Abstract
Background and Purpose
For suspected large vessel occlusion patients efficient transfer to centers that provide endovascular therapy (ET) is critical to maximizing treatment opportunity. Our objective was to examine associations between transfer time, modes of transfer, ET and outcomes within a hub-and-spoke telestroke network.
Methods
Patients with ischemic stroke were included if transferred to a single hub hospital between January 2011 and October 2015 with NIHSS>6, onset<12 hours from hub arrival with complete clinical, imaging, and transfer data. Transfer time was the interval between initiation of telestroke consult and arrival at the hub. Algorithms were created for ideal transfer times; ideal time was subtracted from actual time to calculate delay. We examined bivariate relationships between transfer time and several clinical outcomes and used multivariable regression modeling to explore possible predictors of delay.
Results
Of 234 patients that met inclusion criteria, 51% were transferred by ambulance and 49% by helicopter; 27% underwent ET (36% achieved mRS 0-2 at 90 days). Median actual transfer time was 132 min (IQR 103-165), compared to median ideal transfer time at 102 (96-123). Longer transfer time was associated with decreased likelihood of undergoing ET (OR=0.990, p=0.003). Nocturnal transfer (1800-0600hrs) was associated with significantly longer delay (β=20.5, p<0.0005), while intravenous tPA delivery at spoke hospital was not. The median delay for nocturnal transfer was 31 min (11-51), compared to daytime at 14 (−9-36).
Conclusions
Within a large telestroke network, there was an association between longer transfer time and decreased likelihood of undergoing ET. Nocturnal transfers were associated with substantial delay relative to daytime transfers. In contrast, delivery of tPA was not associated with delays, underscoring the impact of effective protocols at spoke hospitals. More efficient transfer may enable higher ET treatment rates. Metrics and protocols for transfer, especially at night, may improve transfer times.
Keywords: stroke care, stroke delivery, telemedicine, thrombectomy, endovascular, recanalization
Subject Terms: Ischemic Stroke, Cerebrovascular Disease/Stroke, Cerebrovascular Procedures, Quality and Outcomes, Revascularization
Introduction
Current models of acute stroke care have been designed to ensure rapid delivery of intravenous (IV) tPA to all eligible patients. Telestroke, the remote use of audio-video conferencing to assist with care decisions for patients presenting to environments with limited resources, is a highly effective method of improving stroke care delivery and increasing tPA utilization.1,2 The hub and spoke model, where a large tertiary care center serves as the “hub” for smaller community “spoke” hospitals, is widely practiced and is the model used at our center.3
While present telestroke models are safe and efficient in administering tPA to eligible patients, a prominent concern has been time delays to the delivery of endovascular thrombectomy (ET). Given the benefit of ET for patients with strokes secondary to large vessel occlusions (LVO),4 developing systems of care that effectively transport such patients from telestroke spokes to ET-capable centers is essential.5,6 While there are growing data to help determine how to triage patients that require transport to a hub capable of ET,7 characteristics of patient transfer between spoke and hub are poorly understood.
We therefore sought to analyze our real-world experience within our hub and spoke academic telestroke network. Our primary objective was to determine whether patient transfer time was associated with clinical outcomes, including likelihood of receiving ET. Secondarily, we aimed to identify predictors of transfer delay.
Methods
Study Setting
This study was IRB approved and HIPAA compliant, and is a retrospective cohort study of prospectively acquired data. The authors declare that all supporting data are available within the article and its online supplementary files. We evaluated consecutive patients transferred from spoke hospitals to our hub hospital, utilizing data from the local Get with the Guidelines-Stroke (GWTG-Stroke) registry, institutional telestroke logs, and the institutional ET database. GWTG-Stroke is a voluntary, continuous registry and performance improvement initiative.8 90-day modified Rankin Scale (mRS) data was collected prospectively on patients who underwent ET.
The hub and spoke telestroke network is composed of 40 spoke hospitals; hospitals transferred patients by either ground ambulance or helicopter (Supplemental Figure I). The hub is located in a city of 650,000 people, with population density of 13,000 per square mile in 2015. However, the wider functional metropolitan area based on commuting patterns is 8.2 million.9 Within our institutional GWTG-Stroke registry, we identified all ischemic stroke patients who were transferred to our hub center from January 2011 to October 2015 and identified as potential ET candidates at the time of transfer initiation (Figure 1). This was determined by selecting those with NIH Stroke Scale (NIHSS) > 6 and last known well (LKW) < 12 hours by hub arrival (n=618). For patients who meet these criteria, our protocol is established such that the goal of transfer is always presumed ET evaluation. Of these 618 patients, 325 had digital computed tomography (CT) images available for review at the spoke hospital to determine Alberta Stroke Program Early CT Score (ASPECTS), which was calculated by two independent raters (GB, AL). At the time of this study, our system discouraged CT angiography (CTA) or additional more advanced imaging at the spoke hospital as it would have been repeated at the hub, per protocol. Of these 325 patients, 234 were transferred after telestroke consult. Of the 234, 114 were transferred by helicopter, and 120 were transferred by ground ambulance. Sixty-three were treated with ET, and 90-day mRS was available for 53.
