Abstract
Organophosphate esters (OPEs) are often used as flame retardants and plasticizers. Animal data suggest exposure OPEs could impact children’s growth and development, yet impacts on human birth outcomes are understudied. We evaluate impacts of OPE exposure on the timing of delivery and infant’s birthweight in the Pregnancy Infection and Nutrition Study (PIN). North Carolina women enrolled in PIN in early pregnancy and conducted follow-up through delivery. Analyses were limited to mothers recruited 2002–2005, whose children participated in follow-up (n=349). Mothers collected urine samples in which OPE metabolites were assessed and birth outcomes were abstracted from medical records. Bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), diphenyl phosphate (DPHP), isopropyl-phenyl phenyl phosphate (ip-PPP), bis(1-chloro-2-propyl) 1-hydroxy-2-propyl phosphate (BCIPHIPP) were detected in >80% of samples. Average birthweight and gestational age were 3326 g and 39.1 weeks, respectively. As data suggest that the mechanisms of action by which OPEs impact birth outcomes may be fetal sex dependent, we conducted sex-stratified statistical analyses. Women with the highest ip-PPP concentrations delivered girls 1 week earlier than women with lower levels (95% Confidence Interval (CI): −1.9, −0.2). Women with BDCIPP levels above the median had 3.99 (95% CI: 1.08, 14.78) times the odds of delivering their daughters preterm. Similarly, higher ip-PPP levels were associated with lower birthweight, but not after standardizing for gestational age. Among males, maternal ip-PPP was associated with decreased odds of preterm birth (OR=0.21, 95% CI: 0.06, 0.68). DPHP and BCIPHIPP levels were not associated with outcomes in either sex. Results indicate that prenatal OPE exposure may impact timing of birth, though results are imprecise. Given the widespread OPEs exposure and the urgent need to identify and mitigate causes of preterm birth, further investigation is warranted.
Introduction
Organophosphate compounds are commonly detected in indoor environments, owing in part to their widespread use as flame retardant chemicals (FRs). For example, tris (1,3-dichloropropyl) phosphate (TDCIPP) is commonly detected in polyurethane foam samples from residential furniture and baby products (e.g. car seats and nursing pillows 1–4). Other organophosphates, including triphenyl phosphate (TPHP) are used as FRs in furniture, electronics, and construction materials and are also used in other applications as plasticizers.1,5–9 Organophosphate esters (OPEs) are not chemically bound to the products in which they are used and as a result they are predisposed to migrate into the environment, chronically exposing the vast majority of the general population. OPEs are thought to be metabolized relatively quickly by the human body (t½~hours)9–11 and measures of urinary metabolites are used as indicators of exposure. Numerous studies demonstrate that >90% of the population has detectable levels of OPE metabolites in their urine, e.g. 12–15 and some data indicate that pregnant women may have higher levels of OPE metabolites in their urine.12 Data suggest OPEs can be transferred from the mother to her child in utero. 16–18 TPHP was detected in placenta samples of dose rats and both TDCIPP and TPHP have been detected in human placenta samples.17,18 OPEs also have been detected in human chronic villi and deciduae, suggesting transfer early in gestation (i.e. prior to the formation of a mature placenta).16
Toxicological studies suggest that exposure to OPEs may affect reproductive health and children’s early-life growth and development.19–32 For example, chicken embryos exposed to TDCIPP were observed to have a 7% decrease in weight at hatching29 and prenatal exposure to TDCIPP has been shown to increase the number of visibly small rat pups (i.e. runt pups) and significantly impacted weight gain through weaning.30 In addition, perinatal exposure to Firemaster® 550, a flame retardant mixture containing organophosphate [TPHP and isopropylated triaryl phosphates (ITPs)] and brominated compounds (2-ethylhexyl-2,3,4,5-tetrabromobenzoate and bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate) has been associated with rapid weight gain in rat pups and obesity in adult rats, raising concern about potential impacts of FM 550’s components, including OPEs on early growth.19 Gestational duration and potential impacts of OPEs on preterm birth risk have not been investigated in toxicological studied, due in part to the tightly controlled timing of parturition in most animal species. Epidemiologic data investigating potential reproductive and development impacts of OPE exposure are limited. Endocrine disruption has been reported 33–35, suggesting a possible mechanism by which OPEs could alter fetal growth or impact the gestational duration. In the only study of human birth outcomes, OPE metabolite concentrations were negatively associated with pregnancy outcomes (i.e. proportions of successful fertilization, implantation, clinical pregnancy, and live birth) among women undergoing fertility treatment urinary.36
We hypothesize that prenatal exposure to OPEs alters the timing of labor or fetal growth. Using data from the Pregnancy Infection and Nutrition Study (PIN) we test this hypothesis by investigating potential associations between biomarkers of prenatal OPE exposures and birth outcomes (i.e. gestational timing and preterm birth as well as birthweight-for-gestational age). Given the demonstrated male vulnerability to prenatal environmental exposures, the sex-specific adaptation of the placenta, and established differences in the impact of endocrine perturbations by sex, we evaluated the potential for OPEs to impact birth outcomes in a sex specific manner 37–40. To our knowledge, these are the first data investigating relationships between OPEs and fetal growth and the timing parturition.
Methods
Study population
The PIN Study enrolled a cohort of central North Carolina women in early pregnancy and conducted follow-up through delivery.41 Pregnant women were recruited from the University of North Carolina prenatal care clinic, and delivered their infants at University of North Carolina hospitals between 2001 and 2006 (n=2006). The analysis sample for this work is limited to children who were singleton births, free from major birth defects, and whose mothers provided a prenatal urine sample. In addition, as this work is part of a larger project investigating post-natal development, urine samples were only analyzed from mothers with children participating in growth follow-up after birth as a part of the PIN Babies Study. PIN Babies began in January 2004 to follow the children of women participating in the later years of the PIN study through 3 years of age (n = 585 with n = 349 having growth information and included in the present analyses). Questionnaires and medical records were used to collect information about the pregnancy.41 All study protocols were approved by the institutional review board at the University of North Carolina at Chapel Hill and all mothers provided informed consent prior to completing any study activities.
