Abstract
Background and Purpose
We evaluated whether lacunes in centrum semiovale (lobar lacunes) were associated with cerebral amyloid angiopathy (CAA) markers in an Asian intracerebral hemorrhage (ICH) population.
Methods
One hundred and ten patients with primary ICH were classified as CAA-ICH (n=24, mean age 70.9 ± 13.9) or HTN-ICH (n=86, mean age 59.3 ± 13.0) according to the presence of strictly lobar (per modified Boston criteria) or strictly deep bleeds (both ICH and CMBs), respectively. Lacunes were evaluated in the supratentorial area and classified as lobar or classical deep based on the location. A subgroup of 36 patients also underwent PiB-PET to measure cerebral amyloid deposition and global standardized uptake value ratio (SUVR) were calculated.
Results
Lobar lacunes were more frequent in CAA-ICH than HTN-ICH (29.2 vs 11.6%, p=0.036). In multivariable models, lobar lacunes were associated with lobar CMB (Odds ratio 6.8, 95 % confidence interval 1.6-29.9, p=0.011) after adjustment for age, sex, hypertension and WMH. In 15 CAA-ICH and 21 HTN-ICH patients with PiB PET, correlation analyses between lobar lacune counts and global SUVR showed positive association (ρ=0.40, p=0.02) and remained significant after adjustment for age (r=0.34, p=0.04).
Conclusion
Our findings expand upon recent work showing that lobar lacunes are more frequent in CAA-ICH than HTN-ICH. Their independent association with lobar CMBs and brain amyloid deposition suggests a relationship with CAA even in an Asian cohort with overall higher hypertensive load.
INTRODUCTION
Lacunar infarcts have been regarded as one of the imaging features for cerebral small vessel disease (SVD).1 Recently, the distribution of lacune location, similar to cerebral microbleed (CMB) topography, has been linked with different underlying SVD in whites with primary intracerebral hemorrhage (ICH).2 Lacunes located in centrum-semiovale (CSO) or lobar regions, collectively called lobar lacunes were more frequent in cerebral amyloid angiopathy (CAA), while lacunes located in deep locations (basal ganglia, internal capsule, caudate, thalamus, pons) were more frequent in hypertensive SVD (HTN-SVD).2 This important finding needs to be validated in other cohorts that include different ethnic backgrounds. To confirm that lobar lacunes can be regarded as a potential marker of CAA is even more important in Asians, in whom the burden of SVD, especially the prevalence of lacunes and CMBs, was suggested to be higher than whites.3
In this study, we hypothesized that lobar lacunes were associated with core CAA markers such as lobar CMBs in Asian ICH patients. In a subgroup of patients who also received 11C-Pittsburgh Compund B (PiB) PET as a marker of cerebral amyloid deposition, we also tested the hypothesis that lobar lacunes correlate with cerebral amyloid load.
METHODS
We retrospectively collected patients of primary ICH from September 2014 to March 2017 at National Taiwan University Hospital (Supplementary figure I). Patients were classified as CAA-ICH or HTN-ICH according to the presence of strictly lobar (per modified Boston criteria) or strictly deep bleeds (both ICH and CMBs). Neuroimaging markers of SVD were rated according to STRIVE criteria.4 Lacunes were defined as “round or ovoid, subcortical, fluid-filled (similar signal as CSF) cavity, between 3 mm and 15mm in diameter”.2, 4 We classified supratentorial lacunes as lobar (when located in CSO, frontal, parietal, insular/subinsular, temporal, and occipital lobes) or deep (when located in thalamus, basal ganglia, caudate, internal and external capsule) (Figure 1A and 1C).2 Detailed description of patient selection, MRI and PET analyses is reported in in the supplementary material. The authors declare that all supporting data are available within the article/online-only Data Supplement. This study was performed with the approval of and in accordance with the guidelines of the institutional review board and with the informed consent of all subjects or their family members.
Figure 1. Figure representatives of lacune location and amyloid scan findings.
