Abstract
Background/Aim: Matrix metalloproteinases (MMPs) play important roles in inflammation and carcinogenesis, but the genotypic role of MMP-7 has never been investigated in colorectal cancer (CRC) among the Taiwanese. Therefore, in this study we aimed to evaluate the contribution of MMP-7 genotypes to the risk of CRC in Taiwan. Materials and Methods: In this case-control study, MMP-7 A-181G and C-153T promoter genotypes were determined and their association with CRC risk were investigated among 362 CRC patients and 362 age- and gender-matched healthy controls. In addition, the interaction of MMP-7 genotypes and personal behaviors were also examined. Results: The percentages of variant AG and GG for MMP-7 A-181G genotypes were 10.5% and 1.7% in the CRC group and 11.9% and 2.2% in the control group, respectively (p for trend=0.7145). The allelic frequency distribution analysis showed that the variant G allele of MMP-7 A-181G conferred a slight but non-significant decreased CRC susceptibility to the wild-type C allele (odds ratio (OR)=0.86, 95% confidence interval (CI)=0.64-1.31, p=0.37). Taiwanese all harbour the CC genotype at MMP-7 C-153T. As for the gene–lifestyle interaction, there were no obvious joint effects of MMP-7 A-181G genotype on the risk of CRC among ever smoker, alcohol drinker, non-smoker or non-drinker subgroups. No statistically significant correlation was observed between MMP-7 A-181G genotypic distributions and age, gender, tumor size, location or metastasis status. Conclusion: The genotypes of MMP-7 A-181G may play an indirect role in determining personal susceptibility to CRC and prognosis. The further genotyping work on MMP-7 and other genes (such as other MMPs, oncogenes and tumor suppression genes) on CRC susceptibility and prognosis, should be taken into consideration spontaneously in the precision medicine era.
Keywords: Colorectal cancer, genotype, MMP-7, polymorphism, Taiwan
Colorectal cancer (CRC) has been one of the leading causes of cancer associated with significant morbidity and mortality worldwide (1,2). Globally, CRC is the third most common cancer among males and the second among females. Nevertheless, CRC incidence and mortality rates vary markedly across the globe with regional differences that can sometimes be 10-fold (1,2). In Taiwan, the incidence and mortality of CRC has occupied the first and third places among the common types of cancer for many years and its high incidence has been proposed to be closely associated with dietary changes to Western food style and a decreased consumption of dietary fiber or grain-made foods. From the epidemiological viewpoint, studies have attributed more than 85% of CRC etiology to risk environmental factors, particularly meat consumption, cigarette smoking, exposure to carcinogenic aromatic amines, such as arylamines and heterocyclic amines (3,4). Statistically, 15-20% of CRC cases are with strong familial cancer history that have interested the molecular epidemiologists to figure out additional genomic susceptibility factors (5-7). In Taiwan, although specific biomarkers for CRC prediction and detection have keeping on being reported in the decade (8-12), the interactions among the genomic and environmental risk factors, such as smoking and alcohol drinking are mostly unknown.
In our body, MMP-7 has been found to be constitutively produced from the epithelial cells of mammary and parotid glands, pancreas, liver, prostate and peribronchial glands of the lung (13). From the results of the promoter assay in 2001, the basal promoter activity was higher in promoter constructs harboring the combination of the two rare alleles of MMP-7 at A-181G (rs11568818) and C-153T (rs11568819) (14), and these genotypic polymorphisms were associated with altered risk of coronary artery dimensions (14). In cancer genomic association studies, the genotypic polymorphisms of MMP-7 were investigated of their association with many types of cancer, including oral, lung, breast, esophageal, gastric, gallbladder, bladder, cervical cancer, astrocytoma, childhood leukemia and renal cell carcinoma (15-27), and colorectal cancer (28,29).
Horvat and colleagues found that higher frequency of GG genotype at MMP-7 A-181G (rs11568818) was found among the CRC patients with T3/T4 stage, N1/N2 stage and with lymphovascular invasion (29). Although the genotype and allele frequencies of MMP-7 A-181G did not differ between control and colorectal cancer groups or any clinico-pathological parameters, the combined MMP7-181A-MMP1-1,607dupG-MMP3-1,171A-MMP12-82A haplotypes were significantly more frequent in CRC patients than in healthy controls (28). It seems that there may be differential effects of MMP-7 genotypes on CRC susceptibility among different ethics, and more investigations are needed to figure out the contribution of MMP-7 genotypes to CRC risk. In light of the above, we conducted a hospital-based case-control study to investigate the genotypes of MMP-7 firstly among Taiwanese and examine the association of MMP-7 genotypes with the risk of CRC in a representative Taiwanese population.
