Abstract
Objective
To determine if daily respiratory status improved more in extremely low gestational age premature infants after diuretic exposure compared with those not exposed in modern neonatal intensive care units (NICU).
Study design
The Prematurity and Respiratory Outcomes Program (PROP) was a multi-center observational cohort study of 835 extremely premature infants, gestational ages 23 0/7–28 6/7 weeks, enrolled in the first week of life from 13 U.S. tertiary NICUs. We analyzed the PROP study daily medication and respiratory support records of infants up to 34 weeks postmenstrual age. We determined whether there was a temporal association between administration of diuretics and an acute change in respiratory status in premature infants in the NICU, using an ordered categorical ranking of respiratory status.
Results
Infants in the diuretic exposed group of PROP were of lower mean gestational age and lower mean birth weight (p<0.0001). Compared with infants unexposed to diuretics, the probability (adjusted for infant characteristics including gestational age, birth weight, sex, and respiratory status prior to receiving diuretics) that the exposed infants were on a higher level of respiratory support was significantly greater (odds ratio > 1) for each day after the initial day of diuretic exposure.
Conclusions
Our analysis did not support the ability of diuretics to substantially improve the extremely premature infant’s respiratory status. Further study of both safety and efficacy of diuretics in this setting are warranted.
Trial Registration
Clinicaltrials.gov NCT01435187
Keywords: Prematurity, respiratory distress, diuretic
Premature neonates are at increased risk of respiratory distress syndrome (RDS) and chronic lung disease (CLD) with respiratory insufficiency and failure due primarily to lung immaturity and insufficient surfactant production. The sole approved treatment for RDS has been airway instillation of liquid surfactant. RDS and evolving CLD are marked by inflammation of the lung, and it has been hypothesized that this inflammation increases fluid infiltration into the lung parenchyma. Diuretics have been commonly used in neonatal intensive care units (NICUs) to treat these infants despite little evidence of efficacy. A recent paper described the wide assortment of medications currently used in the NICU to treat and prevent long term pulmonary complications 1.
Diuretic prescribing patterns for premature infants receiving NICU care have been highly variable. Studies suggested short-term physiological benefit of diuretics with improved measures of lung compliance, airways resistance, and ventilator support 2, 3. However, there has been a paucity of information about acute responses to diuretics of extremely low gestational age/birth weight newborns (ELGAN/ELBW) managed in the modern NICU, with routine use of prenatal corticosteroids, postnatal surfactant, and advanced ventilatory support. Wide variations in practice suggested there was insufficient evidence to support guidelines for use of diuretics in premature infants 4, 5, 6, 7. For example, analysis of the Pediatrix database indicated that approximately a third of premature neonates received a diuretic at some point during their NICU stay 5. These variations in practice may be due in part to limited efficacy studies and confusion over the short- vs long-term goals of therapy in this vulnerable population.
The National Heart, Lung, and Blood Institute (NHLBI/NIH) supported the Prematurity and Respiratory Outcomes Program (PROP) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH) supported detailed medication data collection. PROP was an observational prospective cohort study of premature infants. The purpose of PROP was to identify mechanisms and associated functional and molecular biomarkers of respiratory disease risk of premature infants (https://grants.nih.gov/grants/guide/rfa-files/RFA-HL-10-007.html). Details on the study design and standardized prospective data collection were previously reported 8, 9. Given that many premature infants are exposed to diuretics, we sought to determine if diuretics provided some benefit in tertiary NICU settings. The objective of this study was to determine if daily respiratory status improved more in extremely low gestational age premature infants after diuretic exposure compared with those not exposed.
METHODS
We analyzed the daily medication and respiratory support records from a contemporary cohort of ELGAN infants in PROP. PROP investigators enrolled 835 infants in the first week of life with gestational ages 23 0/7 to 28 6/7 weeks at 13 tertiary U.S. NICUs from August 2011 to November 2013 8, 9. Infants were excluded if viability was a concern, if the infant had a significant birth defect, or if the family was unlikely to be available for follow up to the primary one-year outcome. An NHLBI Observational and Safety Monitoring Board provided human subjects oversight in addition to Institutional Review Boards for each participating site. At least one parent or guardian provided informed consent for each child participant. The study was registered on clinicaltrials.gov (NCT01435187).
