Abstract
Background
Sepsis is a common diagnosis in critical care with inpatient mortality rates up to 50%. Sepsis care is organized around source control, antibiotics, and supportive care. Drug disposition is deranged by changes in volume of distribution and regional blood flow, as well as multiple organ failure. Thus, assuring that each patient with sepsis attains pharmacokinetic targets is challenging. There is currently no commercially available FDA-approved assay to measure piperacillin-tazobactam, very commonly used as a beta-lactam/beta-lactamase inhibitor combination antibiotic in the intensive care unit (ICU).
Methods
Samples were prepared by ultrafiltration of plasma collected in lithium heparin Vacutainers. Separation was achieved by gradient elution on a C-18 column followed by UV detection at 214 nm. The method is validated in residual blood samples allowing investigators to exploit a waste product to develop insight into beta-lactam pharmacokinetics in the ICU.
Results
Accuracy and precision were within the 25% CLIA error standard for other antibiotic assays. Free piperacillin concentrations were also in good agreement with total piperacillin concentrations measured in the same plasma by an assay in clinical use outside the United States.
Conclusion
We describe a method for measuring piperacillin and tazobactam that meets clinical validation standards. Quick turnaround time and excellent accuracy on a low-cost platform make this method more than adequate for use as a routine therapeutic drug monitoring tool.
Keywords: Piperacillin, Tazobactam, High Pressure Liquid Chromatography, Pharmacokinetics, Sepsis, Critical Care
1. Introduction
The pharmacological effect of antibiotics on the critically ill is difficult to study without first considering the changes in physiological dynamics of the patient [1]. Roberts and Lipman [2] reviewed some of the specific effects sepsis and severe illnesses have on the clearance, distribution and effectiveness of hydrophilic and lipophilic antibiotics. Examples include vasoconstriction/dilation and increased capillary permeability that affect distribution, increased cardiac output leading to increased drug clearance and organ dysfunction leading to decreased drug clearance. The degree to which these changes take place in individuals varies and is monitored using an overall clinical assessment and laboratory results for metabolic indices. Examples of this include use of vasopressors, ventilation, hemodialysis or continuous renal replacement therapy, and lab values such as bilirubin, creatinine and blood urea nitrogen. A practical approach to individualizing and streamlining treatment could incorporate demographic, clinical and pharmacokinetic/dynamic information with patient-specific measured concentrations of antibiotic to direct individual dosing.
Population pharmacokinetic studies regarding piperacillin-tazobactam have previously been performed on pediatric patients and healthy adults [3,4]. Piperacillin and tazobactam disposition agreed with linear kinetics, and was not affected by gender. In order to effectively tailor therapy for the critically ill population, sufficient data is needed to uncover which factors affect specific patients and their response to current dosing regimens. We must then determine the correlation of these factors to necessary dosing changes.
Studies exist in analytical formats that use high-performance liquid chromatography (HPLC) to measure concentrations of multiple antibiotics simultaneously, including piperacillin and tazobactam [5–7]. These antibiotics are commonly given together as Zosyn or Tazocin to ICU patients and are effective against a wide range of microorganisms that cause sepsis. Tazobactam is a beta-lactamase inhibitor and as such can extend the effectiveness of piperacillin by preventing beta-lactamase producing organisms from degrading piperacillin. Small-scale studies of critically ill patients on dialysis have begun to investigate Zosyn kinetics in fragile populations by examining the relationship between plasma and dialysate drug concentrations [8,9]. Currently, there is no commercial assay that measures these antibiotics for therapeutic drug monitoring.
We developed a reversed-phase HPLC method for measuring piperacillin and tazobactam at clinically relevant concentrations in plasma. This employed a polar (aqueous) mobile phase A and a non-polar (C18) column as the stationary phase. These conditions, combined with a programmed gradient introduction of organic mobile phase B, allow for simultaneous elution of piperacillin and tazobactam within a convenient run time. The method was validated in accordance with FDA guidelines [10].