Materials and Methods
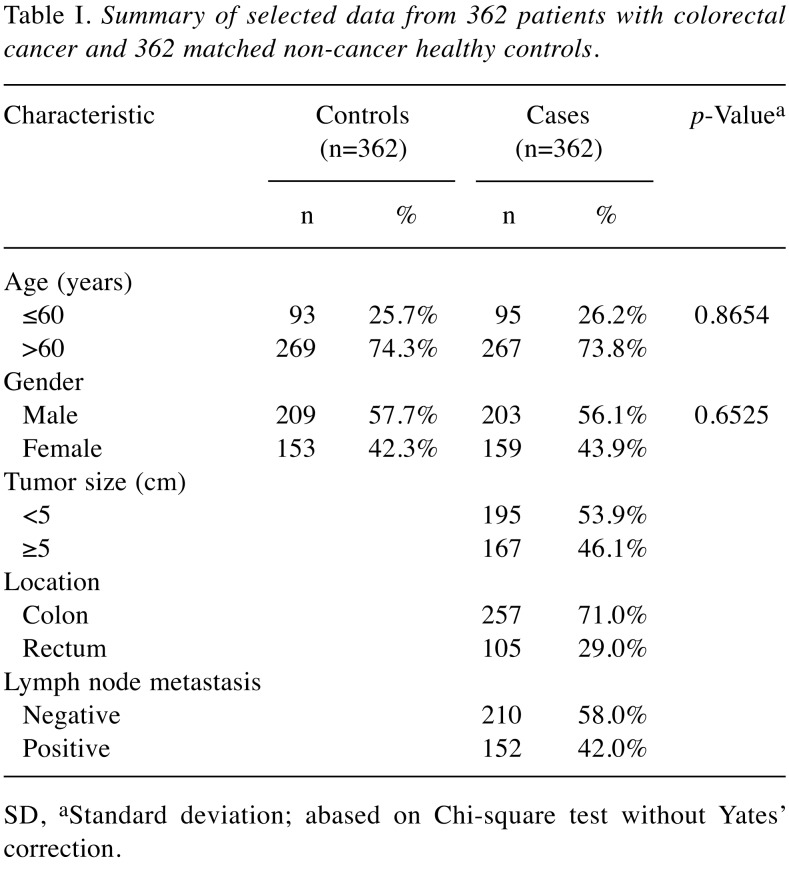
Investigated population. The target population included 724 subjects (362 CRC patients and 362 controls). Patients diagnosed with CRC were recruited at the outpatient clinics of general surgery during the period of 2002 and 2008 at the China Medical University Hospital by the excellent surgery teams under the supervision of Jeng L.B. and Yang M.D. The clinical characteristics of patients, including histological details, were all graded and defined by expert surgeons (8-11). All participants have completed a self-administered questionnaire and provided a 5-ml sample of peripheral blood for genotyping work. An equal number of non-cancer healthy volunteers were selected as controls by matching for age, gender and some indulgences after initial random sampling from the Health Examination Cohort of the Hospital with the help of colleagues in the Department of Family Medicine. The exclusion criteria of the control group included previous malignancy, metastasized cancer from other or unknown origin and any familial or genetic diseases. This study was approved by the Institutional Review Board of the China Medical University Hospital (IRB project identification coding number: DMR99-IRB-108) and written informed consents were obtained from all the participants with the help of Tissue Bank of China Medical University Hospital. The selective demographic information for the participants is summarized in Table I.
Table I. Summary of selected data from 362 patients with colorectal cancer and 362 matched non-cancer healthy controls.
SD, aStandard deviation; abased on Chi-square test without Yates’ correction.
Genotyping conditions. Genomic DNA was extracted from peripheral blood leukocytes with a QIAamp Blood Mini Kit (Blossom, Taipei, Taiwan), stored long-term at –80˚C, diluted and aliquoted for genotyping as a working stock at –20˚C as we frequently conducted (30-32). The MMP-7 genotyping methodology including the designing of the specific primers and the selection of restriction enzymes, were exactly the same as our currently published papers (17,25,27). Concisely, the polymerase chain reaction (PCR) cycling conditions were: one cycle at 94˚C for 5 min; 35 cycles of 94˚C for 30 s, 59˚C for 30 s and 72˚C for 30 s, and a final extension at 72˚C for 10 min. The genotyping PCR for MMP-7 A-181G was conducted using the forward 5’-TGGTACCA TAATGTCCTGAATG-3’ and the reverse 5’-TCGTTATTGGCA GGAAGCACACAATGAATT-3’ primer pairs. The obtained 150 bp PCR products were then digested with EcoRI and resulted in two fragments of 120 and 30 bp when the G allele was present. In the presence of the A allele, the 150 bp fragment remained intact. As for the MMP-7 C-153T, direct sequencing PCR was conducted with the same primers as for MMP-7 A-181G. After amplification, the PCR products were subject to digestion and separation using 3% agarose gel electrophoresis. All the genotypic processing was repeated by two expert researchers independently and blindly, and their results were 100% concordant to each other. In addition, the success rate of PCR-restrictive fragment length polymorphism (RFLP) is 100%, and the genotypes of 5% of the participants in both the control and CRC patient groups were analyzed by PCR direct sequencing (Genomics BioSci & Tech Co). The concordance between direct sequencing and PCR-RFLP methods was 100%.