Daily data included respiratory medications and respiratory support measures administered by neonatal intensive care unit (NICU) clinicians according to their usual care practices. Respiratory medications included inhaled bronchodilators, inhaled corticosteroids, systemic corticosteroids, methylxanthines, pulmonary vasodilators, and diuretics. Diuretics recorded were furosemide, bumetanide, chlorothiazide, hydrochlorothiazide, and spironolactone 10. Respiratory support measures recorded included invasive ventilation with an endotracheal tube (ETT), non-invasive support without ETT, and nasal cannula. Level of flow and fractional inspired oxygen (FiO2) were also recorded.
Statistical Analyses
We asked if there was a temporal association between administration of diuretics and an acute change in respiratory status in premature infants in the NICU. Respiratory status was ranked categorically as: (1) room air only, (2) nasal cannula with < 2 liter per minute (lpm) flow, (3) non-invasive mechanical ventilation or nasal cannula with ≥ 2 lpm flow, (4) invasive mechanical ventilation with endotracheal tube, and (5) deceased. Daily respiratory status was recorded for each baby from birth up to 34 weeks postmenstrual age (PMA). Observations were censored at 34 weeks, as transfer/discharge of PROP infants occurred as early as this time. Diuretic exposure was categorized in the model as unexposed, consecutive exposure day 1 through 7, consecutive exposure day >7, one-day course, and 3-day washout period. A separate model term was used for one-day courses of diuretics, due to uncertainty about the indication for use (eg, prevention of blood transfusion-induced fluid overload). The 3-day washout period model term was used to capture washout effects. We used a generalized linear model of the outcome fit via generalized estimating equations to account for correlation among observations from the same baby. The model gave predicted odds under a proportional odds assumption of a worse outcome (in the direction of outcome 5, deceased) for those in each exposed group (consecutive day 1 through 7, consecutive day >7) compared with unexposed days, adjusting for infant birth weight, infant gestational age at birth, infant race, infant sex, site, and multiplicity of birth. The baby’s current age (days) was included in the model as a linear term. To account for the current status and trajectory of outcome at the time of exposure, the baby’s outcome on the previous day (day 0) (included as categorical term), the change of outcome from day −2 to day 0 (included as linear term), and the change of outcome from day −1 to day 0 (included as linear term) were also included in the model. Current exposure to caffeine, any bronchodilator drugs, any inhaled corticosteroids, or any systemic corticosteroids were also adjusted for by separate terms in the model. SAS (version 9.3) procedure GENMOD was used to analyze the data.
We also analyzed a matched cohort to confirm our findings. The matched cohort was selected as follows: (1) All babies were aligned by postnatal day, (2) Babies became eligible for the exposed cohort on the second consecutive day of diuretic use, (3) When a potential baby became eligible for the exposed cohort we looked for unexposed babies that matched the exposed baby in four categories (5-level respiratory support status on cohort day 1, 5- level respiratory support status on cohort day 0, completed gestational age in weeks, sex), (4) One baby was selected at random from eligible matched babies to be entered into the unexposed cohort, and (5) We continued until no more matches were found, yielding 245 match pairs of babies, each with one exposed and one unexposed baby. The matched cohorts were compared by Sign test to see if the difference in respiratory support status on cohort days 1 and 2 for the pairs (exposed-unexposed) had positive median and hence a higher level of respiratory support.
RESULTS
Among 835 infants enrolled in PROP 8, 483 were exposed at least once to a diuretic and 352 were never exposed (unexposed). There were 3 babies without any medication information that did not enter the statistical model or impact the results. Table I shows the PROP cohort characteristics on the first day of life by diuretic exposure group.
Table 1.