OPE analysis
Urine collection and OPE analyses methods have been described in detail for these women previously.42 In brief, during the late-second or early-third trimester (gestational week 24–30), women collected a spot urine sample which was aliquoted into polyethylene storage tubes and frozen at −80° C until analysis. Levels of six OPE metabolites were assessed in urine samples: BDCIPP, diphenyl phosphate (DPHP), isopropyl-phenyl phenyl phosphate (ip-PPP), bis(1-chloro-2-propyl) 1-hydroxy-2-propyl phosphate (BCIPHIPP), bis(2-chloro-isopropyl) phosphate (BCIPP), and tert butyl-phenyl phenyl phosphate (tb-PPP). Samples were extracted and analyzed as previously described.42–44
Specific gravity (SG) was measured in each urine sample prior to analysis using a digital handheld refractometer (Atago). To investigate the impacts of differences in urine dilution on results, we conducted analyses of urinary metabolites using raw OPE metabolite measures as well as using SG-corrected concentrations.45 Corrected and uncorrected concentrations were very highly correlated (rs>0.82 for all metabolites) and results were very similar using both methods. Here we present only the results obtained with the SG corrected concentrations to facilitate comparison with other study populations.
Birth outcomes
Gestational age in days was estimated using a combination of last menstrual period and earliest-ultrasounds data. When both measures were available and agreed within 14 days, the last menstrual period was used to assign gestational age; otherwise, ultrasound data were used. Birthweight was obtained from the medical record. Sex-specific birthweight-for-gestational age z-scores were assigned using a calculator based on the INERTGROWTH-21st standards.46,47 In addition to continuous measures of gestational age, we used preterm birth (defined as <37 weeks gestation) as an outcome in analyses.
Statistical analyses
Previous analyses indicated that urinary OPE metabolite levels were positively skewed.42 Relationships between OPEs and outcomes were evaluated when detection frequencies of metabolites were >80%. For these metabolites, samples with concentrations below the method limit of detection (MDL) were replaced with the MDL/2 prior to adjustment for SG.
We used multivariable regression models to assess relationship between OPEs and continuous measures of gestational duration and birthweight-for-gestational age z-scores. To allow for more flexible relationships between OPEs and birth outcomes, models were constructed with quartiles of OPE exposure. Birth outcome models were adjusted for maternal age, race, education, pre-pregnancy BMI, parity and season of urine samples collection, factors which we have previously shown are related to urinary OPE metabolite concentrations in this population and which may be related to fetal growth and development. 42 We also considered potential confounding by maternal smoking during pregnancy; however, smoking data were missing for 13 women. Among women with complete data, the inclusion of maternal smoking during pregnancy (yes or no in months 1–6 of pregnancy) did not alter associations or conclusions. Thus, we have chosen to present associations which do not adjust for smoking to preserve our larger sample size.
We additionally investigated relationships between OPEs and dichotomous preterm birth using logistic regression models. Although our sample size was relatively large, preterm birth impacted only a small number of infants (n=43). To avoid over fitting models with limited data, dichotomized categories of exposure (above vs below the median) were used in preterm birth models rather than quartiles.
We hypothesize the potential pathway by which OPEs may impact birth could vary based on the sex of the child. In preliminary analyses we included a multiplicative interaction term (exposure * sex) in statistical models. We considered p<0.2 statistically suggestive of a sex difference the impact of OPE exposures. Based on interaction analyses, which suggested differences in associations by sex for several OPE metabolites (i.e. interaction p<0.2) and the prior literature suggesting sex-specific differences in the impact of endocrine disrupting chemicals on birth outcomes e.g. 40,48, we stratified all analyses to investigate sex-specific impacts of exposure. All analyses were conducted in SAS (Version 9.4; SAS Institute Inc, Cary, NC).
Results
Mothers averaged 29.6 years of age at the time of enrollment and were highly educated, with nearly 70% having a college education (Table 1). This was the first pregnancy for approximately half of the women included in analyses (47.6%); and the majority (55.6%) had a BMI within the normal range at the start of their pregnancy. Women included in the present study (Table 1) were more likely to be white, have higher educational attainment, and be older than mothers in the larger PIN cohort.41,42,49 Infants were born at an average of 39.1 weeks gestation (range 29.0–42.6 weeks; standard deviation 1.84 weeks) and weighed an average of 3326 grams at birth (range 1422–4760 grams; standard deviation 526 grams). Birthweight-for-gestational age z-scores averaged 0.36 (range −2.02, 3.27; standard deviation 0.93). Women included in these analyses tended to be older and more highly educated than the PIN population as a whole and a greater percentage reported white race. A slightly smaller percentage of children in our sample were born preterm (12.3% vs. 14.6%) and a slightly higher percentage of children were born with low birthweight (9.9% vs 10.6%) than in the larger PIN Study cohort.
Table 1.
