Abstract
The objective of this study was to explore the role of the aryl hydrocarbon receptor (AhR) in ambient particulate matter (PM)-mediated activation of dendritic cells (DCs) and Th17-immune responses in vitro. To assess the potential role of the AhR in PM-mediated activation of DCs, co-stimulation, and cytokine expression, bone marrow (BM)-derived macrophages and DCs from C57BL6 wildtype or AhR knockout (AhR−/−) mice were treated with PM. Th17 differentiation was assessed via co-cultures of wildtype or AhR−/− BMDCs with autologous naive T cells. PM2.5 significantly induced AhR DNA binding activity to dioxin responsive elements (DRE) and expression of the AhR repressor (AhRR), cytochrome P450 (CYP) 1A1, and CYP1B1, indicating activation of the AhR. In activated (OVA sensitized) BMDCs, PM2.5 induced interleukin (IL)-1β, CD80, CD86, and MHC class II, suggesting enhanced DC activation, co-stimulation, and antigen presentation; responses that were abolished in AhR deficient DCs. DC-T cell co-cultures treated with PM and lipopolysaccharide (LPS) led to elevated IL-17A and IL-22 expression at the mRNA level, which is mediated by the AhR. PM-treated DCs were essential in endowing T cells with a Th17-phenotype, which was associated with enhanced expression of MHC class II and cyclooxygenase (COX)-2. In conclusion, PM enhances DC activation that primes naive T cell differentiation towards a Th17-like phenotype in an AhR-dependent manner.
Keywords: Aryl hydrocarbon Receptor (AhR), Dendritic Cells (DCs), Particulate Matter (PM), Polycyclic Aromatic Hydrocarbons (PAHs), Th17 cells
Graphical Abstract

1. Introduction
Particulate matter (PM) air pollution has garnered considerable attention in the past few decades as a significant public health concern. Air pollution exposure has been firmly associated with pulmonary, cardiovascular, and neoplastic disease (Dominici et al., 2006; Laden et al., 2006; Loomis et al., 2013). As the lung is the primary site of PM deposition, PM exacerbates respiratory disease, including asthma and chronic obstructive pulmonary disease (COPD) (Atkinson et al., 2001). With respect to asthma, PM facilitates allergen sensitization and worsens asthmatic symptoms (Bowatte et al., 2015; Fuertes and Heinrich, 2015; Fuertes et al., 2013; Mortimer et al., 2008). Exposure to traffic-related air pollution, composed primarily of fuel combustion emissions, is strongly associated with greater incidence of asthma in individuals with no history of atopy (McConnell et al., 2006). Numerous animal studies have corroborated these findings, as PM has been shown to enhance allergic airway inflammation (Acciani et al., 2013; Li et al., 2010; Saravia et al., 2014).
Understanding how PM modulates the development of immunological memory towards allergens may help explain the higher incidence of asthma in highly polluted environments. PM’s ability to worsen allergies has been attributed to its ability to promote inflammation by initiating oxidative stress processes in epithelial cells and alveolar macrophages of the lung (Brown et al., 2006). The current hierarchical model of oxidative stress is outlined as follows: (1) PM promotes oxidative stress via direct generation of reactive oxygen species (ROS), resulting in cells upregulating antioxidant and detoxification enzymes to restore the cellular redox balance; (2) failure to restore the cellular redox balance initiates the production of pro-inflammatory mediators (cytokines and chemokines), which leads to immune cell recruitment; and (3) chronic oxidative stress promotes cytotoxic cell death and immune system activation, which initiates or enhances disease processes (Ayres et al., 2008). This model accurately reflects the biological response to PM exposure, including innate immune responses, but does not account for the effects of PM exposure in shaping the development of long-lasting immunological memory responses (i.e., adaptive immune responses) that occur in diseases such as asthma.
Given the highly complex chemical composition of PM, oxidative stress alone does not fully explain the ability of PM to enhance the allergic response; rather, other unresolved mechanisms may likely be at play in conjunction with oxidative stress processes. Although PM has been shown to possess immunological adjuvant activity that promotes allergic responses (Li et al., 2010; Li et al., 2009), the precise molecular mechanisms through which this occurs have not been determined. How PM enhances the development of long-term towards allergens during sensitization is not unknown. In both ovalbumin (OVA)- and house dust mite (HDM)-allergen animal models of allergic airway inflammation, the combination of allergen with various fractions of PM, including diesel exhaust particles (DEPs), agricultural dust particles, and combustion-derived particles, have enhanced inflammation over allergen-alone treatment (Acciani et al., 2013; Robbe et al., 2014; Wang et al., 2011). In fact, in all these studies, the enhanced inflammatory response from the combined treatment of PM and allergen was characterized by markedly elevated Th2- and Th17-responses. These findings have also been supported in human studies. Diesel-enriched particles enhanced expression of co-stimulatory molecules in human dendritic cells (DCs) and pro-inflammatory cytokine secretion (Porter et al., 2007). Human DCs treated with urban dust particles were also capable of stimulating Th2- and Th17-differentiation of autologous naive T cells in-vitro (Matthews et al., 2016). Th17-immune responses play a critical role in maintaining mucosal barriers and are generated against extracellular bacteria and fungal pathogens. Why Th17-immune responses are generated during allergic sensitization with simultaneous exposure to PM is not understood given that immune responses are typically generated against small amino-acid peptides from proteins and not small molecule chemicals in PM. Furthermore, although ambient PM can contain lipopolysaccharide (LPS), previous studies by our group have used PM with LPS levels that fall below the limits of detection in endotoxin quantification assays (<0.005 EU/mL), ruling out LPS-mediated development of Th17-immune responses in-vivo (Castaneda et al., 2016). An important research avenue that may shed light to these observations is to study how PM shapes the development of adaptive immune responses at the DC-T cell interface.
A prospective link that may explain the development of Th17-immune responses during simultaneous allergen sensitization and PM exposure is the aryl hydrocarbon receptor (AhR). The AhR binds a variety of ligands, including environmental pollutants such as certain polycyclic aromatic hydrocarbons (PAHs), dioxins, and polychlorinated biphenyls (PCBs) (Denison and Nagy, 2003), which are components commonly found in PM derived from fossil fuel combustion and organic matter. The AhR-dependent induction of pro-inflammatory cytokines by organic extracts from diesel and urban dust particles in human-derived macrophages has been shown previously (Vogel et al., 2005). Through the AhR, PM has been demonstrated to promote Th17-polarization and secretion of IL-17A from T cells in-vitro, an observation attributed to PAH content (van Voorhis et al., 2013). A recent study found that vehicular ultrafine PM exacerbated the allergic response in mice through the AhR-Notch signaling cascade in DCs, and elevated IL-17 responses in vivo (Xia et al., 2015). Deletion of AhR lineage-specific CD11c+ cells conferred protection against PM-mediated exacerbation of the allergic response and PM-mediated IL-17 increase, highlighting the key role the AhR in DCs plays in these responses. Interestingly, studying how PM modulates DC activation and subsequent T cell polarization may shed light into understanding how PM modulates the development of the adaptive immune response to exacerbate allergic immune responses.
In this study we aim to characterize how PM affects the activation of innate immune cells and explore if these effects in turn enhance the adaptive immune response at the DC-T cell interface. To understand how PM enhances allergic immune responses, we test the hypothesis that PM enhances the activation of antigen presenting cells, which in turn augments the degree of T cell activation. Bone marrow (BM)-derived macrophages and DCs were treated with PM, OVA, or OVA+PM to investigate if PM enhances activation of these cells. We also focused on understanding how PM promotes the development of Th17-immune responses and if PM mediates its effects through DCs in an AhR-dependent manner.