Statistical analyses. The Student’s t-test was applied for the comparison of ages between the CRC cases and the control groups. Pearson’s Chi-square or Fisher’s exact test (when any cell analyzed was less than 5, such as those in Tables IV-VI) was applied to compare the distribution of the MMP-7 genotypes among the subgroups. The associations between MMP-7 genotypes and CRC risk were estimated by computing odds ratios (ORs) and their 95% confidence intervals (CIs) from logistic regression analysis. Statistically, any difference at p<0.05 was taken as significant between the two groups compared.
Results
The frequency distributions of selected demographic characters, including age and gender for the 362 CRC patients in the case group and 362 non-cancer healthy subjects in the control group, are summarized and compared in Table I. In addition, tumor size, location, and lymph node metastasis status are also summarized and compared in Table I. Since we applied frequency matching to recruit non-cancer healthy subjects as controls, the results showed that there was no difference in respects of the distributions of age and gender between the control and case groups (p=0.8654 and 0.6525, respectively) (Table I). The patients with tumor size <5 cm and ≥5 cm were 195 and 167, respectively. The patients with tumor location at colon and rectum were 257 and 105, respectively. The patients with and without lymph node metastasis were 152 and 210, respectively (Table I).
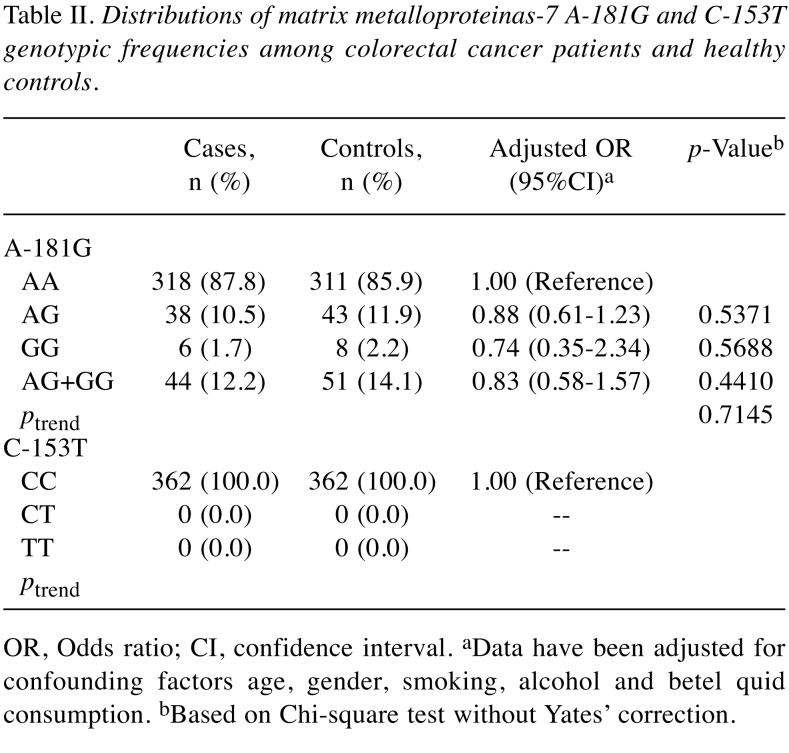
The distributions of the MMP-7 A-181G and C-153T genotypes among the 362 non-cancer healthy controls and the 362 CRC patients are presented and analyzed in Table II. First, the results showed that the genotype of MMP-7 C-153T were the same among all the Taiwanese (Table II lower panel). Second, the genotypes of MMP-7 A-181G were not differently distributed between case and control groups (p for trend=0.7145) (Table II upper panel). In detail, the MMP-7 A-181G homozygous GG and heterozygous AG were not associated with altered CRC risk, compared with wild-type CC genotype (adjusted OR=0.74 and 0.88, 95%CI=0.35-2.34 and 0.61-1.23, p=0.5688 and 0.5371, respectively; Table II upper panel). In the dominant model, there was no association between the AG+GG genotype of MMP-7 A-181G and CRC risk, compared with AA wild-type genotype (adjusted OR=0.83, 95%CI=0.58-1.57, p=0.4410, Table II upper panel).