Figure 1.
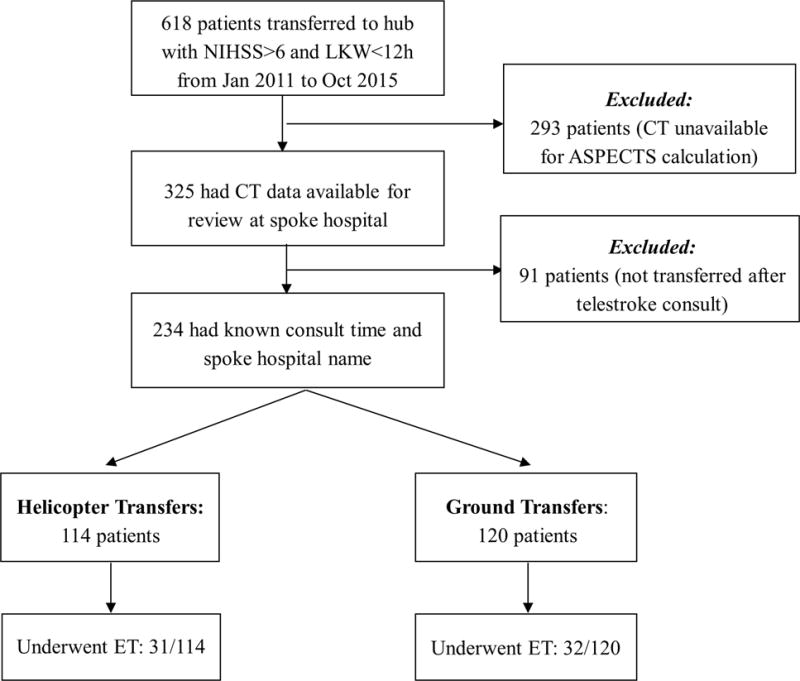
Flow chart of patients who met inclusion/exclusion criteria. NIHSS (NIH Stroke Scale), LKW (Last Known Well), CT (Computed Tomography), ASPECTS (Alberta Stroke Program Early CT Score), ET (Endovascular Thrombectomy).
Key Variables
Actual transfer time was defined as the time from initiation of the telestroke consult response (derived from the telestroke log) to arrival at the hub (emergency room triage data derived from GWTG-Stroke). Ideal transfer time was calculated using Google Maps (Mountain View, CA), correcting for both date and time to account for traffic for ground ambulance transfers and calculated using straight-line distance for helicopter transfers. For ground ambulance transfers, 60 min was added to the average driving time to account for the telestroke consult time, treatment and stabilization of patient, and preparation of patient for transport.10 For helicopter transfers, the straight-line distance was divided by 3.5 km/min, the average helicopter speed.11 To this value, 75 min was added to account for the increased dispatch time of helicopter transport12 and patient processing. Ideal time was subtracted from actual time to calculate delay. Weather Underground (San Francisco, CA), which utilizes data from the National Weather Service, was used to collect historical weather data based on date and time of transport to hub.
Statistical Analyses
Lilliefors test was used to assess normality on non-categorical variables. Median and interquartile range (IQR) were reported for continuous variables. Percent and count were reported for categorical variables. Differences were assessed using non-parametric Wilcoxon rank sum for continuous variables and χ2 tests for categorical variables. A binary logistic regression model was fit to investigate the association between transfer time and probability of undergoing ET. Linear regression models were fit to investigate the association between transfer time and either change in ASPECTS or 90-day mRS. A multivariable regression model was fit to explore three possible predictors of delay (night vs. day, weekend vs. weekday, tPA delivery at spoke). Spearman correlation was used to assess the relationship between distance and transfer time. P-values < 0.05 for two-sided tests were interpreted as statistically significant. Parameter estimates (β) and correlation coefficients (ρ) were reported where appropriate. Analyses were performed with SPSS version 21.0 (IBM Corp.) and Matlab R2015a Statistics Toolbox (Mathworks, Inc.).