Characteristics at Birth: Mean* (SD) or Median^ (IQR) or N (% of non-missing)
| Characteristic | Unexposed (N=352) | Exposed (n=483) | p-value |
|---|---|---|---|
| Gestational Age* (weeks) | 27.2 (1.2) | 26.1 (1.4) | <0.0001 |
| Birth Weight* (grams) | 1002 (229) | 825 (212) | <0.0001 |
| Female Sex | 185 (53) | 223 (46) | 0.06 |
| Race | 0.82 | ||
| Caucasian | 217 (62) | 282 (58) | |
| African American | 123 (35) | 184 (38) | |
| Asian | 9 (3) | 12 (2) | |
| Other/unknown | 3 (1) | 5 (1) | |
| Hispanic Ethnicity | 45 (13) | 47 (10) | 0.18 |
| Multiple Birth | 101 (29) | 109 (23) | 0.04 |
| Apgar Score at 1 min9 | 5 (3,7) | 4 (2,6) | <0.0001 |
| Apgar Score at 5 min^ | 7 (6,8) | 7 (5,8) | <0.0001 |
| Resuscitation | |||
| Supplemental Oxygen | 310 (91) | 418 (90) | 0.54 |
| CPAP | 218 (68) | 179 (44) | <0.0001 |
| PPV | 116 (43) | 235 (58) | 0.0003 |
| T-piece resuscitator | 77 (31) | 141 (37) | 0.10 |
| Intubation | 234 (75) | 420 (90) | <0.0001 |
| Chest Compressions | 26 (11) | 72 (19) | 0.005 |
| Cardiac Drugs | 7 (3) | 32 (9) | 0.004 |
| Surfactant | 182 (60) | 324 (74) | 0.0001 |
CPAP = Continuous Positive Airway Pressure
PPV = Positive Pressure Ventilation
PROP infants in the exposed group were of lower mean gestational age (exposed 26.1 vs unexposed 27.2 weeks GA; P < .0001) and lower mean birth weight (exposed 825 grams vs. unexposed 1002 grams; p<0.0001). Sex, race, and ethnicity did not differ significantly between the diuretic exposed and unexposed. Babies who were exposed to diuretics had significantly lower APGAR scores at 1 and 5 minutes (p<0.0001), were less likely to be resuscitated with continuous positive airway pressure (CPAP) (44% vs 68% respectively, p<0.0001), more likely to have a history of initial resuscitation with positive pressure ventilation (58% vs 43%, p = 0.0003), intubation (90% vs 75%, p<0.0001), chest compressions (19% vs. 11%, p = 0.005), treatment with cardiac drugs (9% vs 3%, p = 0.004), and surfactant (74% vs 60%, p = 0.0001). About ninety percent of infants in either group were treated with supplemental oxygen.
At the time of initial diuretic exposure (median age 14 days, Table 2) 90% received supplemental oxygen compared with 59% of the unexposed infants (p < 0.0001). At a median postnatal age of 14 days, the level of respiratory support was significantly more invasive for exposed infants than for unexposed infants (unadjusted p < 0.0001), with 56% of exposed infants on ventilatory support with an ETT, compared with only 11% of the unexposed. At the time of first exposure, 28% exposed vs 38% of unexposed infants were on CPAP or >2 lpm nasal cannula, 7% exposed vs. 10% of unexposed infants were on nasal cannula < 2 lpm, and only 9% exposed vs. 40% of unexposed infants were on no support.
Table 2.
Characteristics on Day Prior to First Exposure or Median Such Day: Median^ (IQR) or N (% of non-missing)
| Characteristic | Unexposed (N=327) | Exposed (N=480) | p-value |
|---|---|---|---|
| Postnatal Age^ (days) | 14 (14,14) | 14 (9,28) | 0.29 |
| Postmenstrual Age^ (wks) | 294 (285,292) | 284 (266,306) | <0.0001 |
| Any Supplemental Oxygen | 194 (59) | 430 (90) | <0.0001 |
| FIO2^ | 25 (23,30) | 33 (26,44) | <0.0001 |
| Respiratory Support | <0.0001 | ||
| Deceased | 2 (1) | 0 (0) | |
| Endotracheal Tube | 36 (11) | 266 (56) | |
| CPAP or > 2 lpm Nasal Cannula | 125 (38) | 135 (28) | |
| Nasal Cannula <2 lpm | 33 (10) | 31 (7) | |
| No Support | 131 (40) | 45 (9) | |
| Any Caffeine | 300 (92) | 323 (67) | <0.0001 |
| Any Bronchodilator Drugs | 1 (0) | 21 (4) | 0.003 |
| Any Systemic Steroids | 6 (2) | 24 (5) | 0.03 |
| Any Inhaled Steroids | 5 (2) | 16 (3) | 0.15 |
Because the NICU prescribing practice for diuretics had no specific guidance on course length, we calculated length and number of courses of diuretic exposure. Single day exposures might have been related to “prophylaxis” for transfusions 11, or to achieve short-term improvements in respiratory status, however the indication for treatment was not available. Of the 483 infants exposed to diuretics, the median length of the first course was 1 day (Table 3). Over the NICU course, these infants had a median cumulative length of exposure of 18 days. Multi-day courses (2 or more consecutive days) varied from none (n = 110, 23% of exposed) to more than 4 (n = 24, 5% of exposed) with most infants exposed once or twice to a multi-day course (n = 144, 30% and n = 106, 22% respectively). Of babies who received a multi-day course (n = 373) the number (%) who received furosemide was 315 (84%), bumetanide was 10 (2.7%), chlorothiazide was 42 (11.3%), hydrochlorothiazide was 16 (4%) and spironolactone was 17 (5%). A baby could receive more than one type of diuretic, so these are not mutually exclusive categories.