Table II. Distributions of matrix metalloproteinas-7 A-181G and C-153T genotypic frequencies among colorectal cancer patients and healthy controls.
OR, Odds ratio; CI, confidence interval. aData have been adjusted for confounding factors age, gender, smoking, alcohol and betel quid consumption. bBased on Chi-square test without Yates’ correction.
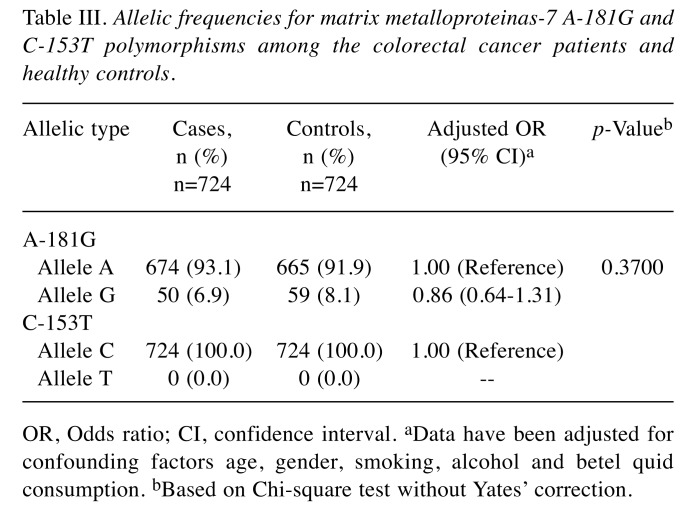
To confirm the results in Table II, analysis of allelic frequency distribution for the MMP-7 A-181G and C-153T was further conducted and the results are presented in Table III. Supporting the findings that genotype of MMP-7 A-181G was not associated with CRC risk, the variant allele G was found at 6.9% in the case group, non-significantly of similar level with that of 8.1% in the control group (adjusted OR=0.86, 95% CI=0.64-1.31, p=0.3700) (Table III upper panel). At the same time, there was no T allele found in any of the subject investigated (Table III lower panel).
Table III. Allelic frequencies for matrix metalloproteinas-7 A-181G and C-153T polymorphisms among the colorectal cancer patients and healthy controls.
OR, Odds ratio; CI, confidence interval. aData have been adjusted for confounding factors age, gender, smoking, alcohol and betel quid consumption. bBased on Chi-square test without Yates’ correction.
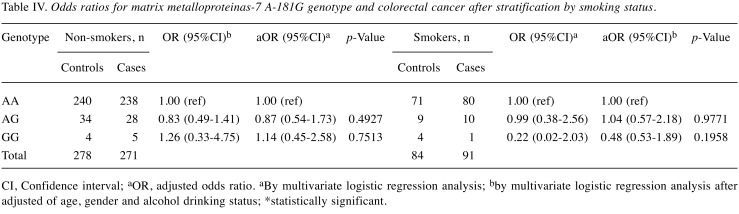
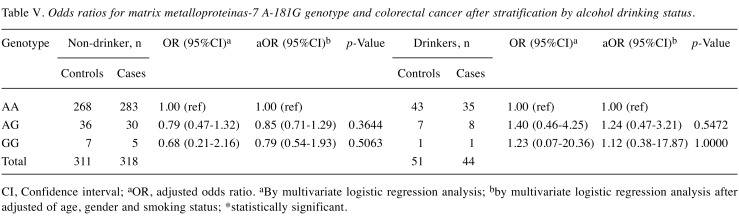
Since smoking and alcohol drinking habits are well-known risk factors for CRC in Taiwan, we were interested in investigating the interactions between the genotype of MMP-7 A-181G and personal cigarette smoking and alcohol drinking behaviors. Firstly, among smokers, those with AG and GG genotypes at MMP-7 A-181G were at 0.83- and 1.26-fold odds of having CRC (95% CI=0.49-1.41 and 0.33-4.75, p=0.4927 and 0.7513, respectively) conferring no risky effect, while this non-significant effect was also the case for non-smokers (Table IV). After adjusting for age, gender and alcohol drinking status, statistical non-significance still existed at a similar level (Table IV). Secondly, among alcohol drinkers, those with AG and GG genotypes at MMP-7 A-181G were at 0.79- and 0.68-fold odds of having CRC (95% CI=0.47-1.32 and 0.21-2.16, p=0.3644 and 0.5063, respectively) conferring no risky effect, while this non-significant effect was also the case for non-drinkers (Table V). After adjusting for age, gender and smoking status, results were still non-significant either (Table V).