2. Material and Methods
2.1 Ambient PM Collection, Extraction, and Chemical Characterization
Ambient PM was collected in the summer of 2011 at an urban sampling site located on the rooftop of a two-story building at the northeast corner of T St. and 13th St. in downtown Sacramento, CA. The sampling site is surrounded by a mixture of residential, commercial and industrial sources and within a quarter mile of a major freeway interchange. In brief, summertime PM2.5 in Sacramento was dominated by organic carbon (49% composition by mass), including PAHs and nonaromatic hydrocarbons, and water soluble inorganic ions (21% composition by mass). Elemental carbon accounted for 1.4% of PM mass, and various metals ranging from lithium to lead were detected at levels significantly above detection limits. PM samples were collected in field studies conducted in an urban setting using a high-volume PM2.5 sampler (Tisch Environmental Inc., TE-6070V-2.5-HVS), operating at a flow rate of 40 cfm. The fine PM fraction (PM2.5 ¼ Dp50 < 2.5 mm) was collected using Teflon coated borosilicate glass microfiber filters (Pall Corporation, TX40H120WW-8X10) followed by a multisolvent extraction method. Detailed descriptions of how PM was collected, extracted, and characterized can be found in the literature (Bein and Wexler, 2014, 2015) as well as federal regulation methods for collecting PM2.5 (EPA, 2016; Homolya and Rice, 1999; Winberry, 1999). Lipopolysaccharide (LPS) levels were quantified by the Lonza Kinetic Chromogenic LAL Endotoxin Assay (Basel, Switzerland). Endotoxin levels in the collected PM sample were found to be below the limit of detection (LOD) of <0.005 endotoxin units.
2.2 DRE Luciferase Reporter Assay
HepG2 cells (ATCC HB-8065, Manassas, VA) were used for transient transfection assays as HepG2 cells have a high transfection efficiency and provide a useful tool to detect activation of AhR by various ligands. Cells were seeded at a density of 1.2 × 104 cells/well onto 24 well plates in DMEM media (Gibco Life Technologies, Grand Island, NY). Cell culture media was supplemented with 10% fetal bovine serum, 100 units of penicillin and 100 μg/mL streptomycin. After 24 hrs, cells reached 50–60% confluency and were transfected with 1.0 μg/well of plasmid DNA with a dioxin response element (DRE) luciferase reporter using jetPRIME (Polyplus-transfection SA, Illkirch, France), as described previously (Vogel et al., 2007). The plasmid DRE reporter construct was amplified and purified with ZymoPURE-EndoZero plasmid isolation kit (Zymo Research, Irvine, CA). In brief, 2 μL/well of JetPrime reagent was used over the recommended 1 μL/well volume. Following transfection, cells were incubated for 24 hrs at 37°C. Cells were then treated in triplicate with PBS (vehicle control), PM (50 μg/mL), the standard reference material (SRM) 1649a composed of urban dust particles (UDP; 50 μg/mL), or 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD; 10 nM) and incubated at 37°C for 4 hrs. UDP and the prototypical AhR ligand TCDD served as positive controls. Luciferase activity was measured with the luciferase reporter assay system (Promega Corp., Madison, WI) according to the manufacturer’s instructions. Chemiluminescence was measured using 20 μL of cell lysate and a luminometer (Berthold Lumat LB 9501/16, Pittsburg, PA). Data is expressed as relative light units (RLU).
2.3 Electrophoretic Mobility Shift Assay (EMSA)
Nuclear extracts were isolated from BMDCs, as described previously (Vogel et al., 2014). In brief, 5 × 106 cells were treated with PM or TCDD for 60 min and harvested in Dulbecco’s PBS containing 1 mM phenylmethylsulfonyl fluoride (PMSF) and 0.05 μg/μl of aprotinin. After centrifugation, the cell pellets were gently resuspended in 1 ml of hypotonic buffer (20 mM HEPES, 20 mM NaF, 1 mM Na3VO4, 1 mM Na4P2O7, 1 mM EDTA, 1 mM EGTA, 0.5 mM PMSF, 0.13 μM okadaic acid, 1 mM dithiothreitol, pH 7.9, and 1 μg/ml each leupeptin, aprotinin, and pepstatin). The cells were allowed to swell on ice for 15 min and then homogenized by 25 strokes of a Dounce homogenizer. After centrifugation for 1 min at 16,000 g, nuclear pellets were resuspended in 300 μl ice-cold high-salt buffer (hypotonic buffer with 420 mM NaCl and 20% glycerol). The samples were passed through a 21-gauge needle and stirred for 30 min at 4°C. The nuclear lysates were microcentrifuged at 16,000 g for 20 min, separated into aliquots, and stored at −80°C. DNA-protein binding reactions were carried out in a total volume of 15 μl containing 10 μg nuclear protein, 60,000 counts/min of DRE oligonucleotide (5′-gcc ccg gag ttg cgt gag aag agc ctg g-3′), 25 mM Tris buffer (pH 7.5), 50 mM NaCl, 1 mM EDTA, 0.5 mM dithiothreitol, 5% glycerol, and 1 μg poly(dI-dC). The samples were incubated at room temperature for 20 min. Competition experiments were performed in the presence of a 100-fold molar excess of unlabeled DNA fragments. Protein-DNA complexes were resolved on a 4% nondenaturating polyacrylamide gel and visualized by exposure of the dehydrated gels to X-ray films. For quantitative analysis, respective bands were quantified using a ChemiImager 4400 (Alpha Innotech, San Leandro, CA).
2.4 Isolation, Differentiation, and Treatment of Bone Marrow-Derived Macrophages and Dendritic Cells
Primary BM progenitor cells were isolated and differentiated from wildtype or AhR knockout (AhR−/−) C57BL/6 mice, as described earlier (Vogel et al., 2013). Briefly, femurs were isolated under sterile conditions, and BM cells were extracted via a Roswell Park Memorial Institute (RPMI) media-loaded syringe. Cells were passed through a 30 μm cell strainer, and the supernatant was centrifuged for 5 min at 1000 × g. The supernatant was decanted, and the pellet was resuspended and cultured in RPMI medium. Cells were plated in 100 mm cell culture dishes. Differentiation of BM-derived macrophages was performed in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF; 20 ng/mL; Tonbo Biosciences, San Diego, CA) whereas BMDCs were differentiated in the presence of GM-CSF (20 ng/mL) and IL-4 (20 ng/mL; Tonbo Biosciences). Differentiation of both macrophages and DCs occurred over 6 days and nonadherent BMDCs were purified (≥85–90%) as described previously (Vogel et al., 2013), and the appropriate media was replenished every 2 days. On day 6, cells were transferred to a 24 well plate at 5 × 104 cells/well and treated in triplicate with PBS, PM (50 μg/mL), OVA (10 μg/mL), or OVA+PM for 24 hours followed by RNA isolation to analyze gene expression.
2.5 Dendritic Cell – T Cell Co-Culture Assay
BMDCs from C57BL/6 wildtype or AhR−/− mice were generated as described above and cultured in RPMI media for 5 days. On day 6, BMDCs were resuspended at a concentration of 1×106 cells/mL in RPMI media with either PBS or LPS (10 ng/mL) for 6 hrs. Wildtype and AhR−/− BMDCs were either (1) cultured in a 96-well plate in a media volume of 250 μL (2.5×105 cells/well) and treated in triplicate with PBS, PM (50 μg/mL), UDP (50 μg/mL), or TCDD (2 nM) for 24 hrs; or (2) cultured in a 96-well plate in a media volume of 25 μL (2.5×104 cells/well) and then co-cultured with wildtype autologous CD4+CD62L+ naive T cells (as described below).
CD4+CD62L+ naive T cells were isolated using Miltenyi Biotec’s mouse CD4+ naive T cell isolation kit following the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA). On day 6, after 6 hrs PBS- or LPS-treatment of wildtype or AhR−/− BMDCs (2.5×104 cells/well), BMDCs were co-cultured with CD4+CD62L+ naive T cells at a 1:10 ratio (2.5×105 T cells/well in a total media volume of 225 μL). Co-cultures were immediately treated in triplicate with PBS, PM (50 μg/mL), UDP (50 μg/mL), or TCDD (2nM). On day 8, co-cultures were retreated, and on day 10 cell culture supernatants were collected to assess protein levels and cell RNA was isolated to analyze gene expression.
