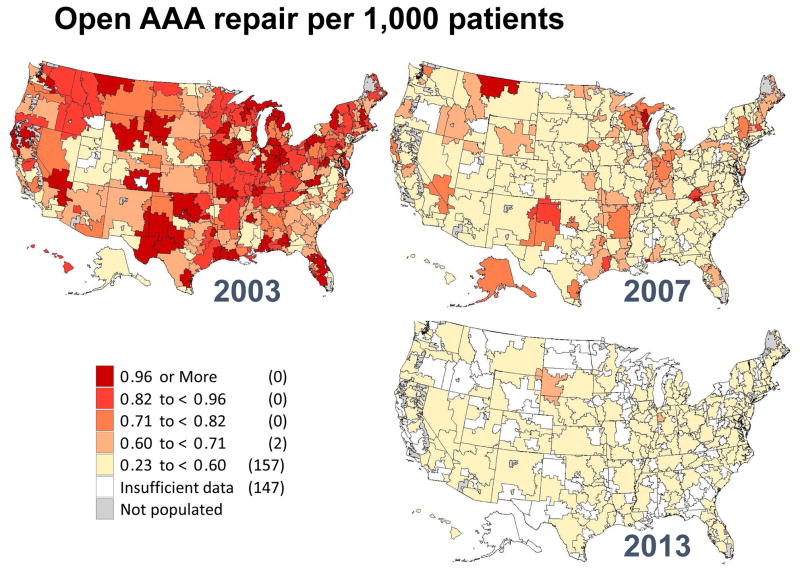
Figure III.
Regional rates of open abdominal aortic aneurysm repair in 2003, 2007 and 2013 by hospital referral region.
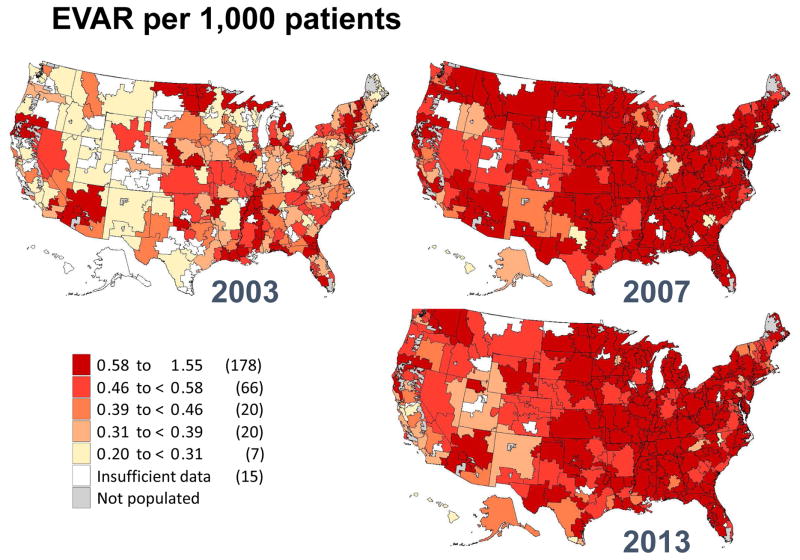
Figure IV.
Regional rates of endovascular aneurysm repair in 2003, 2007 and 2013 by hospital referral region.
Figure V.
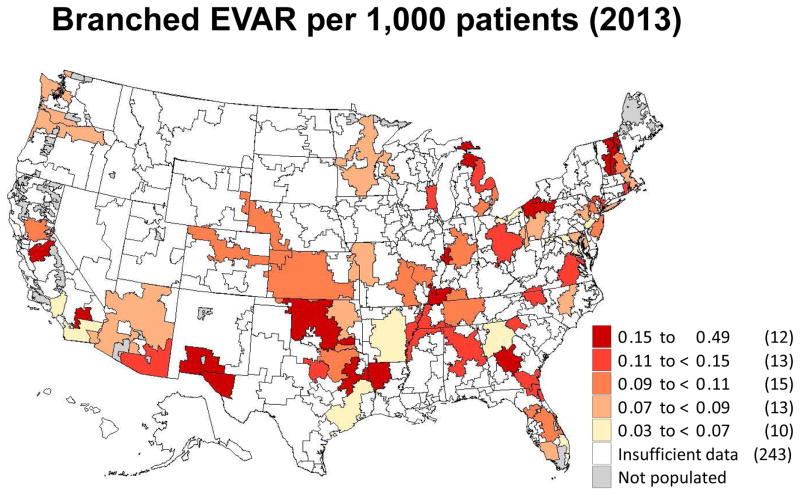
Regional rates of branched endovascular aneurysm repair in 2013 by hospital referral region.