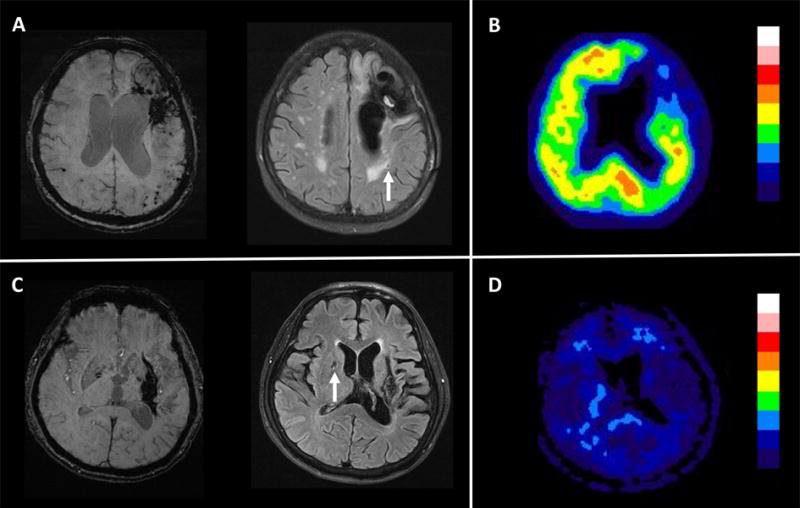
(A and B) Patient with CAA-ICH. (A) SWI showed left frontal ICH and numerous microbleeds restricted to lobar regions. Fluid-attenuated MRI sequence showing a lobar lacune (arrow). (B) PiB PET showed increased cortical amyloid retention (SUVR=1.43). (C and D) Patient with HTN-ICH. (C) SWI showed a left putaminal ICH without additional microbleed. Fluid-attenuated MRI sequence showed one deep lacune in the basal ganglia (arrow) (D) PiB PET showed absence cortical amyloid retention (SUVR=1.01).
RESULTS
Table 1 presents the comparison of the baseline characteristics between CAA-ICH and HTN-ICH patients. Presence of lobar lacunes was more common in CAA-ICH than in HTN-ICH (29.2% vs 11.6%, p=0.036), while deep lacunes were equally prevalent in patients with CAA-ICH and HTN-ICH (16.7% vs 19.8%, p=0.733). The prevalence of lobar CMB was higher in patients with lobar lacunes vs. without lobar lacunes (41.2% vs. 8.6%, p<0.001), while the prevalence of deep CMB was higher in patients with deep lacunes vs. without deep lacunes (61.9% vs. 35.9%, p=0.03). In univariable analyses, lobar lacunes were also associated with older age, higher WMH volume and CAA-ICH (Supplementary table I). In multivariable regression model, lobar lacunes remained independently associated with the presence of lobar CMB after adjustment for age, sex, hypertension, and WMH volume (Odds ratio 6.8, 95 % confidence interval 1.6-29.9, p=0.011).
Table 1.
Demographics and image characteristics in patients with CAA and hypertensive ICH.
| CAA-ICH (n=24) | HTN-ICH (n=86) | |
|---|---|---|
| Female, n (%) | 13 (54.2%) | 29 (33.7%) |
| Age, mean (SD), y | 70.9 ± 13.9 | 59.3 ± 13.0§ |
| Hypertension, n (%) | 15 (62.6%) | 81 (94.2%)§ |
| Diabetes | 2 (8.3%) | 21 (24.4%) |
| Dyslipidemia, n (%) | 5 (20.8%) | 31 (36.0%) |
| eGFR, mean (SD), mL/min/1.73m2 | 96.6 ± 31.2 | 95.2 ± 36.0 |
| Previous ischemic stroke, n (%) | 2 (8.3%) | 8 (9.3%) |
| Previous ICH, n (%) | 4 (16.7%) | 3 (3.4%)* |
| LVH, n (%) | 1 (4.8%) | 18 (21.5%) |
| Cerebral microbleed | ||
| Presence of lobar CMB, n (%) | 15 (62.5%) | 0 (0%)§ |
| Presence of deep CMB, n (%) | 0 (0%) | 45 (51.1%)* |
| White matter hyperintensity | ||
| Fazekas scale ≥ 2, n(%) | 17 (70.8%) | 55 (64.0%) |
| Volume, median (IQR), mL | 19.5 (3.5-27.2) | 6.2 (1.9-18.9) |
| Cortical superficial siderosis, n (%) | 4 (16.7%) | 1 (1.2%)* |
| Enlarged perivascular space (≥20) | ||
| Centrum Semiovale, n (%) | 9 (37.5%) | 23 (26.7%) |
| Basal ganglia, n (%) | 6 (25%) | 16 (18.6%) |
| Presence of any lacune, n (%) | 10 (41.7%) | 23 (26.7%) |
| Presence of lobar lacune, n (%) | 7 (29.2%) | 10 (11.6%)* |
| Lobar lacune count, median (IQR) | 0 (0-1) | 0 (0-0) * |
| Presence of deep lacune, n (%) | 4 (16.7%) | 17 (19.8%) |
| Deep lacune count, median (IQR) | 0 (0-0) | 0 (0-0) |
P-value <0.05,
P-value < 0.001, all other comparisons not significant.