Results
In our sample of 234 patients, median age was 72 years, 54% were male, and 85% were Caucasian. Helicopter was used for 49% of transfers, ground ambulance for 51%. Patient demographic and past medical history were similar for those transferred by ground and by helicopter (Table 1). In a year-to-year comparison from January 2011 to October 2015, 23 patients were transferred in 2011, 56 patients in 2012, 43 patients in 2013, 62 patients in 2014, and 50 patients in 2015. The median measured actual transfer time was 132 min; median calculated ideal transfer time was 102 min, with median calculated delay of 25 minutes (Table 2). The median LKW at the time of hub arrival was 4.6 hours. The median road distance from spoke to hub was 86 km, and median straight-line distance was 74 km. 28% of transfers occurred on weekends, and 52% occurred at night (1800-0600 hours).
Table 1.
Demographic data separated by mode of transport. IQR (Interquartile Range), TIA (Transient Ischemic Attack).
| Total (n=234) | Helicopter (n=114) | Ground (n=120) | ||
|---|---|---|---|---|
|
|
||||
| Median (IQR)/Count (%) | Median (IQR)/Count (%) | Median (IQR)/Count (%) | P | |
| Age | 72 (59-83) | 70 (57-83) | 74 (62-85) | 0.0530 |
| Male | 127 (54) | 64 (56) | 63 (53) | 0.5760 |
| White | 199 (85) | 99 (87) | 100 (83) | 0.4519 |
| Medicare/Private Insurance | 223 (95) | 107 (94) | 116 (97) | 0.3106 |
| Atrial Fibrillation | 75 (32) | 36 (32) | 39 (33) | 0.8800 |
| Coronary Artery Disease | 55 (23) | 36 (32) | 19 (16) | 0.0045 |
| Diabetes Mellitus | 45 (19) | 24 (21) | 21 (18) | 0.4907 |
| Dyslipidemia | 97 (41) | 52 (46) | 45 (38) | 0.2079 |
| Stroke Family History | 3 (1) | 3 (3) | 0 (0) | 0.0737 |
| Heart Failure | 22 (9) | 10 (9) | 12 (10) | 0.7477 |
| Hypertension | 165 (71) | 79 (69) | 86 (72) | 0.6180 |
| Previous Stroke/TIA | 47 (20) | 25 (22) | 22 (18) | 0.4925 |
| Smoker | 30 (13) | 16 (14) | 14 (12) | 0.5880 |
Table 2.
Transfer data separated by mode of transport. IQR (Interquartile Range), N (sample size), NIHSS (NIH Stroke Scale), ASPECTS (Alberta Stroke Program Early CT Score), tPA (tissue Plasminogen Activator), LKW (Last Known Well), BP (Blood Pressure).
| Total | Helicopter | Ground | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Median (IQR)/Count (%) | N | Median (IQR)/Count (%) | N | Median (IQR)/Count (%) | N | P | |
| NIHSS at Spoke | 15 (11-20) | 139 | 16 (11-20) | 67 | 13 (10-19) | 72 | 0.0632 |
| ASPECTS at Spoke | 10 (8-10) | 234 | 10 (8-10) | 114 | 10 (9-10) | 120 | 0.2184 |
| Dense Vessel Sign at Spoke | 76 (37) | 207 | 42 (42) | 101 | 34 (32) | 106 | 0.1560 |
| tPA given at Spoke | 156 (67) | 234 | 81 (71) | 114 | 75 (63) | 120 | 0.1654 |
| Road Distance (km) | 86 (66-97) | 234 | 97 (83-111) | 114 | 69 (34-97) | 120 | >0.0001 |
| Line Distance (km) | 74 (54-83) | 234 | 83 (74-96) | 114 | 55 (21-83) | 120 | >0.0001 |
| Weekend | 66 (28) | 234 | 27 (24) | 114 | 39 (33) | 120 | 0.1342 |
| Night | 121 (52) | 234 | 56 (49) | 114 | 65 (54) | 120 | 0.4403 |
| Low Visibility (<16 km) | 33 (14) | 234 | 6 (5) | 114 | 27 (23) | 120 | 0.0002 |
| Wind Speed (km/h) | 17 (11-22) | 234 | 17 (13-22) | 114 | 15 (9-22) | 120 | 0.2471 |
| Ideal Transfer Time (min) | 102 (96-123) | 234 | 99 (96-102) | 114 | 118 (99-130) | 120 | >0.0001 |
| Actual Transfer Time (min) | 132 (103-165) | 234 | 128 (103-148) | 114 | 137 (101-175) | 120 | 0.1009 |
| Delay (min) | 25 (2-45) | 234 | 30 (4-45) | 114 | 16 (-2-45) | 120 | 0.1245 |