2.6 Naive CD4+ CD62L+ T Cell Activation and Expansion
CD4+CD62L+ naive T cells were isolated from C57BL/6 wildtype mice using Miltenyi Biotec’s mouse CD4+naïve T cell isolation kit following the manufacturer’s instructions and seeded into wells of a round-bottom 96-well plate at a density of 2.5 × 105 cells/well in a media volume of 250 μL/well. T cells were activated with Miltenyi Biotec’s mouse T cell activation/expansion kit. MACSiBeads conjugated with CD3ε and CD28T antibodies were cultured at a 1:1 ratio with T cells. Culture media was supplemented with IL-2 (30 U/mL) and 2-mercaptoethanol (0.01 mM), as well as IL-6 (20 ng/mL) and TFGβ (5 ng/mL) to simulate Th17 conditions. CD4+CD62L+ naive T cells were isolated, cultured with activation beads, and treated in triplicate with PBS, PM (50 μg/mL), UDP (50 μg/mL), or TCDD (2nM) on day 1. Cells were treated again on day 3, and RNA was isolated on day 5 to analyze gene expression.
2.7 Gene Expression Analysis
RNA was isolated from cultured cells using an RNA isolation kit (Zymo Research, Irvine, CA)according to the manufacturer’s instructions. RNA was converted to complimentary DNA (cDNA) using Applied Biosystems’ High-Capacity cDNA Reverse Transcription Kit (Foster City, CA). Gene-specific primers (0.2 μM of forward or reverse primer), cDNA (2 μL/reaction), and SYBR Green (10 μL/reaction; Applied Biosystems) were used for quantitative PCR (qPCR) via a LightCycler LC480 Instrument (Roche Diagnostics, Indianapolis, IN). Gene expression was assessed using the ΔΔ-Ct method and standardized to the expression of Gapdh or Efe1a1 housekeeping genes. Gene primers were designed using Primer3 primer design software (Untergasser et al., 2012).
2.8 Protein Levels
Cell culture supernatants from BMDC-T cell co-cultures and BMDC-only cultures were used to quantify IL-22 and IL-17A protein levels using BioLegend’s (San Diego, CA) mouse ELISA kit according to the manufacturer’s protocol.
2.9 Statistical Methods
Data are expressed as mean ± standard error of the mean (SEM). Inter-group (wildtype vs. AhR−/−) comparisons were performed using two-way ANOVA followed by Bonferroni’s post-test. Intra-group comparisons (within the wildtype treatment groups) were assessed by one-way ANOVA followed by post hoc Tukey’s multiple comparison test using GraphPad PRISM 5 software. A value of P<0.05 was considered statistically significant.
3. Results
3.1 DRE Luciferase Reporter Assay and EMSA
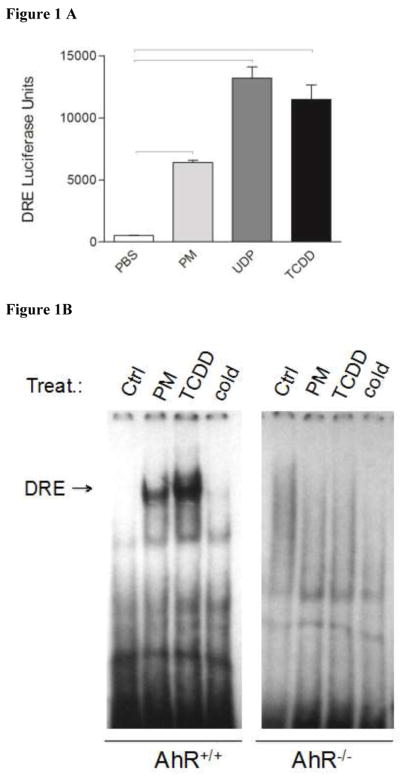
Analysis of the chemical composition of Sacramento PM2.5 demonstrated a relatively high level of PAH content compared to PM from a rural source (Bein and Wexler, 2015). To test whether PM had AhR inducing activity, HepG2 cells were transfected with the DRE-luciferase reporter construct and treated for 4 hr with Sacramento PM, the standard reference material 1649a urban dust particles (UDP), or TCDD, a prototypical AhR agonist (Fig. 1A). The AhR is expressed in lung cells and has been shown to be activated by PAHs (Chan et al., 2013; FitzGerald et al., 1996), however we opted to use HepG2 cells rather than lung-derived cell lines, as HepG2 cells have a higher transfection efficiency. Although the data from HepG2 cells do not allow a direct extrapolation to lung cells, the HepG2 cell line is very useful to detect activation of AhR using the DRE reporter assay in a transient transfection model. PM treatment led to a significant increase in DRE-luciferase reporter activity compared to the PBS-treated control cells and approximately half of UDP- and TCDD-induced DRE activity, demonstrating that Sacramento PM is able to induce AhR activity, a feature that is most likely attributed to PAH content. This is in line with a recent report showing activation of the AhR pathway by PM samples obtained from industrial areas in Taiwan using an in vitro reporter assay (Chou et al., 2017). We hypothesized that PM induces DRE-reporter activity and DRE-dependent gene expression by activating DNA binding activity of AhR. EMSA, used to confirm whether the Sacramento PM was capable of activating AhR, showed that induction of DRE reporter activity was associated with increased AhR binding activity to the DRE consensus element, which regulates the expression of CYP1A1, CYP1B1, and the AhR repressor (AhRR) (Fig. 1B).
Figure 1.
Sacramento PM2.5 induces DRE luciferase reporter activity in HepG2 cells. HepG2 cells were incubated with PBS (control), PM (50 μg/mL), the standard reference material (SRM) 1649a urban dust particles (UDP; 50 μg/mL), or TCDD (10 nM), an AhR agonist, for 4 hrs (A). Data are from three independent experiments and presented as mean ± SEM. Bars indicate a significant difference of p < 0.05 between groups. Sacramento PM2.5 activated AhR and DNA binding activity in BM-derived macrophages from wildtype (AhR+/+), but not from AhR−/− C57BL/6 mice (B). Macrophages were incubated with PBS (control), PM (50 μg/mL) or TCDD (10 nM, positive control) for 1 h. Nuclear extracts were used in electrophoretic-mobility-shift assay (EMSA) with a consensus DRE-binding element.
3.2 Role of the Aryl Hydrocarbon Receptor in PM-Mediated Activation of Bone Marrow-Derived Macrophages
BM-derived macrophages from wildtype or AhR−/− mice were treated with PBS, PM, OVA, or OVA+PM for 24 hr to assess the role the AhR plays in macrophage activation and PM-mediated inflammation (Fig. 2). Cytochrome P450 1A1 (CYP1A1), a gene under transcriptional regulation of the AhR, is a critical enzyme involved in PAH metabolism and elimination. CYP1A1 was significantly enhanced by PM treatment (369-fold increase) and OVA+PM (437-fold increase) in wildtype BM-derived macrophages compared to AhR−/− BM-derived macrophages (Fig. 2A). CYP1A1 levels in AhR−/− macrophages remained unchanged from wildtype control PBS-treated macrophages, demonstrating that the AhR is essential in PM-mediated expression of this enzyme.
Figure 2.
Macrophages fail to become activated by PM or OVA in the absence of the AhR. BM-derived macrophages from wildtype (wt, white bars) or AhR−/− (black bars) C57BL/6 (B6) mice were treated for 24 hrs with PBS, PM (50 μg/mL), OVA (10 μg/mL), or OVA+PM. Gene expression of various activation markers (A–F) was assessed via qPCR and normalized to Gapdh or Efe1a1. Data represent three independent experiments and are presented as mean ± SEM. A significant difference of p < 0.05 is indicated by solid bars between wildtype vs. AhR−/− genotype groups, asterisks between wildtype PBS control vs. wildtype treatment groups, or by dashed lines between wildtype OVA vs. wildtype OVA+PM treatment groups.
Heme oxygenase-1 (HO-1) has been shown to correlate well with particle oxidative potential as this enzyme as this enzyme has been shown to be induced under conditions of oxidative stress (Ayres et al., 2008; Choi and Alam, 1996; Lee et al., 1996; Poss and Tonegawa, 1997; Serpero et al., 2013; Vile et al., 1994), including the lung more recently (Carosino et al., 2015). We assessed how AhR-deficiency modulates HO-1 expression as an indicator of oxidative stress (Fig. 2B). Both PM and OVA+PM treatments enhanced HO-1 expression in wildtype macrophages. Remarkably, HO-1 expression in AhR−/− macrophages treated with PM remained unchanged from PBS-treated control cells, suggesting an AhR- and CYP450 monooxygenase-dependent generation of oxidative stress. The potential role of CYP1A1 in oxidative stress pathways induced by PAHs has been recently shown (Ranjit et al., 2016). The importance of the AhR signaling pathway mediating oxidative stress and inflammation induced by PM has been recently reviewed (Lawal, 2017).