These declines are even more apparent when focusing on open AAA repair (Figure 3). In 2003, 36 regions in the United States performed more than 1.0 AAA repair per 1,000 Medicare patients. However, by 2013, no regions had more than 0.5 open AAA repairs per 1,000 Medicare patients. Furthermore, 163 of the 307 hospital referral regions performed too few repairs to allow calculation of a rate for open AAA repair. Finally, we created a map depicting regional rates of branched/fenestrated EVAR. Since this procedure was not counted in Medicare claims date prior to 2011, the maps only depicts geographic rates for 2013 (Figure 5). Rates in that year overall were low across the country, and insufficient data was available for rate calculation in 243 of the 307 hospital referral regions. Regions with higher overall rates were evident in New England, Florida, the central and southwestern United States, and northern California.
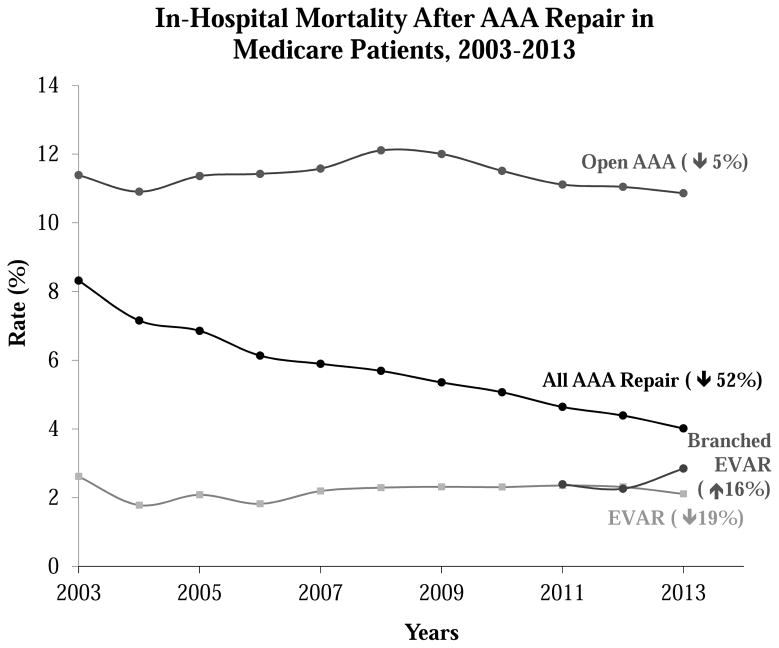
Trends in in-hospital mortality over time, by procedure type
In-hospital mortality rates were more than 10% in this cohort of open aneurysm repair patients, which included both infrarenal, juxtarenal, and paravisceral repairs of non-ruptured but either elective or urgent aneurysms. Mortality rates for EVAR were lower, between 2–3% (p<0.001) over time, and did not change significantly during the study period. Mortality rates for branched/fenestrated EVAR, as with age, were similar to EVAR (Figure 6). As depicted in Table I, the Charlson comorbidity index increased over time for each type of aneurysm repair, be it open or endovascular, from an average score of 2.8 in 2003 to 3.4 in 2013 (p<0.001). Despite this worsening in the comorbid state of aneurysm repair patients, in-hospital mortality rates remained unchanged over the same time period.
Figure VI.