Table IV. Odds ratios for matrix metalloproteinas-7 A-181G genotype and colorectal cancer after stratification by smoking status.
CI, Confidence interval; aOR, adjusted odds ratio. aBy multivariate logistic regression analysis; bby multivariate logistic regression analysis after adjusted of age, gender and alcohol drinking status; *statistically significant.
Table V. Odds ratios for matrix metalloproteinas-7 A-181G genotype and colorectal cancer after stratification by alcohol drinking status.
CI, Confidence interval; aOR, adjusted odds ratio. aBy multivariate logistic regression analysis; bby multivariate logistic regression analysis after adjusted of age, gender and smoking status; *statistically significant.
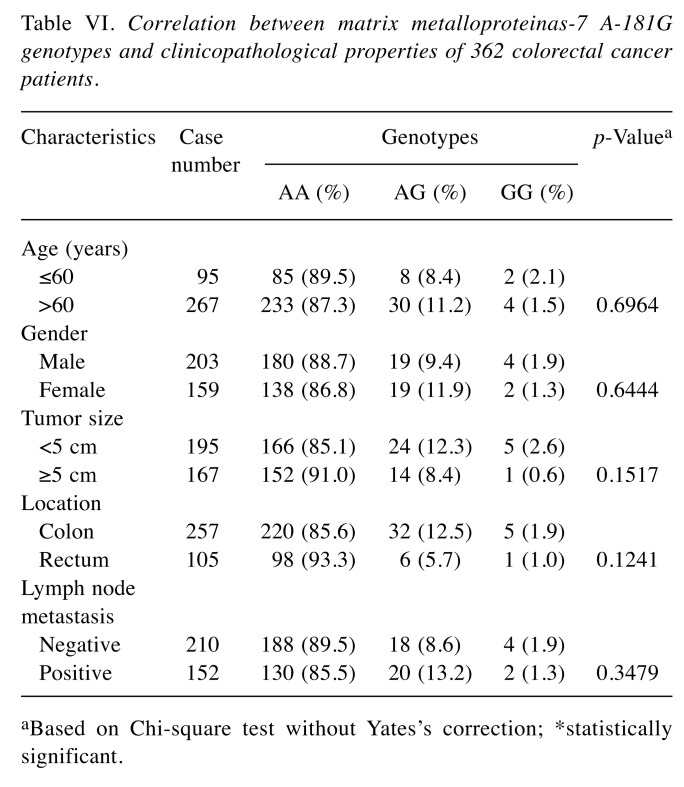
The correlations between genotypes of MMP-7 A-181G and clinicopathological features of 362 CRC patients were further stratified and analyzed in Table VI. No statistically significant correlation was observed between MMP-7 A-181G genotypic distributions and age, gender, tumor size, location or metastasis status (all p>0.05) (Table VI).
Table VI. Correlation between matrix metalloproteinas-7 A-181G genotypes and clinicopathological properties of 362 colorectal cancer patients.
aBased on Chi-square test without Yates’s correction; *statistically significant.
Discussion
Under normal conditions, MMP-7 is in charge of degrading several ECM macromolecules such as casein, type I-V gelatins, fibronectins and proteoglycans (33). There are a few investigations specifically examine the phenotypic role of MMP-7 in CRC carcinogenesis. Firstly, the MMP-7 protein is found to be highly expressed in the luminal surface of dysplastic glands in human CRC patients (33). Secondly, from the clinical practice viewpoint, MMP-7 inhibitors can potentially be applied to control the invasive capacity of cancers (34). Thirdly, MMP-7 was found to be highly expressed in advanced colorectal adenomatous and involved in converting colorectal adenomas into malignant state and facilitating the cancer growth (35). According to the highlights above, it is reasonable that hereditary genomic information coded by various polymorphisms on MMP-7 may determine differential responses and personal susceptibility to CRC in the processes in inflammation, tumor initiation, invasion and metastasis.
In the current study, we firstly examined the contribution of MMP-7 genotypes to CRC susceptibility in Taiwan, where CRC is the highest prevalent cancer in the country for many years. The polymorphisms at the promoter region of the genes are most likely to determine the expression level of it. Thus, in the research design for selection the polymorphic sites, we aimed at investigating the most common examined promoter polymorphism at MMP-7 A-181G. In addition, we have also investigated the genotypes at MMP-7 C-153T, which is also a polymorphic site at promoter region while less examined by genomic scientists. The results showed that the G allele of MMP7 A-181G were non-significantly associated with altered risk to CRC (Tables II and III). There were no variant genotypes at MMP-7 C-153T for Taiwanese (Tables II and III). As far as we are aware, the current study is the first to reveal the genotypic contribution of MMP-7 promoter genotypes to CRC in Taiwan. Different from the previous findings that higher frequency of GG genotype at MMP-7 A-181G among the CRC patients with T3/T4 stage, N1/N2 stage and with lymphovascular invasion (29), we did not find similar correlation between the MMP-7 A-181G genotype and age, gender, tumor size, location or metastasis status (Table VI). This may be due to different target populations investigated, and the small percentages and numbers of those with GG genotypes at MMP-7 A-181G among Taiwanese.