We further assessed three pro-inflammatory cytokines associated with PM-mediated macrophage activation: IL-1β, IL-6, and TNFα (Fig 2C–E) (Mitschik et al., 2008). PM treatment, alone or with OVA, did not led to a significant expression of IL-1 β in wildtype macrophages compared to AhR−/− macrophages (Fig. 2C); this trend was also reflected in IL-6 expression (Fig. 2D). Interestingly, the basal IL-1β and IL-6 expression in AhR−/− macrophages was markedly lower compared to their respective wildtype PBS-control, suggesting that the AhR plays a critical role in the regulation of these cytokines in macrophages. TNFα patterns mirrored those of IL-1β and IL-6; however, wildtype vs. AhR−/− comparisons failed to reach significance (Fig. 2E). Lastly, expression of CXCL2, a chemokine important in neutrophil chemoattractive activity, was elevated by PM treatment (alone and with OVA) in wildtype macrophages and reduced in AhR−/− macrophages (Fig. 2F). Overall, these findings demonstrate that the AhR is an important target of PM containing PAHs.
3.3 Role of the Aryl Hydrocarbon Receptor in PM-Mediated Activation of Bone Marrow-Derived Dendritic Cells
DCs are the professional antigen presenting cells that play a major role in priming adaptive immune responses, particularly in allergy as they induce sensitization to allergens and long-lasting memory. Given that PM enhances the allergic adaptive immune response, we wanted to explore the role the AhR plays in PM-mediated activation of DCs (Fig. 3). Expression of the AhRR was significantly elevated in PM and OVA+PM treated wildtype DCs but markedly decreased in AhR−/− DCs (Fig. 3A). Levels of the PAH-metabolizing CYP450 enzymes of the AhR gene battery, such as CYP1A1 and CYP1B1, were significantly elevated in wildtype DCs treated with PM (with or without OVA) and completely suppressed in AhR−/− DCs (Fig. 3B & C), clearly supporting PM-mediated activation of AhR.
Figure 3.
PM enhances DC maturation and antigen presentation capabilities. BMDCs from wildtype (wt, white bars) or AhR−/− (black bars) C57BL/6 (B6) mice were treated for 24 hrs with PBS, PM (50 μg/mL), OVA (10 μg/mL), or OVA+PM. Gene expression of various activation markers (A–I) was assessed via q-PCR and normalized to Gapdh or Efe1a1. Data represent three independent experiments and are presented as mean ± SEM. A significant difference of p < 0.05 is indicated by solid bars between wildtype vs. AhR−/− genotype groups, asterisks between wildtype PBS control vs. wildtype treatment groups, or by dashed lines between wildtype OVA vs. wildtype OVA+PM treatment groups.
The basal expression levels of the pro-inflammatory cytokines IL-1β and IL-6 were repressed in AhR−/− DCs compared to wildtype DCs (Fig. 3D & E) indicating a physiological role of the AhR in the regulation of IL-1β and IL-6 supporting previous studies (DiNatale et al., 2011; Lahoti et al., 2013). PM treatment, with or without OVA, significantly enhanced expression of COX-2 (Fig. 3F), an effect attributed to the AhR, as COX-2 expression in AhR−/− DCs was comparable to PBS-control treated wildtype DCs. The co-stimulatory molecules CD80 and CD86 serve as markers of DC maturation and activation. Wildtype DCs treated with PM, alone or with OVA, had significantly upregulated CD80 and CD86 expression (Fig. 3G & H). This response was completely attenuated in AhR−/− DCs. Expression of the major histocompatibility complex (MHC) class II, an essential cell surface molecule that presents antigen molecules to the T cell receptor and is necessary for T cell activation, was significantly elevated by the combination of OVA+PM treatment, but not significantly induced in AhR deficient DCs (Fig. 3I). Taken together, these findings demonstrate that (1) the AhR is essential in PM-mediated activation of DCs, and (2) PM treatment significantly enhanced AhR activation, IL-1β and COX-2 expression, antigen presentation and co-stimulatory activity, indicating that PM enhances DC’s antigen presenting capabilities. Notably, PM introduced in the presence of an antigen (i.e OVA) also significantly enhances DC’s antigen presentation and co-stimulatory activity.
3.4 The Effects of PM on Dendritic Cell Priming of Naive CD4+ T Cells
To explore the role PM plays in DC-mediated activation of naive CD4 +T cells during adaptive memory responses and the relevance the AhR plays in this interaction, wildtype or AhR−/− LPS-stimulated DCs were co-cultured with wildtype naive CD4+CD62L+ T cells for 4 days (Fig. 4). LPS-stimulated co-cultures were treated with PBS (LPS/PBS), PM (LPS/PM), UDP (LPS/UDP), or TCDD (LPS/TCDD) on day 1 and day 3 to assess the phenotype of T cell differentiation; in addition, a negative assay control (PBS/PBS) was used in which DCs were pre-treated with PBS instead of LPS and co-cultured with T cells in the presence of PBS. In wildtype DC-T cell co-cultures, CYP1A1 expression was significantly enhanced by LPS/PM, LPS/UDP, and LPS/TCDD treatment compared to both the wildtype PBS control and the respective AhR−/− DC-T cell co-culture (Fig. 4B). CYP1A1 expression in AhR−/− DC-T cell co-cultures is likely driven by the AhR in wildtype CD4+ T cells given the fact that PM treatment failed to induce CYP1A1 in AhR−/− DCs (Fig. 3B).
Figure 4.
PM acts through DCs to enhance IL-17A gene expression in T cells, an effect mediated completely by the AhR in DCs. Wildtype (wt, white bars) or AhR−/− (black bars) BMDCs derived from C57BL/6 (B6) mice pre-treated with PBS (PBS/−) or LPS (10 ng/mL; LPS/−) for 6 hrs were co-cultured with autologous naive CD4+CD62L+ T cells at a 1:10 ratio (2.5×104 DCs: 2.5×105 T cells/well) and treated with PBS (PBS/PBS or LPS/PBS), PM (LPS/PM, 50 μg/mL), UDP (LPS/UDP, 50 μg/mL), or TCDD (LPS/TCDD, 2nM) for 4 days with re-stimulation on day 3. On day 4, cellular RNA was isolated to analyze gene expression patterns (A–D). Gene expression was assessed via q-PCR and normalized to Efe1a1. Data represent three independent experiments and are presented as mean ± SEM. A significant difference of p < 0.05 is indicated by solid bars between wildtype vs. AhR−/− genotype groups or asterisks between wildtype PBS control vs. wildtype treatment groups.
PM treatment (LPS/PM) strongly induced the expression of IL-17A, a characteristic cytokine of Th17 cells, in wildtype DC-T cell co-cultures (Fig. 4C). Wildtype DCs co-cultured with naive CD4+ T cells in the presence of LPS/PM expressed significantly more IL-17A compared to the negative assay control (43-fold increase expression vs. PBS/PBS control) and its respective control (8-fold increase expression vs. LPS/PBS control). Expression of IL-17A was completely abolished in AhR−/− DC-T cell co-cultures treated with LPS/PM (0.84-fold expression vs. PBS/PBS control), suggesting that PM promotes T cell differentiation towards a Th17-like phenotype that is completely mediated by the AhR. Interestingly, the high-affinity AhR ligand TCDD clearly induced IL-17A expression confirming a recent report showing that the dose and the duration of AhR activation may determine the differentiation into Tregs or Th17 cells (Ehrlich et al., 2017). No significant changes in expression of RAR-related orphan receptor gamma (RORγT) were noted within treatment groups or across genotype comparisons (data not shown). The expression level of the Th17 cytokine IL-22 in LPS-activated wildtype DC-T cell co-cultures was elevated by PM, UDP, and TCDD treatment compared to AhR−/− DC-T cell co-cultures. However, PM treatment did not lead to a statistically significant increase of IL-22 over its respective LPS/PBS control (Fig. 4D). These results confirm recent findings in BMDCs that TCDD induces IL-22 in TLR4-activated BMDCs (Vogel et al., 2013).