Trends of in-hospital mortality for open AAA repair, EVAR and branched EVAR from 2003 to 2013 in the adult Medicare population. AAA, abdominal aortic aneurysm; EVAR, endovascular aneurysm repair.
Discussion
In this study, we describe national treatment patterns for open surgical repair, EVAR, and branched/fenestrated EVAR in an observational cohort of Medicare patients treated in fee-for-service practice over the last decade. We found that while traditional EVAR declined slightly and branched/fenestrated EVAR rapidly disseminated into national practice, the number of open AAA repairs fell by nearly 80% during the last decade. When compared to practice patterns a decade earlier, the regions with the highest volume of open AAA repair in 2013 would be in the lowest 20th percentile in 2003, and more than a third of regions of the United States failed to perform enough open aortic aneurysm cases to meet regional reporting criteria according to Medicare privacy standards, which require 10 cases per year
Our findings align with those noted in Europe and the United States, that rates of aneurysm repair and diagnosis do appear to be declining.18, 19 Reasons for this change in epidemiology remain unclear and were beyond the scope of our investigation. Some argue that the declining incidence of smoking noted both in Europe and the United States as well as improved management of hypertension and hypercholesterolemia could be related to a decline in aneurysm incidence and hence repair numbers.18, 20, 21 It is also feasible that cost constrains, less repair of small aneurysms, or patient desire against repair could play into the reason as to why repair numbers are declining. Our belief is that the true causality is likely multi-factorial and a combination of some of these variables. Given the continued trend noted over a decade, however, we feel that this is not a “blip in time”, but that open repair rates especially will remain low, if not continue to decline.
Measuring quality in surgery is a difficult task, and accomplishing this goal in vascular surgery is no exception. Using open AAA repair as a marker for quality has had an intrinsic and intuitive appeal for many years, given the commonality with which it was performed, and the clarity with which good and bad outcomes could be measured.5 The number of AAA repairs with its associated mortality rate continues to be endorsed as an inpatient quality indicator by the Agency for Healthcare Research and Quality (AHRQ)22 and vascular surgery trainees are required to perform at least thirty major open abdominal/aortic vascular cases prior to being eligible for Vascular Surgery Board certification by the American Board of Surgery.23 When open AAA repair was performed with good results, patients left the hospital within 7–10 days, and prolonged ICU stays, ventilator dependence, reoperation, and death were unlikely.24–26 However, when open AAA repair was complicated, each of these events – prolonged time in the ICU, tracheostomy, further surgical procedures, and death – were common. Each of these events was also easily measured in a variety of forums, including registry data as well as administrative data.27 Finally, for vascular surgical trainees, open aortic surgery represented the most complex procedure, often performed only at the apex of one’s training years.8 Open aortic surgery rates are therefore carefully investigated by potential applicants to training programs and often used by applicants to discriminate between programs. Additionally, programs are devising simulator-based training to ensure that open surgical skills are taught to an adequate extent in the face of the declining exposure to open aortic surgery.28 Given the importance of this procedure, both in quality measurement as well as surgical residency training, annual volume thresholds have been proposed for hospitals and surgeons for open AAA. Generally these thresholds begin at approximately 20 procedures per year.29–31
Our data suggest that in current practice, few hospitals and surgeons will meet these, or even approach, these thresholds for open AAA repair. Rapid adoption of EVAR and branched/fenestrated EVAR, accompanied by an overall decline in the total number of AAA repairs performed overall, has limited the ability of open AAA to represent quality of care in vascular surgery for all centers. In some centers where rates of open repair have dropped in number, this operation may therefore not serve as a good quality indicator. This change has significant implications, as substituting EVAR as a marker for quality in AAA repair is not a simple solution. Mortality (a clear, easily measured outcome in both registries and administrative datasets) is uncommon after EVAR,24–26 and it is difficult to measure surveillance, reintervention, and efficacy, especially in the long term. Even achieving long term assessment after EVAR has proven to be challenging.2 Perhaps exactly for this reason one may consider implementing the rate of patients under active surveillance following EVAR as a surrogate quality marker for aneurysm care, rather than mortality after open AAA repair.