2. Materials and Methods
2.1 Materials
Certified reference antibiotic powders of tazobactam (Sigma-Aldrich) and piperacillin (Sigma-Aldrich) were used as test analytes for calibrators and quality control (QC) materials. Penicillin G (MP Biomedicals) was the internal standard. Phosphate buffered saline (PBS) used in mobile phase and in calibrators was prepared from tablets (Sigma Aldrich). All water used for reagents was filtered using a Millipore Milli-Q system, however reagents that were not purchased pre-formulated or certified HPLC grade were filtered a second time using 0.22 um filters (Corning/Millipore). Mobile phase A was prepared with above mentioned PBS tablets and Optima HPLC grade methanol (Fisher Chemical). Mobile phase B of acetonitrile with 0.1%TFA was HPLC grade (Fisher). Acetonitrile for wash steps was also Optima HPLC grade (Fisher). Bovine plasma for QC levels was purchased as citrated whole blood from Lampire Biologicals and was prepared in-house in Bio-one conical tubes (Greiner,).
Calibrators were prepared by diluting stock solutions. These were made by accurately measuring aliquots of powdered piperacillin and tazobactam. An equal volume (ml) of PBS was added to provide 1.0mg/ml. Stock solutions were maintained for one week at −80C. New calibrators were prepared every day using PBS to dilute to desired concentration. Quality control materials were prepared by combining appropriate concentrations of antibiotic stock with bovine plasma. Three levels were used with the first being within 3 times the LLOQ (3.0 ug/ml). 500uL of QC material were added to VivaFree 30K centrifuge filters (Viva Products) and spun for 30 minutes at 2.5 rcf to remove large proteins and produce filtrate for analysis.
2.2 Chromatographic Conditions
Chromatography was performed using an Agilent 1200 series HPLC system. Eluent concentrations were determined using a diode array detector (Agilent G1315C, Santa Clara, CA) at 214nm. Separation was performed using gradient elution and a Kinetex 2.6u XB-C18 100A, 100 × 3 mm’ Column (Phenomenex) with SecurityGuard ULTRA guard column (Phenomenex). The column was maintained at 30°C in a column oven. The flow rate was set to 0.8 ml/min and the method drew up 1uL of internal standard and 9uL of sample independently. This was then injected for analysis to measure free drug concentration. A binary pump (Agilent) controlled A and B mobile phases as follows: Mobile phase B linearly increased from 0% to 30% in the first 5 min. The mobile phase returned to 0% B by 5.1 min for column re-equilibration. The total run time was 7 min and the flowrate was constant.
2.3 Patient Sample Preparation
This study was approved by the Vanderbilt University Medical Center Institutional Review Board. Use of residual blood samples was permitted under a waiver of informed consent. We measured a large sample set (378 unique patients, 1887 samples) of patient plasma to model kinetics in an ICU population using residual blood. The hospital Institutional Review Board approved this observational under a waiver of individual informed consent and recognized that within the facility, consent for treatment explicitly grants consent for research use of specimens obtained for clinical care. Plasma was maintained at 4°C and was no more than 72 hours old. Based on freezing stability studies reporting that piperacillin and tazobactam are stable at −80 for up to 3 months, samples could reasonably remain frozen at −80C for at least a week so as to facilitate batch testing [6]. Sample plasma was filtered in Vivafree micro centrifuge filters with a 30kD MWCO using the same procedure as that used above to prepare QC material.
2.4 Stability
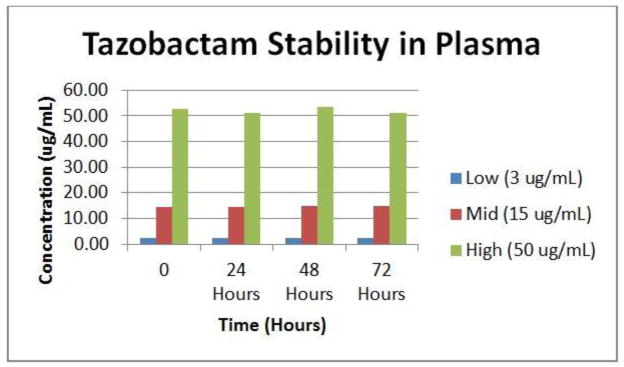
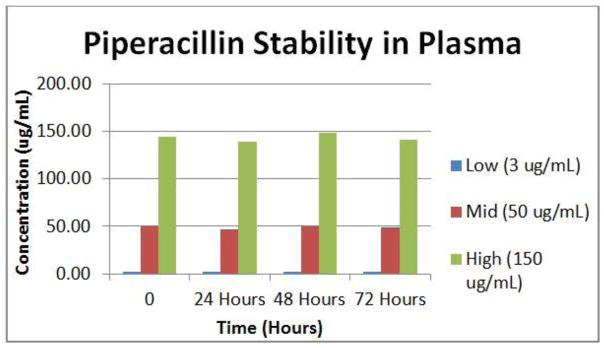
Stability of both antibiotics at 4°C was examined. Aliquots of our low, mid and high concentrations used for QC in bovine plasma were processed and measured at 0, 24, 48 and 72 h.