| Hub Arrival-LKW Time (h) | 4.2 (3.5-5.2) | 234 | 4.0 (3.3-5.0) | 114 | 4.5 (3.7-5.3) | 120 | 0.0250 |
| Hub Arrival Within 6h | 202 (86) | 234 | 98 (86) | 114 | 104 (87) | 120 | 0.8759 |
| Systolic BP at hub | 153 (135-169) | 232 | 152 (135-169) | 112 | 155 (136-169) | 120 | 0.8394 |
| Atrial Fibrillation at hub | 76 (32) | 234 | 39 (34) | 114 | 37 (31) | 120 | 0.5814 |
| Hemiparesis at hub | 214 (91) | 234 | 100 (88) | 114 | 114 (95) | 120 | 0.0465 |
| Altered Consciousness at hub | 75 (32) | 234 | 38 (33) | 114 | 37 (31) | 120 | 0.6821 |
| Aphasia at hub | 167 (71) | 234 | 77 (68) | 114 | 90 (75) | 120 | 0.2073 |
| NIHSS at hub | 15 (10-20) | 234 | 16 (10-20) | 114 | 13 (9-19) | 120 | 0.0764 |
| ASPECTS at hub | 8 (5-9) | 200 | 7 (4-9) | 98 | 8 (6-9) | 102 | 0.0346 |
| tPA given at hub | 7 (3) | 234 | 4 (4) | 114 | 3 (3) | 120 | 0.6507 |
Median NIHSS was 15 at spoke hospitals, with a median NIHSS improvement of 2 during transfer. Median ASPECTS was 10 at spoke hospitals, with a median decrease of 2 (Table 3). Upon arrival to the hub, 91% of patients had hemiparesis and 71% had aphasia. IV tPA was administered to 67% of patients while at the spoke hospital (Table 2). ET was performed on 27% of transferred patients at the hub (Table 3). Of all transferred patients, symptomatic intracranial hemorrhage occurred in 7% and treatment was withdrawn by family in 21% during hospitalization. Median mRS at the time of discharge was 4, with 24% of patients discharged home. Of the patients who underwent ET, 36% achieved mRS 0-2 and median mRS was 4 at 90 days (Table 3).
Table 3.
Outcome data separated by mode of transport.
| Total | Helicopter | Ground | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Median (IQR)/Count (%) | N | Median (IQR)/Count (%) | N | Median (IQR)/Count (%) | N | |
| ASPECTS Decrease (spoke-hub)* | 2 (0-3) | 200 | 2 (0-3) | 98 | 1 (0-3) | 102 |
| NIHSS Change (hub-spoke) | −2 (−6-0) | 139 | −1 (−5-1) | 67 | −2 (−6-0) | 72 |
| Underwent ET at hub | 63 (27) | 234 | 31 (27) | 114 | 32 (27) | 120 |
| Symptomatic ICH | 17 (7) | 234 | 10 (9) | 114 | 7 (6) | 120 |
| Withdrew Care | 49 (21) | 234 | 27 (24) | 114 | 22 (18) | 120 |
| mRS at Discharge | 4 (3-5) | 193 | 4 (2-5) | 94 | 4 (3-5) | 99 |
| Discharged Home | 46 (24) | 189 | 25 (28) | 89 | 21 (21) | 100 |
| mRS at 90-day (ET patients) | 4 (2-4) | 53 | 3 (2-5) | 27 | 4 (2-4) | 26 |
There were no significant differences comparing ground to helicopter except for ASPECTS (Alberta Stroke Program Early CT Score) decrease (*P = 0.0495).
IQR (Interquartile Range), N (sample size), NIHSS (NIH Stroke Scale), ET (Endovascular Thrombectomy), ICH (Intracranial Hemorrhage), mRS (modified Rankin Scale).
Longer transfer time was associated with decreased odds of undergoing ET in logistic regression (OR 0.990, 95% CI 0.983-0.997, p = 0.004) (Table 4). The probability of ET after a transfer time of 60 min is 0.44. This probability decreases by 1% for each additional minute of transfer time. Median transfer time was significantly shorter among those that underwent ET versus those that did not (124 vs 138 min, IQR 93-104 vs 107-174, p = 0.004). In linear regression, transfer time was not significantly associated with ASPECTS decrease during transfer (n = 200, β = 0, p = 0.961). Furthermore, for those patients undergoing ET, transfer times were not associated with functional outcomes as measured by 90-day mRS (n = 53, β = 0.005, p = 0.480).