Surgical training will also face difficult challenges as open AAA repair fades from its role as a primary major case for vascular surgery trainees. Who will perform open aortic surgery in the years to come will remain an increasingly important question, as fewer surgeons with extensive expertise are available. Who will train other surgeons is a similarly important question, especially in the endovascular era where many programs focus heavily on endovascular treatments for aortic aneurysm. The question arises whether open aortic surgery may require to be, or inherently become, centralized to be performed at centers of excellence.
Our study has limitations. First, our study is based primarily on administrative data and thus lacks some granularity regarding patient risk factors and anatomic variables. These include examples such as aneurysm size, or the extent of aneurysm involvement in the visceral segment for open repair as the open repair codes could include pararenal, juxtarenal, and/or visceral components. Therefore, risk adjustment, especially for mortality assessment, remains difficult. However, our primary findings, in terms of the absolute number of procedures performed and the secular changes over time, remains largely unaffected by the need for risk adjustment. Second, more patients in recent years have entered non-fee-for-service Medicare programs, such as Medicare advantage. These patients, in some markets, may represent up to 20% of the patients available for analysis. Furthermore, our cohort does not account for AAA repairs performed outside of Medicare claims. In our own practice, <5% of AAA repairs are performed outside of Medicare, such as private insurance, however, other practices may perform more AAA repairs that are not covered by Medicare. Such patients are not considered in our current report and therefore the overall number of AAA repairs in the United States is likely higher than the numbers we report here. Nonetheless, we feel strongly that the trends of repair, especially the decline in open and overall all AAA repairs is an accurate representation of the current paradigm in AAA repairs in the United States. Finally, codes for branched/fenestrated EVAR have only recently become available, and many have performed this procedure outside of standard available procedural coding mechanisms. However, any changes in our data conferred by this limitation would only add to the differences seen in our analyses.
In summary, the number of open AAA repairs fell by nearly 80% during the last decade, while traditional EVAR declined slightly and branched/fenestrated EVAR rapidly disseminated into national practice. These results suggest that open AAA repair is now performed too infrequently to be used as a metric in the assessment of hospital and surgeon quality in cardiovascular care. Further, surgical training paradigms will need to reflect the changing dynamics necessary to ensure surgeons and interventionists can safely perform these high-risk surgical procedures. Vascular surgeons, quality assessment experts, and surgical educators all need to consider the effect of these changes in future the best measure performance of vascular surgery as well as train the next generation of invasive vascular specialists.
Take Home Message.
Between 2003 and 2013, the total number of AAA repairs in Medicare patients declined by 26% from 31,582 to 23,421. The number of open AAA repairs fell by 76%, from 20,533 in 2003 to 4,916 in 2013, endovascular repair (EVAR) increased from 11,049 in 2003 to 19,247 in 2011, then declined to 16,362 in 2013, and branched EVAR increased from 335 in 2011 to 2,143 in 2013.
Acknowledgments
Funding Sources:
Dr. Goodney was supported by funding from the Society for Vascular Surgery Foundation and the National Heart, Lung, and Blood Institute (NHLBI 1K08HL05676) and FDA U01 FD005478 (Sedrakyan, PI). Dr. Sedrakyan was supported by U01 FD005478 (Sedrakyan, PI).
Appendix. ICD-9 Codes by Diagnosis
| Diagnosis | ICD-9 Codes |
|---|---|
| All AAA repair | 3844; 3925; 3971; 3978 |
| Open AAA Repair | 3844; 3925 |
| EVAR | 3971 |
| Branched EVAR | 3978 |
ICD-9, international classification of diseases, ninth revision; AAA, abdominal aortic aneurysm; EVAR, endovascular aneurysm repair.