Cell culture supernatants from DC-T cell co-cultures were used to confirm protein levels of IL-17A (Fig. 5A) and IL-22 (Fig. 5B). Both IL-17A and IL-22 protein levels were consistent with mRNA levels (Fig. 4C & D). In the presence of PM, UDP, or TCDD, LPS-activated wildtype DCs were able to stimulate T cells to produce greater levels of IL-17A and IL-22 compared to LPS-activated and PM-treated AhR−/− DCs. Although PM-treatment of wildtype DC-T cell co-cultures produced higher levels of IL-17A and IL-22 compared to their respective LPS/PBS control, significance was not reached (p = 0.0696 and p = 0.1106, respectively). Furthermore, DC-only cultures treated identically to DC-T cell co-cultures failed to produce the levels of IL-22 (data not shown) observed in co-cultures (Fig. 5B), confirming that DCs were not the source of IL-22. It is noteworthy that LPS-activated expression of IL-17A and IL-22 was significantly repressed in AhR deficient cells, indicating the critical role of AhR in LPS-mediated cytokine regulation as reported earlier (Kado et al., 2017; Muku et al., 2017; Vogel et al., 2014; Vogel et al., 2013; Wu et al., 2011).
Figure 5.
PM acts through DCs to enhance IL-17A protein levels in T cells, an effect mediated completely by the AhR in DCs. Wildtype (wt, white bars) or AhR−/− (black bars) BMDCs derived from C57BL/6 (B6) mice pre-treated with PBS (PBS/−) or LPS (10 ng/mL; LPS/−) for 6 hrs were co-cultured with autologous naive CD4+CD62L+ T cells at a 1:10 ratio (2.5×104 DCs: 2.5×105T cells/well) and treated with PBS (PBS/PBS or LPS/PBS), PM (LPS/PM, 50 μg/mL), UDP (LPS/UPD, 50 μg/mL), or TCDD (LPS/TCDD, 2nM) for 4 days with re-stimulation on day 3. On day 4, cell culture supernatants were analyzed via ELISA to determine (A) IL-17A and (B) IL-22 protein levels. Data represent three independent experiments and are presented as mean ± SEM. A significant difference of p < 0.05 is indicated by solid bars between wildtype vs. AhR−/− genotype groups or asterisks between wildtype PBS control vs. wildtype treatment groups.
3.5 The Direct Effects of PM on Naive CD4+ T Cells During Activation in the Absence of DCs
To investigate whether PM acted through DCs or directly on T cells to induce a Th17 phenotype, wildtype naive CD4+CD62L+ T cells were activated via anti-CD3 and anti-CD28 in the absence of DCs (Fig. 6). AhRR and CYP1A1 expression in PM, UDP, and TCDD treatment groups support AhR activation (Fig. 6A). Nonetheless, PM and UDP treatment of T cells-alone failed to enhance IL-17A or IL-22 expression (Fig. 6A), suggesting that DC-derived signals and/or cytokines mediated through the AhR are essential for PM-mediated differentiation towards a Th17-like phenotype. IL-17A protein levels also support these findings (Fig. 6B), as treatment of T cells-alone with PM did not induce significant changes in IL-17A protein levels compared to the control (81.41 vs. 58.58 pg/mL); by comparison, PM treatment of DC-T cell co-cultures produced (117.94 vs. 305.59 pg/mL; Fig. 5A).
Figure 6.
In the absence of DCs, CD4+CD62L+ T cells treated with PM fail to differentiate towards a Th17-like phenotype despite AhR activation. CD4+CD62L+ T cells derived from C57BL/6 (B6) mice were activated using MACSiBeads conjugated to anti-CD3ε and anti-CD28 to mimic antigen presenting cell activation signals in the presence of PBS, PM (50 μg/mL), UDP (50 μg/mL), or TCDD (2nM) for 4 days with re-stimulation on day 3. On day 4, cellular RNA was isolated to analyze gene expression patterns (A). Gene expression was assessed via q-PCR and normalized to Efe1a1. Cell culture supernatants were analyzed via ELISA to determine IL-17A protein levels (B). Data represent three independent experiments and are presented as mean ± SEM. Bars indicate a significant difference of p < 0.05 between groups.
3.6 The Effects of PM on Dendritic Cell Activation
Finally, to explore how DCs enhance T cell differentiation towards a Th17-like phenotype, wildtype or AhR−/− DCs were cultured under identical conditions as in DC-T cell co-cultures but in the absence of T cells and only for 24 hrs. LPS/PM treatment significantly enhanced expression of the AhRR in wildtype DCs suggesting AhR activation (Fig. 7A). The expression patterns of the Th17-inducing cytokines IL-1β, IL-6, IL-23, and TGF-β in DCs treated with LPS/PM (Fig. 7D–G) do not explain the observed Th17-like phenotype seen in DC-T cell co-cultures (Fig. 4 & 5). Levels of the DC co-stimulatory molecules CD80 and CD86 also did not differ between wildtype LPS/PBS vs. LPS/PM treatments (Fig 7J & K).
Figure 7.
PM treatment of DCs does not lead to secretion of Th17-inducing cytokines, rather PM enhances MHC class II and COX-2 expression in DCs. Wildtype (wt, white bars) or AhR−/− (black bars) BMDCs derived from C57BL/6 (B6) mice were pre-treated with PBS (PBS/−) or LPS (10 ng/mL; LPS/−) for 6 hrs and then treated with PBS (PBS/PBS or LPS/PBS), PM (LPS/PM, 50 μg/mL), UDP (LPS/UPD, 50 μg/mL), or TCDD (LPS/TCDD, 2nM) for 24 hrs. Cellular RNA was isolated to analyze gene expression patterns (A–L). Gene expression was assessed via q-PCR and normalized to Efe1a1. Data represent three independent experiments and are presented as mean ± SEM. A significant difference of p < 0.05 is indicated by solid bars between wildtype vs. AhR−/− genotype groups or asterisks between wildtype PBS control vs. wildtype treatment groups.
Expression of COX-2 was elevated in LPS-activated wildtype DCs treated with PM and significantly elevated after UPD- and TCDD-treatment compared to control (Fig. 7I), while COX-2 expression was significantly lower in AhR−/− DCs in the same treatments groups. The enhanced expression of COX-2 infers increased production of the pro-inflammatory eicosanoid, prostaglandin E2 (PGE2), which has been shown to regulate Th17-immune cell differentiation and function (Boniface et al., 2009). MHC class II expression in LPS-activated wildtype DCs treated with PM, UDP and TCDD was significantly elevated compared to the control (Fig. 7L). The expression of MHC class II was completely abolished in AhR−/− DCs across all treatment groups. These results suggest that activation of Th17-like phenotype of T cells by DCs involves increased antigen presentation on MHC Class II but not Th17-inducing cytokine secretion.
4. Discussion
In this study we investigated how ambient PM from an urban source rich in PAHs leads to the modulation of the adaptive immune response. The results of the current study demonstrate that PM promotes DC priming of naive T cells towards a Th17-like phenotype. The AhR appears to be an essential mediator of these responses since AhR-deficiency completely abolished the PM-induced Th17-like phenotype in naive T cells. Furthermore, the activation of naive T cells in the presence of PM, but in the absence of DCs, failed to induce a Th17-phenotype, suggesting that DC-derived signals are responsible for eliciting these effects.
The PM used in this experiment was collected from the city of Sacramento, CA, near the intersection of three major highways. Characterization of the Sacramento PM demonstrated a high level of PAH content coming from byproducts of fossil fuel and organic matter combustion (Bein and Wexler, 2015). Exposure to traffic-related air pollution is known to increase the incidence of asthma (McConnell et al., 2006); however, the molecular mechanisms involved in PM-mediated immunotoxicity are not well understood, therefore the current study focuses on the important role of AhR in PM-mediated activation of DC and T cells. However, the findings of this study warrant caution, as PM chemical composition is highly influenced by anthropogenic activity and weather patterns. Therefore, the effects observed in this study cannot be fully extrapolated to other seasons or years due to the highly variable chemical composition of PM.