Table 4.
A. Longer transfer time was associated with decreased likelihood of undergoing endovascular thrombectomy (ET). Odds ratio (OR) are from a binary logistic regression model and parameter estimates (β) are from linear regression models. Sample size (N), ASPECTS (Alberta Stroke Program Early CT Score), mRS (modified Rankin Scale).
B. Nocturnal transfer was associated with significantly longer delay. βs are from a multivariable regression model with time of transfer, day of transfer, and tPA administration as predictors, and with delay in transfer as the outcome. The overall model fit was significant (N = 234, R2 = 0.078, P <0.0005).
| A. Associations with transfer time | OR/β | N | P |
|---|---|---|---|
| Odds of undergoing ET | 0.990 | 234 | 0.004 |
| ASPECTS decrease during transfer | 0.000 | 200 | 0.961 |
| 90-day mRS for ET patients | 0.005 | 53 | 0.480 |
| B. Predictors of delay | β | Partial eta | P |
|
| |||
| Time (Night/Day) | 20.5 | 0.255 | <0.0005 |
| Day (Weekend/Weekday) | 8.3 | 0.096 | 0.145 |
| tPA (Yes/No) | 6.3 | 0.076 | 0.248 |
Associations between delay in transfer and time of transfer (night or day), day of transfer (weekend or weekday), and tPA administration were explored using a multivariable regression model (Table 4). Overall, the model explained a significant amount of variance (n = 234, R2 = 0.078, p < 0.0005). Nocturnal transfer was associated with significantly longer delay in transfer (β = 20.5, p < 0.0005), with an additional 20.5 min delay associated with transfer at night. The median delay for nocturnal transfer was 31 min (IQR 11-51), compared to daytime transfer at 14 min (IQR −9-36). However, neither weekend (β = 8.3, p = 0.145), nor IV tPA delivery at spoke hospital (β = 6.3, p = 0.248) contributed significantly to the model variance. We also examined predictors of delay stratified separately by helicopter and ground transport. Our findings were unchanged (Supplemental Table I).
Helicopter transfers had longer median road (97 vs. 69 km) and median straight-line (83 vs. 55 km, Table 2) distances compared to ground transfers. There was no difference in wind speed comparing helicopter and ground transfers. However, there were significantly more patients transferred by ground when visibility was less than 16 km (Table 2).
There was a significant association between longer transfer time and both greater road distance (ρ = 0.484, p < 0.0005) and greater straight-line distance (ρ = 0.497, p < 0.0005) between hospitals. In calculating delay, we controlled for this using the calculated ideal transfer time. Actual transfer time was better than ideal transfer time (i.e. negative delay) for 23% of patients, of whom 58% were ground transfers. In a sensitivity analysis, multivariable regression models were fit to test the associations between time of transfer, day of transfer, and tPA administration with delay in transfer. An additional covariate (straight distance or driving distance) was added to account for remaining variability in ideal transfer time that may not be accounted for by our estimate. These models continued to show a significant effect of day/night transfer time on transfer delay (Supplemental Table II).
Discussion
In this analysis of data from a hub-and-spoke academic telestroke network, we found that potential LVO stroke patients with longer transfer times had lower odds of undergoing ET. This probability decreases by 1% for each additional minute of transfer time over 60 minutes. Patients undergoing ET after transfer had a median transfer time of 124 min, compared to a median transfer time of 138 min for patients who did not. A similar finding was demonstrated in a Chicago-based study, where the odds of ET decreased by 2.5% for every minute of transfer time.13 Additionally, in our data nocturnal transfers were associated with substantial delay relative to daytime transfers, highlighting the recognized impact of care discrepancies during night hours and the potential impact on outcome.14 Rapid care delivery for LVO patients is a healthcare priority given the impact of time on ET outcomes.15 Since the majority of patients first present to local hospitals and require transfer to ET-capable facilities,16 it is crucial to evaluate and refine the process of transfer. Applying metrics and protocols for transfer, especially at night, will likely have a positive impact on transfer times and subsequently patient outcomes.