Table 3.
Distribution of Exposed Babies by the Number of Multi-Day Courses of Diuretics Median^ (IQR) or N (% of non-missing)
| Characteristic of Diuretic Exposure | N=483 |
|---|---|
| Length of First Course^ (days) | 1 (1,3) |
| Cumulative Length of Exposure^ (days) | 18 (3,48) |
| Number of Multi-Day Courses | |
| 0 | 110 (23) |
| 1 | 144 (30) |
| 2 | 106 (22) |
| 3 | 61 (13) |
| 4 | 38 (8) |
| ≥5 | 24 (5) |
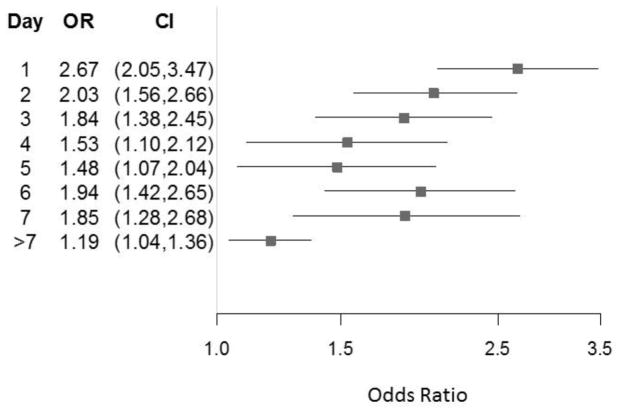
The Figure is a forest plot showing the odds ratio of significantly worse respiratory outcome (more support) for exposed days 1–7 and >7 compared with unexposed days from our repeated measures model. This model adjusted for infant birth weight, infant gestational age at birth, infant race, infant sex, site, and multiplicity of birth, and accounted for respiratory status on the first day of exposure. Compared with infants never exposed to diuretics, the probability that the exposed infants’ respiratory status had a higher level of support was greater (odds ratio >1) for each day after the initial day of diuretic exposure.
Figure. Effect of Diuretic Exposure: Odds Ratio of Having More Respiratory Support Than Unexposed.
Forest plot of the odds ratio of worse respiratory outcome (greater support) for exposed days 1–7 and >7 compared with unexposed days. Model adjusted for infant birth weight, infant gestational age at birth, infant race, infant sex, site, and multiplicity of birth, and accounts for respiratory status on the first day of exposure.
The adjusted odds ratio (OR) for a diuretic exposure of one day only was 2.14 (CI 1.59 to 2.87), and for those with multiple day exposures, the OR for greater level of respiratory support status was 2.67 (CI 2.05–3.47) for the first day, 2.03 (CI 1.56 to 2.66) for the second day, 1.84 (CI 1.38 to 2.45) for the third day, etc. (see Figure 1). There was no period when the probability of greater respiratory support status of exposed infants was lower than for the unexposed infants. Analysis for an eight-level outcome [(1) no respiratory support, (2) nasal cannula with FiO2=0.21, (3) nasal cannula plus FiO2 >0.21, (4) non-invasive support with FiO2=0.21, (5) non-invasive support plus FiO2 >0.21, (6) invasive support with FIO2=0.21, (7) invasive support plus FiO2>0.21, and (8) death] had similar results as the five-level outcome analysis (data not shown).