Selected characteristics of 349 pregnant North Carolina women and their respective children (2002–2005).
| N (%) | |
|---|---|
| Total | 349 (100) |
| Age (years) | |
| ≤25 | 76 (21.8) |
| 26–30 | 126 (36.1) |
| 31–35 | 107 (30.7) |
| ≥36 | 40 (11.5) |
| Race | |
| white | 278 (79.7) |
| non-white | 71 (20.3) |
| Education (years) | |
| ≤15 | 106 (30.4) |
| ≥16 | 243 (69.6) |
| Parity | |
| 0 | 166 (47.6) |
| ≥1 | 183 (52.4) |
| Pre-pregnancy BMI | |
| BMI<18.5 - Underweight | 46 (13.2) |
| 18.5≤BMI<24.9 - Normal range | 194 (55.6) |
| 24.9≤BMI<29.9 - Overweight | 42 (12.0) |
| 29.9≤BMI - Obese | 67 (19.2) |
| Smoking During Pregnancy | |
| Yes | 29 (8.3) |
| No | 307 (88.0) |
| Missing | 13 (3.7) |
| Sex of the Child | |
| Male | 192 (55.0) |
| Female | 157 (45.0) |
Table 2 provides a summary of urinary OPE metabolite concentrations during pregnancy. Four urinary OPE metabolites, BDCIPP, DPHP, ip-PPP and BCIPHIPP, were detected in >83% of samples and concentrations varied considerably between women but distributions were similar for male and female infants. Additional analyses were not conducted for BCIPP and tb-PPP because they were detected infrequently in these samples (2.00–48.70% detection).
Table 2.
Detection frequency, geometric mean and quartile cut points (ng/mL) for urinary OPE metabolites (N=349).
| Metabolite | % Detect | GMa | 25% Percentile | Median | 75th Percentile | Maximum |
|---|---|---|---|---|---|---|
| BCPP | 48.70 | -- | -- | -- | 1.11 | 6.10 |
| BDCIPP | 92.80 | 1.80 | 0.81 | 1.85 | 3.62 | 140 |
| DPHP | 83.70 | 1.42 | 0.79 | 1.31 | 2.67 | 112 |
| ip-PPP | 99.40 | 6.80 | 4.25 | 7.06 | 10.9 | 69.0 |
| tb-PPP | 2.00 | -- | -- | -- | -- | 8.61 |
| BCIPHIPP | 98.30 | 0.51 | –0.24 | –0.42 | 0.82 | 98.0 |
GM: geometric mean
Birth Outcomes
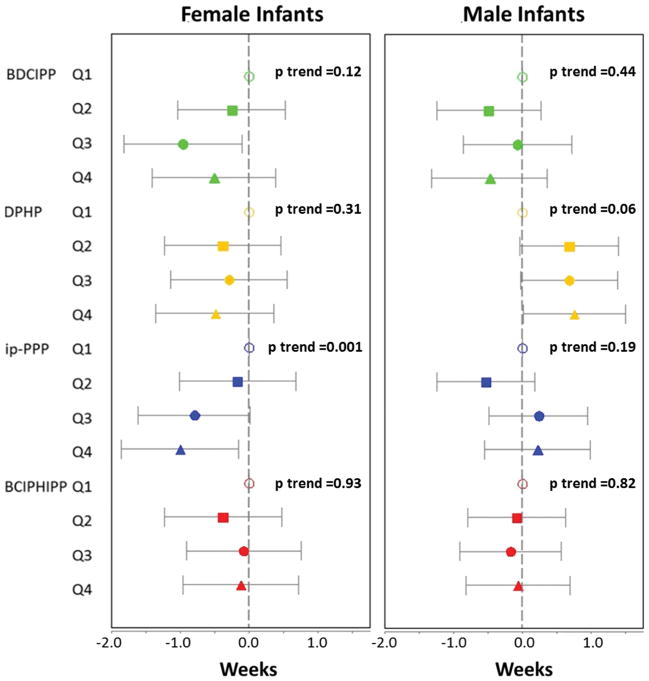
Patterns of association between prenatal OPE metabolites and birth outcomes varied by infant sex. Female infants in the highest quartile of exposure to ip-PPP were born 1 week earlier than those in the first quartile (β=−1.00 week; 95% confidence interval (CI): −1.85, −0.15 weeks; p=0.02; Figure 1 and Supplemental Table 1). While similar patterns were observed for DPHP and BDCIPP, associations did not reach statistical significance. Among male infants, DPHP was associated with a modest increase in gestational duration. For example, baby boys with the highest levels of prenatal exposure were born approximately 5 days later than those with the lowest levels of exposure (β=0.75 weeks; 95% CI: 0.01, 1.50 weeks; p=0.05). Other metabolites were generally not associated with gestational age at delivery among males (i.e. associations were imprecisely estimated, did not follow a consistent pattern across the exposure gradient, and were not statistically significant).
Figure 1.
Mean difference in gestational age at the time of delivery by quartile of OPE exposure (indicated by maternal urinary metabolite concentrations). Analyses adjusted for maternal age, race, education, parity, pre-pregnancy BMI and season of urine sample collection. A table format of these data is shown in Supplemental Table 1.
Additional analyses were conducted to assess relationship between OPE metabolite levels and the odds of preterm birth. Among females, maternal ip-PPP and BDCIPP concentrations were associated with increased odds of preterm birth (Table 3). For example, women with ip-PPP concentrations above the median were 4.58 times as likely to deliver daughters preterm compared to those with lower maternal levels of ip-PPP (95% CI: 1.23, 17.06). Conversely, maternal urinary ip-PPP concentrations were associated with decreased odds of preterm birth among male infants (odds ratio (OR)=0.21; 95% CI: 0.06, 0.68).
Table 3.
Odds ratio for preterm birth (<37 weeks gestation) comparing maternal OPE metabolite levels above the median (Quartile 3+Quartile 4) to those below (Quartile 1+ Quartile 2). Analyses adjusted for maternal age, race, education, parity, pre-pregnancy BMI and season of urine sample collection.
| Female Infants N=19 Preterm |
Male Infants N=24 Preterm |
|||
|---|---|---|---|---|
| OPE Metabolite | OR | 95% CI | OR | 95% CI |
| BDCIPP | 3.99 | 1.08, 14.78 | 0.76 | 0.25, 2.32 |
| DPHP | 1.11 | 0.37, 3.38 | 0.46 | 0.17, 1.25 |
| ip-PPP | 4.58 | 1.23, 17.06 | 0.21 | 0.06, 0.68 |
| BCIPHIPP | 0.64 | 0.20, 2.02 | 1.06 | 0.40, 2.82 |
Similar patterns were observed between OPE metabolites and birthweight (among all births; Table 4); however we found little evidence for associations between prenatal exposure to OPEs and birthweight-for-gestational age z-scores for either male or female infants suggesting any growth impacts were driven by shorter gestational duration (Table 5). Among females, z-scores decreased with increasing ip-PPP quartile, but all 95% confidence intervals crossed the null. No clear patterns were observed for other metabolites or among males.