Efforts to speed transit to the nearest ET-capable center include choice of transport mode. In one system, helicopter provides the fastest means for distances over 10 miles.11 Despite the cost of such transport, the benefit in time saved and disability spared makes this a cost-effective transport option for treatment.17 Consistent with this, we found helicopter utilized more frequently for transfer of patients from greater distances (Table 2, Supplemental Figure I). Our calculations factor in the time cost of geography, by accounting for vehicular ground or air speed. No additional statistical differences comparing helicopter to ground ambulance for transfer delays were found. Further, the impact of weather was limited, despite flight restrictions in bad weather (as evidenced by reduced flights during conditions of poor visibility). Regional emergency transport services operate under strict protocols and frequent quality control, which likely explains the consistency of this result.
Beyond fundamental telestroke hub-and-spoke models there is renewed focus on certification for centers that treat stroke. Traditional procedural quality standards are increasingly being associated with time-based metrics.18,19 Using such metrics to improve both spoke and hub hospital emergency room workflow is a major focus of expediting ET. However, activation or mobilization time for transfer from various geographies is an additional source of delay and presents a challenge to benchmark. We controlled for this using an algorithm based on existing data regarding mobilization for transport.10–12 We propose separate air and ground algorithms that factor in activation time, geographic distance, and typical speeds (Figure 2) to allow target time metrics customized for each center within a referral network. Formulation of target transfer times will allow metrics to encompass the entire transfer process, not just the emergency room process at the spoke hospital, and further aid in evaluating areas of potential quality improvement.
Figure 2.

Algorithms for target transfer times for both helicopter and ground transfers. Air speed was assigned a constant value of 3.5km/min (average medical helicopter airspeed), ground speed was individualized, retrospectively calculated using Google Maps specific for the date and time of each transfer. For metric purposes a single averaged value could be applied.
The present sample was selected on the basis of LSW and NIHSS, providing a clinical suspicion for ET candidacy. Median NIHSS was 15 at transfer, supporting the presumption that the sample reflects mostly LVO patients, further supported by the high percentage of patients with cortical signs.20 Despite patients presenting with high NIHSS, LSW < 6h, and ASPECTS > 6, only 27% of patients in our study underwent ET. For these patients there was no association between transfer time and 90-day mRS. Considering advanced imaging selection at time of presentation this is not surprising. Patients are relatively standardized by candidacy for ET, irrespective of time from onset, and the more selective the institutional protocol the more standardized the treated sample. Our protocol specifically targets the presence of LVO with limited infarct core volume for treatment inclusion.21
Many transferred patients are not candidates for intervention. Our 27% treated with ET is lower than another similar study.13 However, IV tPA was given to more patients (67%) in our sample, and hence resolution of LVO due to thrombolysis may be a contributor. Another contributor is the exclusion of patients with large core infarcts given the use of MRI selection.21 The importance of this exclusion criteria for LVO patients in early timeframes remains uncertain. Several recent trials22–24 have demonstrated treatment benefit with selectivity limited to identification of LVO and onset within 6 hours of groin access, ambivalent to infarct core. That data expanded our institutional approach from October 2015. Selection for treatment can also occur at the spoke hospital level, with recent efforts demonstrating positive impact on patient selection from CTA triage at the spoke hospital.7 While refining LVO detection and selection at both hub and spoke will be a crucial step in the next iteration of stroke care, it remains independent of the speed and efficacy of transfer.
There was no association between longer transfer time and ASPECTS decrease, confirming a previous finding.6 Additionally, delivery of tPA at the spoke hospital was not associated with transfer delay in this study, underscoring the impact of effective protocols in place, a particular focus of telestroke network efforts.1,2 While there likely exists a distance around the hub for each telestroke network where direct triage is more appropriate,25,26 this support the argument for tPA delivery to all eligible ET candidates if a patient presents first to a spoke hospital without ET capabilities.
Not all patients suffer equally from the impact of transfer delays. Recent results from DAWN and DEFUSE 3 support the value of imaging selection in late time windows, selecting for small core infarct and significant clinical deficit.27,28 It is anticipated that this will increase the total number of patients evaluated for ET, providing access to therapy to a potentially far larger population. However, while a subset of patients will sustain ischemic penumbra into late time windows,5 this has little practical impact on transfer expectations. For all patients with unknown vessel occlusion or collateral status, it is reasonable to minimize the time of transfer to maximize the possible role of ET. Therefore, these findings will apply to a large population.