The characteristics of the babies in the matched cohort analysis (data not shown) were very similar to the characteristics of the exposed PROP babies in Table 1. The matched cohort analysis of 245 matched pairs of babies found 28 pairs with the exposed baby needing more respiratory support than the unexposed baby on cohort day 1 and only 13 pairs had the unexposed baby needing more support than the exposed baby. This represented a significantly positive difference in respiratory support status (exposed - unexposed) on cohort day 1 (Sign test p-value=0.03), indicating more support was needed for the exposed babies than for the unexposed babies. On cohort day 2 this difference was even more pronounced with 54 pairs having the exposed baby needing more support than the unexposed baby and only 17 had the unexposed baby needing more support than the exposed baby (Sign test p-value<0.0001).
Discussion
Diuretics have long been used in the NICU, however utilization patterns in the U.S. have varied considerably. A retrospective analysis of more than 1400 infants in 35 U.S. hospitals reported that over a 4-year period (2007–2011), 83% of infants with bronchopulmonary dysplasia (BPD) had received diuretics during their NICU course6. Dosing and duration of therapy varied as well. In the PROP observational cohort, 58% of 832 ELGAN infants were exposed to diuretics and use varied by site from ~40–80%. The rate of exposure was highest for PROP infants with gestational ages (GA) 23–24 weeks (82%) and lowest for those at 28 weeks GA (35%). The most commonly used diuretic was furosemide (57% of the cohort), and furosemide use started earlier (median 2 weeks) than other diuretics.
The reported mechanism of action of furosemide is diuresis through inhibition of ion flux of the Na+-K+-2Cl− cotransporter in the loop of Henle 12, however early studies in anephric lambs suggested direct effects in the lungs as well13. Neonates with respiratory distress are often treated with diuretics with the expectation of removal of increased lung water and blood volume. Although diuresis in adults is reported to occur within one hour of dosing, with duration of effect of 6–8 hours, clinical studies in premature neonates have been limited. Renal function was thought not fully mature at birth, particularly in ELGANs. Pharmacokinetic studies have been insufficient to direct optimal dosing, though handbooks have provided guidance14, 15. There has been no FDA labeling indication for furosemide or other diuretics in premature infants, a common limitation of medications used in NICUs. There was, however, specific warning in premature infants that furosemide may precipitate nephrocalcinosis/nephrolithiasis16. Furosemide has also been reported to delay closure of the patent ductus arteriosus (PDA) in neonates17.
Studies of acute responses to diuretics in small numbers of premature infants were published in the 1980s and 1990s. Within hours of furosemide administration, 16 infants, born 27–32 weeks gestation with BPD on supplemental oxygen had improved lung compliance and minute ventilation, and decreased airways resistance2. Similar results were found in small studies of intubated premature BPD infants <30 weeks GA18,19. Yeh et al found significant diuresis occurred in 29 intubated premature infants with RDS, but no long-term benefit in survival, BPD, or patent ductus arteriosus (PDA).20 Lacking are Phase II or III clinical trials assessing benefit of diuretics that might guide short and long-term use of diuretics in ELGANs, such as ventilator-free days, survival without BPD, or decreased length of stay. Adverse effects of diuretics in ELGAN populations have not been systematically reported.
The PROP study design included daily recording of medications to assess the relationship between medical treatment and pulmonary disease outcomes at 36 weeks and 12 months corrected age. These data afforded an opportunity to examine whether there was short- term respiratory response to diuretics as defined by a reduction in supportive measures. As fluctuations in FiO2 and inspiratory and expiratory pressures might indicate subtle but unclear changes in respiratory status, and continuous outcomes of FiO2 are unreliably impacted by mode of respiratory support and flow, we used an approach that ordered categorical outcome of respiratory status with the assumption that changes of respiratory support from positive pressure ventilation to less invasive support and no respiratory support are desirable and reflect substantive and clinically relevant changes in health. Almost half of the PROP cohort were never exposed to diuretics, so comparisons of respiratory status by 5 outcome categories were possible. Indications for clinicians prescribing diuretics were not captured in the PROP data collection protocol.
Because one-day courses of diuretics might have reflected prophylactic use of diuretic at the time of red blood cell transfusion, these were analyzed separately. The average day of initial exposure to diuretics was early in the NICU course, when respiratory distress syndrome is most prevalent. We did not examine diuretic effects in infants after 34 weeks PMA, as some babies were discharged or transferred out of the NICU as early as 34 weeks PMA. Kao et al21–23 have shown physiologic improvement in small BPD cohorts before routine surfactant and advances in neonatal care.