Table 4.
Mean difference in birthweight by quartile of OPE exposure (indicated by maternal urinary metabolite concentrations). Analyses adjusted for maternal age, race, education, parity, pre-pregnancy BMI and season of urine sample collection.
| OPE Metabolite | Female Infants | Male Infants | |||
|---|---|---|---|---|---|
| Mean Difference (95% CI) | p-value | Mean Difference (95% CI) | p-value | ||
| BDCIPP | Q1 | Reference | -- | Reference | -- |
| Q2 | 103 (−136, 342) | 0.40 | −127 (−356, 102) | 0.28 | |
| Q3 | −16 (−277, 244) | 0.90 | −73 (−311, 166) | 0.55 | |
| Q4 | 43 (−231, 318) | 0.76 | −66 (−318, 186) | 0.61 | |
| trend | 0.91 | trend | 0.76 | ||
| DPHP | Q1 | Reference | -- | Reference | -- |
| Q2 | −109 (−363, 145) | 0.40 | 44 (−174, 262) | 0.69 | |
| Q3 | −94 (−346, 158) | 0.46 | 89 (−122, 300) | 0.41 | |
| Q4 | −158 (−411, 95) | 0.22 | 117 (−107, 341) | 0.31 | |
| trend | 0.35 | trend | 0.26 | ||
| ip-PPP | Q1 | Reference | -- | Reference | -- |
| Q2 | −66 (−318, 186) | 0.61 | −100 (−314, 113) | 0.35 | |
| Q3 | −243 (−488, 2) | 0.05 | 44 (−173, 261) | 0.69 | |
| Q4 | −348 (−601, −94) | 0.01 | 21 (−211, 252) | 0.86 | |
| trend | 0.005 | trend | 0.51 | ||
| BCIPHIPP | Q1 | Reference | -- | Reference | -- |
| Q2 | −76 (−334, 181) | 0.56 | −168 (−377, 42) | 0.12 | |
| Q3 | −90 (−342, 162) | 0.48 | −56 (−273, 161) | 0.61 | |
| Q4 | −107 (−361, 146) | 0.40 | −8 (−229, 213) | 0.94 | |
| trend | 0.33 | trend | 0.72 | ||
Table 5.
Mean difference in birthweight-for-gestational age z-scores by quartile of OPE exposure (indicated by maternal urinary metabolite concentrations). Analyses adjusted for maternal age, race, education, parity, pre-pregnancy BMI and season of urine sample collection.
| OPE Metabolite | Female Infants | Male Infants | |||
|---|---|---|---|---|---|
| Mean Difference (95% CI) | p-value | Mean Difference (95% CI) | p-value | ||
| BDCIPP | Q1 | Reference | -- | Reference | -- |
| Q2 | 0.31 (−0.10, 0.73) | 0.14 | −0.05 (−0.45, 0.35) | 0.80 | |
| Q3 | 0.46 (0.00, 0.91) | 0.05 | −0.13 (−0.54, 0.29) | 0.54 | |
| Q4 | 0.31 (−0.17, 0.79) | 0.20 | 0.05 (−0.39, 0.48) | 0.83 | |
| trend | 0.91 | trend | 0.76 | ||
| DPHP | Q1 | Reference | -- | Reference | -- |
| Q2 | −0.15 (−0.60, 0.29) | 0.50 | −0.17 (−0.55, 0.20) | 0.36 | |
| Q3 | −0.08 (−0.52, 0.37) | 0.73 | −0.05 (−0.41, 0.32) | 0.80 | |
| Q4 | −0.15 (−0.59, 0.30) | 0.52 | −0.02 (−0.41, 0.37) | 0.91 | |
| trend | 0.35 | trend | 0.26 | ||
| ip-PPP | Q1 | Reference | -- | Reference | -- |
| Q2 | −0.15 (−0.61, 0.30) | 0.51 | 0.00 (−0.37, 0.37) | 0.98 | |
| Q3 | −0.28 (−0.72, 0.16) | 0.22 | 0.00 (−0.38, 0.37) | 0.98 | |
| Q4 | −0.37 (−0.82, 0.09) | 0.12 | −0.03 (−0.44, 0.37) | 0.87 | |
| trend | 0.005 | trend | 0.51 | ||
| BCIPHIPP | Q1 | Reference | -- | Reference | -- |
| Q2 | −0.11 (−0.56, 0.34) | 0.63 | −0.40 (−0.76, −0.04) | 0.03 | |
| Q3 | −0.22 (−0.67, 0.22) | 0.32 | −0.13 (−0.51, 0.24) | 0.48 | |
| Q4 | −0.14 (−0.59, 0.30) | 0.53 | −0.02 (−0.40, 0.36) | 0.90 | |
| trend | 0.33 | trend | 0.72 | ||
Discussion
The vast majority of pregnant women included in our study population had detectable levels of several OPE metabolites in samples of their urine. Our results suggest that prenatal exposure to some of these OPEs could be associated with altered timing of birth. Among girls, patterns suggested decreased gestational duration and increased odds of preterm (birth at <37 weeks gestation), particularly for ip-PPP and BDCIPP. Clinical preterm birth and decreased gestational duration--even among full-term infants--is associated with increased risk of numerous adverse health outcomes.50–53 In addition to being the primary cause of mortality in the first year of life, preterm birth has been associated with poorer neurodevelopment, respiratory disease, cardiovascular disease and metabolic disorders later in life. 50–58
Conversely, our results suggest that maternal DPHP concentrations may be associated with increased gestational duration among male infants. Although increased gestational duration is generally considered beneficial, post-term birth (occurring after 42 weeks gestation) carries health risks for both the infants and mother.59 Although boys with higher levels of exposure to DPHP were born later, births at 42 weeks gestation or after were extremely uncommon in this cohort, potentially because included mothers had access to medical care and interventions to prevent post-term birth.