The current findings may encourage genomic scientists to figure out the combinative effects among several genes on CRC and other diseases. Also, the contribution of MMP-7 protein to the ECM alteration during carcinogenesis in complex and should be taken into consideration with other MMPs. Our findings in cancer or clinicopathological parameter association and those of Woo are negative while analyzing only MMP-7 A-181G itself, while the combined MMP7-181A-MMP1-1,607dupG-MMP3-1,171A-MMP12-82A haplotypes were significantly more frequent in CRC patients than in healthy controls (28).
In conclusion, this study investigated the contribution of MMP-7 promoter genotypes and the interaction of them with smoking and alcohol drinking status to determine personal susceptibility to CRC. The genotypes of MMP-7 A-181G and C-153T may play an indirect role in determining personal susceptibility to CRC and the prognosis, the combinative effects of MMP-7 and other genes (such as other MMPs) on CRC susceptibility and prognosis, should be taken into consideration spontaneously in the precision medicine era.
Conflicts of Interest
The Authors declare no conflicts of interest in regard to this study.
Acknowledgements
We appreciate the Tissue-bank of China Medical University Hospital for their excellent technical assistance and all the subjects, doctors, nurses and colleagues. This study was supported mainly by the Taichung Armed Forces General Hospital (106A14) to Dr. Yueh, partially by Taichung Armed Forces General Hospital (106A05) to Dr. Fu, Taichung Veterans General Hospital (TCVGH-CTUST1077701) to Dr. Hung and Dr. Wu, and partially by research grant from Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW107-TDU-B-212-123004).
References
- 1.Douaiher J, Ravipati A, Grams B, Chowdhury S, Alatise O, Are C. Colorectal cancer-global burden, trends, and geographical variations. J Surg Oncol. 2017;115:619–630. doi: 10.1002/jso.24578. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 3.Nagini S. Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol. 2012;4:156–169. doi: 10.4251/wjgo.v4.i7.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jayasurya R, Sathyan KM, Lakshminarayanan K, Abraham T, Nalinakumari KR, Abraham EK, Nair MK, Kannan S. Phenotypic alterations in Rb pathway have more prognostic influence than p53 pathway proteins in oral carcinoma. Mod Pathol. 2005;18:1056–1066. doi: 10.1038/modpathol.3800387. [DOI] [PubMed] [Google Scholar]
- 5.Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer. 2006;42:216–227. doi: 10.1016/j.ejca.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Houlston RS, Tomlinson IP. Polymorphisms and colorectal tumor risk. Gastroenterology. 2001;121:282–301. doi: 10.1053/gast.2001.26265. [DOI] [PubMed] [Google Scholar]
- 7.Rasool S, Rasool V, Naqvi T, Ganai BA, Shah BA. Genetic unraveling of colorectal cancer. Tumour Biol. 2014;35:5067–5082. doi: 10.1007/s13277-014-1713-7. [DOI] [PubMed] [Google Scholar]
- 8.Yueh TC, Chou AK, Gong CL, Fu CK, Pei JS, Wu MH, Tsai CW, Chang WS, Hsiao CL, Yen ST, Li HT, Bau DT. The contribution of excision repair cross-complementing group 1 genotypes to colorectal cancer susceptibility in Taiwan. Anticancer Res. 2017;37:2307–2313. doi: 10.21873/anticanres.11568. [DOI] [PubMed] [Google Scholar]
- 9.Huang CY, Tsai CW, Hsu CM, Chang WS, Shui HA, Bau DT. The significant association of CCND1 genotypes with colorectal cancer in Taiwan. Tumour Biol. 2015;36:6533–6540. doi: 10.1007/s13277-015-3347-9. [DOI] [PubMed] [Google Scholar]