Footnotes
Disclosure/Acknowledgement
No pertinent disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kurosawa K, Matsumura JS, Yamanouchi D. Current status of medical treatment for abdominal aortic aneurysm. Circ J. 2013;77(12):2860–6. doi: 10.1253/circj.cj-13-1252. [DOI] [PubMed] [Google Scholar]
- 2.Schermerhorn ML, Buck DB, O’Malley AJ, Curran T, McCallum JC, Darling J, et al. Long-Term Outcomes of Abdominal Aortic Aneurysm in the Medicare Population. N Engl J Med. 2015;373(4):328–38. doi: 10.1056/NEJMoa1405778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schanzer A, Messina L. Two decades of endovascular abdominal aortic aneurysm repair: enormous progress with serious lessons learned. Journal of the American Heart Association. 2012;1(3):e000075. doi: 10.1161/JAHA.111.000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agency for Healthcare Research and Quality. Abdominal aortic aneurysm (AAA) repair mortality: percentage of in-hospital deaths per 1,000 discharges with AAA repair, ages 18 years and older. 2015 [cited 2016 October 8th]; Available from: https://www.qualitymeasures.ahrq.gov/summaries/summary/49507?
- 5.Dimick JB, Upchurch GR., Jr Measuring and improving the quality of care for abdominal aortic aneurysm surgery. Circulation. 2008;117(19):2534–41. doi: 10.1161/CIRCULATIONAHA.107.726836. [DOI] [PubMed] [Google Scholar]
- 6.Cronenwett JL. Vascular surgery training: is there enough case material? Semin Vasc Surg. 2006;19(4):187–90. doi: 10.1053/j.semvascsurg.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Sachs T, Schermerhorn M, Pomposelli F, Cotterill P, O’Malley J, Landon B. Resident and fellow experiences after the introduction of endovascular aneurysm repair for abdominal aortic aneurysm. Journal of vascular surgery. 2011;54(3):881–8. doi: 10.1016/j.jvs.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schanzer A, Steppacher R, Eslami M, Arous E, Messina L, Belkin M. Vascular surgery training trends from 2001–2007: A substantial increase in total procedure volume is driven by escalating endovascular procedure volume and stable open procedure volume. Journal of vascular surgery. 2009;49(5):1339–44. doi: 10.1016/j.jvs.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Sternberg WC, York JW, Conners MS, Money SR. Trends in aortic aneurysm surgical training for general and vascular surgery residents in the era of endovascular abdominal aortic aneurysm repair. Journal of vascular surgery. 2002;36(4):685–9. doi: 10.1067/mva.2002.128308. [DOI] [PubMed] [Google Scholar]
- 10.Dua A, Kuy S, Lee CJ, Upchurch GR, Jr, Desai SS. Epidemiology of aortic aneurysm repair in the United States from 2000 to 2010. Journal of vascular surgery. 2014;59(6):1512–7. doi: 10.1016/j.jvs.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Semmens JB, Lawrence-Brown MM, Hartley DE, Allen YB, Green R, Nadkarni S. Outcomes of fenestrated endografts in the treatment of abdominal aortic aneurysm in Western Australia (1997–2004) Journal of endovascular therapy: an official journal of the International Society of Endovascular Specialists. 2006;13(3):320–9. doi: 10.1583/05-1686.1. [DOI] [PubMed] [Google Scholar]
- 12.British Society for Endovascular Therapy the Global Collaborators on Advanced Stent-Graft Techniques for Aneurysm Repair Registry. Early results of fenestrated endovascular repair of juxtarenal aortic aneurysms in the United Kingdom. Circulation. 2012;125(22):2707–15. doi: 10.1161/CIRCULATIONAHA.111.070334. [DOI] [PubMed] [Google Scholar]
- 13.Quinones-Baldrich WJ, Holden A, Mertens R, Thompson MM, Sawchuk AP, Becquemin JP, et al. Prospective, multicenter experience with the Ventana Fenestrated System for juxtarenal and pararenal aortic aneurysm endovascular repair. Journal of vascular surgery. 2013;58(1):1–9. doi: 10.1016/j.jvs.2012.12.065. [DOI] [PubMed] [Google Scholar]