Reasons for differences in the pattern of association between males and females are unclear. In general, male infants are more likely to be born preterm than females, which has been attributed to a variety of factors, including the relatively greater weight of male infants at earlier gestational ages, increased susceptibility to infection or pregnancy complication among women carrying male fetuses, or differential impacts of environmental exposure by fetal sex.60–64 However, our findings suggest that female infants may be more susceptible to OPE exposure, at least with respect to preterm birth. Recent work investigating brominated flame retardants suggests that the placenta responds differently to exposure to exogenous chemicals based on the sex of the infant. 65 Placenta samples associated with male infants had higher concentrations of brominated flame retardants, despite no differences in maternal serum concentrations. Impacts on thyroid hormones and metabolic enzymes in placenta also differed by sex, suggesting the placenta may mediate the impacts of environmental chemicals. 65 Other research demonstrates that exposure to phthalates is associated with molecular changes in placental tissue in sexually dimorphic ways. 66 Though OPEs have been reported in placental tissues16–18, it remains unclear whether sex specific impacts extend to OPEs or preterm birth.
Although patterns of association were similar for birthweight, associations appeared to be driven by differences in gestational age; birthweight-for-gestational age z-scores were not significantly associated with measures of OPE exposure. This suggests that impact of OPEs on birthweight is mediated by pregnancy duration and not decreased growth. To our knowledge, impacts of OPE exposure on preterm birth risk have not been investigated previously in a human population. Among chicken embryos, higher exposure to TDCIPP appeared to delay pipping time (i.e. number of hours of incubation required by the embryo to form a pipping star), although relationships were not statistically significant. 29 The same study demonstrated decreased body weight and a shorter head plus bill length among highly exposed chicks, suggesting that early exposure could impact growth and development.29 Past studies investigating impacts of gestational exposure in rodents have reported decreased birthweight with exposure to OPEs, but did not specifically considered the potential drivers of lower body weights (e.g. growth restriction or earlier birth). Differences in species and dosing complicate comparisons of our findings with these results. It is possible that our results are consistent with these studies; however, additional data using animal systems designed to investigate timing of birth is needed to elucidate potential relationships as are data from other human cohorts.
Given the potential relationships identified with altered gestational duration in this cohort, it is important to consider recent data suggesting that levels of exposure to OPE may have increased since 2002–2005 (when samples were collected in this cohort). We recently reported that urinary BDCIPP concentrations were 15 times higher in samples collected in 2015 than those collected in 2002.12 Increases in DPHP were less dramatic, but suggest that levels of exposure in the 2010s likely exceed those observed for the majority of women in the PIN cohort. Temporal trends for other OPE metabolites, including ip-PPP, have yet to be evaluated; however, urinary ip-PPP concentrations among the women in this cohort were substantially higher than those observed among women in the CHAMACOS cohort, a pregnancy cohort of Mexican American women from California giving birth 2000–2001 and providing urine samples at approximately the same gestational week as the women in the PIN cohort (i.e. geometric mean ip-PPP PIN=6.80 and CHAMACOS=0.33 ng/mL).67 There are certainly many differences between PIN and CHAMACOS which could explain differences in urinary ip-PPP and as such we caution against the over interpretation of higher urinary levels among PIN women.
Although our samples size was relatively large, there were only 43 preterm births in our cohort. As a result, we treated preterm birth as a single outcome which is likely an oversimplification of a complex outcome. McElrath et al. 2008 hypothesized that preterm birth could be divided into two sets of proximal causes, intrauterine inflammation (preterm labor, preterm membrane rupture, placental abruption, and cervical insufficiency) and abnormal placentation (preeclampsia and intrauterine growth restriction). 68 Investigating preterm birth as a heterogeneous outcome would require a large study population but could provide additional insights regarding the potential mechanisms leading to altered gestational timing and preterm birth.69
Our analyses are additionally limited by our reliance on a single spot urine sample to characterize exposure to OPEs during the prenatal period. Although our previous work among pregnant women suggests that spot urine BDCIPP and DPHP levels are moderately correlated throughout the course of pregnancy,70 the reliability of spot ip-PPP and BCIPHIPP concentrations has yet to be evaluated. In addition, the timing of the urine sample collection (between 24 and 30 weeks gestation) for OPE assessment could have resulted in some preterm births being excluded from these analyses. If higher levels of exposures are truly related to preterm birth, it is possible that the most impacted infants were excluded (reducing estimated odds ratios). The PIN women included in our current analyses are not representative of the general population (e.g. they were highly educated mainly non-Hispanic white women), suggesting that the generalizability of our results to other populations could be limited. However, we do not anticipate that this would limit the validity of our findings. In fact, we consider the homogeneity of our study population a possible strength as it may reduce the impact of unmeasured confounding. Nonetheless, residual confounding remains a possible explanation for our results. In addition, we used urinary metabolite levels as indicators of exposure to OPEs, an assumption that is more reasonable for some OPEs than others. For example, BDCIPP is a primarily metabolite of TDCIPP and is not known to be used in consumer products or applications. However, DPHP can be formed from metabolism of several organophosphate compounds, including TPHP, but is also produced and used in consumer products. Regardless of the source or pathway of exposure, our results suggest a relationship between measures of these compounds in urine and birth outcomes. Additional research is needed to understand sources of exposure for pregnant women as well as the potential health impacts of exposure among these women and their unborn children.