- 10.Yang MD, Tsai CW, Chang WS, Tsou YA, Wu CN, Bau DT. Predictive role of XRCC5/XRCC6 genotypes in digestive system cancers. World J Gastrointest Oncol. 2011;3:175–181. doi: 10.4251/wjgo.v3.i12.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang MD, Tsai RY, Liu CS, Chang CH, Wang HC, Tsou YA, Wang CH, Lin CC, Shyue SK, Bau DT. Association of Caveolin-1 polymorphisms with colorectal cancer susceptibility in Taiwan. World J Gastrointest Oncol. 2010;2:326–331. doi: 10.4251/wjgo.v2.i8.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi MD, Chen JH, Sung HT, Lee JS, Tsai LY, Lin HH. CXCL12-G801A polymorphism modulates risk of colorectal cancer in Taiwan. Arch Med Sci. 2013;9:999–1005. doi: 10.5114/aoms.2013.39211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saarialho-Kere UK, Crouch EC, Parks WC. Matrix metalloproteinase matrilysin is constitutively expressed in adult human exocrine epithelium. J Invest Dermatol. 1995;105:190–196. doi: 10.1111/1523-1747.ep12317104. [DOI] [PubMed] [Google Scholar]
- 14.Jormsjo S, Whatling C, Walter DH, Zeiher AM, Hamsten A, Eriksson P. Allele-specific regulation of matrix metallo-proteinase-7 promoter activity is associated with coronary artery luminal dimensions among hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 2001;21:1834–1839. doi: 10.1161/hq1101.098229. [DOI] [PubMed] [Google Scholar]
- 15.Lu Z, Wang Y, Zhang Q, Zhang X, Wang S, Xie H, Li Y, Jiao B, Zhang J. Association between the functional polymorphism in the matrix metalloproteinase-7 promoter and susceptibility to adult astrocytoma. Brain Res. 2006;1118:6–12. doi: 10.1016/j.brainres.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Jin X, Fang S, Wang R, Li Y, Wang N, Guo W, Wang Y, Wen D, Wei L, Dong Z, Kuang G. The functional polymorphism in the matrix metalloproteinase-7 promoter increases susceptibility to esophageal squamous cell carcinoma, gastric cardiac adenocarcinoma and non-small cell lung carcinoma. Carcinogenesis. 2005;26:1748–1753. doi: 10.1093/carcin/bgi144. [DOI] [PubMed] [Google Scholar]
- 17.Chou AK, Hsiao CL, Shih TC, Wang HC, Tsai CW, Chang WS, Liu LC, Way TD, Chung JG, Bau DT. The contribution of matrix metalloproteinase-7 promoter genotypes in breast cancer in Taiwan. Anticancer Res. 2017;37:4973–4977. doi: 10.21873/anticanres.11908. [DOI] [PubMed] [Google Scholar]
- 18.Malik MA, Sharma KL, Zargar SA, Mittal B. Association of matrix metalloproteinase-7 (-181A>G) polymorphism with risk of esophageal squamous cell carcinoma in Kashmir Valley. Saudi J Gastroenterol. 2011;17:301–306. doi: 10.4103/1319-3767.84480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang WL, Liang WB, Gao LB, Zhou B, Xiao FL, Zhang L. Genetic polymorphisms in Matrix Metalloproteinases -1 and -7 and susceptibility to gastric cancer: an association study and meta-analysis. Iran J Allergy Asthma Immunol. 2013;12:203–210. [PubMed] [Google Scholar]
- 20.Moreno-Ortiz JM, Gutierrez-Angulo M, Partida-Perez M, Peregrina-Sandoval J, Ramirez-Ramirez R, Muniz-Mendoza R, Suarez-Villanueva S, Centeno-Flores M, Maciel-Gutierrez V, Cabrales-Vazquez JE, Ayala-Madrigal ML. Association of MMP7-181A/G and MMP13-77A/G polymorphisms with colorectal cancer in a Mexican population. Genet Mol Res. 2014;13:3537–3544. doi: 10.4238/2014.February.14.1. [DOI] [PubMed] [Google Scholar]
- 21.Sharma KL, Misra S, Kumar A, Mittal B. Higher risk of matrix metalloproteinase (MMP-2, 7, 9) and tissue inhibitor of metalloproteinase (TIMP-2) genetic variants to gallbladder cancer. Liver Int. 2012;32:1278–1286. doi: 10.1111/j.1478-3231.2012.02822.x. [DOI] [PubMed] [Google Scholar]
- 22.Wieczorek E, Reszka E, Wasowicz W, Grzegorczyk A, Konecki T, Sosnowski M, Jablonowski Z. MMP7 and MMP8 genetic polymorphisms in bladder cancer patients. Cent European J Urol. 2014;66:405–410. doi: 10.5173/ceju.2013.04.art3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie B, Zhang Z, Wang H, Chen Z, Wang Y, Liang H, Yang G, Yang X, Zhang H. Genetic polymorphisms in MMP 2, 3, 7, and 9 genes and the susceptibility and clinical outcome of cervical cancer in a Chinese Han population. Tumour Biol. 2016;37:4883–4888. doi: 10.1007/s13277-015-4204-6. [DOI] [PubMed] [Google Scholar]