Our model adjusted for infant weight and GA at birth, race, sex, site, multiplicity of birth, and concomitant respiratory medication use as these might have had impact on responsiveness to diuretics. Diuretic exposed PROP infants had significantly lower birth weight and GA and were significantly more ill from the time of NICU admission. To minimize potential indication bias, the model also adjusted for outcome on the previous day, and change from two days prior to that, as trajectories of respiratory support status might have impacted the probability of reduction in the level of support. The predominant diuretic in the multi-day course analyses was furosemide, accounting for 84% of the cases. Analyses by individual drugs would not be meaningful so were not reported.
Contrary to our expectations, we found the probability of respiratory support requirements did not improve compared with infants unexposed to diuretics, but rather significantly increased in the 1–7 days after diuretics were started. We do not claim that the diuretics led to worse outcomes. Our results rather could be interpreted that infants treated with diuretics were given more support afterwards, whether the support was needed or not. Alternatively, administration of diuretics may have reflected the recognition of an infant with deteriorating respiratory status which failed to respond to diuretic therapy or responded incompletely to this intervention.
We performed a second analysis of matched pairs which supported the primary model that used the entire cohort. These findings differed from previous studies, and might have been due to the focus on extremely low gestational age neonates, whose lungs were more immature than those previously studied and who were treated routinely with surfactant and caffeine.
Limitations of this study included the lack of information about the indications for starting diuretics and why they were stopped. In addition, recording of acute responses was limited to 24-hour reporting periods. Transient improvements in respiratory status lasting shorter than 24 hours were not captured in the PROP database. This study has reinforced growing concerns about the risk and benefit of one of the most commonly prescribed classes of medications in the NICU24. Routine or sustained use of diuretics was not supported by this analysis from the PROP cohort of extremely preterm infants managed in contemporary tertiary care NICUs. This was a secondary analysis of an observational cohort data set, so more evidence is needed to inform clinical care. We support the need for a clinical trial to evaluate whether there is sufficient benefit for clinically important outcomes such as reduction in mechanical support and supplemental oxygen that can contribute to bronchopulmonary dysplasia and childhood chronic diseases. We suggest that the benefits of diuretics should outweigh the risks of side effects such as nephrocalcinosis, patent ductus arteriosus, and metabolic alkalosis.
Acknowledgments
Supported by the National Institutes of Health grant numbers U01 HL101794, U01 HL101456, U01 HL101798, U01 HL101813, U01 HL101465, U01 HL101800, and 5RO1 HL105702 from the National Heart, Lung, and Blood Institute and support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development under the Best Pharmaceuticals for Children Act to U01 HL101794. C.B., J.T., and A.Z. are employees of the National Institutes of Health (NIH) and have no competing financial interests nor other conflicts of interest. The NIH, as employment agency, had no role in (1) study design; (2) the collection, analysis, and interpretation of data; (3) the writing of the report; and (4) the decision to submit the paper for publication.
PROP Investigators
Cincinnati Children’s Hospital Medical Center site: Claire Chougnet, PhD, James M. Greenberg, MD, William Hardie, MD, Alan H. Jobe, MD, PhD, Karen McDowell, MD; Cincinnati, OH.