2.5 Clinical Comparison Patient Samples
To validate this method against another method that is being used clinically, we obtained ~40 patient samples from Ghent University Hospital in Belgium. These heparinized samples were drawn from ICU patients in the early morning and were maintained at 4C for no more than 3 h after which they were centrifuged and plasma frozen at −80C. They were then batch tested weekly using their validated piperacillin-tazobactam assay. Samples delivered to VUMC were kept frozen and shipped on dry ice. They were stored immediately at −80C and tested within 2 months of initial phlebotomy.
2.6 Method Validation
Linearity was tested using six calibrators. Our calibration curve defaulted to ignore the origin and was a weighted linear regression of amount ratio to area ratio. The acceptable correlation coefficient was ≥0.99. LLOQ was determined by measuring low level spiked plasma samples over 5 injections. To determine accuracy, 3 levels of known concentration in matrix were injected. Each was replicated 5 times within a day and was repeated on three different days. For precision, the same setup applied. Precision included interday and intraday variability. To determine that the method was selective, 6 lots of human plasma were analyzed using 5 injections for each lot. To address problems of interference, the assay was validated against pooled plasma from critically ill patients receiving a wide variety of agents. Additionally, bovine plasma was spiked with an interference mix containing Acetaminophen, 1,000 μg/ml, Caffeine, 1,000 μg/ml, (−)-cotinine, 1,000 μg/ml, ibuprofen, 1,000 μg/ml, naproxen, 1,000 μg/ml, (−)-nicotine, 1,000 μg/ml and phentermine, 100 μg/ml (Sigma-Aldrich).
2.7 System Requirements
According to FDA guidelines for chromatographic methods specifically, we defined system suitability parameters and run acceptance cutoffs. In our analysis of patient samples we processed raw data with criteria for resolution of target peaks in plasma and absorbance beyond reliable report for our method [13]. Resolution factor was difference in retention time divided by half height peak widths: Rs=1.18x (t2-t1)/(w0.5h1+w0.5h2). There are often other peaks near the retention time for tazobactam, so a resolution factor of 2 was required when the resolution was deemed inadequate for reporting. Piperacillin concentrations were higher than tazobactam and patient samples that produced peaks above an absorbance of 2500mAU were not reported.
Before analyzing patient samples, at least 5 calibrators were tested. This was to observe peak shape prior to committing patient samples to the chromatograph. For system suitability requirements when there was a change in peak shape or measurement output, evaluation of three factors took place. RSD was to be less than or equal to 1% for a minimum of 5 injections. Tailing factor was to ≤2. Peak Capacity (run time divided by peak width) was to be consistent throughout and between runs for a method and was to be between 100–400. For this method it was shown to have a range of 250–350. Troubleshooting of the system followed deviations from these values.
Quality control materials determined run acceptance. 67% (4 out of 6) of QC at three levels, replicated at least once was within 15% nominal value. SOPs for general procedures, instruments and specific tests were created and maintained according to WHO Good Laboratory Practice guidelines [14].