The AhR has been shown to play a critical role in the development of various immune responses (Marshall and Kerkvliet, 2010). As a cytosolic receptor protein and transcription factor, the AhR binds a diverse array of ligands, resulting in different immune outcomes depending on ligand binding characteristics, such as ligand binding strength, binding duration, route of exposure, and cytokine milieu (Julliard et al., 2014). Activation of the AhR has been shown to enhance Th17-immune responses in a ligand-specific manner (Veldhoen et al., 2008), including PAHs in air pollution (van Voorhis et al., 2013). We hypothesized that PM induces a more potent adaptive immune response characterized by a Th17 response, aside the Th2-allergen driven immune response.
To test our hypothesis, we focused on two important innate immune cell types in the pulmonary compartment that function as antigen presenting cells: macrophages and DCs. Both cell types are critically involved in PM uptake and clearance from the lung (Hardy et al., 2013). Moreover, as antigen presenting cells, macrophages and DCs play key roles in the development of adaptive immune responses. As innate immune cells, alveolar macrophages serve as a primary line of defense in the lung, mediate the early inflammatory response to pulmonary insults, and also function as scavenger cells that play an essential role in the clearance of PM deposition (Balhara and Gounni, 2012).
Here, PM treatment of BM-derived macrophages led to an increased expression of pro-inflammatory cytokines, the AhR-regulated gene CYP1A1, and HO-1, an indicator of oxidative stress. Interestingly, treatment with PM did not induce AhR-deficient macrophages to express pro-inflammatory cytokines HO-1 or CYP1A1, highlighting the critical role the AhR plays in PM-mediated activation of macrophages.
Our finding that HO-1 was not expressed in PM treated AhR−/− macrophages is important as it suggests the possibility that it is the PAH content of PM that induces CYP1A1 activity rather than PM itself. The PAH content may cause ROS formation and subsequent induction of HO-1. A recent study shows that CYP1A1 associated generation of ROS by PAHs, such as benzo(a)pyrene, is a major contributor to the toxic effects of PAHs in monocytic cells (Ranjit et al., 2016).
DCs treated with PM exhibited maturation and activation phenotypes that were abolished in AhR−/− DCs. PM-mediated AhR activation in DCs was supported by the increased expression levels of the AhRR, CYP1A1, and CYP1B1 (genes of the AhR gene battery) in addition to the fact that AhR deficiency eliminated the induced expression of these genes. The activation of AhR and AhR DNA binding activity by PM treatment was confirmed in EMSA and luciferase reporter assay. PM treatment induced DC maturation, as noted by greater expression of the co-stimulatory molecules CD80, CD86, antigen presentation (MHC II), and the pro-inflammatory cytokine IL-1β. AhR mediated these effects, as DCs lacking AhR failed to mature after PM treatment. The results are in line with our recent report showing TCDD-induced and AhR-dependent expression of CD80 and CD86 in DCs (Vogel et al., 2013). Collectively, these results demonstrate that PM has the capacity to enhance the T cell activating signals – (1) antigen presentation on MHC class II, (2) co-stimulation via CD80/CD86, and (3) cytokine-guided differentiation – delivered by DCs during priming of the adaptive immune system when PM is given in combination with an antigen (i.e., OVA allergen) vs. antigen-alone. Surprisingly, all three DC signals were completely attenuated in the absence of the AhR.
The enhanced expression of the three signals necessary for T cell activation in DCs led us to question whether PM was able to enhance T cell cytokine secretion, particularly in a Th17-phenotypic fashion via the AhR. In an in-vivo study of house dust mite (HDM) allergic airway inflammation, PM exposure during HDM allergen sensitization led to enhanced expression of the co-stimulatory molecules CD80 and CD86 in whole lung, as well as marked Th17 response, via IL-17 protein levels, and increased AhRR gene expression (unpublished results). To gain a greater understanding of how PM modulates the adaptive immune response and the involvement of the AhR in this respect, LPS-activated wildtype DCs or AhR−/− DCs were co-cultured with autologous wildtype naive CD4+CD62L+ T cells in the presence of PM. PM-treated wildtype DCs were able to markedly and significantly enhance gene expression and protein levels of the Th17 principle cytokine IL-17A in T cells. Remarkably, IL-17A mRNA expression and protein secretion are completely abolished in PM-treated co-cultures containing AhR−/− DCs, demonstrating that PM skews T cell differentiation towards a Th17-phenotype via the AhR. Notably, these effects were not associated with a PM-induced mRNA expression of RORγT (data not shown). Furthermore, IL-22, an additional Th17 cytokine, was elevated both at the mRNA and protein level but failed to reach statistical significance compared to its respective PBS control. These results suggest that PM may act as an immune adjuvant in promoting Th17-immune responses, thereby exacerbating the allergic response.
PM failed to induce a Th17-phenotype in T cells in the absence of DCs. IL-17A and IL-22 expression in PM-treated T cells remained unchanged from PBS-control T cells. However, PM was able to enhance AhRR and CYP1A1 expression in T cells, indicating that the T cells expressed a functional AhR, which is required to respond to AhR ligand exposure. Taken together, results from the DC-T cell co-culture and T cell-anti-CD3ε/CD28 cultures provide evidence that the PM-enhanced Th17-like differentiation requires a functional AhR and the presence of DCs.
To identify how DCs skewed T cell differentiation towards a Th17-phenotype, DCs were cultured and treated in identical conditions as DC-T cell co-cultures but without T cells. PM treatment of LPS pre-stimulated DCs did not significantly induce CYP1A1 expression (Fig. 7B) compared to no LPS pre-stimulation of DCs (Fig. 3B). This effect is likely attributed to LPS pre-stimulation as NFκB activity has been shown to modulate the AhR-mediated induction of CYP1A1 (Ke et al., 2001). LPS pre-stimulation also enhanced IL-6 production in wildtype DCs. None of the DC-derived Th17-inducible cytokines (IL-1β, IL-6, IL-23, and TGF-β) could explain the Th17-differentiating effects of DCs under these conditions. However, PM was able to markedly enhance expression of MHC class II in DCs, making these cells more potent at presenting antigen to T cells. Notably, PM treatment of DCs without LPS pre-stimulation enhanced MHC class II expression, and LPS pre-stimulation of DCs further enhanced MHC class II expression, suggesting LPS-pre-stimulation and PM exposure produce a synergistic effect in MHC class II upregulation. COX-2 expression was also markedly enhanced. Although PGE2 levels were not assessed, increased production of PGE2 is often associated with increased expression of COX-2 (Caughey et al., 2001). Greater levels of PGE2 may provide an explanation for the Th17-like phenotype observed in T cells as PGE2 regulates Th17 cell differentiation (Boniface et al., 2009). In conclusion, our results suggest that PM exerts its Th17-effects via modulation of DCs via enhanced antigen presentation on MHC class II to T cells during activation, and not by co-stimulation via CD80/CD86 or cytokine-guided differentiation.
5. Conclusions
In summary, the results of this study demonstrate that the AhR plays a critical role in PM-mediated activation of DCs, the professional antigen presenting cells that prime T cells. Combined OVA and PM treatment led to enhanced activation of DCs, highlighting PM’s adjuvant properties during allergic responses. Furthermore, PM-treated DCs produce a Th17-like phenotype in naive CD4+ T cells, specifically through the AhR in DCs by enhanced expression of MHC class II and COX-2. These effects are completely abolished in AhR-deficient DCs. Through our findings, we show mechanistic evidence of how ambient pollutants promote dysregulation of the adaptive immune system, which may explain how highly polluted environments promote the exacerbation of diseases, such as asthma and COPD, via Th17-immune responses.
Highlights.