Washington University School of Medicine site: Thomas Ferkol, MD, Mark R. Holland, PhD, James Kemp, MD, Philip T. Levy, MD, Phillip Tarr, MD, Gautam K. Singh, MD, Barbara Warner, MD, St. Louis, MO, and Aaron Hamvas, MD, Northwestern University Feinberg School of Medicine, Chicago, IL;
University of California-San Francisco site: Philip L. Ballard, MD, PhD, Roberta A. Ballard, MD, Roberta L. Keller, MD, Amir M. Khan, MD4, Leslie Lusk, MD, Dennis W. Nielson, MD, PhD, Elizabeth E. Rogers, MD, San Francisco, CA; David J. Durand, MD, Children’s Hospital and Research Center Oakland, Oakland, California; Jeffrey D. Merrill, MD, Alta Bates Summit Medical Center, Berkeley, CA; Eric C. Eichenwald, MD, University of Texas Health Science Center- Houston, TX;
Vanderbilt University site: Candice Fike, MD, Tina Hartert, MD, Paul Moore, MD; Judy Aschner, MD, Albert Einstein College of Medicine, Bronx, NY; Scott Guthrie, MD, Jackson-Madison County General Hospital, Jackson, TN; Nathalie Maitre, MD, Nationwide Children’s Hospital, The Ohio State University; Marshall Summar, MD, Children’s National Health System, Washington, DC;
University of Rochester/University at Buffalo site: Carl D’Angio, MD, Vasanth Kumar, MD, Tom Mariani, PhD, Gloria Pryhuber, MD, Anne Marie Reynolds, MD, MPH, Kristin Scheible, MD, Timothy Stevens, MD, MPH Rochester, NY; Clement Ren, MD, Indiana University, Indianapolis, IN; Rita M. Ryan, MD, Medical University of South Carolina, Charleston, SC;
Duke University: C. Michael Cotten, MD, Kim Fisher, PhD, Jack Sharp, MD, Judith A. Voynow, MD, Virginia Commonwealth University; Richmond, VA
Indiana University: Stephanie Davis, MD, Indianapolis, IN; Brenda Poindexter, MD, MS, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH;
University of Pennsylvania, Data Coordinating Center: Jonas Ellenberg, PhD, Rui Feng, PhD, Melissa Fernando, MPH, Howard Panitch, MD, Barbara Schmidt, MD, MSc, Pamela Shaw, PhD, Philadelphia, PA; Scarlett Bellamy, PhD, Drexel University, Philadelphia, PA;
University of Denver, Steering Committee Chair: Lynn M. Taussig, MD, Denver, CO
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Isayama T, Lee SK, Yang J, Lee D, Daspal S, Dunn M, et al. Revisiting the Definition of Bronchopulmonary Dysplasia: Effect of Changing Panoply of Respiratory Support for Preterm Neonates. JAMA Pediatr. 2017;171:271–279. doi: 10.1001/jamapediatrics.2016.4141. [DOI] [PubMed] [Google Scholar]
- 2.Engelhardt B, Elliott S, Hazinski TA. Short- and long-term effects of furosemide on lung function in infants with bronchopulmonary dysplasia. J Pediatr. 1986;109:1034–1039. doi: 10.1016/s0022-3476(86)80295-5. [DOI] [PubMed] [Google Scholar]
- 3.Flemmer A, Simbruner G, Muenzer S, Proquitte H, Haberi C, Nicolai T, et al. Effect of lung water content, manipulated by intratracheal furosemide, surfactant, or a mixture of both, on compliance and viscoelastic tissue forces in lung-lavaged newborn piglets. Crit Care Med. 2000;28:1911–1917. doi: 10.1097/00003246-200006000-00038. [DOI] [PubMed] [Google Scholar]
- 4.Guaman MC, Gien J, Baker CD, Zhang H, Austin ED, Collaco JM. Point Prevalence, Clinical Characteristics, and Treatment Variation for Infants with Severe Bronchopulmonary Dysplasia. Am J Perinatol. 2015;32:960–967. doi: 10.1055/s-0035-1547326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laughon MM, Chantala K, Aliaga S, Herring AH, Hornik CP, Clark RH, et al. Diuretic exposure in premature infants from 1997 to 2011. Am J Perinatol. 2015;32:49–56. doi: 10.1055/s-0034-1373845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slaughter JL, Stenger MR, Reagan PB. Variation in the use of diuretic therapy for infants with bronchopulmonary dysplasia. Pediatrics. 2013;131:716–723. doi: 10.1542/peds.2012-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warrier I, Du W, Natarajan G, Salari V, Aranda J. Patterns of drug utilization in a neonatal intensive care unit. J Clin Pharmacol. 2006;46:449–455. doi: 10.1177/0091270005285456. [DOI] [PubMed] [Google Scholar]