3. Results
3.1 Method Development
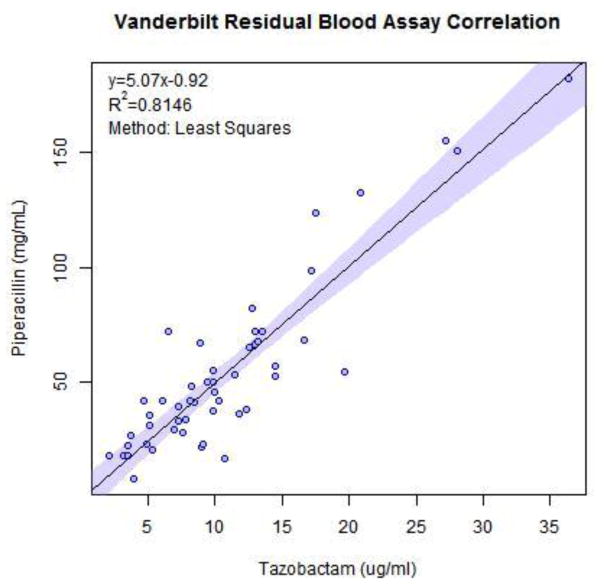
The method produced measurements of piperacillin and tazobactam, using penicillin as the internal standard, with good separation. Fig. 3 is an example of a calibrator in PBS showing adequate resolution of peaks. Through testing, separation of tazobactam, piperacillin and penicillin had been shown to give relative retention times for tazobactam at 2.5 min, penicillin at 5.3 min and piperacillin at 5.5 min. Per FDA suggested guidelines, 6 calibrators were used that span the therapeutic range for our antibiotics. This was 2.0 to 60.0μg/ml for tazobactam and 2.0 to 200.0 μg/ml for piperacillin. The method was linear with a correlation coefficient of 0.99 or higher for both piperacillin and tazobactam.
Fig. 3.
Vanderbilt’s correlation plot for measurements of piperacillin and tazobactam in 50 residual blood samples
3.2 Method Validation
Runs were prepared to show performance of 7 calibrators and 3 levels of QC material. Results showed accuracy and precision within FDA suggested guidelines of 15% for all levels except LLOQ which should be within 20%. LLOQ of 2.0 ug/ml was determined by repeated injection of lower concentration spiked plasma with a CV of ≤15%.
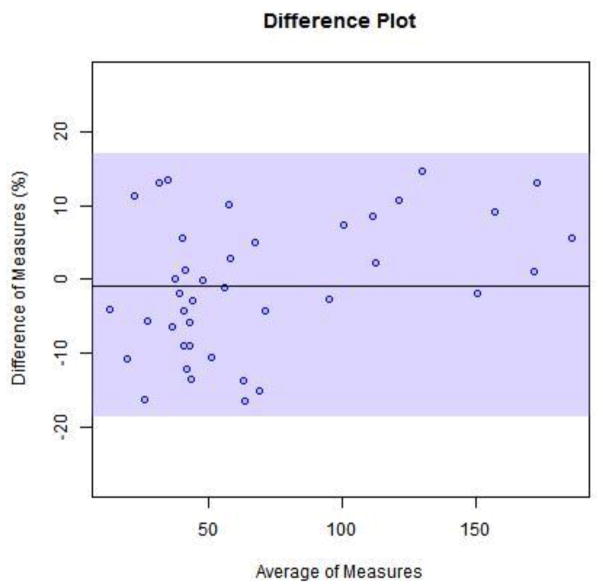
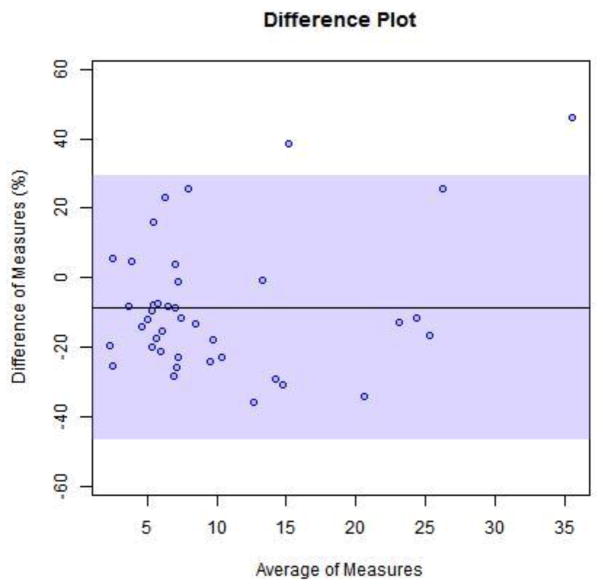
The method was selective for the peaks of nterest with no inherent interfering peaks from the matrix. The interference mix showed no co-eluting peaks for the substances investigated. Both antibiotics were stable in plasma stored at 4C for up to 72 h. For proof of clinical validation potential we tested 40 reference samples from Ghent University Hospital. Ghent’s method produced a good correlation for total measurement of the 2 drugs simultaneously.