- 14.Oderich GS, Greenberg RK, Farber M, Lyden S, Sanchez L, Fairman R, et al. Results of the United States multicenter prospective study evaluating the Zenith fenestrated endovascular graft for treatment of juxtarenal abdominal aortic aneurysms. J Vasc Surg. 2014;60(6):1420–8. e1–5. doi: 10.1016/j.jvs.2014.08.061. [DOI] [PubMed] [Google Scholar]
- 15.Center for Medicare and Medicaid Services. [cited 2016 October 8th]; Available from: http://www.cms.gov/
- 16.The Dartmouth Atlas of Health Care. 2016 [cited 2016 January 3rd]; Available from: http://www.dartmouthatlas.org/
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Anjum A, Powell JT. Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg. 2012;43(2):161–6. doi: 10.1016/j.ejvs.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Dillavou ED, Muluk SC, Makaroun MS. A decade of change in abdominal aortic aneurysm repair in the United States: Have we improved outcomes equally between men and women? J Vasc Surg. 2006;43(2):230–8. doi: 10.1016/j.jvs.2005.09.043. discussion 8. [DOI] [PubMed] [Google Scholar]
- 20.Tynan M, Pechacek T, McKenna M, et al. Centers for Disease Control and Prevention. [Accessed September 11th, 2017];CDC Grand Rounds: Current Opportunities in Tobacco Control. 0 Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5916a3.htm.
- 21.Choke E, Lee K, McCarthy M, Nasim A, Naylor AR, Bown M, et al. Risk models for mortality following elective open and endovascular abdominal aortic aneurysm repair: a single institution experience. Eur J Vasc Endovasc Surg. 2012;44(6):549–54. doi: 10.1016/j.ejvs.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 23.White RA, Hodgson KJ, Ahn SS, Hobson RW, 2nd, Veith FJ. Endovascular interventions training and credentialing for vascular surgeons. J Vasc Surg. 1999;29(1):177–86. doi: 10.1016/s0741-5214(99)70359-9. [DOI] [PubMed] [Google Scholar]
- 24.United Kingdom Evar Trial Investigators. Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D, et al. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010;362(20):1863–71. doi: 10.1056/NEJMoa0909305. [DOI] [PubMed] [Google Scholar]
- 25.De Bruin JL, Baas AF, Buth J, Prinssen M, Verhoeven EL, Cuypers PW, et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2010;362(20):1881–9. doi: 10.1056/NEJMoa0909499. [DOI] [PubMed] [Google Scholar]
- 26.Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT, Jr, Matsumura JS, Kohler TR, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. Jama. 2009;302(14):1535–42. doi: 10.1001/jama.2009.1426. [DOI] [PubMed] [Google Scholar]
- 27.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW, et al. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE) Journal of vascular surgery. 2007;46(6):1093–101. doi: 10.1016/j.jvs.2007.08.012. discussion 101–2. [DOI] [PubMed] [Google Scholar]
- 28.Aziz F. Vascular surgery trainees still need to learn how to sew: importance of learning surgical techniques in the era of endovascular surgery. Front Surg. 2015;2:16. doi: 10.3389/fsurg.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young EL, Holt PJ, Poloniecki JD, Loftus IM, Thompson MM. Meta-analysis and systematic review of the relationship between surgeon annual caseload and mortality for elective open abdominal aortic aneurysm repairs. Journal of vascular surgery. 2007;46(6):1287–94. doi: 10.1016/j.jvs.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 30.Holt PJ, Poloniecki JD, Khalid U, Hinchliffe RJ, Loftus IM, Thompson MM. Effect of endovascular aneurysm repair on the volume-outcome relationship in aneurysm repair. Circ Cardiovasc Qual Outcomes. 2009;2(6):624–32. doi: 10.1161/CIRCOUTCOMES.109.848465. [DOI] [PubMed] [Google Scholar]
- 31.Thompson M, Holt P, Loftus I, Forbes TL. Debate: whether abdominal aortic aneurysm surgery should be centralized at higher-volume centers. Journal of vascular surgery. 2011;54(4):1208–14. doi: 10.1016/j.jvs.2011.07.064. [DOI] [PubMed] [Google Scholar]