- 8.Pryhuber GS, Maitre NL, Ballard RA, Cifelli D, Davis SD, Ellenberg JH, et al. Prematurity and respiratory outcomes program (PROP): study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States. BMC Pediatr. 2015;15:37. doi: 10.1186/s12887-015-0346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poindexter BB, Feng R, Schmidt B, Aschner JL, Ballard RA, Hamvas A, et al. Comparisons and Limitations of Current Definitions of Bronchopulmonary Dysplasia for the Prematurity and Respiratory Outcomes Program. Ann Am Thorac Soc. 2015;12:1822–1830. doi: 10.1513/AnnalsATS.201504-218OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keller RL, Feng R, DeMauro SB, Ferkol T, Hardie W, Rogers EE, et al. Prematurity and Respiratory Outcomes Program. Bronchopulmonary Dysplasia and Perinatal Characteristics Predict 1-Year Respiratory Outcomes in Newborns Born at Extremely Low Gestational Age: A Prospective Cohort Study. J Pediatr. 2017;187:89–97. doi: 10.1016/j.jpeds.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefano JL, Bhutani VK. Role of furosemide therapy after booster-packed erythrocyte transfusions in infants with bronchopulmonary dysplasia. J Pediatr. 1990;117:965–968. doi: 10.1016/s0022-3476(05)80146-5. [DOI] [PubMed] [Google Scholar]
- 12.Huang X, Dorhout Mees E, Vos P, Hamza S, Braam B. Everything we always wanted to know about furosemide but were afraid to ask. Am J Physiol Renal Physiol. 2016;310:F958–971. doi: 10.1152/ajprenal.00476.2015. [DOI] [PubMed] [Google Scholar]
- 13.Bland RD, McMillan DD, Bressack MA. Decreased pulmonary transvascular fluid filtration in awake newborn lambs after intravenous furosemide. J Clin Invest. 1978;62:601–609. doi: 10.1172/JCI109166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drug Dosages. Harriet Lane Handbook. https://www.unboundmedicine.com/harrietlane/view/Harriet_Lane_Handbook/309832/all/Drug_Dosages.
- 15.Ponto LL, Schoenwald RD. Furosemide (frusemide). A pharmacokinetic/pharmacodynamic review (Part I) Clin Pharmacokinet. 1990;18:381–408. doi: 10.2165/00003088-199018050-00004. [DOI] [PubMed] [Google Scholar]
- 16.Furosemide package insert. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d5b9f12e-d1e9-42de-90f2-c9ba33a86457.
- 17.Green TP, Thompson TR, Johnson DE, Lock JE. Furosemide promotes patent ductus arteriosus in premature infants with the respiratory-distress syndrome. N Engl J Med. 1983;308:743–748. doi: 10.1056/NEJM198303313081303. [DOI] [PubMed] [Google Scholar]
- 18.Prabhu VG. Pulmonary effect of inhaled furosemide in ventilated infants with severe bronchopulmonary dysplasia. Pediatrics. 1997;100:420–421. doi: 10.1542/peds.100.3.420a. [DOI] [PubMed] [Google Scholar]
- 19.Prabhu VG, Keszler M, Dhanireddy R. Dose-dependent evaluation of the effects of nebulized furosemide on pulmonary function in ventilated preterm infants. J Perinatol. 1998;18:357–360. [PubMed] [Google Scholar]
- 20.Yeh TF, Shibli A, Leu ST, Raval D, Pildes RS. Early furosemide therapy in premature infants (less than or equal to 2000 gm) with respiratory distress syndrome: a randomized controlled trial. J Pediatr. 1984;105:603–609. doi: 10.1016/s0022-3476(84)80431-x. [DOI] [PubMed] [Google Scholar]
- 21.Kao LC, Durand DJ, McCrea RC, Birch M, Powers RJ, Nickerson BG. Randomized trial of long-term diuretic therapy for infants with oxygen-dependent bronchopulmonary dysplasia. J Pediatr. 1994;124:772–781. doi: 10.1016/s0022-3476(05)81373-3. [DOI] [PubMed] [Google Scholar]
- 22.Kao LC, Warburton D, Cheng MH, Cedeno C, Platzker AC, Keens TG. Effect of oral diuretics on pulmonary mechanics in infants with chronic bronchopulmonary dysplasia: results of a double-blind crossover sequential trial. Pediatrics. 1984;74:37–44. [PubMed] [Google Scholar]
- 23.Kao LC, Warburton D, Sargent CW, Platzker AC, Keens TG. Furosemide acutely decreases airways resistance in chronic bronchopulmonary dysplasia. J Pediatr. 1983;103:624–629. doi: 10.1016/s0022-3476(83)80602-7. [DOI] [PubMed] [Google Scholar]
- 24.Stewart A, Brion LP. Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst Rev. 2011:CD001453. doi: 10.1002/14651858.CD001453.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]