Ghent University had observed 20% plasma protein binding for piperacillin (Alain Verstraete, personal communication). Forty samples were measured with our method. There was an average of 75% of the total drug measurement for tazobactam and 80% of the total drug measurement for piperacillin. We used the expected value of 80% total drug measurement to plot bias between methods.
4. Discussion
This method was developed using FDA guidelines for bioanalytical methods. While initial development data was within the suggested 15% and 20% ranges, a more important indicator for clinical validity is what limits CLIA expects for such an assay. As there are no available commercial assays for our drugs of study, we have used the CLIA standard of 25% error allowed for the antibiotic tobramycin [15]. Our method shows adequate linearity, accuracy, precision, sensitivity and selectivity to measure piperacillin and tazobactam in human plasma. A short run time and minimal processing steps make for convenient turnaround time and good control of conditions for stability. Development followed FDA guidelines but some establishment criteria, such as 67% for acceptance of Quality Control, may have justification for wider parameters. Upon clinical validation this can be revisited. Further study of opportunistic samples and possibly direct comparison using bedside samples will facilitate the modeling of dose dynamics.
This method validation meets criteria for medical decision making, not just clinical research. Deployment of a clinically validated pip-tazo assay will enable prospective randomized trials of antibiotic dosing tailored to attain pharmacokinetic targets rather than one-size-fits-all prescriptions. This validated method has minimal sample processing, definite analyte separation and short run duration. This method’s rapid turnaround time will be beneficial following clinical validity as it provides a novel testing option for therapeutic drug monitoring of these drugs.
Stability studies suggest that piperacillin frozen at −80°C should be stable up to 3 months [6]. Our comparison samples were kept at a proper temperature after draw and all measures were taken to keep them at least at 4°C, if not frozen. Our results are consistent with Ghent University’s pre-analytical stability studies which showed stability when measured in plasma in contact with a gel separator stored at proper temperatures [16]. The data presented here show that piperacillin and tazobactam concentrations are also stable when blood is stored at 4°C for at least 72 h. This is fundamentally enabling for pharmacokinetic studies using opportunistic sampling of residual blood specimens. RBS is an innovative approach to clinical research that uses a scarce resource, patient blood, wisely. Exploiting blood, plasma, and serum leftover from clinical testing for research reduces phlebotomy. Finally, as most clinical laboratories hold blood tubes for 72 hours before discarding them, RBS allows “look-back” analysis of drug concentrations prior to a clinical event.
Our analytical HPLC method is being used to develop statistical inferences about antibiotic effectiveness and dose dynamics. We have been able to measure free drug levels with predictable difference from total drug measurement, allowing us to skip extra sample processing steps such as liquid- liquid extraction and solid phase extraction. This provides very quick turnaround time and minimal materials, making this method beneficial for clinical testing. The LOQ of the assay is completely appropriate for clinical use; levels near or below the LOQ are below the MIC of most relevant organisms in the ICU. Should, as many have suggested, TDM of beta lactams prove valuable, it is going to be highly useful and important that assays are widely available at reasonable cost. A reliable HPLC system with UV detection can be purchased new for much less than $100,000; in contrast entry-level mass spec systems cost considerably more. Most hospital clinical laboratories have HPLC systems already and can implement this method with a clinically useful turnaround time, but a mass spec based assay might necessitate a send out test for many hospital labs, which would be much less clinically useful.
5. Conclusion
There are several HPLC methods to analyze tazobactam and piperacillin, but few measure free drug levels. The unbound drug is not only the pharmacologically relevant agent, but is also the fraction of total drug that is removed by extracorporeal therapies such as dialysis and CRRT. In our observations of the critically ill patient population, protein binding of piperacillin is highly variable and ought to be considered carefully in estimation of target attainment. In combination, these factors support our decision to measure free drug instead of total drug.
The primary advantage of this method over other published methods is that it is performed with relatively low-cost and widely available equipment and could thus be integrated into a wide range of clinical and research laboratories. Using this data to streamline dosing procedures is the first step. Once implemented, this method may even expand to include other antibiotics of interest and serve a wider population.
Fig. 1.
Stability of Tazobactam in plasma at 4°C
Fig. 2.
Stability of Piperacillin in plasma at 4°C
Fig. 4.
Bland-Altman plots for Vanderbilt’s method measurements with expected value of 80% total drug measured by Ghent method. (4) piperacillin (5) tazobactam
Fig. 5.