Conclusions
Cumulatively, our results indicate that prenatal OPE exposure may impact timing of birth, suggesting a possible link with life-long health. Given the widespread exposure to these compounds, and predicted increases in their use in the coming decades, further research investigating relationships is warranted.
Supplementary Material
Highlights.
Organophosphate ester metabolites were frequently detected in pregnant women’s urine.
BDCIPP and ip-PPP were associated with shorter gestation among female infants.
Urinary ip-PPP was associated with longer gestation among male infants.
OPEs were associated with birthweight; impacts appear driven by gestational length.
Acknowledgments
This research was supported in part by grants from the National Institute of Environmental Health Sciences (R21 ES023904and P30ES10126) and the U.S. Environmental Protection Agency (RD832736). The work of KH was funded in part by a training grant from the National Institute of Environmental Health Sciences (T32 ES007018).
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stapleton HM, Sharma S, Getzinger G, et al. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol. 2012;46(24):13432–13439. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stapleton HM, Klosterhaus S, Eagle S, et al. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol. 2009;43(19):7490–7495. doi: 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stapleton HM, Klosterhaus S, Keller A, et al. Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol. 2011;45(12):5323–5331. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper EM, Kroeger G, Davis K, Clark CR, Ferguson PL, Stapleton HM. Results from Screening Polyurethane Foam Based Consumer Products for Flame Retardant Chemicals: Assessing Impacts on the Change in the Furniture Flammability Standards. Environmental Science & Technology. 2016;50(19):10653–10660. doi: 10.1021/acs.est.6b01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stapleton HM, Klosterhaus S, Keller A, et al. Identification of Flame Retardants in Polyurethane Foam Collected from Baby Products. Environmental Science & Technology. 2011;45(12):5323–5331. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper EM, Kroeger G, Davis K, Clark CR, Ferguson PL, Stapleton HM. Results from Screening Polyurethane Foam Based Consumer Products for Flame Retardant Chemicals: Assessing Impacts on the Change in the Furniture Flammability Standards. Environmental science & technology. 2016;50(19):10653–10660. doi: 10.1021/acs.est.6b01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballesteros-Gomez A, Brandsma SH, de Boer J, Leonards PE. Analysis of two alternative organophosphorus flame retardants in electronic and plastic consumer products: resorcinol bis-(diphenylphosphate) (PBDPP) and bisphenol A bis (diphenylphosphate) (BPA-BDPP) Chemosphere. 2014;116:10–14. doi: 10.1016/j.chemosphere.2013.12.099. [DOI] [PubMed] [Google Scholar]
- 8.Kajiwara N, Noma Y, Takigami H. Brominated and organophosphate flame retardants in selected consumer products on the Japanese market in 2008. J Hazard Mater. 2011;192(3):1250–1259. doi: 10.1016/j.jhazmat.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 9.Mendelsohn E, Hagopian A, Hoffman K, et al. Nail polish as a source of exposure to triphenyl phosphate. Environ Int. 2016;86:45–51. doi: 10.1016/j.envint.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van den Eede N, Maho W, Erratico C. First Insights in the Metabolism of Phoosphate Flame Retardants and Plastucuzers Using Huan Liver Fractions. Toxicol Lett. 2013;223(1):9–15. doi: 10.1016/j.toxlet.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Carignan C, Fang M, Stapleton HM, Heiger-Bernays W, McClean MD, Webster TF. Urinary biomarkers of flame retardant exposure among collegiate U.S. gymnasts. Environ Int. 2016 doi: 10.1016/j.envint.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman K, Butt C, Webster T, et al. Temporal Trends in Exposure to Organophophate Flame Retardants in the United States. Environ Sci Technol Let. 2017;4(3):112–118. doi: 10.1021/acs.estlett.6b00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van den Eede N, Heffernan AL, Aylward LL, et al. Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ Int. 2015;74:1–8. doi: 10.1016/j.envint.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Cequier E, Sakhi AK, Marce RM, Becher G, Thomsen C. Human exposure pathways to organophosphate triesters - a biomonitoring study of mother-child pairs. Environ Int. 2015;75:159–165. doi: 10.1016/j.envint.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Romano ME, Hawley NL, Eliot M, et al. Variability and predictors of urinary concentrations of organophosphate flame retardant metabolites among pregnant women in Rhode Island. Environ Health. 2017;16(1):40. doi: 10.1186/s12940-017-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao FR, Chen M, Gao FM, Shen H, Hu JY. Organophosphorus Flame Retardants in Pregnant Women and Their Transfer to Chorionic Villi. Environmental Science & Technology. 2017;51(11):6489–6497. doi: 10.1021/acs.est.7b01122. [DOI] [PubMed] [Google Scholar]
- 17.Baldwin KR, Phillips AL, Horman B, et al. Sex Specific Placental Accumulation and Behavioral Effects of Developmental Firemaster 550 Exposure in Wistar Rats. Sci Rep-Uk. 2017:7. doi: 10.1038/s41598-017-07216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding JJ, Xu ZM, Huang W, Feng LM, Yang FX. Organophosphate ester flame retardants and plasticizers in human placenta in Eastern China. Science of the Total Environment. 2016;554:211–217. doi: 10.1016/j.scitotenv.2016.02.171. [DOI] [PubMed] [Google Scholar]