- 24.Lievre A, Milet J, Carayol J, Le Corre D, Milan C, Pariente A, Nalet B, Lafon J, Faivre J, Bonithon-Kopp C, Olschwang S, Bonaiti-Pellie C, Laurent-Puig P, members of the Ag Genetic polymorphisms of MMP1, MMP3 and MMP7 gene promoter and risk of colorectal adenoma. BMC Cancer. 2006;6:270. doi: 10.1186/1471-2407-6-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao CH, Chang WS, Hu PS, Wu HC, Hsu SW, Liu YF, Liu SP, Hung HS, Bau DT, Tsai CW. The contribution of MMP-7 promoter polymorphisms in renal cell carcinoma. In Vivo. 2017;31:631–635. doi: 10.21873/invivo.11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vairaktaris E, Serefoglou Z, Yapijakis C, Vylliotis A, Nkenke E, Derka S, Vassiliou S, Avgoustidis D, Neukam FW, Patsouris E. High gene expression of matrix metalloproteinase-7 is associated with early stages of oral cancer. Anticancer Res. 2007;27:2493–2498. [PubMed] [Google Scholar]
- 27.Pei JS, Chou AK, Hsu PC, Tsai CW, Chang WS, Wu MF, Wu MH, Hsia TC, Cheng SP, Bau DT. Contribution of matrix metalloproteinase-7 genotypes to the risk of non-solid tumor, childhood leukemia. Anticancer Res. 2017;37:6679–6684. doi: 10.21873/anticanres.12126. [DOI] [PubMed] [Google Scholar]
- 28.Woo M, Park K, Nam J, Kim JC. Clinical implications of matrix metalloproteinase-1, -3, -7, -9, -12, and plasminogen activator inhibitor-1 gene polymorphisms in colorectal cancer. J Gastroenterol Hepatol. 2007;22:1064–1070. doi: 10.1111/j.1440-1746.2006.04424.x. [DOI] [PubMed] [Google Scholar]
- 29.Horvat M, Potocnik U, Repnik K, Kavalar R, Zadnik V, Potrc S, Stabuc B. Single nucleotide polymorphisms in Genes MACC1, RAD18, MMP7 and SDF-1a as prognostic factors in resectable colorectal cancer. Radiol Oncol. 2017;51:151–159. doi: 10.1515/raon-2016-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pei JS, Chang WS, Hsu PC, Hung YW, Cheng SP, Tsai CW, Bau DT, Gong CL. The contribution of MMP-8 promoter genotypes to childhood leukemia. In Vivo. 2017;31:1059–1064. doi: 10.21873/invivo.11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu PS, Chang WS, Chou AK, Hsia NY, Hung YW, Lin CW, Wu CW, Huang CY, Wu MF, Liao CH, Tsai CW, Bau DT, Gong CL. The association of MMP-8 genotypes with Pterygium. In Vivo. 2018;32:41–46. doi: 10.21873/invivo.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen TC, Chang WS, Tsai CW, Chao CY, Lin YT, Hsiao CL, Hsu CL, Chen WC, Hsia TC, Bau DT. The contribution of matrix metalloproteinase-1 promoter genotypes in Taiwan lung cancer risk. Anticancer Res. 2018;38:253–257. doi: 10.21873/anticanres.12215. [DOI] [PubMed] [Google Scholar]
- 33.Yokoyama Y, Grunebach F, Schmidt SM, Heine A, Hantschel M, Stevanovic S, Rammensee HG, Brossart P. Matrilysin (MMP-7) is a novel broadly expressed tumor antigen recognized by antigen-specific T cells. Clin Cancer Res. 2008;14:5503–5511. doi: 10.1158/1078-0432.CCR-07-4041. [DOI] [PubMed] [Google Scholar]
- 34.Edman K, Furber M, Hemsley P, Johansson C, Pairaudeau G, Petersen J, Stocks M, Tervo A, Ward A, Wells E, Wissler L. The discovery of MMP7 inhibitors exploiting a novel selectivity trigger. ChemMedChem. 2011;6:769–773. doi: 10.1002/cmdc.201000550. [DOI] [PubMed] [Google Scholar]
- 35.Qasim BJ, Ali HH, Hussein AG. Immunohistochemical expression of matrix metalloproteinase-7 in human colorectal adenomas using specified automated cellular image analysis system: a clinicopathological study. Saudi J Gastroenterol. 2013;19:23–27. doi: 10.4103/1319-3767.105916. [DOI] [PMC free article] [PubMed] [Google Scholar]