Bland-Altman plots for Vanderbilt’s method measurements with expected value of 80% total drug measured by Ghent method. (4) piperacillin (5) tazobactam
Table 1.
Accuracy and Precision data for the method over 3 days with 5 injections per day
| Intraday | ||||
|---|---|---|---|---|
| Low | Mid | High | ||
| Tazo | Mean | 2.95 | 16.3 | 47.5 |
| StdDev | 0.29 | 0.86 | 1.59 | |
| CV | 10% | 5% | 3% | |
| Acc | −2% | 8% | −5% | |
| Low | Mid | High | ||
| Pip | Mean | 2.33 | 49.9 | 134 |
| StdDev | 0.26 | 1.54 | 2.15 | |
| CV | 11% | 3% | 2% | |
| Acc | −22% | 0% | −10% | |
| Interday | ||||
| Low | Mid | High | ||
| Tazo | Mean | 2.61 | 15.0 | 49.0 |
| StdDev | 0.30 | 1.28 | 2.39 | |
| CV | 11% | 9% | 5% | |
| Acc | −13% | 0% | −2% | |
| Low | Mid | High | ||
| Pip | Mean | 2.45 | 46.9 | 125 |
| StdDev | 0.22 | 4.78 | 8.13 | |
| CV | 9% | 10% | 6% | |
| Acc | −18% | −6% | −16% | |
Table 2.
Percent change in measured concentration of Tazobactam and Piperacillin in bovine plasma after
| Tzb | Pip | ||
|---|---|---|---|
| 3 ug/ml | 1% | 3 ug/ml | 11% |
| 15 ug/ml | 2% | 50 ug/ml | 0% |
| 50 ug/ml | 3% | 150 ug/ml | 2% |
Highlights.
Measurement of plasma free piperacillin and tazobactam concentrations by HPLC
Minimal sample preparation
Application to therapeutic drug monitoring
Acknowledgments
This work was funded by the NIH, Baxter Healthcare Clinical evidence Councel for Renal Failure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sylvia M. Verhoven, Division of Nephrology and Hypertension, Vanderbilt University Medical Center, Nashville, Tennessee
Joseph J. Groszek, Division of Nephrology and Hypertension, Vanderbilt University Medical Center, Nashville, Tennessee
William H. Fissell, Division of Nephrology and Hypertension, Vanderbilt University Medical Center, Nashville, Tennessee
Adam Seegmiller, Pathology Microbiology and Immunonology, Vice Chair for Clinical Pathology, Director of Laboratory Medicine and Hematopathology: Vanderbilt University Medical Center.
Jennifer Colby, Pathology Microbiology and Immunology, Assistant Director Clinical Chemistry: Vanderbilt University Medical Center.
Pratish Patel, Therapeutic Drug Monitoring/Antimicrobial Stewardship: Vanderbilt University Medical Center.
Alain Verstraete, Department of Clinical Chemistry Microbiology and Immunology: Ghent University Hospital, Belgium.
Matthew Shotwell, Department of Biostatistics and Department of Anesthesiology: Vanderbilt University Medical Center.
References
- 1.Roberts Jason A, et al. Individualized Antibiotic Dosing for Patients Who Are Critically Ill: Challenges and Potential Solutions. The Lancet Infectious Diseases. 2014;14(6):498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts Jason, Lipman Jeffrey. Pharmacokinetic issues for antibiotics in the critically ill patient. Critical care medicine. 2009;37:840–51. doi: 10.1097/CCM.0b013e3181961bff. [DOI] [PubMed] [Google Scholar]
- 3.Chen Yewei, Lu Jinmiao, Dong Min, Wu Dan, Zhu Yiqing, Li Qin, Chen Chao, Li Zhiping. Target Attainment Analysis and Optimal Sampling Designs for Population Pharmacokinetic Study on Piperacillin/tazobactam in Neonates and Young Infants. European Journal of Clinical Pharmacology. 2016;72(12):1479–488. doi: 10.1007/s00228-016-2131-0. Web. [DOI] [PubMed] [Google Scholar]
- 4.Xia Chun-Hua, Xiong Yu-Qing, Wang Guang-Ji. An Improved High-performance Liquid Chromatographic Method with a Solid-phase Extraction for the Determination of Piperacillin and Tazobactam: Application to Pharmacokinetic Study of Different Dosage in Chinese Healthy Volunteers. Biomedical Chromatography. 2007;21(7):680–686. doi: 10.1002/bmc.800. [DOI] [PubMed] [Google Scholar]