CAA: Cerebral amyloid angiopathy; CMB: Cerebral microbleed; eGFR: Estimated glomerular filtration rate;
HTN: Hypertension; ICH: Intracerebral hemorrhage; LVH: Left ventricular hypertrophy
In 15 CAA-ICH and 21 HTN-ICH patients with PiB PET scans, the amyloid retention was higher in CAA-ICH compared to HTN-ICH patients (median SUVR: 1.26 [1.03-1.57] vs. 1.09 [1.00-1.16], p=0.018) (Supplementary table II). Figure 1 shows representative images of PiB PET in two patients with CAA-ICH and HTN-ICH respectively. Correlation analyses between lobar lacune counts and global SUVR showed positive association (ρ=0.40, p=0.02) and remained significant after adjustment for age (r=0.34, p=0.04).
DISCUSSION
Our study confirms the recently described association between lobar lacunes and CAA and the first one that including an Asian population that also received amyloid PET imaging. The prevalence of lacunar infarcts is reported to be very common in Asians whereas research into its underlying etiologies (HTN-SVD vs CAA) has been scarce in that particular population. We analyzed one hundred and ten Asian patients with primary ICH, and found that lobar lacunes were more frequent in CAA-ICH than HTN-ICH. In addition, lobar lacunes were significantly related to lobar CMB, the key imaging marker of CAA.5 The number of lobar lacunes correlated with the cerebral amyloid load, suggesting a direct association between lobar lacunes and amyloid angiopathy.
Our results confirm and expand on recent data showing that lobar lacunes are related to vascular amyloid deposition in patients with primary ICH. The overall prevalence of lobar lacunes seemed to be higher in our patients compared to previous report.2 This result is consistent with the observations that Asian patients tend to have a higher overall SVD load.3 Surprisingly, deep lacunes were equally prevalent in our patients with CAA and HTN-ICH (16.7% vs 19.8%), which could represent consequences of older age and higher frequency/severity of hypertension in our patient population. The influence of hypertensive SVD on CAA should thus be seriously considered but is beyond the scope of current study.
Our study had some limitations. First, we excluded patients who had mixed cerebral hemorrhage in both lobar and deep regions. The predominant etiology of ICH in these patients is mainly attributed to HTN-SVD, but the actual etiology is more difficult to determine with certainty when coexisting different vascular pathologies are a consideration.6, 7 Second, in order to have more patients with amyloid PET, we included patients treated in outside hospitals during the acute phase of ICH (n=20). Including these patients enabled us to test our hypothesis of association between lobar lacunes and CAA using the gold standard technique of in vivo amyloid imaging. We performed sensitivity analyses excluding this group to prevent any bias that might result from referral or early management practices. The significant findings did not change.
In conclusion, our study showed that lobar lacunes were more frequent in Asian patients with CAA-related strictly lobar hemorrhages, and that they significantly correlated with lobar CMBs. The correlation between lobar lacune counts and brain amyloid load supports the proposed relationship between CAA pathology and lobar lacunes formation. Further work should aim to clarify the physiopathological mechanisms of lacunar infarction in patients with CAA, with a particular emphasis on vascular dysfunction as a potential mediator.
Supplementary Material
Acknowledgments
Sources of funding: This work was supported by grants from National Taiwan University Hospital (Yen, NTUH-104-N01), Ministry of Science and Technology, Taiwan (Yen, MOST-106-2314-B-002 -066 -) and National Institute of Health (Gurol, NS083711).
Footnotes
Disclosures: None.
References
- 1.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013;12:483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasi M, Boulouis G, Fotiadis P, Auriel E, Charidimou A, Haley K, et al. Distribution of lacunes in cerebral amyloid angiopathy and hypertensive small vessel disease. Neurology. 2017;88:2162–2168. doi: 10.1212/WNL.0000000000004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau KK, Li L, Schulz U, Simoni M, Chan KH, Ho SL, et al. Total small vessel disease score and risk of recurrent stroke: Validation in 2 large cohorts. Neurology. 2017;88:2260–2267. doi: 10.1212/WNL.0000000000004042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Etten ES, Auriel E, Haley KE, Ayres AM, Vashkevich A, Schwab KM, et al. Incidence of symptomatic hemorrhage in patients with lobar microbleeds. Stroke. 2014;45:2280–2285. doi: 10.1161/STROKEAHA.114.005151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yakushiji Y, Yokota C, Yamada N, Kuroda Y, Minematsu K. Clinical characteristics by topographical distribution of brain microbleeds, with a particular emphasis on diffuse microbleeds. J Stroke Cerebrovasc Dis. 2011;20:214–221. doi: 10.1016/j.jstrokecerebrovasdis.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Pasi M, Charidimou A, Boulouis G, Auriel E, Ayres A, Schwab KM, et al. Mixed-location cerebral hemorrhage/microbleeds: Underlying microangiopathy and recurrence risk. Neurology. 2018;90:e119–e126. doi: 10.1212/WNL.0000000000004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