This study has several limitations, beyond being a single center, retrospective analysis. The hub in this study is a large academic medical center in a metropolitan area, treating predominantly medically-insured Caucasian patients; this may limit generalizability, though there is inherent universality in the impact of delays and protocols to minimize them. Further, thrombectomy technology evolved during the study period, with stentrievers utilized since June of 2012. However, small numbers of patients prior to this timepoint limit the impact of this on our findings, and thrombectomy technique is independent of transfer metrics. Our established protocol and our data support that the majority of transfers were for ET evaluation, but we cannot exclude the possibility that a handful of patients may have been transferred for other reasons (consideration of hemicraniectomy, care of post thrombolysis complications, or other work-up). Finally, the 2013 and 2015 ET trials were published during the study period, and we cannot exclude that they may have impacted decisions regarding transfer and management of patients with LVO. A recent analysis has shown that there has been a national increase in ET since the 2015 trials.29 Strengths of this study include the large sample size of patients, detailed information about transfer conditions, and the novel perspective of examining transfer time from initiation of telestroke consult.
Conclusion
This study identifies that longer transfer time is associated with decreased likelihood of undergoing ET, with a 44% chance of ET if transfer time is 60 mins, but with a 1% decrease in the likelihood of ET with every subsequent passing minute. Nocturnal transfers were associated with greatest transfer delay, presenting a clear target for system based interventions. An algorithm to calculate target transfer times was used to provide data customizable to each individual center for the entire process of transfer and may aid quality metric calculations in future work. Refining system wide transfer protocols will offer treatment access for more LVO patients, and represents a frontier to explore with future studies.
Supplementary Material
Acknowledgments
None
Sources of Funding: RWR was supported in part by NIH R25 NS065743; APM was supported in part by NIH K23 AG057794; KSZ was supported in part by NIH K08 HS024561; LHS was supported in part by NIH U10 NS086729.
Footnotes
Disclosures: None
References
- 1.Akbik F, Hirsch JA, Chandra RV, Frei D, Patel AB, Rabinov JD, et al. Telestroke-the promise and the challenge. Part one: growth and current practice. J Neurointerv Surg. 2017;9:357–360. doi: 10.1136/neurintsurg-2016-012291. [DOI] [PubMed] [Google Scholar]
- 2.Akbik F, Hirsch JA, Chandra RV, Frei D, Patel AB, Rabinov JD, et al. Telestroke-the promise and the challenge. Part two-expansion and horizons. J Neurointerv Surg. 2017;9:361–365. doi: 10.1136/neurintsurg-2016-012340. [DOI] [PubMed] [Google Scholar]
- 3.Silva GS, Farrell S, Shandra E, Viswanathan A, Schwamm LH. The status of telestroke in the United States: a survey of currently active stroke telemedicine programs. Stroke. 2012;43:2078–2085. doi: 10.1161/STROKEAHA.111.645861. [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 5.Regenhardt RW, Das AS, Stapleton CJ, Chandra RV, Rabinov JD, Patel AB, et al. Blood Pressure and Penumbral Sustenance in Stroke from Large Vessel Occlusion. Front Neurol. 2017;8:317. doi: 10.3389/fneur.2017.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulouis G, Lauer A, Siddiqui AK, Charidimou A, Regenhardt RW, Viswanathan A, et al. Clinical Imaging Factors Associated With Infarct Progression in Patients With Ischemic Stroke During Transfer for Mechanical Thrombectomy. JAMA Neurol. 2017;74(11):1361–1367. doi: 10.1001/jamaneurol.2017.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulouis G, Siddiqui KA, Lauer A, Charidimou A, Regenhardt RW, Viswanathan A, et al. Immediate Vascular Imaging Needed for Efficient Triage of Patients With Acute Ischemic Stroke Initially Admitted to Nonthrombectomy Centers. Stroke. 2017;48:2297–2300. doi: 10.1161/STROKEAHA.117.017607. [DOI] [PubMed] [Google Scholar]
- 8.Ali SF, Singhal AB, Viswanathan A, Rost NS, Schwamm LH. Characteristics and outcomes among patients transferred to a regional comprehensive stroke center for tertiary care. Stroke. 2013;44:3148–3153. doi: 10.1161/STROKEAHA.113.002493. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Census Bureau. Boston City, Massachusetts. 2015 Available at: https://www.census.gov/quickfacts/fact/table/bostoncitymassachusetts/PST045216. Accessed 2/17, 2018.