- 5.Augey Valerie, Grosse Pierre-Yves, Albert Gregory, Audran Michel, Bressolle Françoise. High-performance liquid chromatographic determination of tazobactam and piperacillin in human plasma and urine. Journal of Chromatography B: Biomedical Sciences and Applications. 1996;682(1):125–136. doi: 10.1016/0378-4347(96)00049-7. [DOI] [PubMed] [Google Scholar]
- 6.Ocampo Ap, Hoyt Kd, Wadgaonkar N, Carver Ah, Puglisi Cv. Determination of Tazobactam and Piperacillin in Human Plasma, Serum, Bile and Urine by Gradient Elution Reversed-phase High-performance Liquid Chromatography. Journal of Chromatography B: Biomedical Sciences and Applications. 1989;496:167–79. doi: 10.1016/s0378-4347(00)82563-3. Web. [DOI] [PubMed] [Google Scholar]
- 7.Veni P Rama Krishna, Sharmila N, Narayana Kjp, Hari Babu B, Satyanarayana Pvv. Simultaneous Determination of Piperacillin and Tazobactam In pharmaceutical Formulations by RP-HPLC Method. Journal of Pharmacy Research. 2013;7(1):127–31. Web. [Google Scholar]
- 8.Connor MJ, Salem C, Bauer SR, Hofmann CL, Groszek J, Butler R, Rehm SJ, Fissell WH. Therapeutic Drug Monitoring of Piperacillin-Tazobactam Using Spent Dialysate Effluent in Patients Receiving Continuous Venovenous Hemodialysis. Antimicrobial Agents and Chemotherapy. 2010;55(2):557–60. doi: 10.1128/AAC.00548-10. Web. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arzuaga A, Isla A, Gascón AR, Maynar J, Martín A, Solinís MA, Toral D, Pedraz JL. Quantitation and Stability of Piperacillin and Tazobactam in Plasma and Ultrafiltrate from Patients Undergoing Continuous Venovenous Hemofiltration by HPLC. Biomedical Chromatography. 2005;19(8):570–78. doi: 10.1002/bmc.482. Web. [DOI] [PubMed] [Google Scholar]
- 10.FDA CDER. [accessed January 2017];Guidance for Industry: Bioanalytical Method Validation. 2001 May; https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf.
- 11.Veillette John J, Alexander Winans S, Forland Steven C, Maskiewicz Victoria K. A Simple and Rapid RP-HPLC Method for the Simultaneous Determination of Piperacillin and Tazobactam in Human Plasma. Journal of Pharmaceutical and Biomedical Analysis. 2016;131:80–86. doi: 10.1016/j.jpba.2016.08.010. Web. [DOI] [PubMed] [Google Scholar]
- 12.Carlier Mieke, Stove Veronique, Roberts Jason A, Van De Velde Eric, DeWaele Jan J, Verstraete Alain G. Quantification of Seven Beta-lactam Antibiotics and Two Beta-lactamase Inhibitors in Human Plasma Using a Validated UPLC-MS/MS Method. International Journal of Antimicrobial Agents. 2012;40(5):416–22. doi: 10.1016/j.ijantimicag.2012.06.022. Web. [DOI] [PubMed] [Google Scholar]
- 13.Ng, Linda L., PhD [accessed Feb. 2017];Validation of Chromatographic Methods-FDA. 1994 Web. https://www.fda.gov/downloads/Drugs/Guidances/UCM134409.pdf.
- 14. [accessed Dec 2016];World Health Organization Laboratory Quality Stepwise Implementation Tool. http://www.who.int/ihr/lyon/hls_lqsi/en/
- 15.Allowable Total Error Table. [accessed March 2017];Data Innovations. https://datainnovations.com/allowable-total-error-table.
- 16.Carlier Mieke, DeWaele Jan J, Verstraete Alain G, Stove Veronique. Exploration of the Pre-analytical Stability of Beta-lactam Antibiotics in Plasma and Blood:Implications for Therapeutic Drug Monitoring and Pharmacokinetic Studies. Clinical Chemistry and Laboratory Medicine (CCLM) 2015;53(9) doi: 10.1515/cclm-2014-0833. [DOI] [PubMed] [Google Scholar]