- 19.Patisaul HB, Roberts SC, Mabrey N, et al. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster(R) 550 in rats: an exploratory assessment. J Biochem Mol Toxicol. 2013;27(2):124–136. doi: 10.1002/jbt.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farhat A, Crump D, Chiu S, et al. In Ovo Effects of Two Organophosphate Flame Retardants--TCPP and TDCPP--on Pipping Success, Development, mRNA Expression, and Thyroid Hormone Levels in Chicken Embryos. Toxicol Sci. 2013 doi: 10.1093/toxsci/kft100. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Liang K, Liu J, et al. Exposure of zebrafish embryos/larvae to TDCPP alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic-pituitary-thyroid axis. Aquat Toxicol. 2013;126:207–213. doi: 10.1016/j.aquatox.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Ji K, Choi K. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat Toxicol. 2012;114–115:173–181. doi: 10.1016/j.aquatox.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Crump D, Chiu S, Kennedy SW. Effects of tris(1,3-dichloro-2-propyl) phosphate and tris(1-chloropropyl) phosphate on cytotoxicity and mRNA expression in primary cultures of avian hepatocytes and neuronal cells. Toxicol Sci. 2012;126(1):140–148. doi: 10.1093/toxsci/kfs015. [DOI] [PubMed] [Google Scholar]
- 24.Kawashima K, Tanaka S, Nakaura S, et al. Effect of phosphoric acid tri-esters flameretardants on the prenatal and postnatal developments of the rats. J Toxicol Sci. 1983;8(339) (Abstract) [Google Scholar]
- 25.Babich MA. Preliminary Risk Assessment of Flame Retardant (FR) Chemicals in Upholstered Furniture Foam. Bethesda, MD 20814: U.S. Consumer Product Safety Commission; 2006. [Google Scholar]
- 26.Ferrante J. Toxicity review of aromatic phosphate plasticizers. Bethesda, MD 20814: U.S. Consumer Product Safety Commission; 1999. [Google Scholar]
- 27.National Research Council (NRC) Toxicological Risks of Selected Flame-Retardant Chemicals. Subcommittee on Flame Retardant Chemicals, National Research Council, National Academy of Sciences. National Academy Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- 28.Welsh JJ, Collins TF, Whitby KE, Black TN, Arnold A. Teratogenic potential of triphenyl phosphate in Sprague-Dawley (Spartan) rats. Toxicol Ind Health. 1987;3(3):357–369. doi: 10.1177/074823378700300308. [DOI] [PubMed] [Google Scholar]
- 29.Farhat A, Crump D, Chiu S, et al. In Ovo effects of two organophosphate flame retardants--TCPP and TDCPP--on pipping success, development, mRNA expression, and thyroid hormone levels in chicken embryos. Toxicol Sci. 2013;134(1):92–102. doi: 10.1093/toxsci/kft100. [DOI] [PubMed] [Google Scholar]
- 30.Moser VC, Phillips PM, Hedge JM, McDaniel KL. Neurotoxicological and thyroid evaluations of rats developmentally exposed to tris(1,3-dichloro-2-propyl)phosphate (TDCIPP) and tris(2-chloro-2-ethyl)phosphate (TCEP) Neurotoxicol Teratol. 2015;52(Pt B):236–247. doi: 10.1016/j.ntt.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Mcgee SP, Cooper EM, Stapleton HM, Volz DC. Early Zebrafish Embryogenesis Is Susceptible to Developmental TDCPP Exposure. Environmental Health Perspectives. 2012;120(11):1585–1591. doi: 10.1289/ehp.1205316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu J, Han J, Zhou BS, et al. Toxicogenomic Responses of Zebrafish Embryos/Larvae to Tris(1,3-dichloro-2-propyl) Phosphate (TDCPP) Reveal Possible Molecular Mechanisms of Developmental Toxicity. Environmental Science & Technology. 2013;47(18):10574–10582. doi: 10.1021/es401265q. [DOI] [PubMed] [Google Scholar]
- 33.Preston EV, McClean MD, Claus Henn B, et al. Associations between urinary diphenyl phosphate and thyroid function. Environ Int. 2017;101:158–164. doi: 10.1016/j.envint.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meeker JD, Cooper EM, Stapleton HM, Hauser R. Urinary metabolites of organophosphate flame retardants: temporal variability and correlations with house dust concentrations. Environ Health Perspect. 2013;121(5):580–585. doi: 10.1289/ehp.1205907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meeker JD, Cooper EM, Stapleton HM, Hauser R. Exploratory analysis of urinary metabolites of phosphorus-containing flame retardants in relation to markers of male reproductive health. Endocrine Disruptors. 2013 doi: 10.4161/endo.26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carignan CC, Minguez-Alarcon L, Butt CM, et al. Urinary Concentrations of Organophosphate Flame Retardant Metabolites and Pregnancy Outcomes among Women Undergoing in Vitro Fertilization. Environmental Health Perspectives. 2017;125(8) doi: 10.1289/EHP1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vatten LJ, Skjaerven R. Offspring sex and pregnancy outcome by length of gestation. Early Hum Dev. 2004;76(1):47–54. doi: 10.1016/j.earlhumdev.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Voegtline KM, Costigan KA, Kivlighan KT, Henderson JL, DiPietro JA. Sex-specific associations of maternal prenatal testosterone levels with birth weight and weight gain in infancy. J Dev Orig Health Dis. 2013;4(4):280–284. doi: 10.1017/S2040174413000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clifton VL. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl):S33–39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Veiga-Lopez A, Kannan K, Liao C, Ye W, Domino SE, Padmanabhan V. Gender-Specific Effects on Gestational Length and Birth Weight by Early Pregnancy BPA Exposure. J Clin Endocrinol Metab. 2015;100(11):E1394–1403. doi: 10.1210/jc.2015-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.PIN. PIN — Pregnancy, Infection, and Nutrition Study. 2012 http://www.cpc.unc.edu/projects/pin.