Air pollution PM can shape CD4+ T cell immune responses via the AhR in DCs
PAHs in PM activate the AhR in DCs
PM enhances DC activation by increasing CD80, CD86, and MHC class II expression
DCs prime T cell differentiation towards a Th17-like phenotype in an AhR-dependent manner
DCs are essential in Th17-like differentiation as PAHs act via the AhR in DCs, not T cells
Acknowledgments
Funding Information
This work was supported by funding through the National Institute for Occupational Safety and Health [NIOSH OHO7550] to K.E.P.; the National Institute of Environmental Health Sciences [NIEHS R01 ES019898] to C.F.A.V.; the National Institutes of Environmental Health Sciences [NIEHS R21 ES025560] to P.A.; the UC Davis Western Center for Agricultural Health and Safety Graduate Student Seed Grant, the UC Davis Graduate Research Mentorship Fellowship, and the National Institute of General Medical Sciences at the National Institute of Health, Pharmacology T32 Training Grant [NIH T32 GM099608] to A.R.C.
We would like to acknowledge Dale Uyeminami, Alexa Pham, and Sarah Kado for their valuable help through this experiment. We also acknowledge Suzette Smiley-Jewell and Maria Paz Prada for editing the manuscript.
Footnotes
Author Contributions
ARC, CFAV, KJB, AMM and HTY performed experiments. KJB collected, extracted, and analyzed particulate matter samples. ARC analyzed data and interpreted the results with help from CFAV. The manuscript was written by ARC with feedback from CFAV, KEP, and KJB. The article was read, revised, and approved by all authors.
Competing Interests
The authors do not have competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acciani TH, Brandt EB, Khurana Hershey GK, Le Cras TD. Diesel exhaust particle exposure increases severity of allergic asthma in young mice. Clin Exp Allergy. 2013;43:1406–1418. doi: 10.1111/cea.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RW, Anderson HR, Sunyer J, Ayres J, Baccini M, Vonk JM, Boumghar A, Forastiere F, Forsberg B, Touloumi G, Schwartz J, Katsouyanni K. Acute effects of particulate air pollution on respiratory admissions: results from APHEA 2 project. Air Pollution and Health: a European Approach. Am J Respir Crit Care Med. 2001;164:1860–1866. doi: 10.1164/ajrccm.164.10.2010138. [DOI] [PubMed] [Google Scholar]
- Ayres JG, Borm P, Cassee FR, Castranova V, Donaldson K, Ghio A, Harrison RM, Hider R, Kelly F, Kooter IM, Marano F, Maynard RL, Mudway I, Nel A, Sioutas C, Smith S, Baeza-Squiban A, Cho A, Duggan S, Froines J. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential--a workshop report and consensus statement. Inhal Toxicol. 2008;20:75–99. doi: 10.1080/08958370701665517. [DOI] [PubMed] [Google Scholar]
- Balhara J, Gounni AS. The alveolar macrophages in asthma: a double-edged sword. Mucosal Immunol. 2012;5:605–609. doi: 10.1038/mi.2012.74. [DOI] [PubMed] [Google Scholar]
- Bein K, Wexler A. A high-efficiency, low-bias method for extracting particulate matter from filter and impactor substrates. Atmos Environ. 2014;90:87–95. [Google Scholar]
- Bein K, Wexler A. Compositional variance in extracted particulate matter using different filter extraction techniques. Atmos Environ. 2015;107:24–34. [Google Scholar]
- Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, McClanahan TK, McKenzie BS, Kastelein RA, Cua DJ, de Waal Malefyt R. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, Matheson M, Dharmage SC. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy. 2015;70:245–256. doi: 10.1111/all.12561. [DOI] [PubMed] [Google Scholar]
- Brown D, Hutchison G, Stone V, Barlow P. Particle Toxicology. Taylor & Francis Group; Boca Raton, FL: 2006. [Google Scholar]
- Carosino CM, Bein KJ, Plummer LE, Castaneda AR, Zhao Y, Wexler AS, Pinkerton KE. Allergic airway inflammation is differentially exacerbated by daytime and nighttime ultrafine and submicron fine ambient particles: heme oxygenase-1 as an indicator of PM-mediated allergic inflammation. J Toxicol Environ Health A. 2015;78:254–266. doi: 10.1080/15287394.2014.959627. [DOI] [PubMed] [Google Scholar]
- Castaneda AR, Bein KJ, Smiley-Jewell S, Pinkerton KE. Fine Particulate Matter (PM2.5) Enhances Allergic Sensitization in BALB/c Mice. Journal of Toxicology and Environmental Health. 2016 doi: 10.1080/15287394.2016.1222920. In Press (Accepted 8 August 2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey GE, Cleland LG, Penglis PS, Gamble JR, James MJ. Roles of cyclooxygenase (COX)-1 and COX-2 in prostanoid production by human endothelial cells: selective up-regulation of prostacyclin synthesis by COX-2. J Immunol. 2001;167:2831–2838. doi: 10.4049/jimmunol.167.5.2831. [DOI] [PubMed] [Google Scholar]
- Chan JK, Vogel CF, Baek J, Kodani SD, Uppal RS, Bein KJ, Anderson DS, Van Winkle LS. Combustion derived ultrafine particles induce cytochrome P-450 expression in specific lung compartments in the developing neonatal and adult rat. Am J Physiol Lung Cell Mol Physiol. 2013;304:L665–677. doi: 10.1152/ajplung.00370.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- Chou WC, Hsu CY, Ho CC, Hsieh JH, Chiang HC, Tsou TC, Chen YC, Lin P. Development of an in Vitro-Based Risk Assessment Framework for Predicting Ambient Particulate Matter-Bound Polycyclic Aromatic Hydrocarbon-Activated Toxicity Pathways. Environ Sci Technol. 2017 doi: 10.1021/acs.est.7b02002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- DiNatale BC, Schroeder JC, Perdew GH. Ah receptor antagonism inhibits constitutive and cytokine inducible IL6 production in head and neck tumor cell lines. Mol Carcinog. 2011;50:173–183. doi: 10.1002/mc.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich AK, Pennington JM, Bisson WH, Kolluri SK, Kerkvliet NI. TCDD, FICZ, and other high affinity AhR ligands dose-dependently determine the fate of CD4+ T cell differentiation. Toxicol Sci. 2017 doi: 10.1093/toxsci/kfx215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA. Quality Assurance Guidance Document 2.12. EPA: U.S. Environmental Protection Agency; Office of Air Quality Planning and Standards; Air Quality Assessment Division; Research Triangle Park, NC: 2016. Monitoring PM2.5 in Ambient Air Using Designated Reference or Class I Equivalent Methods. (EPA-454/B-16–001, January 2016) [Google Scholar]
- FitzGerald CT, Fernandez-Salguero P, Gonzalez FJ, Nebert DW, Puga A. Differential regulation of mouse Ah receptor gene expression in cell lines of different tissue origins. Arch Biochem Biophys. 1996;333:170–178. doi: 10.1006/abbi.1996.0378. [DOI] [PubMed] [Google Scholar]
- Fuertes E, Heinrich J. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization. Allergy. 2015;70:1350–1351. doi: 10.1111/all.12611. [DOI] [PubMed] [Google Scholar]
- Fuertes E, Standl M, Cyrys J, Berdel D, von Berg A, Bauer CP, Kramer U, Sugiri D, Lehmann I, Koletzko S, Carlsten C, Brauer M, Heinrich J. A longitudinal analysis of associations between traffic-related air pollution with asthma, allergies and sensitization in the GINIplus and LISAplus birth cohorts. PeerJ. 2013;1:e193. doi: 10.7717/peerj.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy CL, Lemasurier JS, Mohamud R, Yao J, Xiang SD, Rolland JM, O’Hehir RE, Plebanski M. Differential uptake of nanoparticles and microparticles by pulmonary APC subsets induces discrete immunological imprints. J Immunol. 2013;191:5278–5290. doi: 10.4049/jimmunol.1203131. [DOI] [PubMed] [Google Scholar]
- Homolya J, Rice J. EPA: Particulate Matter (PM2.5) Speciation Guidance. 1999. Prepared for the U.S. Environmental Protection Agency; Office of Air Quality Planning and Standards; Emissions, Monitoring, and Analysis Division; Monitoring and Quality Assurance Group; Research Triangle Park, NC. [Google Scholar]