- 10.Demaerschalk BM, Miley ML, Kiernan TE, Bobrow BJ, Corday DA, Wellik KE, et al. Stroke telemedicine. Mayo Clin Proc. 2009;84:53–64. doi: 10.4065/84.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz MA, Hendey GW, Bivins HG. When is the helicopter faster? A comparison of helicopter and ground ambulance transport times. J Trauma. 2005;58:148–153. doi: 10.1097/01.ta.0000124264.43941.41. [DOI] [PubMed] [Google Scholar]
- 12.Svenson JE, O’Connor JE, Lindsay MB. Is air transport faster? A comparison of air versus ground transport times for interfacility transfers in a regional referral system. Air Med J. 2006;25:170–172. doi: 10.1016/j.amj.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Prabhakaran S, Ward E, John S, Lopes DK, Chen M, Temes RE, et al. Transfer delay is a major factor limiting the use of intra-arterial treatment in acute ischemic stroke. Stroke. 2011;42:1626–1630. doi: 10.1161/STROKEAHA.110.609750. [DOI] [PubMed] [Google Scholar]
- 14.Reeves MJ, Smith E, Fonarow G, Hernandez A, Pan W, Schwamm LH, et al. Off-hour admission and in-hospital stroke case fatality in the get with the guidelines-stroke program. Stroke. 2009;40:569–576. doi: 10.1161/STROKEAHA.108.519355. [DOI] [PubMed] [Google Scholar]
- 15.Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA, et al. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73:1066–1072. doi: 10.1212/WNL.0b013e3181b9c847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith EE, Schwamm LH. Endovascular clot retrieval therapy: implications for the organization of stroke systems of care in North America. Stroke. 2015;46:1462–1467. doi: 10.1161/STROKEAHA.115.008385. [DOI] [PubMed] [Google Scholar]
- 17.Silbergleit R, Scott PA, Lowell MJ, Silbergleit R. Cost-effectiveness of helicopter transport of stroke patients for thrombolysis. Acad Emerg Med. 2003;10:966–972. doi: 10.1197/s1069-6563(03)00316-6. [DOI] [PubMed] [Google Scholar]
- 18.Sun CH, Nogueira RG, Glenn BA, Connelly K, Zimmermann S, Anda K, et al. “Picture to puncture”: a novel time metric to enhance outcomes in patients transferred for endovascular reperfusion in acute ischemic stroke. Circulation. 2013;127:1139–1148. doi: 10.1161/CIRCULATIONAHA.112.000506. [DOI] [PubMed] [Google Scholar]
- 19.Ng FC, Low E, Andrew E, Smith K, Campbell BCV, Hand PJ, et al. Deconstruction of Interhospital Transfer Workflow in Large Vessel Occlusion: Real-World Data in the Thrombectomy Era. Stroke. 2017;48:1976–1979. doi: 10.1161/STROKEAHA.117.017235. [DOI] [PubMed] [Google Scholar]
- 20.Heldner MR, Zubler C, Mattle HP, Schroth G, Weck A, Mono ML, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44:1153–1157. doi: 10.1161/STROKEAHA.111.000604. [DOI] [PubMed] [Google Scholar]
- 21.Leslie-Mazwi TM, Hirsch JA, Falcone GJ, Schaefer PW, Lev MH, Rabinov JD, et al. Endovascular Stroke Treatment Outcomes After Patient Selection Based on Magnetic Resonance Imaging and Clinical Criteria. JAMA Neurol. 2016;73:43–49. doi: 10.1001/jamaneurol.2015.3000. [DOI] [PubMed] [Google Scholar]
- 22.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 23.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 24.Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138–1147. doi: 10.1016/S1474-4422(16)30177-6. [DOI] [PubMed] [Google Scholar]
- 25.Goyal M, Almekhlafi MA, Fan L, Menon BK, Demchuk AM, Yeatts SD, et al. Evaluation of interval times from onset to reperfusion in patients undergoing endovascular therapy in the Interventional Management of Stroke III trial. Circulation. 2014;130:265–272. doi: 10.1161/CIRCULATIONAHA.113.007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheth KN, Smith EE, Grau-Sepulveda MV, Kleindorfer D, Fonarow GC, Schwamm LH. Drip and ship thrombolytic therapy for acute ischemic stroke: use, temporal trends, and outcomes. Stroke. 2015;46:732–739. doi: 10.1161/STROKEAHA.114.007506. [DOI] [PubMed] [Google Scholar]
- 27.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 28.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith EE, Saver JL, Cox M, Liang L, Matsouaka RA, Xian Y, et al. Increase in Endovascular Therapy in Get With The Guidelines-Stroke After The Publication of Pivotal Trials. Circulation. 2017;136(24):2303–2310. doi: 10.1161/CIRCULATIONAHA.117.031097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