- 42.Hoffman K, Lorenzo A, Butt CM, et al. Predictors of urinary flame retardant concentration among pregnant women. Environ Int. 2017;98:96–101. doi: 10.1016/j.envint.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van den Eede N, Neels H, Jorens PG, Covaci A. Analysis of organophosphate flame retardant diester metabolites in human urine by liquid chromatography electrospray ionisation tandem mass spectrometry. J Chromatogr A. 2013;1303:48–53. doi: 10.1016/j.chroma.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 44.Butt CM, Hoffman K, Chen A, Lorenzo A, Congleton J, Stapleton HM. Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environ Int. 2016;94:627–634. doi: 10.1016/j.envint.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54(10):615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- 46.INTERGROWTH- 21ST. [Accessed: October 26, 2017];Newborn Size. Available: https://intergrowth21.tghn.org/newborn-size-birth/#c4.
- 47.Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 48.Wolff MS, Engel SM, Berkowitz GS, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116(8):1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daniels JL, Pan IJ, Jones R, et al. Individual characteristics associated with PBDE levels in U.S. human milk samples. Environ Health Perspect. 2010;118(1):155–160. doi: 10.1289/ehp.0900759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359(3):262–273. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 51.MacDorman MF, Kirmeyer SE, Wilson EC. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2012. Fetal and Perinatal Mortality, United States, 2006. [PubMed] [Google Scholar]
- 52.Reddy UM, Bettegowda VR, Dias T, Yamada-Kushnir T, Ko CW, Willinger M. Term pregnancy: a period of heterogeneous risk for infant mortality. Obstet Gynecol. 2011;117(6):1279–1287. doi: 10.1097/AOG.0b013e3182179e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Souza JP, Gulmezoglu AM, Carroli G, Lumbiganon P, Qureshi Z, Group WR. The world health organization multicountry survey on maternal and newborn health: study protocol. BMC Health Serv Res. 2011;11:286. doi: 10.1186/1472-6963-11-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. New Engl J Med. 2008;359(3):262–273. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 55.Saigal S, Doyle LW. Preterm birth 3 - An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 56.Arpino C, Compagnone E, Montanaro ML, et al. Preterm birth and neurodevelopmental outcome: a review. Child Nerv Syst. 2010;26(9):1139–1149. doi: 10.1007/s00381-010-1125-y. [DOI] [PubMed] [Google Scholar]
- 57.Pike KC, Lucas JS. Respiratory consequences of late preterm birth. Paediatr Respir Rev. 2015;16(3):182–188. doi: 10.1016/j.prrv.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 58.Moss TJ. Respiratory consequences of preterm birth. Clin Exp Pharmacol Physiol. 2006;33(3):280–284. doi: 10.1111/j.1440-1681.2006.04359.x. [DOI] [PubMed] [Google Scholar]
- 59.Galal M, Symonds I, Murray H, Petraglia F, Smith R. Postterm pregnancy. Facts Views Vis Obgyn. 2012;4(3):175–187. [PMC free article] [PubMed] [Google Scholar]
- 60.McGregor JA, Leff M, Orleans M, Baron A. Fetal gender differences in preterm birth: findings in a North American cohort. Am J Perinatol. 1992;9(1):43–48. doi: 10.1055/s-2007-994668. [DOI] [PubMed] [Google Scholar]
- 61.Ghidini A, Salafia CM. Gender differences of placental dysfunction in severe prematurity. Bjog-Int J Obstet Gy. 2005;112(2):140–144. doi: 10.1111/j.1471-0528.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- 62.Goldenberg RL, Andrews WW, Faye-Petersen OM, Goepfert AR, Clivera SP, Hauth JC. The Alabama preterm birth study: Intrauterine infection and placental histologic findings in preterm births of males and females less than 32 weeks. American Journal of Obstetrics and Gynecology. 2006;195(6):1533–1537. doi: 10.1016/j.ajog.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 63.Macgillivray I, Davey DA. The Influence of Fetal Sex on Rupture of the Membranes and Preterm Labor. American Journal of Obstetrics and Gynecology. 1985;153(7):814–815. doi: 10.1016/0002-9378(85)90361-8. [DOI] [PubMed] [Google Scholar]
- 64.Campbell DM, MacGillivray I, Carr-Hill R, Samphier M. Fetal sex and pre-eclampsia in primigravidae. Br J Obstet Gynaecol. 1983;90(1):26–27. doi: 10.1111/j.1471-0528.1983.tb06741.x. [DOI] [PubMed] [Google Scholar]
- 65.Leonetti C, Butt CM, Hoffman K, Hammel SC, Miranda ML, Stapleton HM. Brominated flame retardants in placental tissues: associations with infant sex and thyroid hormone endpoints. Environ Health. 2016;15(1):113. doi: 10.1186/s12940-016-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adibi JJ, Buckley JP, Lee MK, et al. Maternal urinary phthalates and sex-specific placental mRNA levels in an urban birth cohort. Environ Health. 2017;16(1):35. doi: 10.1186/s12940-017-0241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castorina R, Butt C, Stapleton HM, et al. Flame retardants and their metabolites in the homes and urine of pregnant women residing in California (the CHAMACOS cohort) Chemosphere. 2017;179:159–166. doi: 10.1016/j.chemosphere.2017.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McElrath TF, Hecht JL, Dammann O, et al. Pregnancy Disorders That Lead to Delivery Before the 28th Week of Gestation: An Epidemiologic Approach to Classification. American Journal of Epidemiology. 2008;168(9):980–989. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Savitz DA. Invited Commentary: Disaggregating Preterm Birth to Determine Etiology. American Journal of Epidemiology. 2008;168(9):990–992. doi: 10.1093/aje/kwn193. [DOI] [PubMed] [Google Scholar]
- 70.Hoffman K, Daniels JL, Stapleton HM. Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ Int. 2014;63:169–172. doi: 10.1016/j.envint.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.