- Julliard W, Fechner JH, Mezrich JD. The aryl hydrocarbon receptor meets immunology: friend or foe? A little of both. Front Immunol. 2014;5:458. doi: 10.3389/fimmu.2014.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado S, Chang WLW, Chi AN, Wolny M, Shepherd DM, Vogel CFA. Aryl hydrocarbon receptor signaling modifies Toll-like receptor-regulated responses in human dendritic cells. Arch Toxicol. 2017;91:2209–2221. doi: 10.1007/s00204-016-1880-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke S, Rabson AB, Germino JF, Gallo MA, Tian Y. Mechanism of suppression of cytochrome P-450 1A1 expression by tumor necrosis factor-alpha and lipopolysaccharide. J Biol Chem. 2001;276:39638–39644. doi: 10.1074/jbc.M106286200. [DOI] [PubMed] [Google Scholar]
- Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med. 2006;173:667–672. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoti TS, John K, Hughes JM, Kusnadi A, Murray IA, Krishnegowda G, Amin S, Perdew GH. Aryl hydrocarbon receptor antagonism mitigates cytokine-mediated inflammatory signalling in primary human fibroblast-like synoviocytes. Ann Rheum Dis. 2013;72:1708–1716. doi: 10.1136/annrheumdis-2012-202639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal AO. Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: The role of Nrf2 and AhR-mediated pathways. Toxicol Lett. 2017;270:88–95. doi: 10.1016/j.toxlet.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Lee PJ, Alam J, Wiegand GW, Choi AM. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc Natl Acad Sci U S A. 1996;93:10393–10398. doi: 10.1073/pnas.93.19.10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Harkema JR, Lewandowski RP, Wang M, Bramble LA, Gookin GR, Ning Z, Kleinman MT, Sioutas C, Nel AE. Ambient ultrafine particles provide a strong adjuvant effect in the secondary immune response: implication for traffic-related asthma flares. Am J Physiol Lung Cell Mol Physiol. 2010;299:L374–383. doi: 10.1152/ajplung.00115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Wang M, Bramble LA, Schmitz DA, Schauer JJ, Sioutas C, Harkema JR, Nel AE. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environ Health Perspect. 2009;117:1116–1123. doi: 10.1289/ehp.0800319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis D, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K International Agency for Research on Cancer Monograph Working Group I. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013;14:1262–1263. doi: 10.1016/s1470-2045(13)70487-x. [DOI] [PubMed] [Google Scholar]
- Marshall NB, Kerkvliet NI. Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann N Y Acad Sci. 2010;1183:25–37. doi: 10.1111/j.1749-6632.2009.05125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews NC, Pfeffer PE, Mann EH, Kelly FJ, Corrigan CJ, Hawrylowicz CM, Lee TH. Urban Particulate Matter-Activated Human Dendritic Cells Induce the Expansion of Potent Inflammatory Th1, Th2, and Th17 Effector Cells. Am J Respir Cell Mol Biol. 2016;54:250–262. doi: 10.1165/rcmb.2015-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Berhane K, Yao L, Jerrett M, Lurmann F, Gilliland F, Kunzli N, Gauderman J, Avol E, Thomas D, Peters J. Traffic, susceptibility, and childhood asthma. Environ Health Perspect. 2006;114:766–772. doi: 10.1289/ehp.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitschik S, Schierl R, Nowak D, Jorres RA. Effects of particulate matter on cytokine production in vitro: a comparative analysis of published studies. Inhal Toxicol. 2008;20:399–414. doi: 10.1080/08958370801903784. [DOI] [PubMed] [Google Scholar]
- Mortimer K, Neugebauer R, Lurmann F, Alcorn S, Balmes J, Tager I. Early-lifetime exposure to air pollution and allergic sensitization in children with asthma. J Asthma. 2008;45:874–881. doi: 10.1080/02770900802195722. [DOI] [PubMed] [Google Scholar]
- Muku GE, Lahoti TS, Murray IA, Podolsky MA, Smith KJ, Hubbard TD, Kuzu G, Gowda K, Amin SG, Perdew GH. Ligand-mediated cytoplasmic retention of the Ah receptor inhibits macrophage-mediated acute inflammatory responses. Lab Invest. 2017;97:1471–1487. doi: 10.1038/labinvest.2017.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter M, Karp M, Killedar S, Bauer SM, Guo J, Williams D, Breysse P, Georas SN, Williams MA. Diesel-enriched particulate matter functionally activates human dendritic cells. Am J Respir Cell Mol Biol. 2007;37:706–719. doi: 10.1165/rcmb.2007-0199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjit S, Midde NM, Sinha N, Patters BJ, Rahman MA, Cory TJ, Rao PS, Kumar S. Effect of Polyaryl Hydrocarbons on Cytotoxicity in Monocytic Cells: Potential Role of Cytochromes P450 and Oxidative Stress Pathways. PLoS One. 2016;11:e0163827. doi: 10.1371/journal.pone.0163827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe P, Spierenburg EA, Draijer C, Brandsma CA, Telenga E, van Oosterhout AJ, van den Berge M, Luinge M, Melgert BN, Heederik D, Timens W, Wouters IM, Hylkema MN. Shifted T-cell polarisation after agricultural dust exposure in mice and men. Thorax. 2014;69:630–637. doi: 10.1136/thoraxjnl-2013-204295. [DOI] [PubMed] [Google Scholar]
- Saravia J, You D, Thevenot P, Lee GI, Shrestha B, Lomnicki S, Cormier SA. Early-life exposure to combustion-derived particulate matter causes pulmonary immunosuppression. Mucosal Immunol. 2014;7:694–704. doi: 10.1038/mi.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpero LD, Bellissima V, Colivicchi M, Sabatini M, Frigiola A, Ricotti A, Ghiglione V, Strozzi MC, Li Volti G, Galvano F, Gazzolo D. Next generation biomarkers for brain injury. J Matern Fetal Neonatal Med. 2013;26(Suppl 2):44–49. doi: 10.3109/14767058.2013.829688. [DOI] [PubMed] [Google Scholar]
- Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Voorhis M, Knopp S, Julliard W, Fechner JH, Zhang X, Schauer JJ, Mezrich JD. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PLoS One. 2013;8:e82545. doi: 10.1371/journal.pone.0082545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Vile GF, Basu-Modak S, Waltner C, Tyrrell RM. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci U S A. 1994;91:2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Khan EM, Leung PS, Gershwin ME, Chang WL, Wu D, Haarmann-Stemmann T, Hoffmann A, Denison MS. Cross-talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor-kappaB. J Biol Chem. 2014;289:1866–1875. doi: 10.1074/jbc.M113.505578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol Endocrinol. 2007;21:2941–2955. doi: 10.1210/me.2007-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Wong P, Kuzmicky P, Kado N, Matsumura F. Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environ Health Perspect. 2005;113:1536–1541. doi: 10.1289/ehp.8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Wu D, Goth SR, Baek J, Lollies A, Domhardt R, Grindel A, Pessah IN. Aryl hydrocarbon receptor signaling regulates NF-kappaB RelB activation during dendritic-cell differentiation. Immunol Cell Biol. 2013;91:568–575. doi: 10.1038/icb.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Thevenot P, Saravia J, Ahlert T, Cormier SA. Radical-containing particles activate dendritic cells and enhance Th17 inflammation in a mouse model of asthma. Am J Respir Cell Mol Biol. 2011;45:977–983. doi: 10.1165/rcmb.2011-0001OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberry WT. EPA: Sampling of Ambient Air for Total Suspended Particulate Matter (SPM) and PM10 Using High Volume (HV) Sampler. 1999. Prepared for the U.S. Environmental Protection Agency; Office of Research and Development; Center for Environmental Research Information; Cincinnati, OH (EPA/625/R-96/010a, June 1999) [Google Scholar]
- Wu D, Li W, Lok P, Matsumura F, Vogel CF. AhR deficiency impairs expression of LPS-induced inflammatory genes in mice. Biochem Biophys Res Commun. 2011;410:358–363. doi: 10.1016/j.bbrc.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Viera-Hutchins L, Garcia-Lloret M, Noval Rivas M, Wise P, McGhee SA, Chatila ZK, Daher N, Sioutas C, Chatila TA. Vehicular exhaust particles promote allergic airway inflammation through an aryl hydrocarbon receptor-notch signaling cascade. J Allergy Clin Immunol. 2015;136:441–453. doi: 10.1016/j.jaci.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]